Abstract
Background
Intra-abdominal adhesion is one of the most common complications after abdominal surgery. The efficacy of current treatments for intra-abdominal adhesion is unsatisfactory. In this study, we investigated the effect of gallic acid on the prevention and treatment of intra-abdominal adhesions after abdominal surgery using an intra-abdominal adhesion rat model.
Material/Methods
The experimental rats were randomly divided into the sham operation group, the control group, the chitosan group, and 3 gallic acid groups of different concentrations. All rats except those in the sham operation group received cecal abrasion to induce adhesion. From the first postoperative day, the rats in the gallic acid groups were administered different concentrations of gallic acid in a 2-ml gavage daily. All rats were sacrificed on postoperative day 7, and the degree of intra-abdominal adhesion was evaluated by the naked eye. The amount of collagen deposited between the injured peritoneal tissues was assessed by Sirius red staining. Serum levels of interleukin-6 (IL-6), tumor necrosis factor (TNF-α), and transforming growth factor-β (TGF-β) were measured by ELISA. Western blot was used to detect the level of NF-κB phosphorylation in the injured peritoneal or adhesion tissues of the rats.
Results
Compared with the control group, the scores of intra-abdominal adhesions in the rats treated with larger doses of gallic acid were significantly decreased, and the degree of inflammation and fibrosis was also significantly decreased. Gallic acid significantly reduced IL-6, TNF-α, and TGF-β1 serum levels. NF-κB phosphorylation in the higher gallic acid groups was significantly reduced.
Conclusions
Gallic acid inhibits the formation of postoperative intra-abdominal adhesions in rats by inhibiting the inflammatory reaction and fibrogenesis. Gallic acid is a promising drug for preventing intra-abdominal adhesions.
MeSH Keywords: Fibrosis, Gallic Acid, Inflammation, Tissue Adhesions
Background
Intra-abdominal adhesion is one of the most common complications after abdominal surgery, and the incidence rate is 95–100%. In addition, 15–20% of patients experienced corresponding clinical symptoms caused by adhesion [1,2]. Postoperative intra-abdominal adhesion is the most important cause of postoperative intestinal obstruction, chronic abdominal pain, and female infertility, which may also incur additional medical costs [3,4]. Currently, the treatment of intra-abdominal adhesion is very limited. Adhesions can be released by surgery, but the adhesion caused by secondary surgery is even more detrimental to the patient [5,6]. Therefore, the prevention of postoperative intra-abdominal adhesion is very important. Currently, the main anti-adhesion materials are anti-adhesion membrane, drugs, and medical Biogel, but their efficacy remains unsatisfactory [7,8]. Therefore, identifying new effective anti-adhesion drugs is necessary.
Postoperative intra-abdominal adhesion is a complex process involving many types of cells, cytokines, and multi-step biological processes [9,10]. In simple terms, local trauma in the peritoneum can cause bleeding exudation in the local tissue, thus stimulating the inflammatory reaction. Given the increase in local vascular permeability and exudation of fibrinogen-rich fluid, local blood clots can form a local membrane structure on the wound. Subsequently, the inflammatory response system and the fibrogenesis system play an important role. Cytokines and cells in the inflammatory response stimulate the exudation in the local wound tissue to promote the formation of adhesion. Within 5–7 days after the trauma, the fibrin deposition formation gradually degrades with the activation of the fibrinolytic system. If the fibrin deposition is fully degraded, adhesions will not form. If the fibrinolytic system fails to degrade the fibrin deposition in a timely fashion, adhesion formation will occur [11–13].
Gallic acid is a phenolic complex widely found in natural plants, such as Chinese nutgall, rhubarb, lacquer tree, and some fruits [14,15]. Recent research demonstrated that gallic acid down-regulates the expression of matrix metalloproteinase 2 and matrix metalloproteinase 9 and inhibits tumor growth [16,17]. Gallic acid inhibits the inflammatory reaction by inhibiting activation of the p65-NF-κB and IL-6/STAT3 pathways [16,18,19]. Gallic acid inhibits hepatic fibrosis in the CCL4-induced rat model [20]. In addition, gallic acid also inhibits oxidative stress by increasing the expression of reduced glutathione [21].
The inflammatory response plays a role in promoting the process of intra-abdominal adhesion formation. Oxidative stress may further aggravate the inflammatory response, thereby promoting exudation of fiber and adhesion formation. The up-regulation of the fibrinolytic system and extracellular matrix metalloproteinases inhibits adhesion formation. Gallic acid also inhibits the inflammatory reaction, reduces fiber exudation, suppresses extracellular matrix formation, and inhibits oxidative stress. Thus, gallic acid may be used as a drug to inhibit intra-abdominal adhesions after abdominal surgery. In addition, gallic acid is one of the active ingredients of some traditional Chinese medicines, such as rhubarb and peony, that have been proven to prevent peritoneal adhesion [22–24]. Therefore, we hypothesize that gallic acid has efficacy in inhibiting postoperative intra-abdominal adhesions. In this study, we investigated the preventive effect of gallic acid on the formation of adhesions after abdominal surgery in a rat model of intra-abdominal adhesions.
Material and Methods
Experimental animals and reagents
A total of 48 rats were purchased from the Experimental Animal Center of Xi’an Jiaotong University. These rats weighed between 200 and 250 g. All rats were reared in a room temperature environment (22±2°C) with free access to food and drinking water. All animal experiments were approved by the Animal Ethics Committee of Xi’an Jiaotong University. Gallic acid (Cat# G7384) was purchased from Sigma-Aldrich Co. LLC (St. Louis, MO). Gallic acid was dissolved in saline at different concentrations.
