Abstract
Inflammation is emerging as a critical mechanism underlying neurological disorders of various etiologies, yet its role in altering brain function as a consequence of neuroinfectious disease remains unclear. Although acute alterations in mental status due to inflammation are a hallmark of central nervous system (CNS) infections with neurotropic pathogens, post-infectious neurologic dysfunction has traditionally been attributed to irreversible damage caused by the pathogens themselves. More recently, studies indicate that pathogen eradication within the CNS may require immune responses that interfere with neural cell function and communication without affecting their survival. In this Review we explore inflammatory processes underlying neurological impairments caused by CNS infection and discuss their potential links to established mechanisms of psychiatric and neurodegenerative diseases.
Neuroinfectious diseases are associated with acute changes in mental and motor function that are followed by chronic neurological dysfunction that can persist long after recovery from the infectious event1–4. During the acute stage, CNS invasion by neurotropic pathogens activates inflammatory responses that control replication and/or coordinate their elimination. Brain cells, including resident macrophages and microglia, endothelial cells, ependymal cells, neurons and glia (astrocytes and oligodendrocytes) express innate immune molecules that induce the recruitment of leukocytes into infected CNS compartments to promote pathogen clearance. Innate immune responses also induce the expression of inflammatory mediators, which may exert cell- and region-specific influences on brain function (Fig. 1). Thus, initial inflammatory events during pathogen neuroinvasion are often associated with clinical signs that not only identify the CNS site of infection but are the result of pathophysiological events that affect blood–brain barrier (BBB) function, cerebral metabolism, oxygen consumption and blood flow. Clinical signs of acute infection induce adaptive changes in behavior, including fatigue, hypersomnia, depressed activity, decreased social interactions and inability to concentrate5. These behavioral effects are mediated by cytokines and serve to conserve energy and promote survival. However, inflammatory responses during neuroinfectious diseases may progress to focal neurological deficits, delirium, obtundation or even coma. The role of the inflammatory response in worsening morbidity and mortality during acute infections of the CNS is also evidenced by the standard use of anti-inflammatory agents to limit immune system–mediated effects6 and the lack of prodromal and focal symptoms observed in immuno-compromised hosts7.
Figure 1.
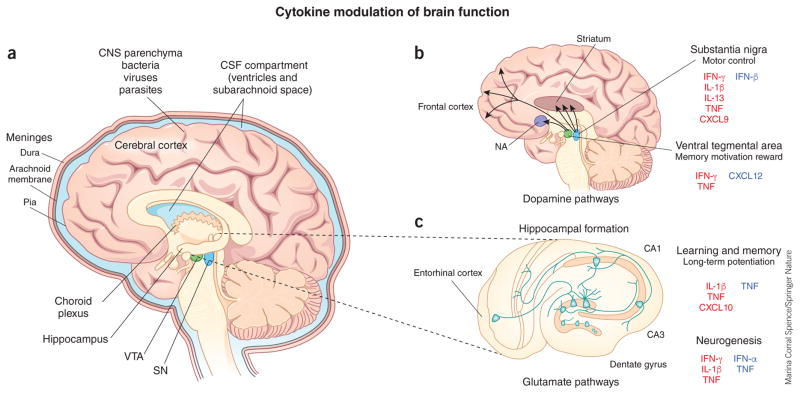
Neuroinfectious diseases and cytokine modulation of brain functions. (a) Neurotropic pathogens gain access to the CNS parenchyma (bacteria, viruses and parasites) or CSF compartments (bacteria, viruses and fungi), the latter of which includes the subarachnoid space and the ventricular system. (b, c) Increased expression of cytokines and chemokines modulate brain function via effects on dopaminergic pathways (b) and glutamatergic pathways (c). Dopamine is manufactured in the substantia nigra (SN), which affects motor function, and in the ventral tegmental area (VTA), which is involved in memory, motivation and reward. Dopaminergic neurons of the SN project to the dorsal striatum, and those of the VTA project to the nucleus accumbens (NA) and the prefrontal cortex. Glutamatergic pathways are involved in learning and memory formation, which also require long-term potentiation, characterized by a persistent increase in synaptic strength following high-frequency stimulation, and genesis of new neurons within the dentate gyrus (adult neurogenesis). Molecules labeled in red have inhibitory effects; those labeled in blue have stimulatory effects.
