Abstract
The human immunodeficiency virus (HIV) envelope protein gp120 promotes axonal damage and neurite pruning, similar to that observed in HIV-positive subjects with neurocognitive disorders. Thus, gp120 has been used to examine molecular and cellular pathways underlying HIV-mediated neuronal dysfunction. Gp120 binds to tubulin beta III, a component of neuronal microtubules. Microtubule function, which modulates the homeostasis of neurons, is regulated by polymerization and post-translational modifications. Based on these considerations, we tested the hypothesis that gp120 induces dynamic instability of neuronal microtubules. We first observed that gp120 prevents the normal polymerization of tubulin in vitro. We then tested whether gp120 alters the post-translational modifications in tubulin by examining the ability of gp120 to change the levels of acetylated tubulin in primary rat neuronal cultures. Gp120 elicited a time-dependent decrease in tubulin acetylation that was reversed by Helix-A peptide, a compound that competitively displaces the binding of gp120 to neuronal microtubules. To determine whether post-translational modifications in tubulin also occur in vivo, we measured acetylated tubulin in the cerebral cortex of HIV transgenic rats (HIV-tg). We observed a decrease in tubulin acetylation in 5- and 9-month-old HIV-tg rats when compared to age-matched wild type. Neither changes in microglia morphology nor alterations in mRNA levels for interleukin-1β and tumor necrosis factor α were detected in 5-month-old animals. Our findings propose neuronal microtubule instability as a novel mechanism of HIV neurotoxicity, without evidence of enhanced inflammation.
Keywords: HAND, Helix-A peptide, HIV transgenic rats, microglia, microtubules
Despite the introduction of combined antiretroviral therapy, human immunodeficiency virus-1 (HIV) remains present and active in the central nervous system. It is thought that this long-term viral presence contributes to cognitive and motor impairments termed HIV-associated neurocognitive disorders (HAND) (McArthur et al. 2005; Spudich and Gonzalez-Scarano 2012). Impaired cognition in HAND has been proposed to be caused by synaptodendritic injury (Masliah et al. 1997; Carey et al. 2006); nevertheless, the molecular events underlying the synaptic simplification during HIV infection remain poorly understood.
Viral proteins, such as Tat and gp120, replicate the pattern of HIV-mediated synaptic pruning in animal models (Toggas et al. 1994; Kim et al. 2003) which has led to further use of these viral proteins to investigate the molecular mechanisms of HIV neurotoxicity. It has been demonstrated that gp120 can be endocytosed into neurons (Bachis et al. 2003; Berth et al. 2015). Upon entry into the neuron, gp120 is axonally transported (Bachis et al. 2006; Teodorof et al. 2014) and binds, with high affinity, to the carboxy terminal tail of neuronal-specific tubulin β-3 (TUBB3), through a conserved α-helix region (Avdoshina et al. 2016b). TUBB3 is a main component of neuronal microtubules (MTs). MTs regulate intracellular axonal transport of vesicles and other organelles, including mitochondria, which are important for cellular maintenance and survival. Gp120 alters both mitochondrial morphology and their transport along MTs in a manner similar to that observed in post mortem brains of HAND subjects (Avdoshina et al. 2016a). Thus, it is plausible that there is an association between altered MT dynamics and gp120 neurotoxicity. However, this hypothesis has never been tested.
Changes in the integrity and/or dynamics of MTs are sufficient to alter trafficking in neurons and lead to neuronal atrophy (McMurray 2000; Cartelli et al. 2010; Baird and Bennett 2013; Brunden et al. 2014). MTs are formed by α- and β-tubulin heterodimers, which are arranged in a head-to-tail fashion, forming structurally polarized linear protofilaments. Their assembly is a dynamic and tightly controlled process. Post-translational modifications (PTMs) in tubulin regulate the functional specialization of MTs (Wloga and Gaertig 2010; Janke 2014). Moreover, PTMs of tubulin provide structural domains for interaction with motor proteins such as kinesin-1 (Peris et al. 2009; Hammond et al. 2010), as well as MT-associated proteins (Kumar and Flavin 1982; Chapin and Bulinski 1994; Nogales 2000; Erck et al. 2005).
Tubulin undergoes several PTMs including acetylation, which occurs after tubulin polymerization at α-tubulin Lys40 and is preserved in all α-tubulin isoforms. In mature neurons, acetylated tubulin is particularly rich in the proximal site of axons, which is involved in intracellular transport. Increased tubulin acetylation promotes the accumulation of active kinesin-1 and consequently, improves axonal transport (Hammond et al. 2010). In contrast, inhibition of acetylated α-tubulin in cortical neurons inhibits the trafficking of vesicles containing brain-derived neurotrophic factor (Dompierre et al. 2007), a trophic factor that has a potent neuroprotective activity against gp120 (Nosheny et al. 2006). Moreover, inhibition of tubulin acetylation often results in pruning of neuronal processes (Dompierre et al. 2007; Wloga and Gaertig 2010), a pathological hallmark of HAND. Thus, tubulin acetylation may play a role in HIV-mediated neurotoxicity.
In considering the potential interaction between gp120 and MTs as an underlying contributing factor to neuronal dysfunction in HAND, we examined the ability of gp120 to interfere with MT polymerization and change tubulin acetylation. We report that gp120 inhibits normal MTs polymerization and alters typical PTM patterning. We propose that synaptic injury seen in HAND could be caused by gp120 ability to negatively affect neuronal cytoskeletal structure and function.
Materials and methods
Materials
The viral protein gp120IIIB was purchased from Immunodiagnostics Inc., Woburn, MA, USA. Helix-A peptide was synthesized by and purchased from Genscript, Piscataway, NJ, USA, and coupled to nanoparticles as previously described (Avdoshina et al. 2016b). All other chemicals were commercially obtained, reagent grade.