Grouping of the experimental animals
All rats were fasted for 1 day before surgery, and the abdominal hairs of the rats were shaved off. Anesthesia was induced by intraperitoneal injection of phenobarbital sodium (GuideChem, Shanghai, China) at 50 mg/kg. Intra-abdominal adhesion modeling was performed as described in the literature [25]. After successful anesthesia, the rat was placed in the supine position. Skin disinfection was performed 3 times using pyrrolidone iodine, and a 2- to 3-cm incision was created at the middle of the abdomen. Except for the sham operation group, the intra-abdominal adhesion was induced in the rats of all other groups by the peritoneal abrasion method. Briefly, the cecum was removed, and the cecum wall was abraded with dry sterile gauze 30 times. Then, the cecum was placed back into the abdominal cavity. The contralateral peritoneal wall of the cecum was abraded 30 times in the same manner, resulting in a small amount of bleeding exudation on the cecum wall and the peritoneal wall. Then, the position of the abraded cecum was adjusted such that the abraded area of the cecum was facing the abraded peritoneal wall. For the rats in the chitosan group, 2 ml of chitosan gel (No. 2640172, Hairun Biotechnology Co. LTD, Hunan, China) was applied to the cecum and peritoneal walls before closing the abdomen. After disinfecting the skin again, the abdominal cavity was closed by an intermittent suture in 2 layers. Within 1 week after the operation, rats in the gallic acid group were intragastrically administered with 2 ml of the gallic acid solution at different concentrations daily. The intragastric doses were 50 mg/kg for the GA1 group, 100 mg/kg for the GA2 group, and 150 mg/kg for the GA3 group. The rats in the sham operation group, the control group, and the chitosan group were administered 2 ml of saline daily by gavage.
General adhesion assessment for the specimens
One week after the operation, all rats were anesthetized by intraperitoneal injection again and then sacrificed. A U-shaped incision was created for laparotomy. The degree of intra-abdominal adhesion was evaluated by 2 experimentalists unaware of the experimental design, based on the adhesion score system by Nair et al. [26,27] (Supplementary Table 1). Pathological specimens and blood samples were collected from all rats. Specifically, the cecum and abdominal tissues were taken from the most severe adhesion sites of the rats with adhesion, whereas the cecal lesions and corresponding peritoneal tissues were collected from the non-adhesion tissues.
Pathological assessment
After soaking in formalin for 24 h, the pathological specimen was used to prepare continuous pathological sections at a thickness of 4 μm with paraffin embedding. Four incisions were randomly selected from each pathological tissue for hematoxylin and eosin (HE) staining, and inflammation and fibrosis were observed under light microscopy. The evaluation was based on the scoring system used in previous studies [28,29]. The score of the inflammatory response was evaluated as follows (Supplementary Table 2): 0 for cases with no inflammatory cells; 1 for cases with observable macrophages, lymphocytes, and plasma cells; 2 for cases with macrophages, plasma cells, eosinophils, and neutrophils; and 3 for cases with inflammatory cell infiltration and microabscess formation. The fibrogenesis score was evaluated as follows: 0 for cases with no fibrogenesis; 1 for cases with a mild amount of visible fibrogenesis; 2 for cases with a moderate amount of visible fibrogenesis; and 3 for cases with a severe amount of visible fibrogenesis. All scores were evaluated by 2 pathologists from the First Affiliated Hospital of Xi’an Jiaotong University. Five fields under high-magnification microscopy were randomly selected from each pathological section for scoring, and the average score of each rat was used as the final score.
Sirius red picric acid staining
More than 8 pathological sections were randomly selected for Sirius red picric acid staining using the experimental method described in the literature [25]. Staining was performed with 0.1% Sirius red picric acid (Direct Red 80; Sigma-Aldrich, St. Louis, MO), followed by counterstaining with hematoxylin. Five high-magnification fields were randomly selected from each pathological section to measure the width of collagen tissue using ImagePro Plus 5.0 software (Leica Qwin. Plus, Leica Microsystem Imaging Solutions Ltd., Cambridge, UK). The average thickness of each tissue adhesive area was used as the thickness of each tissue adhesive area.
Immunohistochemistry
Immunohistochemistry was performed using the SABC kit (Maxim, Fuzhou, China). All experimental procedures were performed following the operation manual’s instructions. Briefly, 3 pathological sections of each indicator were randomly selected for staining. The sections were incubated with the α-SMA primary antibody (GB13044, Servicebio, Hubei, China, 1: 200) at 4°C overnight. Subsequently, the sections were incubated with a ubiquitinated secondary antibody for 30 min at room temperature, followed by streptavidin peroxidase for 30 min at room temperature. Then, DAB development, hematoxylin counterstaining, further dehydration, and mounting were performed. Five high-magnification fields were randomly selected from each section with HE staining for immunohistochemistry scoring to obtain the average of all the sections of each tissue as the final score. Among them, 0 was scored for tissues with no expression, 1 was scored for tissues with weakly positive expression, 2 was scored for tissues with positive expression, 3 was scored for tissues with strongly positive expression, and 4 was scored for tissues with extremely abundant expression.