Historically, post-infection neurological sequelae have been attributed to acute neuronal death caused by the invading pathogen or the immune response to it. Animal models of neuroinfectious diseases support the idea that CNS injury mediated by pathogens or lymphocytes underlies permanent loss of motor and cognitive function8. Explanations for the focality of neurological symptoms in patients thus rely on the CNS regions acutely infected, as determined on the basis of initial MRI results9. However, neurological dysfunction in patients that survive CNS infections is not always consistent with prior neuroimaging findings10, and rodent models of neuroinfectious diseases do not always show extensive brain damage in survivors11. In addition, chronic complications from neuroinfectious diseases have been linked to ongoing inflammatory processes within the CNS that are acutely induced by innate and adaptive immune responses to infectious agents but may persist in the absence of ongoing pathogen replication and exhibit different cell- or region-specific effects from those of the inciting pathogen12–14. Moreover, events triggered in the acute setting of CNS infection may lead to neurological sequelae via mechanisms similar to those observed with normal aging or in noninfectious, neurological disorders such as Alzheimer’s disease12,15. Thus, in contrast to acute infection, post-infectious, chronic inflammatory processes within the CNS can cause maladaptive behavioral alterations that manifest as depression, decreased cognition, impaired learning or loss of fine adjustments in motor and mental functions. In this Review we explore chronic inflammatory processes underlying neurological impairments due to CNS infection and discuss their potential links to established mechanisms of psychiatric or neurodegenerative disorders.
CNS anatomy dictates immune responses to invading pathogens
The CNS has a high degree of anatomic and cellular heterogeneity that exerts profound effects on most facets of brain function and behavior and may provide insights into various aspects of disease pathogenesis during infections. The meninges, CNS parenchyma and ventricular system each have distinct vascular structural components that may limit the entry of certain pathogens and particular leukocyte subsets while providing abluminal localizing cues that promote immune cell interactions16. Fluids from each of these compartments, along with cerebrospinal fluid (CSF), which is formed continuously by the choroid plexus within the fourth ventricle, drain into cervical lymph nodes via lymphatic vessels that may also contain antigen-specific immune cells17. Parenchymal interstitial fluids drain through paravenous pathways, which have basement membranes that may limit the egress of immune cells18. The CNS parenchyma is divided into forebrain, midbrain and hindbrain regions that contain gray and white matter areas of neuronal cell bodies and their myelinated tracts, respectively. Neurons, astrocytes, oligodendrocytes and microglia within disparate CNS regions show extensive functional and immunological heterogeneity that may result in regional differences in innate and adaptive immune responses19,20. Here we will briefly discuss the vascular specializations and cell- and region-specific proinflammatory responses within CSF and parenchymal compartments that control acute infections and leukocyte recruitment (reviewed in this issue)21. These CNS compartment–specific responses may also promote post-infection complications via inflammatory molecule–mediated alterations in neurological pathways that affect motor and cognitive functions such as motivation and reward, memory, learning behaviors and functional fine tuning (Table 1).
Table 1.
Cytokine effects on neurotransmitter systems and neuronal function
| Cytokine | Effect |
|---|---|
| IL-1β | Degeneration of dopaminergic neurons with age-related decline in motor skills, Parkinsonism143 |
| Inhibition of long-term potentiation144 | |
| Blocking of cerebellar function145 | |
| TNF | Induction of death of dopaminergic neurons via miRNA targeting of mitochondrial complex-1 (ref. 146) |
| Blocking morphine-induced activation of ventral tegmental (reward) dopaminergic neurons147 | |
| Cognitive and motor deficits due to hyperexcitability from increased long-term potentiation148–150 | |
| Enhancement (TNFR2) and inhibition (TNFR1) of adult neurogenesis151 | |
| Cognitive dysfunction via a mechanism that involves TNFR1 signaling in astrocytes, which modify excitatory synapses within the dentate gyrus152 | |
| IFN-γ | Induction of CXCL9-mediated damage to dopaminergic neurons153 |
| Induction of CXCL10-mediated depression of long-term potentiation154 | |
| Enhancement of adult neurogenesis155 | |
| Cerebellar dysfunction156 | |
| IFN-α | Depression induced via activation of indoleamine-2,3-dioxygenase (IDO), which increases kynurenic acid (KA) and decreases quinolinic acid (QA), both neuroactive metabolites; activation of the kynurenine pathway leads to reduced formation of the neurotransmitters serotonin and dopamine157 |