Tubulin polymerization assay
MT assembly was assessed using tubulin polymerization assay according to the manufacturer’s instructions (Cytoskeleton Inc., Denver, CO, USA). Purified porcine tubulin proteins (> 99% purity) were suspended in general tubulin buffer containing 80 mM piperazine-N,N′-bis(2-ethanesulfonic acid), 2 mM MgCl2, 0.5 mM EGTA, 1 mM Guanosine-5′-triphosphate (pH 6.9) and 15% glycerol in the absence or presence of indicated compounds at 4°C. The mixture was immediately transferred to pre-warmed 96-well plates, and the rate and extent of tubulin polymerization was determined by monitoring the turbidity of a solution containing purified porcine brain tubulin at A340. The final concentration of tubulin in the reaction was 3 μg/mL. Paclitaxel, which stabilizes MT polymer and protects it from disassembly (Amos and Lowe 1999), was used as a positive control for the polymerization reaction.
Animals
Animal studies were done in strict accordance with the Laboratory Animal Welfare Act, with National Institutes of Health Guide for the Care and Use of Laboratory Animals following approved animal protocols from Georgetown University Animal Care and Use Committee (ACUC) or National Institute of Environmental Health Sciences ACUC.
Age-matched male HIV transgenic (HIV-tg) or Fischer 344/NHsd wild-type (wt) rats were obtained from Harlan Laboratories (Madison, WI, USA). Animals were housed in an animal facility according to isolator housing conditions to minimize risk of infection, under a 12 h light-dark cycle (6 : 00–18 : 00 Eastern Standard Time) with ad libitum access to reverse osmosis treated drinking water and food (Teklad global 18% protein 2018S diet; Teklad Harlan, Madison, WI, USA; sterilized for controls and gamma irradiated for HIV-tg rats to minimize risk of infection). The diet contained (as % of total fatty acid) 16.7% saturated, 21.8% monounsaturated, 54.8% linoleic acid, 6.2% α-linolenic acid, 0.03% AA, 0.02% eicosapentaenoic (20 : 5 n-3), and 0.06% docosahexaenoic acid (22 : 6 n-3).
Rat cortical neurons
Primary rat cortical neurons were prepared from the cortex of embryonic day 17–18 Sprague–Dawley rats (Charles River, MA, USA) following an established protocol (Avdoshina et al. 2016a,b). Cells were seeded (0.5 ×106/mL) onto poly-L-lysine pre-coated plates or glass coverslips in Neurobasal Medium containing 2% B27 supplement, 25 nM glutamate, 0.5 mM L-glutamine, and 1% antibiotic-antimycotic solution (Invitrogen, Carlsbad, CA, USA). Cultures were grown at 37°C in 5% CO2/95% air for 7 days prior to the experiments. At day 7 in vitro, cell cultures contained 95% neurons as characterized for microtubule-associated protein-2 or class III β-tubulin positivity, as previously described (Avdoshina et al. 2016a,b).
Immunohistochemistry
HIV-tg and age-matched wt rats were lightly anesthetized with CO2, decapitated, and the brain excised from the cranium. Brains were fixed by immersion in 4% paraformaldehyde/0.1 M phosphate buffer for 18 h followed by 30% sucrose cryoprotection. Fifty-μm free-floating coronal serial cryo-sections of the forebrain were stored in solution (FD Neurotechnologies, Baltimore, MD, USA) at −20°C. Five sections (between +1.0 and 0.4 mm from bregma) were washed with phosphate-buffered saline (PBS), equilibrated to 23°C, transferred to 10 mM citrate buffer containing 0.05% Tween-20 and incubated 30 min at 80°C. Sections were then rinsed in PBS and incubated 2 h in blocking solution (2% goat serum, 1% bovine serum albumin, and 0.1% Triton X-100 in automation buffer (Biomedia, Foster City, CA, USA)). Sections were incubated with an antibody against ionized calcium-binding adapter molecule 1 (Iba-1, 1 : 500, Dako, Glostrup, Denmark) in blocking solution for 18 h at 4°C, reequilibrated to 23°C, washed with PBS, and incubated with Alexa Fluor antibody conjugates (1 : 250, Invitrogen) in blocking solution without Triton X-100 for 2 h at 23°C. Defined regions of interest were identified in the motor cortex, somatosensory cortex, and piriform cortex and standardized across plane of cut for each rat. Digital images of immunostaining were collected using a LSM 410 inverted confocal laser-scanning microscope (Carl Zeiss, Oberkochen, Germany). Image stacks were collected at 1.5 mm steps (20 ×) or 1.0 mm steps (63 ×) and displayed as a single image using 3D maximum projection.
Real-time polymerase chain reaction (qPCR)
Five- and 9-month-old HIV-tg and wt rats were lightly anesthetized with CO2, decapitated, and the brain excised from the cranium. The motor and somatosensory cortex were dissected on ice and immediately frozen on dry ice, and stored at −80°C. Cortical tissue (100 mg) was homogenized in Qiagen® lysis solution and total RNA was isolated by phenol-chloroform extraction using a RNeasy® lipid tissue mini-kit (Qiagen, Valencia, CA, USA) and 2 μg total RNA was used for reverse transcription (SuperScript™II, Invitrogen). qPCR was carried out on a Perkin Elmer ABI PRISM 7000 sequence detection system using 2.5 μL cDNA as a template, in combination with 1 Å~ Power SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA), and optimized forward and reverse primers for tumor necrosis factor alpha (TNFα) or interleukin-1 beta (IL1β). The 50 μL reaction mixtures were held at 50°C for 2 min, 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 1 min at 60°C. Amplification curves were generated with sequence detection system 1.9.1 software (Applied Biosystems). Mean-fold change in threshold cycle values over time-matched saline vehicle controls was calculated according to the ΔΔCT method (Livak and Schmittgen 2001) and normalized to ribosomal protein L32. Data (n = 4 per group) are expressed as the relative level of the target gene as fold change from control.