Western blot
Tissue protein extraction was performed using the RIPA Protein Extraction Kit (Thermo Fisher Scientific, USA) following the operation manual. Western blot analysis was performed as described in the literature [30]. The extracted proteins were subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred to a PVDF membrane. After blocking with 5% milk for 1 h, the membrane was incubated with the primary antibody at 4°C overnight. The primary antibodies included anti-NF-κB (catalog no. Ab16502, Abcam, Cambridge, UK, 1: 1000 dilution), anti-NF-κB phospho S536 (catalog number ab86299, Abcam, Cambridge, 1: 1000 dilution), and anti-B-Actin (sc-47778, Santa Cruz Biotechnology, 1: 1000 dilution). After incubation, the PVDF membrane was rinsed and then incubated with the secondary antibody for 1 h at room temperature. Then, protein expression was detected using a chemiluminescence detection system (Millipore, Billerica, MA).
ELISA
The collected blood samples were centrifuged at 3000 rpm for 30 min to obtain the serum of each animal, which was stored at –20°C for later use. ELISA was performed according to the protocol of each kit. The detected indicators included IL-1β (EK30B1/2, MultiSciences (Lianke) Biotech Co., Ltd, Hangzhou, China), TNF-α (88-7340, Affymetrix Biotech Co., Ltd, Shanghai, China), and TGF-β1 (70-EK3812/2, MultiSciences (Lianke) Biotech Co., Ltd, Hangzhou, China).
Statistical methods
All data in this study were analyzed using SPSS 18.0 (Chicago, IL), and all data are expressed as the mean ± standard deviation. The comparison of data with normal distributions between groups was performed using ANOVA, and data without normal distributions were analyzed using the Kruskal-Wallis test. Count data were processed using Fisher’s exact test. A difference with a P-value less than 0.05 was considered statistically significant.
Results
Gallic acid reduces the intra-abdominal adhesion score and the incidence of adhesion
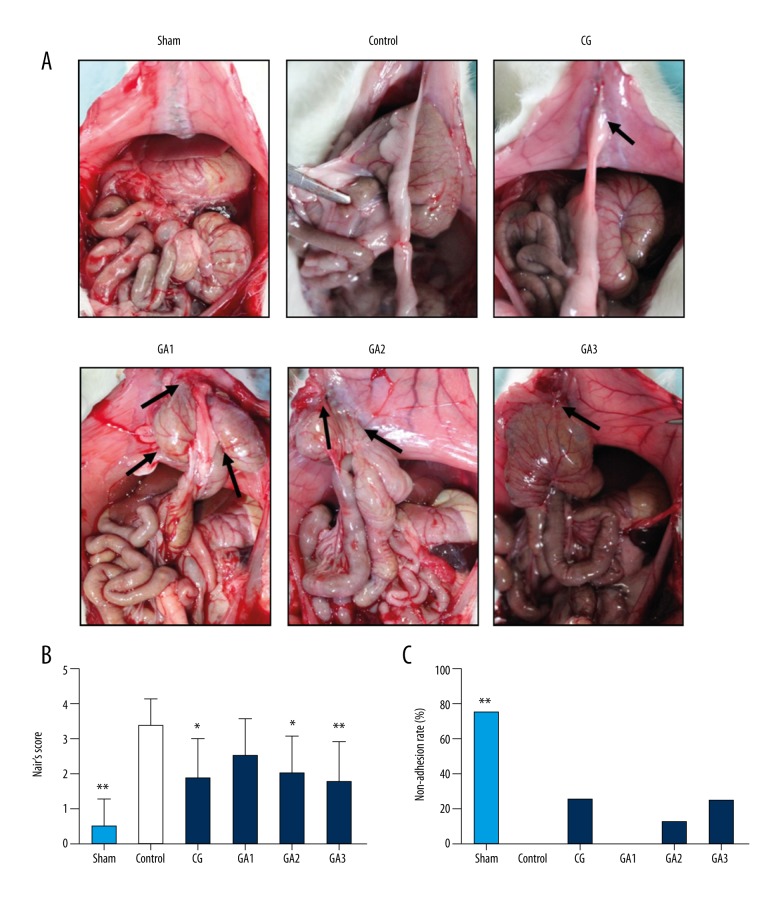
A total of 48 rats completed the experiment. No deaths or infected wounds were noted during the experiment. At the 7th postoperative day, intra-abdominal adhesion was assessed based on the Nair scoring system, and the results are provided in Figure 1. Except for the low-dose group, Gallic acid-treated rats exhibited significantly reduced adhesion scores (Figure 1A, 1B, P<0.05), especially rats in the high-dose group. However, the preventive effect of gallic acid exhibited no significant difference compared with the chitosan group. The non-adhesion rate was the highest in the sham group, following by the chitosan and higher gallic acid-treated groups, and the adhesion rate was highest in the control and lowest in the gallic acid-treated group (Figure 1C, P<0.05).
Figure 1.
Higher doses of gallic acid attenuated postoperative intra-abdominal adhesion in rats (n=8). (A) In the sham operation group, intra-abdominal adhesions occurred in only a few rats. All rats in the control group exhibited severe adhesions. Two rats in the chitosan (CG) group had no adhesions, and the remaining rats exhibited relatively mild adhesions. All rats in the gallic acid 1 (GA1) group had adhesions, but the adhesions were lighter than those in the control group. The adhesions of the rats in the gallic acid 2 and 3 groups (GA2, GA3) were significantly lighter than those in the control group. The black arrows indicate the adhesive area. (B) The Nair score for each group (compared with the control group, * P<0.05 and ** P<0.01, abnormal distribution, Kruskal-Wallis test). (C) The non-adhesion rate of each group (compared with the control group, ** P<0.01, Fisher’s exact test).
