Abstract
Neurons that endocytose the human immunodeficiency virus-1 (HIV) protein gp120 exhibit neurite retraction and activation of caspase-3, suggesting that the endocytic process may be crucial for gp120-mediated neuronal injury. The goal of this study is to demonstrate that internalization and accumulation of gp120 play a role in its neurotoxic effects. In mammalian cells, endocytosis is primarily a dynamin-dependent process. To establish whether gp120 is endocytosed in a dynamin-dependent manner, we used fibroblasts in which deletion of dynamins was induced by tamoxifen. We observed a robust reduction of intracellular gp120 immunoreactivity in tamoxifen-treated cells. To examine whether endocytosis of gp120 is crucial for its neurotoxic effect, we blocked gp120 internalization into primary rat cortical neurons by dynasore, an inhibitor of the dynamin GTPase activity. We found that dynasore blocks both gp120 internalization and neurotoxicity. We then utilized gp120-loaded mesoporous silica nanoparticles to deliver gp120 intracellularly. We established that once internalized, gp120 is neurotoxic regardless of chemokine receptor activation. Our data suggest that dynamin-dependent endocytosis of gp120 is critical for its neurotoxicity.
Keywords: CXCR4, Tat, pErk, Dynasore, Mesoporous silica nanoparticles
Introduction
Human immunodeficiency virus-1 (HIV)-associated neurocognitive disorders (HAND) persist despite the use of combined active antiretroviral therapy (Gelman 2015). HAND subjects may exhibit axonal injury and loss of synapses (Ellis et al. 2007; Spudich and Gonzalez-Scarano 2012). Neurite pruning is caused by the HIV glycoprotein gp120 even in the absence of productive HIV infection. In fact, transgenic mice overexpressing gp120 (Toggas et al. 1994; Toggas and Mucke 1996; Lee et al. 2013) or rats injected with gp120 (Bagetta et al. 1996; Bansal et al. 2000; Nosheny et al. 2004) exhibit widespread neuronal injury. Therefore, it has been suggested that gp120, alone or in combination with other viral proteins or host factors, may be a crucial pathological agent causing direct neuronal cell death. Understanding the detailed mechanisms of gp120-mediated neurotoxicity will ultimately help discover new adjunct therapies for HAND.
Gp120 binds the chemokine co-receptors CXCR4 or CCR5 (Herbein et al. 1998; Rizzuto et al. 1998; Misse et al. 1999), which in turn activate intracellular signals (Davis et al. 1997; Meucci et al. 1998; Kaul and Lipton 1999) that could impair neuronal survival. However, gp120 is also detected inside both central and peripheral neurons (Bachis et al. 2003; Berth et al. 2015). In neurons, gp120 forms a vesicular complex with mannose-binding lectin (Teodorof et al. 2014), binds to microtubules (Avdoshina et al. 2016a), and undergoes anterograde or retrograde axonal trafficking (Bachis et al. 2006; Ahmed et al. 2009; Teodorof et al. 2014). Endocytosed gp120 impairs mitochondrial transport and dynamics (Avdoshina et al. 2016b), and consequently it negatively influences energy distribution within synapses. Thus, revealing the mechanism through which gp120 is endocytosed and the consequences of this internalized gp120, could lead to a new treatment for gp120-mediated neurotoxicity.
Gp120 binds to CXCR4 and CCR5 by the third variable region (V3) (Huang et al. 2005). These chemokine receptors are G protein-coupled receptors (GPCRs) that undergo constitutive, as well as ligand-mediated endocytosis (Amara et al. 1997; Signoret et al. 1997; Tarasova et al. 1998). GPCR internalization is a multiple step process which includes kinase-mediated phosphorylation and β-arrestin binding (Orsini et al. 1999), and G protein uncoupling and formation of coated vesicles around membranes by clathrin (Kirchhausen et al. 2014). The endocytic process continues with the cleavage of clathrin-coated vesicles from the cell membrane by dynamin, a cytosolic protein with GTPase activity (Doherty and McMahon 2009; Ferguson and De Camilli 2012). Thus, dynamin controls the release of clathrin-coated vesicles from the membrane into the intracellular space and plays a crucial in endocytosis. There are three dynamin isoforms that are encoded by three different genes: dynamin I, II, and III (Cao et al. 1998). Both dynamin I and III are expressed in the central nervous system and have several overlapping functions which include the regulation of the endocytosis and recycling of synaptic vesicles (Ferguson et al. 2007; Raimondi et al. 2011), GPCRs (Wolfe and Trejo 2007) and ionotropic receptors (Carroll et al. 1999; Anggono and Huganir 2012). In addition, endocytosis is the main mechanism through which HIV enters immune cells (Bosch et al. 2008; Miyauchi et al. 2009). Thus, gp120 could be endocytosed by a dynamin-mediated event. However, several investigators have demonstrated the presence of non-canonical endocytic pathways in neurons that do not require dynamin and/or clathrin (Doherty and McMahon 2009). For instance, presynaptic vesicle retrieval occurs via clathrin-independent endocytosis (Xu et al. 2008; Chung et al. 2010). In addition, other HIV proteins, including trans-activator of transcription (Tat), are internalized by neurons via a clathrin–independent mechanism (Liu et al. 2000). In this study, we tested the hypothesis that gp120 is internalized by neurons by means of dynamin-dependent endocytosis, initiated by binding to one of the chemokine co-receptors. We report that blocking gp120 internalization prevents its neurotoxicity. Moreover, we provide evidence that gp120 can be neurotoxic regardless of CXCR4 activation.
Materials and Methods
Reagents
Fluorescein isothiocyanate (FITC) conjugated gp120IIIB and gp120IIIB were purchased from Immunodiagnostics, Inc., (Woburn, MA). FITC conjugated Tat was from US Biological (Salem, MA). AlexaFluor488 conjugated transferrin was purchased from ThermoFisher Scientific (Waltham, MA). AMD3100 octahydrochloride hydrate (A5602), dynasore hydrate (D7693), 4-hydroxytamoxifen (H6278), tetraethyl orthosilicate (TEOS), 3-(aminopropyl) triethoxysilane (APTES), hexadecyltrimethylammonium bromide (CTAB), fluorescein 5(6)-isothiocyanate (FITC), 2-propanol (IPA), ethanol, HCl, 2-mercaptoethanol (BME), and N-(3-diethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC) were purchased from Sigma-Aldrich (St. Louis, MO). 2-ethylsulfonic acid (MES), and NaCl from Acros Organics (Fairlawn, NJ); NH4F and N-hydroxysulfosuccinimide (Sulfo-NHS) were from ThermoFisher Scientific (Waltham, MA).
Cell Cultures
Animal studies were done in strict accordance with the Laboratory Animal Welfare Act, with National Institutes of Health Guide for the Care and Use of Laboratory Animals, and after approval from the Georgetown University Animal Care and Use Committee. Primary rat cortical neurons were prepared as previously described (Avdoshina et al. 2010) from the cortex of embryonic (E17–18) Sprague-Dawley rats (Taconic, Derwood, MD). In brief, cells were grown on coverslips (ThermoFisher Scientific) pre-coated with poly-L-lysine in neurobasal medium (NBM) containing 2% B27 supplement, 25 nM glutamate, 0.5 mM-glutamine, and 1% antibiotic-antimycotic solution (ThermoFisher Scientific). Cultures were grown for 7 days at 37 °C in 5% CO2/95% air.
Dynamin triple knock out (TKO) fibroblasts are a generous gift from Dr. De Camilli (Yale University, New Haven, CT). Their characterization has been described elsewhere (Park et al. 2013). Fibroblasts were grown in high glucose DMEM, 10% fetal bovine serum (FBS), 2% antibiotic-antimycotic solution (ThermoFisher Scientific) at 37 °C in 5% CO2/95% air. Fibroblasts were grown in flasks until they were about 50% confluent and then were exposed to 3 μM 4-hydroxytamoxifen on days 1 and 2 (without changing media) to knock out dynamins. On day 3, tamoxifen-treated medium was replaced with unconditioned medium, leaving about 5% of tamoxifen media. On day 5, one flask of untreated and one flask of tamoxifen-treated cells were trypsinized, transferred, and grown on cover slips (ThermoFisher Scientific) at a low density of 12,500 cells/well or were collected for characterization of dynamin expression by Western blot analysis. On day 6, after a complete media exchange with serum free media, cells were exposed to F-gp120IIIB (1:200), FC-transferrin (5 μg/ml), or F-Tat (1:50), kept on ice for 30 min, and transferred to 37 °C for 30 min.