Western blot analysis
Cortical tissues or cortical neurons were homogenized in RIPA buffer (Millipore, MA, USA) containing protease–phosphatase inhibitors (ThermoFisher Scientific Inc., Waltham, MA, USA) by sonication at 4°C. Proteins were separated on a NuPAGE 4–12% Bis-Tris gel (Invitrogen) and transferred to a nitrocellulose membrane using iBlot device (Invitrogen). Membranes were blocked for 30 min with 5% milk in PBS and 0.05% Tween and probed with anti-acetylated α tubulin (1 : 50 000; Sigma Aldrich, St. Louis, MO, USA) in blocking buffer 1 h at 23°C. Immune complexes were detected with the corresponding secondary antibodies (Jackson ImmunoResearch, West Grove, PA, USA; 1 : 10 000 dilution) followed by chemiluminescence reagents (ThermoFisher Scientific Inc.). Membranes were stripped with Restore Western Blot Stripping Buffer (Invitrogen) for 30 min at 37°C and re-probed with anti-α-tubulin (1 : 50 000; Sigma Aldrich) in blocking buffer 1 h at 23°C to serve as a protein loading control. The intensity of immunoreactive bands was quantified as optical density using ImageJ (National Institute of Health, Bethesda, MD, USA). The ratio of optical densities of acetylated tubulin band relative to α-tubulin for each individual sample was calculated for data analysis.
Synthesis and characterization of Helix-A nano
Reagents for and details of the synthesis of mesoporous silica nanoparticles (MSNs) are described elsewhere (Avdoshina et al. 2016b). Briefly, a modified Stöber reaction was used to synthesize MSNs. MSNs were modified with 3-(aminopropyl) triethoxysilane (APTES), 2% APTES and 5% Millipore water by volume in 2-propanol at a concentration of 1 mg nanoparticle/mL APTES solution at 35°C for 2 h under constant and vigorous agitation. MSNs-APTES was conjugated to Helix-A peptide via N-(3-diethylaminopropyl)-N’-ethylcarbodiimide hydrochloride/sulfo-NHS coupling reaction and activated in a solution of 2 mM N-(3-diethylaminopropyl)-N’-ethylcarbodiimide hydrochloride/5 mM sulfo-NHS in 0.1 M MES and 0.5 M NaCl for 15 min at 1 mg/mL, as previously described (Avdoshina et al. 2016b). One mg of MSN-APTES was dispersed in the reaction solution for conjugation for 2 h at 23°C constant and vigorous agitation. The reaction was quenched with the addition of 10 mM hydroxylamine HCl. Dynamic Light Scattering and Zeta (ζ)-potential characterization were performed using a Zetasizer ZEN3600 (Malvern, Worcestershire, UK), as previously described (Avdoshina et al. 2016b).
Statistical analysis
Statistical analyses were performed using GraphPad Prism software (GraphPad Software Inc., San Diego, CA, USA). Results are expressed as mean ± SE. Data were evaluated for homogeneity of variance prior to analysis. Data from two-group comparisons by Student’s t-test or one-way ANOVA followed by multiple comparisons by Tukey’s test. Statistical significance was set at p < 0.05.
Results
Gp120 disrupts tubulin polymerization
To examine whether the reported binding of gp120 to TUBB3 (Avdoshina et al. 2016b) alters MT polymerization, we tested the ability of gp120 to inhibit tubulin polymerization in vitro. As a positive control, we used the anti-mitotic drug paclitaxel, which stabilizes MT structure (Amos and Lowe 1999). Purified tubulin from porcine brain was incubated with gp120IIIB or heat-inactivated gp120 and optical density was measured as absorbance over time as originally described (Shelanski et al. 1973; Lee and Timasheff 1977). While paclitaxel showed a clear elimination of the nucleation phase and increased the Vmax of the growth phase, gp120 elicited a reduction in nucleation, elongation, and equilibrium phases, suggesting a decrease in tubulin polymerization and MT formation (Fig. 1).
Fig. 1.

gp120 inhibits polymerization of microtubules (MTs). Purified tubulin in reaction buffer was incubated at 37°C in the absence or presence of heat-inactivated gp120 (control), gp120IIIB (50 nM) or paclitaxel (10 μM). Samples were run in duplicates. Assembly of MTs was then measured over 1 h at 1 min intervals at an absorbance of 340 nm using a spectrophotometer. The experiment was repeated twice with comparable results.
Gp120 decreases acetylation of tubulin in neurons
The stability of MTs is often associated with changes in PTMs, including tubulin acetylation (Hubbert et al. 2002; Matsuyama et al. 2002). To determine whether gp120 alters levels of acetylated tubulin (Ac-tubulin) in neurons, primary rat cortical neurons were exposed to gp120 (5 nM) at various time points and Ac-tubulin levels were determined by western blot analysis (Fig. 2). Quantitation of optical density showed that gp120 promotes a time-dependent decrease in Ac-tubulin immunoreactivity, relative to α-tubulin. The decrease is significant starting at 6 h and remained significant at least up to 24 h (Fig. 2).
Fig. 2.