Gallic acid inhibits the inflammatory response
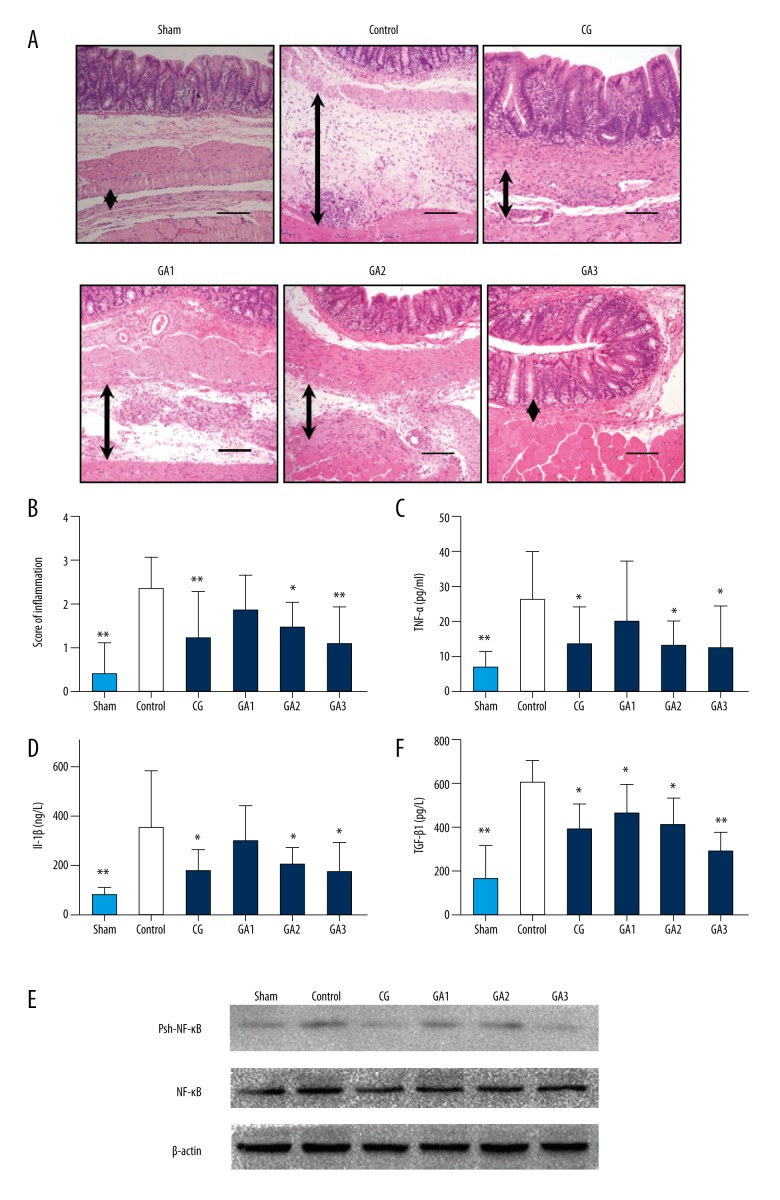
We assessed the changes in the inflammation-related indicators in each group. The inflammation scores of the HE-stained sections in the middle- and high-dose gallic acid-treated groups were significantly reduced compared with the control group (Figures 2A, 2B, and Supplementary Figure 1A). The serum TNF-α, TGF-β, and IL-1β expression levels in each group were further detected by ELISA. The result demonstrated that the level of TNF-α and IL-1β was significantly reduced in the gallic acid-treated group when compared with the control group and its effect was concentration dependent (Figure 2C, 2D; P<0.05). The TGF-β level in the higher gallic acid-treated group was significance decreased compared to the control group (Figure 2E; P<0.05). Phosphorylated NF-κB and non-phosphorylated NF-κB levels were detected by Western blot in each group, and phosphorylated NF-κB levels were significantly reduced in the gallic acid groups compared with the control group (Figure 2F; P<0.05).
Figure 2.
Higher doses of gallic acid treatment can reduce the inflammation in the adhesion tissue in the rat model on the 7th postoperative day (n=8; compared with the control group, * P<0.05 and ** P<0.01). (A) HE staining of each group at 100× magnification. The black arrows indicate tissue with adhesion. (B) The inflammatory score of each group based on HE staining (compared with the control group, * P<0.05 and ** P<0.01, abnormal distribution, Kruskal-Wallis test). (C) TNF-α expression in each group (compared with the control group, * P<0.05 and ** P<0.01, abnormal distribution, Kruskal-Wallis test). (D) Expression of IL-1β in each group (compared with the control group, * P<0.05 and ** P<0.01, abnormal distribution, Kruskal-Wallis test). (E) TGF-β expression in each group (compared with the control group, * P<0.05 and ** P<0.01, abnormal distribution, Kruskal-Wallis test). (F) Western blot detection of NF-kB and phosphorylated NF-κB expression in each group.