Immunocytochemistry
After treatment with fluorescent compounds, cells were rinsed gently with ice-cold 1× Phosphate Buffered Saline (PBS) and then fixed in 4% paraformaldehyde (PFA) for fibroblasts and 4% PFA/4% sucrose for neurons, for 10 min at room temperature (RT). Cells were permeabilized with a 0.02% Triton X-100 solution in PBS for 10 min and then blocked with 1% bovine serum albumin (BSA) in PBS for 1 h at RT. Primary antibodies were used in the following concentrations and incubated at 4 °C overnight: gp120IIIB (cat#1301, 1:200, Immunodiagnostics, Inc.) and anti-microtubule associated protein-2 (MAP2) (cat#M4403, 1:5000, Sigma-Aldrich). Corresponding goat anti-mouse secondary antibodies AlexaFluor488 (cat#A11029, 1:1000) and AlexaFluor594 (cat#A11005, 1:1000), goat anti-rabbit AlexaFluor 594 (cat#A11012, 1:1000) all from ThermoFisher Scientific were incubated for 1 h at RT. Staining for actin was done by incubating cells for 20 min at RT with AlexaFluor594 Phalloidin (cat#A12381, ThermoFisher Scientific) diluted in 1% BSA in PBS according to manufacturer instructions at a final concentration of approximately 160 nM. Coverslips were washed three times with 1× PBS and then incubated with 4′,6′-diamidino-2-phenylindole (DAPI) (Cat#D9542, 1:5000, ThermoFisher Scientific) for 10 min at RT. After a final three washes with 1× PBS, coverslips were mounted with Fluoro-Gel with TES Buffer (Electron Microscopy Sciences, Hatfield, PA), and imaged with Zeiss LSM880 microscope.
Analysis of Internalization
Internalization of gp120 and transferrin was calculated using ImageJ (NIH, Bethesda, MD) in four to five coverslips per treatment. First, the region of interest was selected using the Phalloidin staining. Then, intensity of fluorescence of either gp120 or transferrin was measured and normalized to the area of each cell body. After subtracting baseline autofluorescence typically seen in untreated cells, fluorescence in cells treated with gp120 or transferrin was set arbitrarily as 100%. The effect of treatments on gp120 and transferrin internalization was calculated as the difference between 100% and the fluorescence remaining after AMD3100 pretreatment or dynamin TKO.
Neuronal Processes and Cell Death
Experimenters were blinded to all treatments during analyses. The length of neuronal processes was measured by 2D Sholl analysis using ImageJ as previously described (Bachis et al. 2012). In brief, cortical neurons were grown on coverslips and fixed as described in immunocytochemistry. Fixed cells were then blocked, permeabilized and incubated overnight at 4 °C with a MAP2 antibody (1:5000; Sigma-Aldrich). Coverslips were washed and then incubated for 1 h at room temperature with the corresponding secondary antibody (1:2000; ThermoFisher Scientific). Cells were imaged with a Zeiss LSM880 microscope as described above. Image scale was calibrated and length of MAP2 positive processes was measured in three randomly selected fields (10 neurons per field) using ImageJ. Experiments were repeated two to four times using different preparations of primary cortical neurons.
Cell death was measured using Hoechst/Propidium Iodide staining (cat#94403, Sigma Aldrich) as previously described (Avdoshina et al. 2016a). Cells were counted with ImageJ.
Western Blot Analysis
Cells were kept on ice throughout all processing. Conditioned media was aspirated and cells were gently rinsed with 1× PBS. Cells were then collected in 1× Radioimmunoprecipitation assay (RIPA) buffer (Merk Millipore Corp., Billerica, MA) with 1× Halt Proteinase/Phosphatase inhibitors (ThermoFisher Scientific). After microtip sonication on ice, lysates were centrifuged at ≥10,000 g at 4 °C for 10 min. Protein concentration was determined by BCA protein assay (ThermoFisher Scientific). Lysates were loaded onto 4–12% Bis-tris gels (ThermoFisher Scientific) for gel electrophoresis. After transfer to a nitrocellulose membrane using the iBlot2 transfer machine (ThermoFisher Scientific), the membrane was blocked for 30–60 min in 5% BSA in Tris-buffered saline with 0.05% Tween-20 (TBST). Membranes were incubated sequentially with the following antibodies overnight at 4 °C: pErk (cat#9101, 1:1000, Cell Signaling Technology, Danvers, MA), Erk (cat#9102, 1:1000, Cell Signaling) or with Dynamin clone 41 (cat#610245, 1:1000, BD Biosciences, San Jose, CA) and β-actin as a loading control (cat#A2228, 1:20,000, Sigma-Aldrich). After washing 3 × 5 minutes with TBST, incubation with corresponding HRP conjugated anti-rabbit and anti-mouse secondary antibodies (cat# 111–035-003, 1:10,000, cat#115–035-003, 1:10,000, Jackson ImmunoResearch, West Grove, PA) occurred for 1 h at RT. Between each step, blots were stripped with Restore™ PLUS Western Blot Stripping Buffer (ThermoFisher Scientific) for 30 min at 37 °C and then examined for remaining chemiluminescence before re-blocking and probing with the next antibody.
Mesoporous Silica Nanoparticles Synthesis and Characterization
Mesoporous Silica Nanoparticles (MSNs) were prepared by hydrolysis and condensation of tetraethyl orthosilicate (TEOS) following a Stober modified method as we previously reported (Parodi et al. 2014). In details, 170 mg ammonium fluoride (base) and 150 mg hexadecyltrimethylammonium bromide (template) were dissolved in 48 ml water (80 °C for 1 h). 1 ml of TEOS were then added drop-wise to the solution followed by a 0.72 ml of 3-(aminopropyl) triethoxysilane and the reaction proceeded for 2 h at 80 °C. MSNs were washed twice with ethanol and then let overnight in a 2% HCl/ethanol solution to remove surfactants. The particles were finally washed with 50% ethanol and stored in 2-propanol. MSNs were conjugated to gp120 via N-(3-diethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC)/sulfo-NHS coupling reaction. The gp120 was activated in a solution of 2 mM EDC/5 mM sulfo-NHS in 0.1 M MES and 0.5 M NaCl for 15 min at 1 mg/ml. 1 mg of MSNs was dispersed in the reaction solution. The conjugation took place at room temperature for 2 h under agitation. Hydroxylamine HCl was added to the solution at a concentration of 10 mM to quench the reaction. The final loading efficiency was determined to be the 80% of the total gp120 used in the reaction.
Transmission electron microscope (TEM) and scanning electron microscope (SEM) images were used to determine the porosity and the dimension of the particles after synthesis. TEM samples were prepared by drying nanoparticles onto 300 mesh carbon-coated copper grids. Samples were prepared for SEM by drying nanoparticles on a stage and sputter coating the sample with a 5 nm thick layer of platinum/palladium using a Sputter Coater 208HR (Cressington Scientific, Watford, U.K.). Microscopy images were taken using Nova NanoSEM 230 (FEI, Hillsboro, Oregon).
Statistical Analysis
Statistical analyses were performed using GraphPad Prism software (GraphPad Software, Inc. La Jolla, CA). Results are depicted as mean ± standard error of mean. For a comparison of more than two groups, an ANOVA test, followed by a proper post-hoc test for multiple comparisons, was applied. P values of <0.05 indicate statistical significance.