| Excitotoxicity or decreased glutamatergic signaling caused by binding of NMDA receptors by QA (produced by microglia) or KA (produced by astrocytes), respectively, inhibiting synaptic plasticity and adult neurogenesis157,158 |
Bacteria, fungi, viruses and parasites may initially gain access to the CSF across vessels within the meninges and ventricular system, which are fenestrated and nonrestrictive22. In comparison, CNS parenchymal vessels have specializations that provide a barrier to blood-borne pathogens, cells and large molecules. The meningeal arachnoid membrane and ventricular choroid plexus epithelial cells have intercellular tight junctions, comprising claudins 1, 2 and 3 (ref. 23), that help restrict the invasion of pathogens into the CNS parenchyma. CSF circulates throughout the ventricular system and subarachnoid space of the meninges via apertures between these compartments24, allowing detection of pathogens via meningeal CSF sampling. During pathogen invasion, meningeal cells express the cellular adhesion molecule ICAM-1 and the neutrophil chemoattractant CXCL2 (refs. 25,26), and brain endothelial cells upregulate ICAM-2 and the neutrophil chemoattractants leukotriene B4 and complement component C5a27,28. The infiltration of neutrophils into these compartments is critical for pathogen clearance but leads to the clinical signs of meningitis, a classic triad of headache, neck stiffness (meningismus) and photophobia. These signs are the result of catecholamine expression by phagocytes exposed to bacterial products29, which promotes mydriasis, leading to excessive transfer of light to the brain and vasospasm30. Antigen-presenting cells (APCs) that reside within the choroid plexus and meninges express major histocompatibility complex class II and the C-type lectin receptor DNGR-1, also known as CLEC9A31, a marker of dendritic cell subsets with functional similarity to lymphoid and tissue dendritic cells. These resident APCs provide a mechanism for local restimulation of infiltrating T cells, which is required for their extravasation into the CNS parenchyma32,33. High concentrations of proinflammatory cytokines within the CSF of patients with infectious meningitis are associated with impaired cognition and correlate with poor outcome34,35. Resolution of meningitis requires both administration of antimicrobials and immunocompetence36, the latter of which enables the recruitment of lymphocytes and monocytes in response to upregulation of vascular cell adhesion molecule 1 (VCAM-1) and expression of chemoattractants such as CCL5, CXCL9, CXCL10 and CXCL11 on vessels37. Although baseline surveillance of the meninges and choroid plexus by interleukin 4 (IL-4)-expressing CD4+ type 2 helper T (TH2) cells is critical for the performance of cognitive tasks38, high concentrations of TH1 cytokines such as IL-1β, tumor necrosis factor (TNF) and interferon-γ (IFN-γ) may result in continued cognitive impairment after bacterial meningitis, as suggested by studies in animal models using agents that target these molecules3. Although the mechanisms that cause cognitive impairment during acute meningitis are incompletely understood, studies suggest involvement of global and regional disruptions in neurogenesis39, synaptic coupling40 and neuronal circuitry41, all of which underlie various aspects of perception, mood, learning and memory formation.
Within the CNS, the BBB functions as a communication conduit with the immune system. It is a highly selective barrier that separates the CNS parenchyma from the blood at the capillary and post-capillary levels while responding to luminal and abluminal immune signals, coordinating interactions between cells at both interfaces during CNS infections32. The cellular constituents of the BBB form the neurovascular unit (NVU), comprising endothelial cells with ensheathing pericytes and astrocyte endfeet, which modulates BBB integrity and responds to infiltrating pathogens, immune cells and the metabolic demands of neurons42. The functional integrity of the BBB is achieved by junctional complexes that prevent permeation of solute, cells and pathogens through paracellular routes and connect BBB endothelial cells to each other and to the cytoskeleton via scaffolding proteins. Adherens junctions are comprised of cadherin proteins and link to actin filaments via α-, β- and γ-catenin, while tight junctions, formed by occludins and claudins, link to the cystokeleton via the scaffolding and regulatory proteins ZO-1, ZO-2, ZO-3 and cingulin. Activation of Rho GTPases regulates the length of actin fibers, which in turn controls the integrity of both adherens junction and tight junction complexes. Claudin 3 or claudin 5 is required for tight junction formation and may be decreased in the setting of certain neuroinfectious diseases43.