Gallic acid inhibited the fibrosis of the adhesion tissue
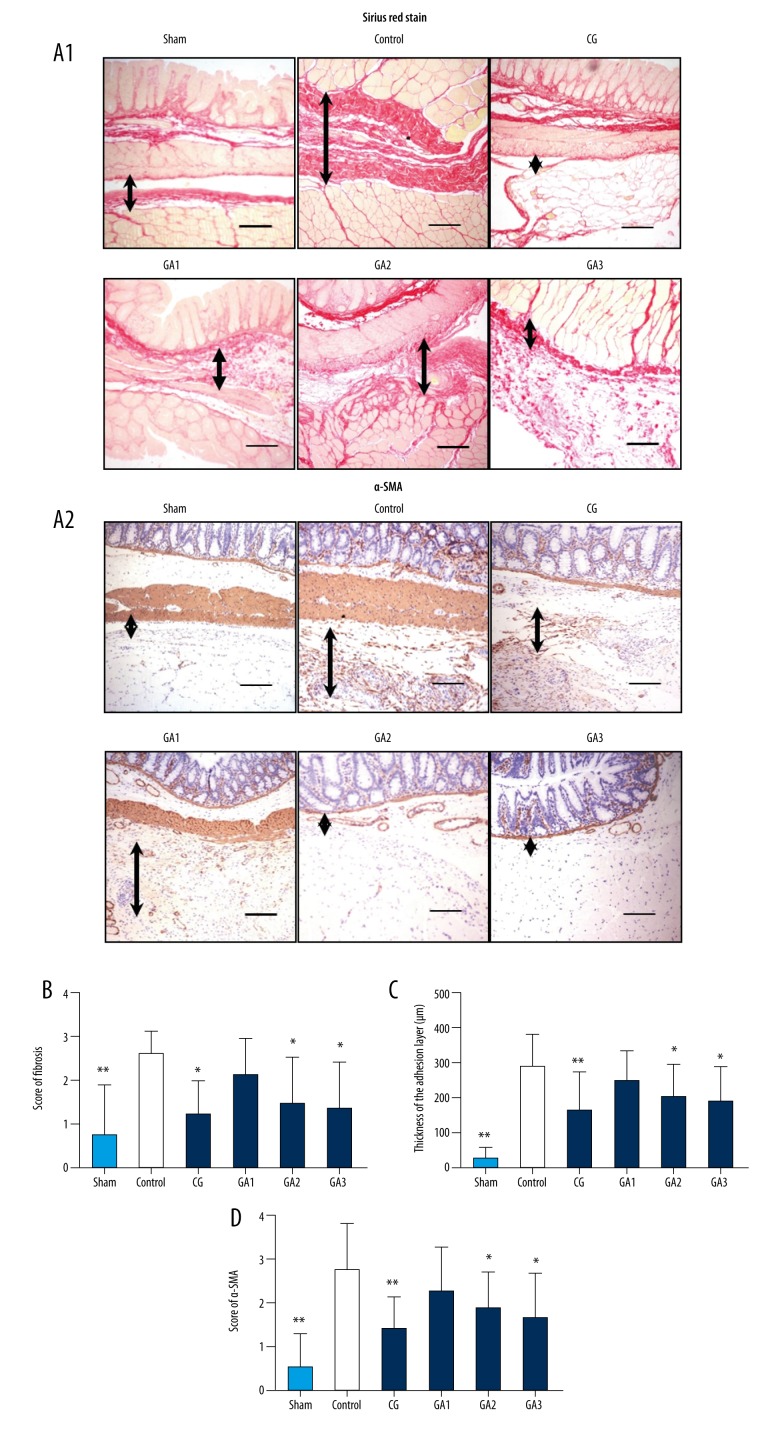
To assess the degree of fibrosis in the adhesion tissue, histological fibrosis scoring, Sirius red staining, and α-SMA immunohistochemical staining were performed on adhesion tissues. The fibrosis scores of the tissues suggested that the fibrosis scores of the rats in the 2 higher-dose gallic acid groups were significantly reduced compared with the control group, and its effect was concentration-dependent (Figures 3B and Supplementary Figure 1B; P<0.05). Sirius red staining results suggested that the fibrin thickness in the 2 higher-dose gallic acid groups were significantly reduced compared with the control group (Figures 3A1, 3C and Supplementary Figure 1B; P<0.05). The results of α-SMA immunohistochemistry suggested that the α-SMA protein expression levels in the 2 higher-dose gallic acid groups were significantly reduced compared with the control group (Figures 3A2, 3D, and Supplementary Figure 1C, P<0.05).
Figure 3.
Higher doses of gallic acid treatment attenuated the collagen deposition of the adhesion tissue in the rat model on the 7th postoperative day (n=8; compared with the control group, * P<0.05 and ** P<0.01). (A) Sirius red picric acid staining and α-SMA staining of each group. (A1) Sirius red picric acid staining of each group at 100× magnification, and the black arrows indicate the tissue in the adhesion area. (A2) α-SMA staining of each group at 100× magnification, and the black arrows indicate the tissue in the adhesion area. (B) The fibrosis score of each group (compared with the control group, * P<0.05 and ** P<0.01, abnormal distribution, Kruskal-Wallis test). (C) Adhesion thickness in each group as assessed by Sirius red picric acid staining (compared with the control group, * P<0.05 and ** P<0.01, abnormal distribution, Kruskal-Wallis test). (D) α-SMA staining scores of each group (compared with the control group, * P<0.05 and ** P<0.01, abnormal distribution, Kruskal-Wallis test).
Discussion
Intra-abdominal adhesion is one of the most common complications after abdominal surgery. Currently, the prevention and treatment of intra-abdominal adhesion remains unsatisfactory, and no standard is available for the prevention and treatment of intra-abdominal adhesion [31,32]. Gallic acid is an active ingredient of some traditional Chinese medicines, including rhubarb and peony. Previous studies had demonstrated that gallic acid exhibits anti-inflammation, anti-oxidative stress, anti-cancer, anti-diabetes, and anti-fibrin formation properties [33,34]. As an anti-inflammatory mechanism, gallic acid can inhibit the inflammatory reaction through activation of the p65-NF-κB and IL-6/STAT3 pathways, and these 2 pathways also play an important role in the formation of intra-abdominal adhesions [16]. In this study, we demonstrated that higher doses of gallic acid effectively inhibited the formation of intra-abdominal adhesions in a rat model, and its effect was concentration-dependent. The mechanism may be related to inhibition of the inflammatory reaction and fiber formation.