Results
Gp120 Internalization is Dynamin-Dependent
To test the hypothesis that gp120 is endocytosed through a dynamin-mediated mechanism, we used fibroblasts generated from mice harboring floxed alleles of all three dynamin genes in which application of 4-hydroxytamoxifen (tamoxifen) causes conditional triple dynamin knock out (TKO) (Park et al. 2013). These fibroblasts without tamoxifen treatment were used as a control. We first confirmed that tamoxifen inhibits expression of dynamin by Western blot analysis of lysates of control and tamoxifen-exposed cells with a dynamin monoclonal antibody recognizing the N terminus of dynamin I and II (Werbonat et al. 2000) with equal affinity. Fig. 1a shows that cells exposed to tamoxifen to knock down dynamin do not exhibit dynamin immunoreactivity. Control and tamoxifen-treated fibroblasts were then exposed to FITC-conjugated gp120IIIB (F-gp120) for 30 min at 4 °C and 30 min at 37 °C. To confirm that these cells exhibit proper endocytosis, fibroblasts were exposed to transferrin conjugated to AlexaFluor488 (AF-transferrin), whose internalization is known to be dynamin-dependent (van Dam and Stoorvogel 2002). In untreated cells, in which dynamin I and II are functional, both F-gp120 and AF-transferrin (Fig. 1b) were observed inside cells. Most of the gp120IIIB immunoreactivity localized perinuclearly as previously demonstrated in neurons (Bachis et al. 2003; Bachis et al. 2006). In contrast, a drastic reduction of immunoreactivity for both F-gp120 (80.5%) and AF-transferrin (78.9%) when compared to untreated cells was obtained in tamoxifen-treated fibroblasts (Fig. 1c), suggesting that conditional TKO of dynamins impairs the endocytic process of gp120, similar to that of transferrin. To ensure that the general process of internalization was not blocked due to the dynamin TKO, we exposed tamoxifen-treated fibroblasts to FITC-conjugated Tat (F-Tat, 1:50), an HIV protein that is internalized by a dynamin-independent mechanism (Liu et al. 2000). F-Tat was endocytosed in TKO fibroblasts (Fig. 1c) confirming that the internalization of Tat is dynamin-independent. Overall, our data show that the endocytic process of gp120IIIB requires dynamin, suggesting a clathrin-mediated event.
Fig. 1. Knockdown of dynamins inhibits the internalization of gp120 in fibroblasts.
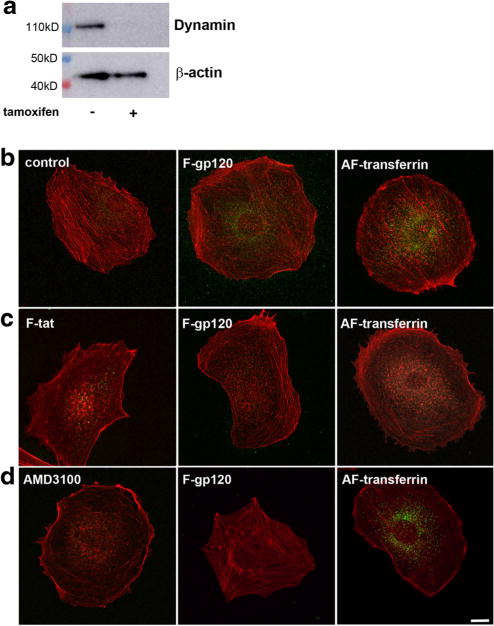
a Fibroblasts were grown for two days in the absence (−) or presence (+) of tamoxifen. Cell lysates were then prepared for Western blot analysis with an antibody against dynamin I and II. The blot was reprobed with β-actin as a loading control. b–d Representative immunofluorescent images of fibroblasts exposed to various stimuli and counterstained for filamentous actin using AlexaFluor594 Phalloidin antibody (red). b Untreated (control) cells exposed to F-gp120 or AF-transferrin or c tamoxifen-treated fibroblasts exposed to F-gp120 (1:200), AF-transferrin (5 μg/ml) or F-Tat (1:50). Fluorescent compounds were added and cells were kept for 30 min on ice and 30 min at 37 °C. Internalization of gp120 and transferrin (green) was visualized by confocal microscope. Scale bar = 10 μm. The experiment was replicated with three independent preparations, in duplicate, with comparable results. Please note that internalization of F-Tat, which is dynamin-independent, occurred even without the presence of dynamins. d) Control fibroblasts were exposed to F-gp120 (1:200, green) or AF-transferrin (5 μg/ml) in the presence of AMD3100 for 30 min at 4 °C and 30 min at 37 °C. AMD3100 pretreatment (500 nM) was initiated 15 min prior to F-gp120 or AF-transferrin application. Images are representative of two independent experiments done in duplicate. Scale Bar = 10 μm
CXCR4 Plays a Role in gp120 Internalization
Gp120IIIB binds to CXCR4 even in the absence of CD4 (Bandres et al. 1998). This receptor is a crucial co-receptor for HIV infection of T-cells (Herbein et al. 1998), but also mediates the neurotoxic effect of gp120 (Hesselgesser et al. 1998; Meucci et al. 1998; Kaul et al. 2007). Thus, CXCR4, which is endocytosed by a clathrin-mediated mechanism (Orsini et al. 1999; Venkatesan et al. 2003), is a valid target to explain gp120 internalization. However, recent data have shown a partial CXCR4-independent internalization of gp120 (Berth et al. 2015). Therefore, we utilized untreated fibroblasts to determine the extent by which CXCR4 mediates gp120 internalization. Fibroblasts were exposed to F-gp120 alone or in the presence of AMD3100, a CXCR4 antagonist that has been used to block both HIV infection (Donzella et al. 1998; De Clercq 2003) and gp120 neurotoxicity (Catani et al. 2000; Bachis et al. 2003; Khan et al. 2005). Preincubation of fibroblasts with AMD3100 for 15 min resulted in a robust 69.4% inhibition of F-gp120 endocytosis but a weak (~10%) reduction of AF-transferrin internalization (Fig. 1d), confirming that CXCR4 plays a crucial role in gp120IIIB endocytosis (Bachis et al. 2006).
Gp120 Endocytosis in Neurons Leads to Neuronal Injury
Endocytosis and subsequent accumulation of intracellular gp120 could be a mechanism to explain its neurotoxicity. While fibroblasts are a useful model to study dynamin-dependence and receptor dynamics, primary neurons are a better experimental model to test this hypothesis. To establish whether the endocytic process has a role in gp120 neurotoxicity, we first determined whether gp120 endocytosis in neurons could be blocked by dynasore, a cell permeable inhibitor of dynamin I and II (Macia et al. 2006). Primary rat cortical neurons were exposed to gp120IIIB alone or in combination with dynasore for 1 or 6 h. Double immunostaining with antibodies against microtubule associated protein-2 (MAP2) and gp120 confirmed that neurons are capable of internalizing gp120 (Fig. 2a). Dynasore inhibited gp120 internalization after 1 h (Fig. 2a) supporting our data in fibroblasts that gp120 endocytosis requires dynamin. We then used dynasore to evaluate the role of gp120 internalization in neuronal survival by determining the effect of gp120 on neurite simplification. Neurite simplification is often used as a pre-apoptotic marker in experimental models of HAND (Toggas et al. 1994; Masliah et al. 1997). Neurons were exposed to dynasore and gp120IIIB, alone or in combination, for 6 h and their MAP2 positive processes analyzed by Scholl analysis as previously described (Bachis et al. 2012). Neurons exposed to gp120 exhibited shorter and fewer MAP2 positive processes (Figs. 2b and c). This phenomenon was prevented by dynasore (Figs. 2b and c), further suggesting that cellular factors involved in membrane/receptor trafficking are responsible for gp120’s toxicity.
Fig. 2. Dynasore prevents the internalization and short-term neurotoxicity of gp120.
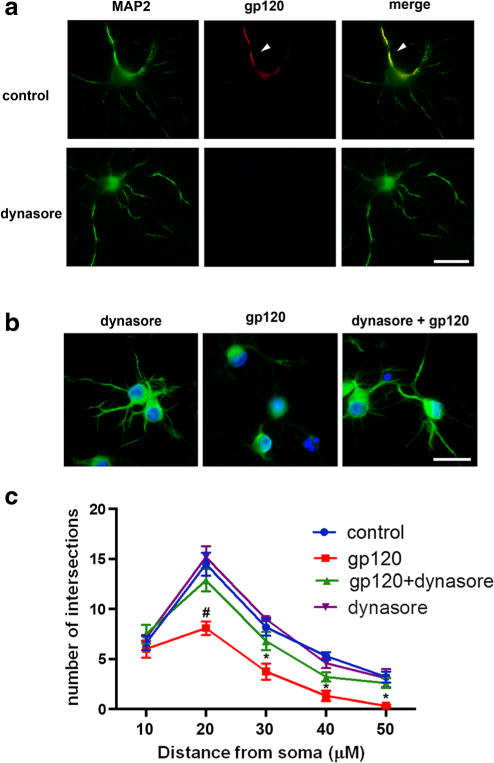
a Primary rat cortical neurons were exposed to gp120 (5 nM, upper panel) for 1 h alone or in combination with dynasore (80 μM, lower panel). Neurons were then stained for MAP2 (green) and gp120 (red). Yellow indicates the presence of gp120 internalization (arrow). Scale bar = 50 μm. b Neurons were exposed to dynasore (80 μM) or gp120 (5 nM) alone, or preincubated with dynasore for 10 min prior to gp120. Six hours later, neurons were fixed and stained for MAP2 (green) and counter stained with DAPI (blue). Scale bar = 50 μm. c Quantification of neurite processes was done on 30 randomly selected neurons per treatment using Sholl’s analysis as described in Materials and Methods. This experiment was repeated with 3 individual preparations of primary neurons with comparable results. Data are presented as mean ± s.e.m. #p < 0.05, *p < 0.01 vs control. Two-way ANOVA and Sheffe’s test
To examine whether dynasore, in addition to neurite pruning, also prevents gp120-mediated cell death, neuronal loss was measured following 8 h incubation with gp120 by Hoescht/Propidium iodide staining. AMD3100 was used as a control to assure that gp120 is neurotoxic through CXCR4. We found that AMD3100 blocked the neurotoxic effect of gp120 (Fig. 3). Importantly, gp120 was significantly less toxic in neurons pretreated with dynasore than those pretreated with vehicle (Fig. 3). Nevertheless, the neuroprotective effect of dynasore was not sustainable or long-lasting because, after 12–14 h of exposure to dynasore, neuronal cultures contained over 50% of propidium iodide positive cells (data not shown), suggesting a dramatic decrease in neuronal survival.