Host detection of pathogen-associated molecular patterns (PAMPs) by pattern recognition receptors (PRRs) at the NVU directly regulates BBB integrity and tight junction formation via induction of innate cytokines (Table 2), including type I i (IFN-α and IFN-β), type III interferon (IFN-λ), TNF and IL-1β, which differentially activate the small GTPases Rac1 and RhoA44,45. Direct signaling of the type I interferon receptor, IFNAR, and of the IFN-λ receptor, IFNLR, at the NVU promotes BBB closure, limiting further entry of neurotropic viruses, whereas TNF and IL-1β open the barrier. Brain endothelial cells and astrocytes also express the TAM receptors Mertk and Axl, receptor tyrosine kinases that diminish host innate immune responses upon binding with their ligands, Gas6 and Protein S. Mertk and Axl recognize phosphatidylserine displayed by the outer membrane of enveloped viruses46. IFNAR and Mertk synergistic signaling at the BBB ‘preferentially’ activates the cytoskeletal regulatory GTPase Rac1, which enhances endothelial barrier function and limits the expression of barrier-disrupting inflammatory cytokines, including IL-1β and TNF47. Cytokine-mediated disruption of the BBB may impair cognition and motor function via increased leukocyte entry into the hippocampus or basal ganglia, as has been observed in post-surgical patients and in those with Parkinson’s disease or CNS lupus48–50. In addition, CXCL10 expressed downstream of IFNAR signaling in brain endothelial cells induces lethargy and cognitive dysfunction during viral infection via impaired presynaptic release of neurotransmitter within the hippocampus, weakening synaptic long-term potentiation51.
Table 2.
Infections of the CSF compartment and NVU cytokine expression
| Pathogen | Brain endothelial cells | Choroid plexus epithelial cells | Astrocytes | Meninges or CSF |
|---|---|---|---|---|
| Bacteria | ||||
| Mtb | NIA | NIA | TNF, IL-6, ROS159 | NIA |
| S. pneumoniae | NO160,161; IL-8, CXCL1, CXCL2, CCL20, IL-6 (ref. 27) | NIA | IL-6, TNF162 | NIA |
| N. meningitidis | IL-6, IL-8 (ref. 163); IL-6, TNF164 | CXCL1–3, IL-6, IL-8, TNF, G-CSF and GM-CSF165 | IL-19 (ref. 166) | IL-6, IL-1, TNF167 |
| Fungi | ||||
| C. neoformans | High-mobility group box 1 protein168 | NIA | NO169 | NIA |
| Viruses | ||||
| Enteroviruses | IL-6, MIF, CCL2 (ref. 170) | CXCL1–3, IL-8, CCL5 (ref. 171) | IL-6, IL-8, CCL5, CXCL10 (ref. 172) | NIA |
| Herpes simplex viruses | NIA | NIA | IFN-α, IFN-β173; TNF, IL-6 (ref. 174) | NIA |
NIA, no information available.
Pathogenic invasion of the CNS parenchyma is met with strong cell- and region-specific local innate immune responses that limit replication and cellular tropism while inducing antimicrobial responses throughout the CNS. The initial immune response depends on the route of entry; viruses may invade via anterograde or retrograde trafficking along neurites from peripheral neurons, including those of the olfactory neuroepithelium and spinal tracts, whereas other pathogens enter via paracellular or transcellular paths across the BBB22. PRRs are expressed along all routes of invasion, inducing interferons upon PAMP-induced activation52 (Box 1).
Box 1. Enhanced innate immune responses of hindbrain neurons and astrocytes.
Astrocytes and neurons in hindbrain regions show enhanced immune signatures at baseline and in response to an inflammatory stimulus. Early in vitro experiments demonstrated that brain stem astrocytes had higher expression of major histocompatibility complex class II and ICAM-I than various forebrain structures58. Cerebellar and spinal cord astrocytes additionally released higher basal levels of many chemokines and cytokines in vitro, including IL-1β, CCL2, IL-6 and IL-9, than cortical astrocytes, and brain region had specific effects on chemokine or cytokine expression after exposure to HIV proteins175. In situ hybridization studies have shown that interferon-stimulated gene ISG-49 (also known as IFIT-3) is highly expressed in the Purkinje cell layer, corpus callosum and choroid plexus after lymphocytic choriomeningitis virus infection. In contrast, ISG-56 (IFIT-1) is most highly expressed in the olfactory bulb and olfactory neurons. Striking differences in ISG expression occur even in neuronal subpopulations within a given region. For example, whereas ISG-49 is present at high concentrations in Purkinje cells and molecular and granule neurons of the cerebellum, ISG-56 is primarily restricted to the granule neuron layer and ISG-54 (IFIT-2) is found in the Purkinje cell layer. Granule cell neurons also express high amounts of ISG-49 and ISG-56 after WNV infection176. Differences in expression of innate immune-associated genes can be seen at baseline. Microarray analysis comparing granule cell neurons of the cerebellum to cortical neurons of the cerebral cortex has shown that expression of interferon-related genes, including Ifit1, Irf7, Stat1 and OasI, is higher in cerebellar granule cell neurons. Granule cell neurons show increased resistance to viral infection at baseline and in response to IFN-β treatment. The kinetics response to IFN-β treatment is also increased in cerebellar granule cell neurons, as several genes including Ifit1, Rsad2 and Oas reached peak expression levels at an earlier time point than did cortical neurons177.