The main mechanism for the formation of intra-abdominal adhesions is that the inflammatory reaction stimulates the exudation of fibrinogen-rich fluid. If the fibrin cannot be immediately dissolved by the fibrinolytic system, an intra-abdominal adhesion will form, and the oxidative stress in this process will aggravate the inflammation [35,36]. In the present study, inflammation-related indicators were tested based on HE staining scoring and the reduction in the inflammatory cytokines TNF-α, TGF-β, and IL-1β. The expression level of NF-κB was further detected by Western blot analysis, and the results suggested that the expression level of phosphorylated NF-κB was significantly reduced in the 2 higher-dose gallic acid-treated groups. The mechanism by which gallic acid reduces the inflammatory reaction may involve suppressing the inflammatory reaction by decreasing the expression of TNF-α, TGF-β, and IL-1β, and inhibiting the expression of phosphorylated NF-κB.
The fibrinolytic system plays an important role in the formation of intra-abdominal adhesions. The fibrinolytic system is activated after the exudation of the fiber-rich fluid, mediating a dynamic equilibrium between the formation and dissolution of the fibers. If the fibrinolytic system functions properly within 5–7 postoperative days, adhesion formation can be inhibited [37,38]. Previous studies demonstrated that gallic acid inhibits MMP-9 and MMP-2 activity and hepatic fibrosis in rats [17,20]. In the present study, the adhesion thickness, the fibrosis score, and the α-SMA levels in the groups treated with higher doses of gallic acid were significantly reduced compared with the control group, indicating that gallic acid reduces fibrosis in the intra-abdominal adhesion and thus inhibits the intra-abdominal adhesion.
In this study, we confirmed that gallic acid can inhibit the formation of abdominal intra-abdominal adhesion in a rat model; however, the clinical application of gallic acid requires further investigation. First, the toxicity of gallic acid is still not fully elucidated and awaits further study [39,40]. Second, this study also confirmed that the inhibitory effect of gallic acid on intra-abdominal adhesions was not significantly enhanced compared with chitosan, and its effect is limited. However, gallic acid is one of the active ingredients in many traditional Chinese medicines [21,41], so this study also provided a theoretical basis for the mechanism of these traditional Chinese medicines, such as rhubarb, in inhibiting the formation of intra-abdominal adhesions.
Conclusions
Gallic acid inhibited intra-abdominal adhesion formation in a rat model by inhibiting the inflammatory reaction and fibrosis. Gallic acid may be a promising drug for preventing intra-abdominal adhesion. As a possible mechanism, gallic acid may inhibit the inflammatory response by reducing TNF-α, TGF-β, and IL-1β expression, and by inhibiting the expression of phosphorylated NF-κB.
Supplementary Files
Supplementary Table 1.
Nair et al. scoring system.
| 0 | No adhesions |
| 1 | Between viscera or between visceral viscus and abdominal wall (one band) |
| 2 | Between viscera or between visceral viscus and abdominal wall (two bands) |
| 3 | Between viscera or between visceral viscus and abdominal wall (more than two bands) or multiple intestinal adhesions without adhesion to the abdominal wall |
| 4 | Adhesion of the viscera directly to the abdominal wall (number of bands or size not important) |
Supplementary Table 2.
Histopathologic criteria for inflammatory scaling.
| Score | Degree of fibrosis | Degree of inflammation |
|---|---|---|
| 0 | None | No inflammation |
| 1 | Mild | Giant cells, lymphocytes, and plasma cells |
| 2 | Moderate | Giant cells, plasma cells, eosinophils, and neutrophils |
| 3 | Severe | Inflammatory cell infiltration and microabscess formation |
HE, Sirius red picric acid, and α-SMA staining at 200× magnification, and the black arrows indicate the tissues in the adhesive area. (A) HE staining. (B) Sirius red picric acid staining. (C) α-SMA staining.