Fig. 3. Gp120-mediated neuronal loss is blocked by dynasore.
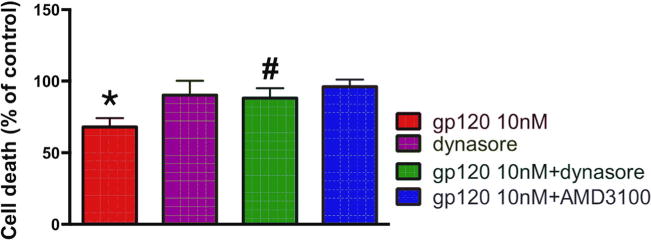
Primary cortical neurons were pre-treated with AMD3100 (5 nM) or dynasore (80 μM) 15 min prior to gp120 (10 nM). Eight hours later, neurons were fixed and cell death was quantified using Hoescht/Propidium Iodide staining. Positive cells were counted using ImageJ. Experiment was repeated with 3 preparations of primary neurons with comparable results. Statistics analyzed using one-way ANOVA with Tukey post-hoc, #p < 0.05 vs gp120, *p < 0.01 vs control
Gp120 Delivered Intracellularly via Mesopourous Silica Nanoparticles is Neurotoxic
The ability of dynasore to prevent short-term gp120-mediated toxicity suggests that the accumulation of gp120 inside cells is a major mechanism for neurotoxicity. However, CXCR4, upon binding to gp120, activates a signal cascade involving extracellular receptor kinase 1/2 (Erk1/2) (Meucci et al. 1998) which may be involved in the neurotoxic pathway utilized by gp120. To further examine the extent to which activation of receptor-mediated signaling cascades are responsible for gp120-mediated neurotoxicity, we designed a gp120 that could enter neurons without binding to CXCR4. We utilized gp120-loaded mesoporous silica nanoparticles (gp120MSNs) because these nanoparticles are readily engulfed via phagocytosis and deliver their contents intracellularly, bypassing receptors on the cell membrane (Parodi et al. 2014). Fig. 4 shows the characteristics of 50 nm gp120MSNs. The localization inside neurons of gp120MSNs was confirmed by electron microscopy (Fig. 4c) as previously described (Avdoshina et al. 2016a). We then examined whether gp120MSNs activate CXCR4 by determining Erk phosphorylation (pErk), a typical signaling molecule associated with CXCR4 (Busillo and Benovic 2007). Rat primary cortical neurons were exposed to gp120IIIB (5 nM) or gp120MSNs (5 μg/ml), which contain an approximately equivalent concentration of uncoupled gp120, for up to an hour. Boiled gp120 and unloaded MSNs were used as controls. Gp120, but not boiled gp120, induced pErk (Fig. 5a), confirming previous data that gp120 activates the MAPK pathway (Meucci et al. 1998). Neither unloaded MSNs nor gp120MSNs showed a significant induction of pErk (Figs. 5b and c), consistent with the notion that gp120MSNs are rapidly internalized without activation of CXCR4.
Fig. 4. Characterization of gp120MSNs.
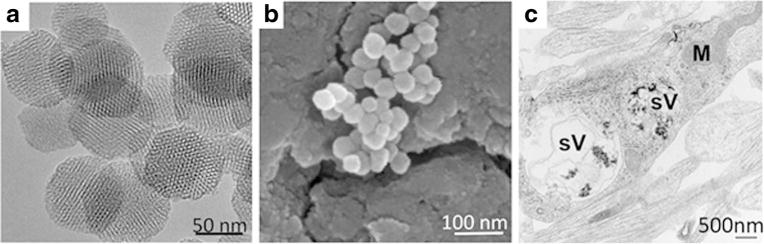
a TEM image of nonmodified MSN with nonordered porosity. b SEM image of loaded gp120MSNs. c EM of cortical neurons exposed to gp120MSNs (5 μg/ml), which contain an approximately equimolar concentration of non conjugated gp120 used in this study. sV = vesicles, M = mitochondria
Fig. 5. gp120MSNs do not activate pErk.
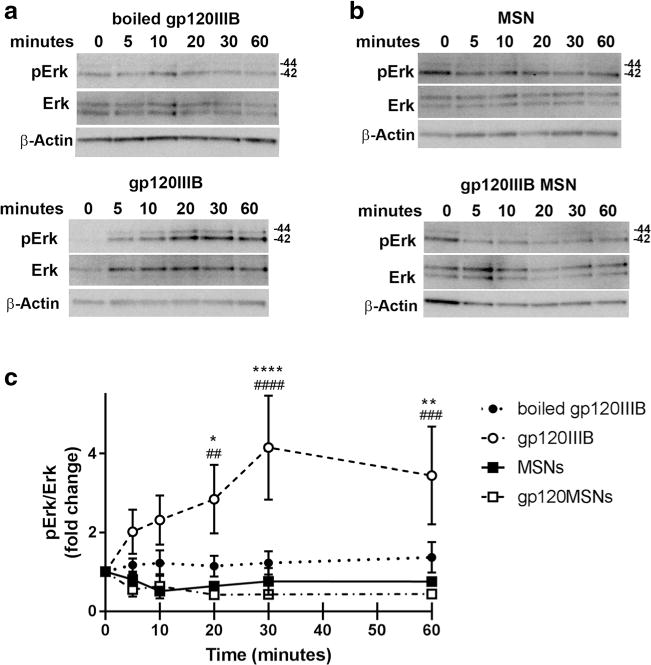
Primary rat cortical neurons were exposed for the indicated time points to a 5 nM boiled gp120IIIB (upper panel) or 5 nM gp120IIIB (lower panel), or b 5 μg/ml of MSNs (upper panel) or gp120MSNs (lower panel). Cell lysates were analyzed for pErk and total Erk by Western blot. c Densitometry analyses of the ratio pErk/total Erk of boiled gp120IIIB (closed circles), gp120IIIB (open circles), MSNs (closed squares), and gp120IIIB MSNs (open squares). Data are presented as mean ± s.e.m. from 5 individual preparations of primary neurons. *p < 0.05, **p < 0.01,****p < 0.0001 MSN v. gp120IIIB; ##p < 0.01, ###p < 0.001, ####p < 0.0001 gp120MSN v. gp120IIIB (Two-way ANOVA with Tukey post-hoc). β-actin was used to confirm protein loading
To investigate the neurotoxic effect of internalized gp120MSNs, we examined neurite pruning. Cortical neurons exposed to gp120MSNs for 24 h exhibited shorter MAP2 positive processes similar to gp120 as compared to control neurons (Figs. 6a and b). Neurite shortening by gp120, but not gp120MSNs, was prevented when cells were preincubated for 15 min with 5 nM AMD3100 (Figs. 6a and b). Thus, gp120, when delivered intracellularly, can be neurotoxic even in the absence of CXCR4 activation.
Fig. 6. AMD3100 does not prevent gp120MSN neurotoxicity.