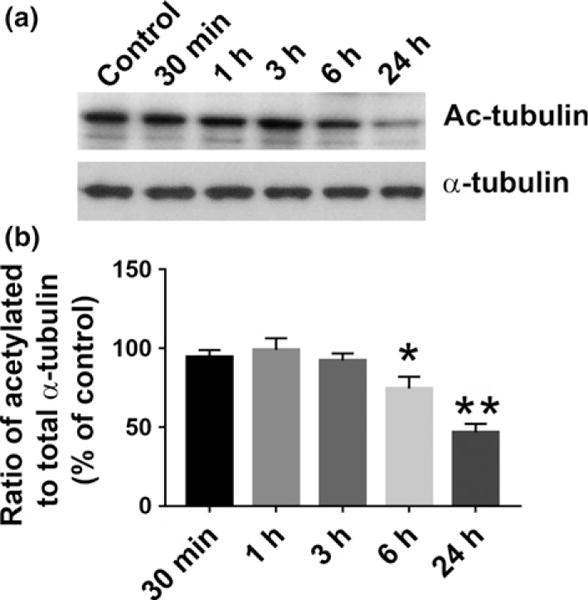
gp120 decreases acetylation of tubulin in neurons. Rat cortical neurons were exposed to gp120IIIB (5 nM) for the indicated time points. Control cells were exposed to heat-inactivated gp120. (a) Lysates (1 μg of total protein) were analyzed for Ac-tubulin by western blot using an antibody against acetylated α-tubulin. Membranes were stripped and re-probed with an antibody to α-tubulin and visualized by chemiluminescence as described in Materials and methods. (b) Semiquantification of Ac-tubulin levels (α-tubulin normalized) was performed by densitometric analysis, using ImageJ. Data, expressed as % of control, represent mean ± SEM (n = 4 each treatment). *p < 0.05, **p < 0.01 versus control (One-way ANOVA followed by Tukey test).
Helix-A peptide reverses gp120-induced tubulin acetylation
gp120 relies on a specific α-helical domain to bind directly to TUBB3 and this binding is prevented by Helix-A nano, a peptide that, when cross-linked to mesoporous silica nanoparticles, penetrates the cell membrane and prevents the binding of gp120 to TUBB3 (Avdoshina et al. 2016b). Moreover, Helix-A nano inhibits the neurotoxic effect of gp120, measured by shrinkage of microtubule-associated protein 2-positive processes (Avdoshina et al. 2016b). Therefore, we used Helix-A nano as a tool to test whether binding of gp120 to TUBB3 leads to a decrease in Ac-tubulin. Exposure of cortical neurons to Helix-A nano alone up to 24 h did not change Ac-tubulin levels when compared to control (Fig. 3). However, co-incubation with Helix-A nano prevented gp120-mediated decrease in Ac-tubulin levels (Fig. 3), supporting the hypothesis that binding of gp120 to MTs is crucial for the reduction in Ac-tubulin.
Fig. 3.

Helix-A nano inhibits gp120-mediated reduction in Ac-tubulin. Primary rat cortical neurons were exposed to heat-inactivated gp120 (control), gp120 (5 nM), Helix-A nano (5 μg/mL), alone or in combination with gp120 for 24 h. (a) One μg of protein was separated on a 4–12% Bis-Tris gel and transferred to a nitrocellulose membrane for western blot analysis of Ac-tubulin as described in the legend of Figure 2. (b) Optical density was determined using ImageJ. Data are expressed as the ratio of Ac-tubulin relative to α-tubulin. *p < 0.05 relative to control as determined by ANOVA and Tukey post hoc comparison (n = 3 each group).
HIV-tg rats exhibit decreased Ac-tubulin
Changes in Ac-tubulin by gp120 might be more relevant to explaining HAND neuropathology if they are observed also in a relevant animal model. To determine Ac-tubulin in vivo, we used HIV-tg rats. These animals express all HIV viral genes except the gag-pol replication genes and exhibits features seen in HIV-positive individuals such as T-cell abnormalities (Reid et al. 2001), alveolar epithelium dysfunction (Lassiter et al. 2009), behavior deficits (Lashomb et al. 2009), synaptic-dendritic alterations (Roscoe et al. 2014), and dopamine dysfunction (Moran et al. 2013). Moreover, HIV-tg rats express several HIV proteins in the brain, including gp120 (Royal et al. 2012). Thus, these animals are considered to be an optimal experimental model to study mechanisms of HAND. Ac-tubulin levels were determined in lysates from the cerebral cortex of 5- and 9-month-old rats because there are age-dependent changes in gp120 levels in the brain (Peng et al. 2010). Significantly lower levels of Ac-tubulin were detected by western blot in HIV-tg rats as compared to age-matched wt at both 5 and 9 months of age (Fig. 4).
Fig. 4.

Tubulin acetylation is decreased in the cortex of human immunodeficiency virus (HIV)-tg rats. Tissue lysates were prepared from the cortex of 5- and 9-month-old wt and HIV-tg rats. (a) One μg of protein was separated on a 4–12% Bis-Tris Gel and transferred to a nitrocellulose membrane. Membranes were probed with an antibody to Ac-tubulin, stripped and re-probed with a α-tubulin antibody as described in Materials and methods. (b) Optical density of each protein-associated band was determined using ImageJ. Data, expressed in arbitrary units (AU), were calculated as the ratio of Ac-tubulin relative to α-tubulin. Data are the mean ± SEM of 6 animals per group. *p < 0.05 compared to age-matched wt control (Student’s t-test).