Footnotes
Source of support: This study was supported by the National Natural Science Foundation of China (No. 81770521), the Scientific and Technological Development Research Project Foundation of Shaanxi Province (No. 2016SF-157and 2016SF-121), and the Joint Foundation of the First Affiliated Hospital of Xi’an Jiaotong University and Buchang Group (No. BC2017-10)
References
- 1.Gomel V, Koninckx PR. Microsurgical principles and postoperative adhesions: Lessons from the past. Fertil Steril. 2016;106(5):1025–31. doi: 10.1016/j.fertnstert.2016.08.040. [DOI] [PubMed] [Google Scholar]
- 2.Ouaïssi M, Gaujoux S, Veyrie N, et al. Post-operative adhesions after digestive surgery: Their incidence and prevention: Review of the literature. J Visc Surg. 2012;149:e104–14. doi: 10.1016/j.jviscsurg.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 3.van den Beukel BA, de Ree R, van Leuven S, et al. Surgical treatment of adhesion-related chronic abdominal and pelvic pain after gynaecological and general surgery: A systematic review and meta-analysis. Hum Reprod Update. 2017;23:276–88. doi: 10.1093/humupd/dmx004. [DOI] [PubMed] [Google Scholar]
- 4.Arung W, Meurisse M, Detry O. Pathophysiology and prevention of postoperative peritoneal adhesions. World J Gastroenterol. 2011;17:4545–53. doi: 10.3748/wjg.v17.i41.4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahimi VB, Shirazinia R, Fereydouni N, et al. Comparison of honey and dextrose solution on post-operative peritoneal adhesion in rat model. Biomed Pharmacother. 2017;92:849–55. doi: 10.1016/j.biopha.2017.05.114. [DOI] [PubMed] [Google Scholar]
- 6.Lin LX, Yuan F, Zhang HH, et al. Evaluation of surgical anti-adhesion products to reduce postsurgical intra-abdominal adhesion formation in a rat model. PLoS One. 2017;12:e0172088. doi: 10.1371/journal.pone.0172088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, Zhu J, He T, et al. Prevention of intra-abdominal adhesion using electrospun PEG/PLGA nanofibrous membranes. Mater Sci Eng C Mater Biol Appl. 2017;78:988–97. doi: 10.1016/j.msec.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Ren G, Zhang W. Reduction of abdominal adhesions with elecrospun fiber membranes in rat models. J Invest Surg. 2017 doi: 10.1080/08941939.2017.1310961. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Hasdemir PS, Ozkut M, Guvenal T, et al. Effect of pirfenidone on vascular proliferation, inflammation and fibrosis in an abdominal adhesion rat model. J Invest Surg. 2017;30:26–32. doi: 10.1080/08941939.2016.1215578. [DOI] [PubMed] [Google Scholar]
- 10.Vipond MN, Whawell SA, Thompson JN, Dudley HA. Peritoneal fibrinolytic activity and intra-abdominal adhesions. Lancet. 1990;335:1120–22. doi: 10.1016/0140-6736(90)91125-t. [DOI] [PubMed] [Google Scholar]
- 11.Arung W, Drion P, Detry O. Sepramesh and postoperative peritoneal adhesions in a rat model. Acta Chir Belg. 2016;116:357–61. doi: 10.1080/00015458.2016.1181322. [DOI] [PubMed] [Google Scholar]
- 12.Bello-Guerrero JA, Cruz-Santiago CA, Luna-Martínez J. Pirfenidone vs. sodium hyaluronate/carboxymethylcellulose as prevention of the formation of intra-abdominal adhesions after colonic surgery. A randomized study in an experimental model. Cir Esp. 2016;94:31–37. doi: 10.1016/j.ciresp.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Beyene RT, Kavalukas SL, Barbul A. Intra-abdominal adhesions: Anatomy, physiology, pathophysiology, and treatment. Curr Probl Surg. 2015;52:271–319. doi: 10.1067/j.cpsurg.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Ryu Y, Jin L, Kee HJ, et al. Gallic acid prevents isoproterenol-induced cardiac hypertrophy and fibrosis through regulation of JNK2 signaling and Smad3 binding activity. Sci Rep. 2016;6:34790. doi: 10.1038/srep34790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pang JS, Yen JH, Wu HT, et al. Gallic acid inhibited matrix invasion and AP-1/ETS-1-Mediated MMP-1 transcription in human nasopharyngeal carcinoma cells. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18071354. pii: E1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen YJ, Lee YC, Huang CH, Chang LS. Gallic acid-capped gold nanoparticles inhibit EGF-induced MMP-9 expression through suppression of p300 stabilization and NFκB/c-Jun activation in breast cancer MDA-MB-231 cells. Toxicol Appl Pharmacol. 2016;310:98–107. doi: 10.1016/j.taap.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Chen YJ, Lin KN, Jhang LM, et al. Gallic acid abolishes the EGFR/Src/Akt/Erk-mediated expression of matrix metalloproteinase-9 in MCF-7 breast cancer cells. Chem Biol Interact. 2016;252:131–40. doi: 10.1016/j.cbi.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 18.Kuo CL, Lai KC, Ma YS, et al. Gallic acid inhibits migration and invasion of SCC-4 human oral cancer cells through actions of NF-κB, Ras and matrix metalloproteinase-2 and -9. Oncol Rep. 2014;32:355–61. doi: 10.3892/or.2014.3209. [DOI] [PubMed] [Google Scholar]
- 19.Dell’agli M, Galli GV, Bulgari M, et al. Ellagitannins of the fruit rind of pomegranate (Punica granatum) antagonize in vitro the host inflammatory response mechanisms involved in the onset of malaria. Malar J. 2010;9:208. doi: 10.1186/1475-2875-9-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Tang L, White J, Fang J. Inhibitory effect of gallic acid on CCl4-mediated liver fibrosis in mice. Cell Biochem Biophys. 2014;69:21–26. doi: 10.1007/s12013-013-9761-y. [DOI] [PubMed] [Google Scholar]
- 21.Park WH. Gallic acid induces HeLa cell death via increasing GSH depletion rather than ROS levels. Oncol Rep. 2017;37:1277–83. doi: 10.3892/or.2016.5335. [DOI] [PubMed] [Google Scholar]