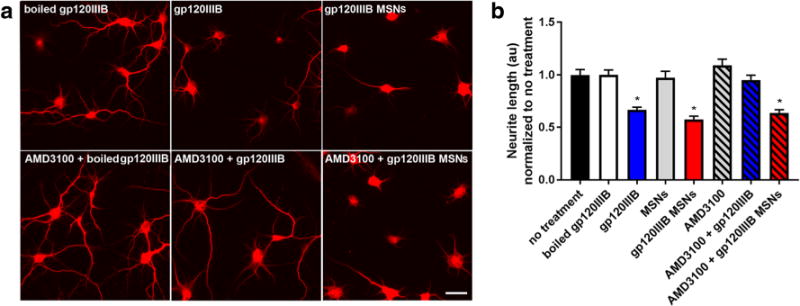
Primary rat cortical neurons were exposed to boiled gp120IIIB (5 nM), gp120IIIB (5 nM), MSNs, or gp120MSNs (5 μg/ml) with or without a 15 min preincubation with AMD3100 (5 nM). Neurons were fixed and imaged 24 h later. a Representive immunofluorescent images of neurons fixed and stained for MAP2. Scale bar = 50 μm. b Neurite lengths were quantified by ImageJ as described in Materials and Methods. The total number of neurons imaged each treatment are as follows: boiled gp120 (81), gp120IIIB (99), MSNs (41), gp120IIIB MSNS (83), AMD3100 (63), AMD3100 + gp120IIIB (96), AMD3100 + gp120IIIBMSNs (90). No significant differences in neurite length were seen with AMD3100 + boiled gp120 or AMD3100 + MSNs (data not shown). Data are shown as mean ± s.e.m. *p < 0.0001 vs boiled gp120IIIB. (One-way ANOVA, and Dunnett post hoc)
Discussion
T-tropic gp120 is highly toxic to neurons both in vitro (Meucci and Miller 1996; Kaul et al. 2007) and in vivo (Bansal et al. 2000; Acquas et al. 2004) through a variety of direct and indirect mechanisms. Furthermore, overexpression of gp120 in transgenic mice leads to neurite simplification and neuronal loss (Toggas et al. 1994; Lee et al. 2013), a clear indication that even without the virus, gp120 alone decreases neuronal survival. Because gp120 binds to chemokine receptors, it has been suggested that its neurotoxicity derives from the activation of signaling pathways linked to these receptors. However, synaptodendritic atrophy, impaired mitochondrial function (Avdoshina et al. 2016b), and activates caspase-3 and other markers of apoptosis (Bachis et al. 2003), are seen in neurons that internalize gp120, suggesting that the endocytic process of gp120 may be crucial for its neurotoxic effects. This direct mechanism could be sufficient to initiate an irreversible neurodegenerative process. The goal of this study was to reveal whether the endocytic process and subsequent intracellular accumulation of gp120 has a role in its neurotoxicity. We first demonstrated that the envelope protein is endocytosed by a dynamin-mediated mechanism. This suggests that gp120 enters via an endocytic pathway, typically associated with GPCRs, which utilizes clathrin-coated vesicles. We then discovered that prevention of gp120 endocytosis abolishes its short-term neurotoxic effects on neurite pruning and neuronal survival. Lastly, we have shown that once internalized, gp120 is neurotoxic irrespective of receptor-mediated signaling activation.
Gp120, which can be shed from HIV (Schneider et al. 1986), has been shown to promote axonal degeneration (Melli et al. 2006) and dendritic injury (Everall et al. 2002; Iskander et al. 2004), two key pathological events that may account for the synaptodendritic atrophy observed in HIV positive subjects (Masliah et al. 1997). In our study, we report that gp120-mediated shortening of neuronal processes as well as neuronal loss are prevented by dynasore, a non-competitive, cell-permeable inhibitor of dynamin I and II (Macia et al. 2006). In addition, the delivery of gp120 by MSNs inside neurons induces neuronal injury similar to that of gp120, suggesting that internalized gp120 triggers a neurotoxic mechanism(s) independently from receptor signaling. However, the mechanism of how internalized gp120 causes neuronal injury remains not fully understood. Once internalized, gp120 is axonally transported and undergoes intracellular trafficking by the microtubular network (Bachis et al. 2006; Teodorof et al. 2014) by a direct binding to neuronal microtubules (Avdoshina et al. 2016a). This binding impairs microtubule function and subsequently the ability to transport mitochondria (Avdoshina et al. 2016b). Reduced trafficking of mitochondria is known to cause axonal and dendritic degeneration (Chang et al. 2006; Shirendeb et al. 2012) because it alters mitochondria function and impairs energy homeostasis. Thus, gp120 could decrease neuronal survival by reducing proper energy supply within neurites. This would be in line with human studies showing altered mitochondrial metabolism in HIV subjects (Opii et al. 2007; Bennett et al. 2014). In this study, we could not examine internalization of R5 strain of gp120 due to technical problems with gp120ADA immunofluorescence. Nevertheless, it is important to note that both X4- and R5-tropic gp120s alter mitochondria function by binding to microtubules (Avdoshina et al. 2016b; Avdoshina et al. 2016a). Thus, it is plausible to suggest that internalization of both X4 and R5 gp120s is a crucial direct mechanism involved in their neurotoxic effects. More experiments are needed to confirm this hypothesis.
We found that gp120 causes neurite pruning and cell death regardless of Erk activation, a typical signaling cascade linked to CXCR4 receptors (Meucci et al. 1998; Luo et al. 2008), leading us to believe that the internalization and accumulation of gp120 are critical cellular mechanisms for neurotoxicity. In this study, we did not address the mechanisms by which internalized gp120 reduces neuronal survival. However, we can speculate about the consequences of accumulated intracellular gp120 based on previous data as well as our knowledge of the existing literature. Intracellular gp120 binds to mannose-binding lectin (Teodorof et al. 2014), a carrier that facilitates glycoprotein trafficking from the endoplasmic reticulum to the Golgi apparatus (Nonaka et al. 2007). Mannose-binding lectin-gp120 complex associates with subcellular vesicles that traffic along neurites and could carry gp120 toward the soma (Teodorof et al. 2014). This suggestion would be consistent with results showing that gp120 is retrogradely transported from the synaptic cleft to the perinuclear region of neurons (Bachis et al. 2006; Berth et al. 2015). The transport is mainly axonal because both colchicine and nocodazole block this event (Bachis et al. 2006; Teodorof et al. 2014). Interestingly, only a small amount of gp120 is seen inside lysosomes (Bachis et al. 2006) or associated with lysosome-associated membrane glycoprotein 2 positive organelles (Berth et al. 2015), suggesting that gp120 is not efficiently degraded by the endogenous autophagic process. This suggestion is supported by recent data showing a limited autophagy in both gp120 transgenic mice (Fields et al. 2013) as well as in cortical neurons exposed to gp120 in culture (Passeri et al. 2014). Therefore, we can hypothesize that gp120 may be forming toxic inclusions in lysosomes or other organelles, which lead to cell death. For instance, gp120 accumulation inside lysosomes could disrupt the metabolism of sphingolipids and produce toxic bio-products, such as ceramide, as described by other investigators (Haughey et al. 2004). However, we cannot ignore the likelihood that gp120 is neurotoxic to a subset of neurons by other mechanisms including the release of glutamate (Bezzi et al. 2001; Kaul et al. 2001), activation of NMDA receptors (Xu et al. 2011), increased proinflammatory cytokines (Milligan et al. 2001), induced oxidative stress (Mattson et al. 2005), and expression of the proapoptotic transcription factor p53 (Garden et al. 2004; Khan et al. 2005). Future experiments are needed to test these hypotheses.
Dynamin has been shown to facilitate GPCR internalization, including CXCR4 (Orsini et al. 1999) suggesting that CXCR4 sequestration is mediated by the endocytic pathway. However, most of the data on CXCR4 internalization thus far have been obtained in non-neuronal cells (Amara et al. 1997; Tarasova et al. 1998; Bhandari et al. 2007). While our work could not demonstrate whether gp120 is endocytosed as a complex with CXCR4, AMD3100 reduced gp120 endocytosis and prevented its toxicity, supporting the suggestion that internalization of gp120 in neurons occurs primarily through a receptor-mediated mechanism implicating dynamin. A recent report (Berth et al. 2015) has shown that CXCR4 is not required for gp120 endocytosis in cultured F11 cells, a cell hybrid of a rat embryonic dorsal root ganglion and mouse neuroblastoma cell line N18TG2 (Platika et al. 1985). These investigators have shown that gp120 is internalized through lipid rafts rather than in a CXCR4- or dynamin-dependent manner (Berth et al. 2015). Lipid rafts are membrane microdomains, which are proposed to facilitate receptor internalization and incorporation into endocytic vesicles. They are rich in cholesterol and exhibit a high concentration of glycosphingolipids (Ewers and Helenius 2011). The cholesterol-rich microdomain is required for gp120 interaction with chemokine receptors (Yi et al. 2006). In addition, gp120 promotes CXCR4 clustering in the raft microdomains (Kamiyama et al. 2009). Even HIV endocytosis can be both dynamin-dependent and receptor-mediated (Bosch et al. 2008; Miyauchi et al. 2009) or receptor independent (Bomsel 1997; Eugenin et al. 2011). In our study, the knockout of dynamins did not fully prevent gp120 internalization as evidenced by the slight immunoreactivity (~20%) remaining after knockdown. Thus, it is plausible to suggest that both lipid rafts and CXCR4 are required for an efficient internalization of gp120. Perhaps two parallel endocytic mechanisms exist for gp120 internalization.