Decreased Ac-tubulin could be a phenomenon related to neuroinflammation that has been described in these animals (Royal et al. 2012). To determine whether 5-month-old HIV-tg rats show evidence of neuroinflammation, microglial morphology and levels of two well-known pro-inflammatory cytokines, IL1β and TNFα, were examined. Microglial morphology was determined using immunohistochemistry with an antibody against Iba-1. Sampling across the somatosensory cortex showed no overt differences in microglia morphology between wt and HIV-tg rats (Fig. 5). Moreover, Iba-1 immunoreactive cells displayed a uniform morphology of fine branching with no indication of cells showing thick, shortened processes or an ameboid morphological phenotype in both wt and HIV-tg rats.
Fig. 5.
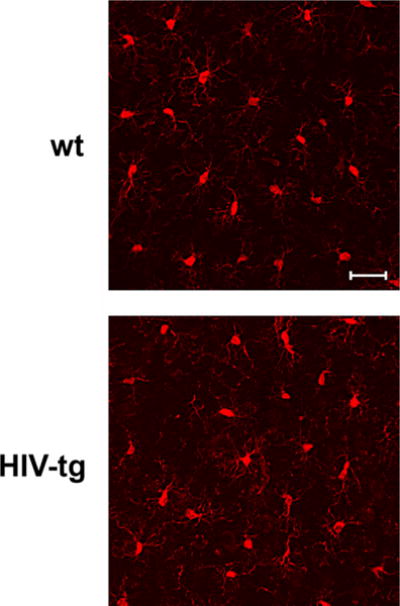
Microglia morphology does not change in human immunodeficiency virus (HIV)-tg animals. Representative immunofluorescence for ionized calcium-binding adapter molecule 1 (Iba-1)-positive cells (red) in layers IV–V of somatosensory cortex of 5-month-old wt control and HIV-tg rats. Images represent compiled z-stack images collected throughout a 50 μm section for 3–5 sections obtained from each of 4 animals per group. Iba-1-positive cells showed fine branching in both controls and HIV-tg rats with no overt changes in thickness or reduction in processes. Scale bar = 10 μm.
Consistent with the absence of a shift in microglia response, mRNA levels for TNFα and IL1β showed no difference in the cortex of HIV-tg as compared to wt control rats (Fig. 6), supporting a previous report showing no neuroinflammatory responses in young HIV-tg rats (Lee et al. 2015).
Fig. 6.
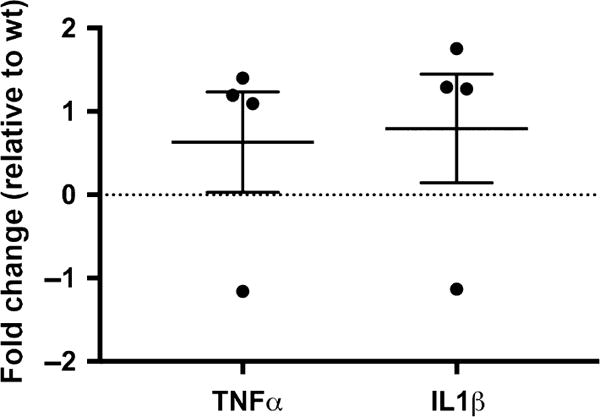
Five-month-old human immunodeficiency virus (HIV)-tg animals do not exhibit increased pro-inflammatory cytokines. Cortical tissues from wt and HIV-tg rat were used to determine tumor necrosis factor alpha (TNFα) or IL1β mRNA levels by real-time polymerase chain reaction (qPCR), as described in Materials and methods. The graph shows the single value for each rat as well as the mean ± SD of the combined data.
Discussion
Despite the lack of direct infection, neurons remain a prime target for the toxic adverse effects of HIV. Knowledge of the mechanisms whereby HIV causes neurodegeneration is invaluable in understanding HAND-associated neuropathology. Although massive neuronal loss may be responsible for the dementia observed in severe cases of HAND, it appears that neuronal apoptosis is a late event and does not represent the main pathological substrate of asymptomatic or mild HAND (Clifford and Ances 2013). Furthermore, the relatively rapid onset and progression of neurological decline does not support neuronal loss as the primary cause of HAND. Therefore, HAND more likely reflects a specific neuronal dysfunction resulting from the combined effects of several mechanisms. Our data suggest that HIV, through gp120, causes an initial “traumatic” event in axons and dendrites that may culminate in retrograde degeneration and synaptic simplifications seen in HAND.
It is well established that gp120 is internalized by neurons (Bachis et al. 2006; Berth et al. 2015), binds to mannose-binding lectin (Teodorof et al. 2014), and is retrogradely transported along the axon to the soma (Bachis et al. 2006). During axonal transport, gp120 binds with high affinity to the carboxy terminal of at least two neuronal-specific tubulin isoforms, TUBB3 and Δ2 isoform of TUB α1/β1 (Avdoshina et al. 2016b). Here, we show that gp120 reduces MT polymerization and acetylation of α-tubulin, suggesting that gp120 binding to MTs results in the destabilization of the cytoskeleton and its function. These results are corroborated by the finding that gp120 decreases the ability of MTs to transport mitochondria (Avdoshina et al. 2016a) in neurons. Abnormal mitochondrial transport has been implicated in the progression of multiple neurodegenerative diseases, including Huntington’s (Chang et al. 2006), Parkinson’s (Wild and Dikic 2010), and Alzheimer’s disease (Anandatheerthavarada et al. 2003). However, altered mitochondria transport in these diseases is brought about by different molecular and cellular mechanisms. For instance, in Huntington’s disease, mutated huntingtin inhibits axonal transport by acting at kinesin-1 motor protein (Morfini et al. 2009) or by interacting with mitochondrial fission GTPase, which regulates mitochondrial transport (Song et al. 2011). Our data suggest that in HAND, gp120 impairs tubulin function by binding to tubulin isoforms and inhibiting proper assembly of MTs. In this manner, gp120 can then disrupt normal axonal transport that is critical for neuronal maintenance and survival.