- 22.Zhou C, Jia P, Jiang Z, et al. Preventive effects of the intestine function recovery decoction, a traditional Chinese medicine, on postoperative intra-abdominal adhesion formation in a rat model. Evid Based Complement Alternat Med. 2016;2016:1621894. doi: 10.1155/2016/1621894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu C. A C8-Modified Graphene@mSiO2 composites based method for quantification of gallic acid in rat plasma after oral administration of changtai granule and its application to pharmacokinetics. Biol Pharm Bull. 2017;40(7):1021–28. doi: 10.1248/bpb.b17-00015. [DOI] [PubMed] [Google Scholar]
- 24.D’Ignazio L, Batie M, Rocha S. Hypoxia and inflammation in cancer, focus on HIF and NF-κB. Biomedicines. 2017;5:1–15. doi: 10.3390/biomedicines5020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei G, Zhou C, Wang G, et al. Keratinocyte growth factor combined with a sodium hyaluronate gel inhibits postoperative intra-abdominal adhesions. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17101611. pii: E1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozerhan IH, Urkan M, Meral UM, et al. Comparison of the effects of Mitomycin-C and sodium hyaluronate/carboxymethylcellulose [NH/CMC] (Seprafilm) on abdominal adhesions. Springerplus. 2016;5:846. doi: 10.1186/s40064-016-2359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nair SK, Bhat IK, Aurora AL. Role of proteolytic enzyme in the prevention of postoperative intraperitoneal adhesions. Arch Surg. 1974;108:849–53. doi: 10.1001/archsurg.1974.01350300081019. [DOI] [PubMed] [Google Scholar]
- 28.Du MH, Luo HM, Tian YJ, et al. Electroacupuncture ST36 prevents postoperative intra-abdominal adhesions formation. J Surg Res. 2015;195:89–98. doi: 10.1016/j.jss.2014.12.043. [DOI] [PubMed] [Google Scholar]
- 29.Parsaei P, Karimi M, Asadi SY, Rafieian-Kopaei M. Bioactive components and preventive effect of green tea (Camellia sinensis) extract on post-laparotomy intra-abdominal adhesion in rats. Int J Surg. 2013;11:811–15. doi: 10.1016/j.ijsu.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 30.Wei G, Chen X, Wang G, et al. Inhibition of cyclooxygenase-2 prevents intra-abdominal adhesions by decreasing activity of peritoneal fibroblasts. Drug Des Devel Ther. 2015;9:3083–98. doi: 10.2147/DDDT.S80221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang H, Song Y, Li Z, et al. Evaluation of ligustrazine on the prevention of experimentally induced abdominal adhesions in rats. Int J Surg. 2015;21:115–21. doi: 10.1016/j.ijsu.2015.06.081. [DOI] [PubMed] [Google Scholar]
- 32.Liu Z, Cheng S, Gu C, et al. Effect of hydrogen-rich saline on postoperative intra-abdominal adhesion bands formation in mice. Med Sci Monit. 2017;23:5363–73. doi: 10.12659/MSM.904669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pandurangan AK, Mohebali N, Esa NM, et al. Gallic acid suppresses inflammation in dextran sodium sulfate-induced colitis in mice: Possible mechanisms. Int Immunopharmacol. 2015;28:1034–43. doi: 10.1016/j.intimp.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 34.An X, Zhou A, Yang Y, et al. Protective effects of gallic acid against NiSO4-induced toxicity through down-regulation of the Ras/ERK signaling pathway in Beas-2B Cells. Med Sci Monit. 2016;22:3446–54. doi: 10.12659/MSM.900460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schnüriger B, Barmparas G, Branco BC, et al. Prevention of postoperative peritoneal adhesions: A review of the literature. Am J Surg. 2011;201:111–21. doi: 10.1016/j.amjsurg.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 36.Kamel RM. Prevention of postoperative peritoneal adhesions. Eur J Obstet Gynecol Reprod Biol. 2010;150:111–18. doi: 10.1016/j.ejogrb.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Diamond MP, El-Hammady E, Wang R, Saed G. Regulation of matrix metalloproteinase-1 and tissue inhibitor of matrix metalloproteinase-1 by dichloroacetic acid in human fibroblasts from normal peritoneum and adhesions. Fertil Steril. 2004;81:185–90. doi: 10.1016/j.fertnstert.2003.05.006. [DOI] [PubMed] [Google Scholar]
- 38.Imai A, Suzuki N. Topical non-barrier agents for postoperative adhesion prevention in animal models. Eur J Obstet Gynecol Reprod Biol. 2010;149:131–35. doi: 10.1016/j.ejogrb.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 39.Verma S, Singh A, Mishra A. Gallic acid: molecular rival of cancer. Environ Toxicol Pharmacol. 2013;35:473–85. doi: 10.1016/j.etap.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 40.Lee W, Lee SY, Son YJ, Yun JM. Gallic acid decreases inflammatory cytokine secretion through histone acetyltransferase/histone deacetylase regulation in high glucose-induced human monocytes. J Med Food. 2015;18:793–801. doi: 10.1089/jmf.2014.3342. [DOI] [PubMed] [Google Scholar]
- 41.Yang YH, Wang Z, Zheng J, Wang R. Protective effects of gallic acid against spinal cord injury-induced oxidative stress. Mol Med Rep. 2015;12:3017–24. doi: 10.3892/mmr.2015.3738. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
Nair et al. scoring system.
| 0 | No adhesions |
| 1 | Between viscera or between visceral viscus and abdominal wall (one band) |
| 2 | Between viscera or between visceral viscus and abdominal wall (two bands) |
| 3 | Between viscera or between visceral viscus and abdominal wall (more than two bands) or multiple intestinal adhesions without adhesion to the abdominal wall |
| 4 | Adhesion of the viscera directly to the abdominal wall (number of bands or size not important) |
Supplementary Table 2.
Histopathologic criteria for inflammatory scaling.
| Score | Degree of fibrosis | Degree of inflammation |
|---|---|---|
| 0 | None | No inflammation |
| 1 | Mild | Giant cells, lymphocytes, and plasma cells |
| 2 | Moderate | Giant cells, plasma cells, eosinophils, and neutrophils |
| 3 | Severe | Inflammatory cell infiltration and microabscess formation |
HE, Sirius red picric acid, and α-SMA staining at 200× magnification, and the black arrows indicate the tissues in the adhesive area. (A) HE staining. (B) Sirius red picric acid staining. (C) α-SMA staining.