Although our data were obtained in an experimental model of HIV infection of the CNS, our results may have a clinical significance in terms of discovering the molecular and cellular mechanisms of HIV neurotoxicity that may lead to a new adjunct therapy. Given the fact that the antiretroviral therapy has not eliminated mild neurocognitive deficits and asymptomatic neurocognitive impairments seen in HIV positive subjects (McArthur et al. 2003), our data could help developing new adjunct therapies to reduce the burden of HIV-mediated neurodegeneration.
Acknowledgments
The authors would like to thank Dr. De Camilli (Dept. of Neuroscience and Cell Biology, Yale University) for the gift of fibroblasts and Dr. Coate (Dept. of Biology, Georgetown University), for allowing us use of the Zeiss LSM880 microscope. The authors acknowledge Peter Johnson, imaging analysis manager at Georgetown University, for advice on quantitation of fluorescence images and Dr. Gu, Director of the Electron Microscopy Core at Houston Methodist Research Institute, for helping with MSN images.
This work was supported by US National Institute of Health grants R21-NS079172 and R01-NS074916. EW is supported by US National Institute of Health grant T32-NS041218. The work with nanoparticles was supported by the Brown Foundation (Project ID: 18130011) and by the Cullen Trust for Health Care Foundation (Project ID: 18130014).
Footnotes
Compliance with Ethical Standards
Conflicts of Interest The authors declare that they have no conflicts of interest.
References
- Acquas E, Bachis A, Nosheny RL, Cernak I, Mocchetti I. Human immunodeficiency virus type 1 protein gp120 causes neuronal cell death in the rat brain by activating caspases. Neurotox Res. 2004;5:605–615. doi: 10.1007/BF03033180. [DOI] [PubMed] [Google Scholar]
- Ahmed F, MacArthur L, De Bernardi MA, Mocchetti I. Retrograde and anterograde transport of HIV protein gp120 in the nervous system. Brain Behav Immun. 2009;23:355–364. doi: 10.1016/j.bbi.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara A, Gall SL, Schwartz O, Salamero J, Montes M, Loetscher P, Baggiolini M, Virelizier JL, Arenzana-Seisdedos F. HIV coreceptor downregulation as antiviral principle: SDF-1alpha-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J Exp Med. 1997;186:139–146. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anggono V, Huganir RL. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr Opin Neurobiol. 2012;22:461–469. doi: 10.1016/j.conb.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avdoshina V, Biggio F, Palchik G, Campbell LA, Mocchetti I. Morphine induces the release of CCL5 from astrocytes: potential neuroprotective mechanism against the HIV protein gp120. Glia. 2010;58:1630–1639. doi: 10.1002/glia.21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avdoshina V, Taraballi F, Dedoni S, Corbo C, Paige M, Saygideger Kont Y, Uren A, Tasciotti E, Mocchetti I. Identification of a binding site of the human immunodeficiency virus envelope protein gp120 to neuronal-specific tubulin. J Neurochem. 2016a;137:287–298. doi: 10.1111/jnc.13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avdoshina V, Fields JA, Castellano P, Dedoni S, Palchik G, Trejo M, Adame A, Rockenstein E, Eugenin E, Masliah E, Mocchetti I. The HIV protein gp120 alters mitochondrial dynamics in neurons. Neurotox Res. 2016b;29:583–593. doi: 10.1007/s12640-016-9608-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Major EO, Mocchetti I. Brain-derived neurotrophic factor inhibits human immunodeficiency virus-1/gp120-mediated cerebellar granule cell death by preventing gp120 internalization. J Neurosci. 2003;23:5715–5722. doi: 10.1523/JNEUROSCI.23-13-05715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Aden SA, Nosheny RL, Andrews PM, Mocchetti I. Axonal transport of human immunodeficiency virus type 1 envelope protein glycoprotein 120 is found in association with neuronal apoptosis. J Neurosci. 2006;26:6771–6780. doi: 10.1523/JNEUROSCI.1054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Avdoshina V, Zecca L, Parsadanian M, Mocchetti I. Human immunodeficiency virus type 1 alters brain-derived neurotrophic factor processing in neurons. J Neurosci. 2012;32:9477–9484. doi: 10.1523/JNEUROSCI.0865-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagetta G, Corasaniti MT, Malorni W, Rainaldi G, Berliocchi L, Finazzi-Agro A, Nistico G. The HIV-1 gp120 causes ultrastructural changes typical of apoptosis in the rat cerebral cortex. Neuroreport. 1996;7:1722–1724. doi: 10.1097/00001756-199607290-00005. [DOI] [PubMed] [Google Scholar]
- Bandres JC, Wang QF, O’Leary J, Baleaux F, Amara A, Hoxie JA, Zolla-Pazner S, Gorny MK. Human immunodeficiency virus (HIV) envelope binds to CXCR4 independently of CD4, and binding can be enhanced by interaction with soluble CD4 or by HIV envelope deglycosylation. J Virol. 1998;72:2500–2504. doi: 10.1128/jvi.72.3.2500-2504.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal AK, Mactutus CF, Nath A, Maragos W, Hauser KF, Booze RM. Neurotoxicity of HIV-1 proteins gp120 and tat in the rat striatum. Brain Res. 2000;879:42–49. doi: 10.1016/s0006-8993(00)02725-6. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Doyle T, Salvemini D. Mitotoxicity in distal symmetrical sensory peripheral neuropathies. Nat Rev Neurol. 2014;10:326–336. doi: 10.1038/nrneurol.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berth S, Caicedo HH, Sarma T, Morfini G, Brady ST. Internalization and axonal transport of the HIV glycoprotein gp120. ASN Neuro. 2015;7:1–15. doi: 10.1177/1759091414568186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- Bhandari D, Trejo J, Benovic JL, Marchese A. Arrestin-2 interacts with the ubiquitin-protein isopeptide ligase atrophin-interacting protein 4 and mediates endosomal sorting of the chemokine receptor CXCR4. J Biol Chem. 2007;282:36971–36979. doi: 10.1074/jbc.M705085200. [DOI] [PubMed] [Google Scholar]
- Bomsel M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat Med. 1997;3:42–47. doi: 10.1038/nm0197-42. [DOI] [PubMed] [Google Scholar]
- Bosch B, Grigorov B, Senserrich J, Clotet B, Darlix JL, Muriaux D, Este JA. A clathrin-dynamin-dependent endocytic pathway for the uptake of HIV-1 by direct T cell-T cell transmission. Antivir Res. 2008;80:185–193. doi: 10.1016/j.antiviral.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Busillo JM, Benovic JL. Regulation of CXCR4 signaling. Biochim Biophys Acta. 2007;1768:952–963. doi: 10.1016/j.bbamem.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Garcia F, McNiven MA. Differential distribution of dynamin isoforms in mammalian cells. Mol Biol Cell. 1998;9:2595–2609. doi: 10.1091/mbc.9.9.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll RC, Beattie EC, Xia H, Luscher C, Altschuler Y, Nicoll RA, Malenka RC, von Zastrow M. Dynamin-dependent endocytosis of ionotropic glutamate receptors. Proc Natl Acad Sci U S A. 1999;96:14112–14117. doi: 10.1073/pnas.96.24.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani MV, Corasaniti MT, Navarra M, Nistico G, Finazzi-Agro A, Melino G. gp120 induces cell death in human neuroblastoma cells through the CXCR4 and CCR5 chemokine receptors. J Neurochem. 2000;74:2373–2379. doi: 10.1046/j.1471-4159.2000.0742373.x. [DOI] [PubMed] [Google Scholar]