In this study, we provide evidence that the HIV envelope protein interferes with normal regulation of the cytoskeleton, both by inhibiting MT polymerization and reducing acetylation of tubulin in neurons. These changes may reduce MT functionality, including trafficking of mitochondria (Avdoshina et al. 2016a), which is crucial for neuronal survival (Belzil et al. 2013; Franker and Hoogenraad 2013; Nahm et al. 2013). We also show that lower levels of Ac-tubulin are present in the cortex of HIV-tg rats when compared to wt even in the absence of a concurrent neuroinflammatory response as measured by cytokine production and changes in microglia morphology. Thus, it appears that the alteration in cytoskeleton function observed in our studies precedes the ongoing neuroinflammation that has been previously proposed as one of the mechanisms of HAND. This consideration may explain why HAND still persists even though the antiretroviral therapy has reduced the cases of encephalitis (Gelman 2015). Thus, we propose that the direct interaction of gp120 with components of the cytoskeleton could significantly contribute to the loss of synaptic connectivity and the slow but progressive neurodegeneration seen in HAND subjects.
Helix-A peptide, which has been shown to displace gp120 from binding to TUBB3, prevents the degeneration of neuronal processes as well as improves neuronal survival (Avdoshina et al. 2016b). In this study, we show that Helix-A peptide reduces the ability of gp120 to decrease Ac-tubulin. Our data provide additional evidence that gp120 binding to TUBB3 promotes neuronal degeneration by disruption of MT stability. The role of decreased Ac-tubulin in the overall neurotoxic effect of gp120, however, is, at present, only speculative. Although acetylation of tubulin is frequently associated with stable MTs, acetylation itself does not stabilize MTs (Haggarty et al. 2003). Nevertheless, acetylation at Lys 40 of α-tubulin plays a role in recruiting molecular motors such as kinesin-1 and dynein/dynactin (Reed et al. 2006; Dompierre et al. 2007), suggesting that acetylation of tubulin activates both anterograde and retrograde trafficking. Whether gp120 inhibits kinesin-1 or other molecular motors still remains a question. In addition, tubulin acetylation may decrease binding of tubulin-associated proteins, such as Tau, which affects microtubule polymerization in neurons. (Popov et al. 2001; Fauquant et al. 2011; Janning et al. 2014). Clearly, this is a complex mechanism that warrants further investigation.
Acknowledgments
This work was supported by US National Institute of Health grants NS079172 and NS074916 (to I.M.), P30AI087714 (pilot award from DC D-CFAR to V.A.), NS041218 (T32 to E.D.W.) and the Division of National Toxicology Program, National Institute for Environmental Health Sciences #1Z01ES101623.
All experiments were conducted in compliance with the ARRIVE guidelines.
Abbreviations
- Ac-tubulin
acetylated tubulin
- APTES
3-(aminopropyl) triethoxysilane
- AU
arbitrary units
- cART
combined antiretroviral therapy
- EDC
N-(3-diethylaminopropyl)-N’-ethylcarbodiimide hydrochloride
- HAND
HIV-associated neurocognitive disorders
- HIV
Human immunodeficiency virus-1
- HIV-tg
HIV transgenic rats
- Iba-1
ionized calcium-binding adapter molecule 1
- IL1β
interleukin-1 beta
- MAP2
microtubule-associated protein 2
- MSNs
mesoporous silica nanoparticles
- MTs
microtubules
- PBS
phosphate-buffered saline
- PTMs
post-translational modifications
- qPCR
real-time polymerase chain reaction
- Sulfo-NHS
NH4F and N-hydroxysulfosuccinimide
- TNFα
tumor necrosis factor alpha
- TUBB3
tubulin β-3
- wt
wild type
Footnotes
Conflict of interest disclosure
The authors declare that they have no conflicts of interest.
References
- Amos LA, Lowe J. How Taxol stabilises microtubule structure. Chem Biol. 1999;6:R65–R69. doi: 10.1016/s1074-5521(99)89002-4. [DOI] [PubMed] [Google Scholar]
- Anandatheerthavarada HK, Biswas G, Robin MA, Avadhani NG. Mitochondrial targeting and a novel transmembrane arrest of Alzheimer’s amyloid precursor protein impairs mitochondrial function in neuronal cells. J Cell Biol. 2003;161:41–54. doi: 10.1083/jcb.200207030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avdoshina V, Fields JA, Castellano P, et al. The HIV protein gp120 alters mitochondrial dynamics in neurons. Neurotox Res. 2016a;29:583–593. doi: 10.1007/s12640-016-9608-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avdoshina V, Taraballi F, Dedoni S, Corbo C, Paige M, Saygideger Kont Y, Uren A, Tasciotti E, Mocchetti I. Identification of a binding site of the human immunodeficiency virus envelope protein gp120 to neuronal-specific tubulin. J Neurochem. 2016b;137:287–298. doi: 10.1111/jnc.13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Major EO, Mocchetti I. Brain-derived neurotrophic factor inhibits human immunodeficiency virus-1/gp120-mediated cerebellar granule cell death by preventing gp120 internalization. J Neurosci. 2003;23:5715–5722. doi: 10.1523/JNEUROSCI.23-13-05715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Aden SA, Nosheny RL, Andrews PM, Mocchetti I. Axonal transport of human immunodeficiency virus type 1 envelope protein glycoprotein 120 is found in association with neuronal apoptosis. J Neurosci. 2006;26:6771–6780. doi: 10.1523/JNEUROSCI.1054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird FJ, Bennett CL. Microtubule defects & Neurodegeneration. J Genet Syndr Gene Ther. 2013;4:203. doi: 10.4172/2157-7412.1000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzil C, Neumayer G, Vassilev AP, et al. A Ca2 + -dependent mechanism of neuronal survival mediated by the microtubule-associated protein p600. J Biol Chem. 2013;288:24452–24464. doi: 10.1074/jbc.M113.483107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berth S, Caicedo HH, Sarma T, Morfini G, Brady ST. Internalization and axonal transport of the HIV glycoprotein gp120. ASN Neuro. 2015;7:1–15. doi: 10.1177/1759091414568186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunden KR, Trojanowski JQ, Smith AB, 3rd, Lee VM, Ballatore C. Microtubule-stabilizing agents as potential therapeutics for neurodegenerative disease. Bioorg Med Chem. 2014;22:5040–5049. doi: 10.1016/j.bmc.2013.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Rippeth JD, Heaton RK, Grant I. Prospective memory in HIV-1 infection. J Clin Exp Neuropsychol. 2006;28:536–548. doi: 10.1080/13803390590949494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartelli D, Ronchi C, Maggioni MG, Rodighiero S, Giavini E, Cappelletti G. Microtubule dysfunction precedes transport impairment and mitochondria damage in MPP+ -induced neurodegeneration. J Neurochem. 2010;115:247–258. doi: 10.1111/j.1471-4159.2010.06924.x. [DOI] [PubMed] [Google Scholar]
- Chang DT, Rintoul GL, Pandipati S, Reynolds IJ. Mutant huntingtin aggregates impair mitochondrial movement and trafficking in cortical neurons. Neurobiol Dis. 2006;22:388–400. doi: 10.1016/j.nbd.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Chapin SJ, Bulinski JC. Cellular microtubules heterogeneous in their content of microtubule-associated protein 4 (MAP4) Cell Motil Cytoskelet. 1994;27:133–149. doi: 10.1002/cm.970270205. [DOI] [PubMed] [Google Scholar]
- Clifford DB, Ances BM. HIV-associated neurocognitive disorder. Lancet Infect Dis. 2013;13:976–986. doi: 10.1016/S1473-3099(13)70269-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dompierre JP, Godin JD, Charrin BC, Cordelieres FP, King SJ, Humbert S, Saudou F. Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington’s disease by increasing tubulin acetylation. J Neurosci. 2007;27:3571–3583. doi: 10.1523/JNEUROSCI.0037-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erck C, Peris L, Andrieux A, et al. A vital role of tubulin-tyrosine-ligase for neuronal organization. Proc Natl Acad Sci USA. 2005;102:7853–7858. doi: 10.1073/pnas.0409626102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauquant C, Redeker V, Landrieu I, Wieruszeski JM, Verdegem D, Laprevote O, Lippens G, Gigant B, Knossow M. Systematic identification of tubulin-interacting fragments of the microtubule-associated protein Tau leads to a highly efficient promoter of microtubule assembly. J Biol Chem. 2011;286:33358–33368. doi: 10.1074/jbc.M111.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franker MA, Hoogenraad CC. Microtubule-based transport - basic mechanisms, traffic rules and role in neurological pathogenesis. J Cell Sci. 2013;126:2319–2329. doi: 10.1242/jcs.115030. [DOI] [PubMed] [Google Scholar]
- Gelman BB. Neuropathology of HAND with suppressive antiretroviral therapy: encephalitis and neurodegeneration reconsidered. Curr HIV/AIDS Rep. 2015;12:272–279. doi: 10.1007/s11904-015-0266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggarty SJ, Koeller KM, Wong JC, Grozinger CM, Schreiber SL. Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc Natl Acad Sci USA. 2003;100:4389–4394. doi: 10.1073/pnas.0430973100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond JW, Huang CF, Kaech S, Jacobson C, Banker G, Verhey KJ. Posttranslational modifications of tubulin and the polarized transport of kinesin-1 in neurons. Mol Biol Cell. 2010;21:572–583. doi: 10.1091/mbc.E09-01-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- Janke C. The tubulin code: molecular components, readout mechanisms, and functions. J Cell Biol. 2014;206:461–472. doi: 10.1083/jcb.201406055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janning D, Igaev M, Sundermann F, et al. Single-molecule tracking of tau reveals fast kiss-and-hop interaction with microtubules in living neurons. Mol Biol Cell. 2014;25:3541–3551. doi: 10.1091/mbc.E14-06-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BO, Liu Y, Ruan Y, Xu ZC, Schantz L, He JJ. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am J Pathol. 2003;162:1693–1707. doi: 10.1016/S0002-9440(10)64304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, Flavin M. Modulation of some parameters of assembly of microtubules in vitro by tyrosinolation of tubulin. Eur J Biochem. 1982;128:215–222. doi: 10.1111/j.1432-1033.1982.tb06954.x. [DOI] [PubMed] [Google Scholar]