- Chang DT, Rintoul GL, Pandipati S, Reynolds IJ. Mutant huntingtin aggregates impair mitochondrial movement and trafficking in cortical neurons. Neurobiol Dis. 2006;22:388–400. doi: 10.1016/j.nbd.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Chung C, Barylko B, Leitz J, Liu X, Kavalali ET. Acute dynamin inhibition dissects synaptic vesicle recycling pathways that drive spontaneous and evoked neurotransmission. J Neurosci. 2010;30:1363–1376. doi: 10.1523/JNEUROSCI.3427-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam EM, Stoorvogel W. Dynamin-dependent transferrin receptor recycling by endosome-derived clathrin-coated vesicles. Mol Biol Cell. 2002;13:169–182. doi: 10.1091/mbc.01-07-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CB, Dikic I, Unutmaz D, Hill CM, Arthos J, Siani MA, Thompson DA, Schlessinger J, Littman DR. Signal transduction due to HIV-1 envelope interactions with chemokine receptors CXCR4 or CCR5. J Exp Med. 1997;186:1793–1798. doi: 10.1084/jem.186.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E. The bicyclam AMD3100 story. Nat Rev Drug Discov. 2003;2:581–587. doi: 10.1038/nrd1134. [DOI] [PubMed] [Google Scholar]
- Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- Donzella GA, Schols D, Lin SW, Este JA, Nagashima KA, Maddon PJ, Allaway GP, Sakmar TP, Henson G, De Clercq E, Moore JP. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat Med. 1998;4:72–77. doi: 10.1038/nm0198-072. [DOI] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, Clements JE, Zink MC, Berman JW. Human immunodeficiency virus infection of human astrocytes disrupts blood-brain barrier integrity by a gap junction-dependent mechanism. J Neurosci. 2011;31:9456–9465. doi: 10.1523/JNEUROSCI.1460-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everall IP, Bell C, Mallory M, Langford D, Adame A, Rockestein E, Masliah E. Lithium ameliorates HIV-gp120-mediated neurotoxicity. Mol Cell Neurosci. 2002;21:493–501. doi: 10.1006/mcne.2002.1196. [DOI] [PubMed] [Google Scholar]
- Ewers H, Helenius A. Lipid-mediated endocytosis. Cold Spring Harb Perspect Biol. 2011;3:a004721. doi: 10.1101/cshperspect.a004721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, De Camilli P. Dynamin, a membrane-remodelling GTPase. Nat Rev Mol Cell Biol. 2012;13:75–88. doi: 10.1038/nrm3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Brasnjo G, Hayashi M, Wolfel M, Collesi C, Giovedi S, Raimondi A, Gong LW, Ariel P, Paradise S, O’Toole E, Flavell R, Cremona O, Miesenbock G, Ryan TA, De Camilli P. A selective activity-dependent requirement for dynamin 1 in synaptic vesicle endocytosis. Science. 2007;316:570–574. doi: 10.1126/science.1140621. [DOI] [PubMed] [Google Scholar]
- Fields J, Dumaop W, Rockenstein E, Mante M, Spencer B, Grant I, Ellis R, Letendre S, Patrick C, Adame A, Masliah E. Age-dependent molecular alterations in the autophagy pathway in HIVE patients and in a gp120 tg mouse model: reversal with beclin-1 gene transfer. J Neuro-Oncol. 2013;19:89–101. doi: 10.1007/s13365-012-0145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garden GA, Guo W, Jayadev S, Tun C, Balcaitis S, Choi J, Montine TJ, Moller T, Morrison RS. HIV associated neurodegeneration requires p53 in neurons and microglia. FASEB J. 2004;18:1141–1143. doi: 10.1096/fj.04-1676fje. [DOI] [PubMed] [Google Scholar]
- Gelman BB. Neuropathology of HAND with suppressive antiretroviral therapy: encephalitis and neurodegeneration reconsidered. Curr HIV/AIDS Rep. 2015;12:272–279. doi: 10.1007/s11904-015-0266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey NJ, Cutler RG, Tamara A, McArthur JC, Vargas DL, Pardo CA, Turchan J, Nath A, Mattson MP. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Ann Neurol. 2004;55:257–267. doi: 10.1002/ana.10828. [DOI] [PubMed] [Google Scholar]
- Herbein G, Mahlknecht U, Batliwalla F, Gregersen P, Pappas T, Butler J, O’Brien WA, Verdin E. Apoptosis of CD8+ T cells is mediated by macrophages through interaction of HIV gp120 with chemokine receptor CXCR4. Nature. 1998;395:189–194. doi: 10.1038/26026. [DOI] [PubMed] [Google Scholar]
- Hesselgesser J, Taub D, Baskar P, Greenberg M, Hoxie J, Kolson DL, Horuk R. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1 alpha is mediated by the chemokine receptor CXCR4. Curr Biol. 1998;8:595–598. doi: 10.1016/s0960-9822(98)70230-1. [DOI] [PubMed] [Google Scholar]
- Huang CC, Tang M, Zhang MY, Majeed S, Montabana E, Stanfield RL, Dimitrov DS, Korber B, Sodroski J, Wilson IA, Wyatt R, Kwong PD. Structure of a V3-containing HIV-1 gp120 core. Science. 2005;310:1025–1028. doi: 10.1126/science.1118398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iskander S, Walsh KA, Hammond RR. Human CNS cultures exposed to HIV-1 gp120 reproduce dendritic injuries of HIV-1-associated dementia. J Neuroinflammation. 2004;1:7. doi: 10.1186/1742-2094-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiyama H, Yoshii H, Tanaka Y, Sato H, Yamamoto N, Kubo Y. Raft localization of CXCR4 is primarily required for X4-tropic human immunodeficiency virus type 1 infection. Virology. 2009;386:23–31. doi: 10.1016/j.virol.2008.12.033. [DOI] [PubMed] [Google Scholar]
- Kaul M, Lipton SA. Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc Natl Acad Sci U S A. 1999;96:8212–8216. doi: 10.1073/pnas.96.14.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kaul M, Ma Q, Medders KE, Desai MK, Lipton SA. HIV-1 coreceptors CCR5 and CXCR4 both mediate neuronal cell death but CCR5 paradoxically can also contribute to protection. Cell Death Differ. 2007;14:296–305. doi: 10.1038/sj.cdd.4402006. [DOI] [PubMed] [Google Scholar]
- Khan MZ, Shimizu S, Patel JP, Nelson A, Le M-T, Mullen-Przeworski A, Brandimarti R, Fatatis A, Meucci O. Regulation of neuronal P53 activity by CXCR4. Mol Cell Neurosci. 2005;30:58–66. doi: 10.1016/j.mcn.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T, Owen D, Harrison SC. Molecular structure, function, and dynamics of clathrin-mediated membrane traffic. Cold Spring Harb Perspect Biol. 2014;6:a016725. doi: 10.1101/cshperspect.a016725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Amin ND, Venkatesan A, Wang T, Tyagi R, Pant HC, Nath A. Impaired neurogenesis and neurite outgrowth in an HIV-gp120 transgenic model is reversed by exercise via BDNF production and Cdk5 regulation. J Neuro-Oncol. 2013;19:418–431. doi: 10.1007/s13365-013-0194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Jones M, Hingtgen CM, Bu G, Laribee N, Tanzi RE, Moir RD, Nath A, He JJ. Uptake of HIV-1 tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nat Med. 2000;6:1380–1387. doi: 10.1038/82199. [DOI] [PubMed] [Google Scholar]
- Luo Y, Lathia J, Mughal M, Mattson MP. SDF1alpha/CXCR4 signaling, via ERKs and the transcription factor Egr1, induces expression of a 67-kDa form of glutamic acid decarboxylase in embryonic hippocampal neurons. J Biol Chem. 2008;283:24789–24800. doi: 10.1074/jbc.M800649200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, Achim CL, McCutchan JA, Nelson JA, Atkinson JH, Grant I. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC group. The HIV neurobehavioral Research Center. Ann Neurol. 1997;42:963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Haughey NJ, Nath A. Cell death in HIV dementia. Cell Death Differ. 2005;12(Suppl 1):893–904. doi: 10.1038/sj.cdd.4401577. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Haughey N, Gartner S, Conant K, Pardo C, Nath A, Sacktor N. Human immunodeficiency virus-associated dementia: an evolving disease. J Neuro-Oncol. 2003;9:205–221. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
- Melli G, Keswani SC, Fischer A, Chen W, Hoke A. Spatially distinct and functionally independent mechanisms of axonal degeneration in a model of HIV-associated sensory neuropathy. Brain. 2006;129:1330–1338. doi: 10.1093/brain/awl058. [DOI] [PubMed] [Google Scholar]
- Meucci O, Miller RJ. gp120-induced neurotoxicity in hippocampal pyramidal neuron cultures: protective action of TGF-beta1. J Neurosci. 1996;16:4080–4088. doi: 10.1523/JNEUROSCI.16-13-04080.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, O’Connor KA, Nguyen KT, Armstrong CB, Twinning C, Gaykema RPA, Holguin A, Martin D, Maier SF, Watkins LR. Intrathecal HIV envelope protein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. J Neurosci. 2001;21:2808–2819. doi: 10.1523/JNEUROSCI.21-08-02808.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misse D, Cerutti M, Noraz N, Jourdan P, Favero J, Devauchelle G, Yssel H, Taylor N, Veas F. A CD4-independent interaction of human immunodeficiency virus-1 gp120 with CXCR4 induces their cointernalization, cell signaling, and T-cell chemotaxis. Blood. 1999;93:2454–2462. [PubMed] [Google Scholar]
- Miyauchi K, Kim Y, Latinovic O, Morozov V, Melikyan GB. HIV enters cells via endocytosis and dynamin-dependent fusion with endosomes. Cell. 2009;137:433–444. doi: 10.1016/j.cell.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka M, Ma BY, Ohtani M, Yamamoto A, Murata M, Totani K, Ito Y, Miwa K, Nogami W, Kawasaki N, Kawasaki T. Subcellular localization and physiological significance of intracellular mannan-binding protein. J Biol Chem. 2007;282:17908–17920. doi: 10.1074/jbc.M700992200. [DOI] [PubMed] [Google Scholar]
- Nosheny RL, Bachis A, Acquas E, Mocchetti I. Human immunodeficiency virus type 1 glycoprotein gp120 reduces the levels of brain-derived neurotrophic factor in vivo: potential implication for neuronal cell death. Eur J Neurosci. 2004;20:2857–2864. doi: 10.1111/j.1460-9568.2004.03764.x. [DOI] [PubMed] [Google Scholar]
- Opii WO, Sultana R, Abdul HM, Ansari MA, Nath A, Butterfield DA. Oxidative stress and toxicity induced by the nucleoside reverse transcriptase inhibitor (NRTI)–2′,3′-dideoxycytidine (ddC): relevance to HIV-dementia. Exp Neurol. 2007;204:29–38. doi: 10.1016/j.expneurol.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini MJ, Parent J-L, Mundell SJ, Benovic JL. Trafficking of the HIV coreceptor CXCR4 Role of arrestins and identificaton of residues in the C-terminas tail that mediate receptor internalization. J Biol Chem. 1999;274:31076–31086. doi: 10.1074/jbc.274.43.31076. [DOI] [PubMed] [Google Scholar]
- Park RJ, Shen H, Liu L, Liu X, Ferguson SM, De Camilli P. Dynamin triple knockout cells reveal off target effects of commonly used dynamin inhibitors. J Cell Sci. 2013;126:5305–5312. doi: 10.1242/jcs.138578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parodi A, Haddix SG, Taghipour N, Scaria S, Taraballi F, Cevenini A, Yazdi IK, Corbo C, Palomba R, Khaled SZ, Martinez JO, Brown BS, Isenhart L, Tasciotti E. Bromelain surface modification increases the diffusion of silica nanoparticles in the tumor extracellular matrix. ACS Nano. 2014;8:9874–9883. doi: 10.1021/nn502807n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passeri E, Mocchetti I, Moussa C. Is human immunodeficiency virus-mediated dementia an Autophagic defect that leads to neurodegeneration? CNS Neurol Disord Drug Targets. 2014;13:1571–1579. doi: 10.2174/1871527313666140806125841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platika D, Boulos MH, Baizer L, Fishman MC. Neuronal traits of clonal cell lines derived by fusion of dorsal root ganglia neurons with neuroblastoma cells. Proc Natl Acad Sci U S A. 1985;82:3499–3503. doi: 10.1073/pnas.82.10.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondi A, Ferguson SM, Lou X, Armbruster M, Paradise S, Giovedi S, Messa M, Kono N, Takasaki J, Cappello V, O’Toole E, Ryan TA, De Camilli P. Overlapping role of dynamin isoforms in synaptic vesicle endocytosis. Neuron. 2011;70:1100–1114. doi: 10.1016/j.neuron.2011.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto CD, Wyatt R, Hernandez-Ramos N, Sun Y, Kwong PD, Hendrickson WA, Sodroski J. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science. 1998;280:1949–1953. doi: 10.1126/science.280.5371.1949. [DOI] [PubMed] [Google Scholar]
- Schneider J, Kaaden O, Copeland TD, Oroszlan S, Hunsmann G. Shedding and interspecies type sero-reactivity of the envelope glycopolypeptide gp120 of the human immunodeficiency virus. J Gen Virol. 1986;67(Pt 11):2533–2538. doi: 10.1099/0022-1317-67-11-2533. [DOI] [PubMed] [Google Scholar]
- Shirendeb UP, Calkins MJ, Manczak M, Anekonda V, Dufour B, McBride JL, Mao P, Reddy PH. Mutant huntingtin’s interaction with mitochondrial protein Drp1 impairs mitochondrial biogenesis and causes defective axonal transport and synaptic degeneration in Huntington’s disease. Hum Mol Genet. 2012;21:406–420. doi: 10.1093/hmg/ddr475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signoret N, Oldridge J, Pelchen-Matthews A, Klasse PJ, Tran T, Brass LF, Rosenkilde MM, Schwartz TW, Holmes W, Dallas W, Luther MA, Wells TN, Hoxie JA, Marsh M. Phorbol esters and SDF-1 induce rapid endocytosis and down modulation of the chemokine receptor CXCR4. J Cell Biol. 1997;139:651–664. doi: 10.1083/jcb.139.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich S, Gonzalez-Scarano F. HIV-1-related central nervous system disease: current issues in pathogenesis, diagnosis, and treatment. Cold Spring Harb Perspect Med. 2012;2:a007120. doi: 10.1101/cshperspect.a007120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasova NI, Stauber RH, Michejda CJ. Spontaneous and ligand-induced trafficking of CXC-chemokine receptor 4. J Biol Chem. 1998;273:15883–15886. doi: 10.1074/jbc.273.26.15883. [DOI] [PubMed] [Google Scholar]
- Teodorof C, Divakar S, Soontornniyomkij B, Achim CL, Kaul M, Singh KK. Intracellular mannose binding lectin mediates subcellular trafficking of HIV-1 gp120 in neurons. Neurobiol Dis. 2014;69:54–64. doi: 10.1016/j.nbd.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toggas SM, Mucke L. Transgenic models in the study of AIDS dementia complex. Curr Top Microbiol Immunol. 1996;206:223–241. doi: 10.1007/978-3-642-85208-4_12. [DOI] [PubMed] [Google Scholar]
- Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367:188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- Venkatesan S, Rose JJ, Lodge R, Murphy PM, Foley JF. Distinct mechanisms of agonist-induced endocytosis for human chemokine receptors CCR5 and CXCR4. Mol Biol Cell. 2003;14:3305–3324. doi: 10.1091/mbc.E02-11-0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werbonat Y, Kleutges N, Jakobs KH, van Koppen CJ. Essential role of dynamin in internalization of M2 muscarinic acetylcholine and angiotensin AT1A receptors. J Biol Chem. 2000;275:21969–21974. doi: 10.1074/jbc.M001736200. [DOI] [PubMed] [Google Scholar]
- Wolfe BL, Trejo J. Clathrin-dependent mechanisms of G protein-coupled receptor endocytosis. Traffic. 2007;8:462–470. doi: 10.1111/j.1600-0854.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- Xu J, McNeil B, Wu W, Nees D, Bai L, Wu LG. GTP-independent rapid and slow endocytosis at a central synapse. Nat Neurosci. 2008;11:45–53. doi: 10.1038/nn2021. [DOI] [PubMed] [Google Scholar]
- Xu H, Bae M, Tovar-y-Romo LB, Patel N, Bandaru VV, Pomerantz D, Steiner JP, Haughey NJ. The human immunodeficiency virus coat protein gp120 promotes forward trafficking and surface clustering of NMDA receptors in membrane microdomains. J Neurosci. 2011;31:17074–17090. doi: 10.1523/JNEUROSCI.4072-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi L, Fang J, Isik N, Chim J, Jin T. HIV gp120-induced interaction between CD4 and CCR5 requires cholesterol-rich microenvironments revealed by live cell fluorescence resonance energy transfer imaging. J Biol Chem. 2006;281:35446–35453. doi: 10.1074/jbc.M607302200. [DOI] [PubMed] [Google Scholar]