- Lashomb AL, Vigorito M, Chang SL. Further characterization of the spatial learning deficit in the human immunodeficiency virus-1 transgenic rat. J Neurovirol. 2009;15:14–24. doi: 10.1080/13550280802232996. [DOI] [PubMed] [Google Scholar]
- Lassiter C, Fan X, Joshi PC, Jacob BA, Sutliff RL, Jones DP, Koval M, Guidot DM. HIV-1 transgene expression in rats causes oxidant stress and alveolar epithelial barrier dysfunction. AIDS Res Ther. 2009;6:1. doi: 10.1186/1742-6405-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Timasheff SN. In vitro reconstitution of calf brain microtubules: effects of solution variables. Biochemistry. 1977;16:1754–1764. doi: 10.1021/bi00627a037. [DOI] [PubMed] [Google Scholar]
- Lee DE, Yue X, Ibrahim WG, et al. Lack of neuroinflammation in the HIV-1 transgenic rat: an [(18)F]-DPA714 PET imaging study. J Neuroinflammation. 2015;12:171. doi: 10.1186/s12974-015-0390-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Masliah E, Heaton RK, Marcotte TD, et al. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group The HIV Neurobehavioral Research Center. Ann Neurol. 1997;42:963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- Matsuyama A, Shimazu T, Sumida Y, et al. In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J. 2002;21:6820–6831. doi: 10.1093/emboj/cdf682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur JC, Brew BJ, Nath A. Neurological complications of HIV infection. Lancet Neurol. 2005;4:543–555. doi: 10.1016/S1474-4422(05)70165-4. [DOI] [PubMed] [Google Scholar]
- McMurray CT. Neurodegeneration: diseases of the cytoskeleton? Cell Death Differ. 2000;7:861–865. doi: 10.1038/sj.cdd.4400764. [DOI] [PubMed] [Google Scholar]
- Moran LM, Booze RM, Webb KM, Mactutus CF. Neurobehavioral alterations in HIV-1 transgenic rats: evidence for dopaminergic dysfunction. Exp Neurol. 2013;239:139–147. doi: 10.1016/j.expneurol.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini GA, You YM, Pollema SL, et al. Pathogenic huntingtin inhibits fast axonal transport by activating JNK3 and phosphorylating kinesin. Nat Neurosci. 2009;12:864–871. doi: 10.1038/nn.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahm M, Lee MJ, Parkinson W, et al. Spartin regulates synaptic growth and neuronal survival by inhibiting BMP-mediated microtubule stabilization. Neuron. 2013;77:680–695. doi: 10.1016/j.neuron.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogales E. Structural insights into microtubule function. Annu Rev Biochem. 2000;69:277–302. doi: 10.1146/annurev.biochem.69.1.277. [DOI] [PubMed] [Google Scholar]
- Nosheny RL, Bachis A, Aden SA, De Bernardi MA, Mocchetti I. Intrastriatal administration of human immunodeficiency virus-1 glycoprotein 120 reduces glial cell-line derived neurotrophic factor levels and causes apoptosis in the substantia nigra. J Neurobiol. 2006;66:1311–1321. doi: 10.1002/neu.20288. [DOI] [PubMed] [Google Scholar]
- Peng J, Vigorito M, Liu X, Zhou D, Wu X, Chang SL. The HIV-1 transgenic rat as a model for HIV-1 infected individuals on HAART. J Neuroimmunol. 2010;218:94–101. doi: 10.1016/j.jneuroim.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Peris L, Wagenbach M, Lafanechere L, Brocard J, Moore AT, Kozielski F, Job D, Wordeman L, Andrieux A. Motor-dependent microtubule disassembly driven by tubulin tyrosination. J Cell Biol. 2009;185:1159–1166. doi: 10.1083/jcb.200902142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov AV, Pozniakovsky A, Arnal I, Antony C, Ashford AJ, Kinoshita K, Tournebize R, Hyman AA, Karsenti E. XMAP215 regulates microtubule dynamics through two distinct domains. EMBO J. 2001;20:397–410. doi: 10.1093/emboj/20.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed NA, Cai D, Blasius TL, Jih GT, Meyhofer E, Gaertig J, Verhey KJ. Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol. 2006;16:2166–2172. doi: 10.1016/j.cub.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Reid W, Sadowska M, Denaro F, et al. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci USA. 2001;98:9271–9276. doi: 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscoe RF, Jr, Mactutus CF, Booze RM. HIV-1 transgenic female rat: synaptodendritic alterations of medium spiny neurons in the nucleus accumbens. J Neuroimmune Pharmacol. 2014;9:642–653. doi: 10.1007/s11481-014-9555-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal W, 3rd, Zhang L, Guo M, Jones O, Davis H, Bryant JL. Immune activation, viral gene product expression and neurotoxicity in the HIV-1 transgenic rat. J Neuroimmunol. 2012;247:16–24. doi: 10.1016/j.jneuroim.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelanski ML, Gaskin F, Cantor CR. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci USA. 1973;70:765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Chen J, Petrilli A, et al. Mutant huntingtin binds the mitochondrial fission GTPase dynamin-related protein-1 and increases its enzymatic activity. Nat Med. 2011;17:377–382. doi: 10.1038/nm.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich S, Gonzalez-Scarano F. HIV-1-related central nervous system disease: current issues in pathogenesis, diagnosis, and treatment. Cold Spring Harb Perspect Med. 2012;2:a007120. doi: 10.1101/cshperspect.a007120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teodorof C, Divakar S, Soontornniyomkij B, Achim CL, Kaul M, Singh KK. Intracellular mannose binding lectin mediates subcellular trafficking of HIV-1 gp120 in neurons. Neurobiol Dis. 2014;69:54–64. doi: 10.1016/j.nbd.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367:188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- Wild P, Dikic I. Mitochondria get a Parkin’ ticket. Nat Cell Biol. 2010;12:104–106. doi: 10.1038/ncb0210-104. [DOI] [PubMed] [Google Scholar]
- Wloga D, Gaertig J. Post-translational modifications of microtubules. J Cell Sci. 2010;123:3447–3455. doi: 10.1242/jcs.063727. [DOI] [PMC free article] [PubMed] [Google Scholar]