Astrocytes have a variety of neuroprotective functions, including buffering of ions, glutamate and other neurotransmitters and regulation of synaptic function, neuronal repair and BBB integrity, in addition to producing neurotrophic factors and anti-inflammatory cytokines such as IL-10 (refs. 53,54). Astrocyte heterogeneity during physiological conditions is based on their morphology, function and localization in white and gray matter and is well established55. However, studies indicate substantial regional heterogeneity in astrocyte function during neuroinflammatory disease and injury41,56. In vitro baseline expression of PRRs, cytokines and chemokines varies among astrocytes derived from different regions of the CNS57,58, supporting in vivo observations of differences in susceptibility and clearance between brain regions during neuroinfectious diseases (Box 1). After infection, astrocytes respond to Toll-like receptor and NOD-like receptor signals by expressing complement components, interferons, IL-1β, IL-6 and chemokines59. Although these responses might augment pathogen clearance, they may also negatively affect neuronal function and survival through alterations in astrocyte homeostasis. For example, impaired glutamate uptake contributes to synaptic loss, impairment of neurogenesis and neurotoxicity60,61, which lead to poor clinical outcome after neuroinflammation.
Microglia, the only resident myeloid cells within the CNS parenchyma, also respond to microbial pathogens via recognition of PAMPs by PRRs, which can result in the transcription of genes encoding IL-1 family cytokines including IL-1β, IL-18 and IL-33 (ref. 62). There is evidence that both local expansion of microglia and recruitment of bone marrow–derived monocytes into the CNS parenchyma can contribute to increased phagocytosis of pathogens during neuroinfectious diseases63,64. Thus, although the relative abundance of microglia differs among brain regions, with lower numbers observed in hindbrain65, this may be altered during CNS infections. However, as microglial activation contributes to local neurodegeneration66, the relative disparity in numbers may provide additional means for limiting the effects of their secreted cytokines within hindbrain regions. Microglia derived from different brain regions also show differences in levels of nitric oxide (NO), glutamate uptake and TNF expression upon activation with lipopolysaccharide (LPS)67 and may remain activated after resolution of CNS infection12.
Infections of CSF compartments and brain function
Acute infection of the CSF compartments by bacteria and fungi causes the classical sickness behaviors described above and, if inadequately treated, can cause permanent CNS injury and damage leading to deficits in vision, hearing, cognition and motor function68. Although antimicrobials are critical for limiting CNS damage by proliferating pathogens, antimicrobial treatment without control of inflammation may result in paradoxically worse outcomes by stimulating the release of bacterial or fungal components that further provoke immunopathological responses adjacent to brain parenchyma69,70. Consistent with this, survivors of acute bacterial meningitis caused by Streptococcus pneumoniae or Neisseria meningitides may show persistent neurological deficits, including cognitive dysfunction or dementia, even if they received a timely diagnosis and treatment71. Monocyte-derived macrophages in the CNS, particularly meningeal and perivascular macrophages, have a protective role during bacterial meningitis72. However, microglial recognition of pathogens or pathogen components through Toll-like receptors induces neurotoxicity through the release of cell death signals, such as oxidants, or activation of inflammasome components, including caspase-1, which cleaves pro-IL-1β to the biologically active IL-1β (ref. 73). Indeed, caspase-1 deficiency or in vivo use of broad caspase inhibitors in experimental models of bacterial meningitis reduces neuronal damage with mitigation of lethargy and improved motor function74,75.
Although the innate immune response has a major role in neuronal damage during acute bacterial meningitis, adaptive immune responses may underlie ongoing neurological deficits during and after recovery from more indolent infections of the CSF compartment, such as those caused by Mycobacterium tuberculosis (Mtb) and Cryptococcus neoformans (Fig. 2). Tuberculous meningitis (TBM) represents approximately 1% of active tuberculosis cases and may present with or without concurrent pulmonary tuberculosis76. Patients with TBM often present with behavioral abnormalities or psychiatric disorders in addition to other neurological symptoms77, with ongoing neurological sequelae occurring at high frequency in survivors. Similarly, chronic meningitis due to C. neoformans, a pathogenic yeast that can infect the meningeal compartment in immunocompetent individuals, who account for one-third of cases78, may present with psychosis or mania; deficits in attention, concentration and visuospatial skills79; or a frontal network syndrome characterized by apathy, disinhibition and decreased executive function80. Cryptococcal meningitis is associated with up to 30% mortality despite optimal antimicrobial therapy81, and survivors may continue to experience neuropsychological consequences despite resolution of initial imaging abnormalities79.
Figure 2.
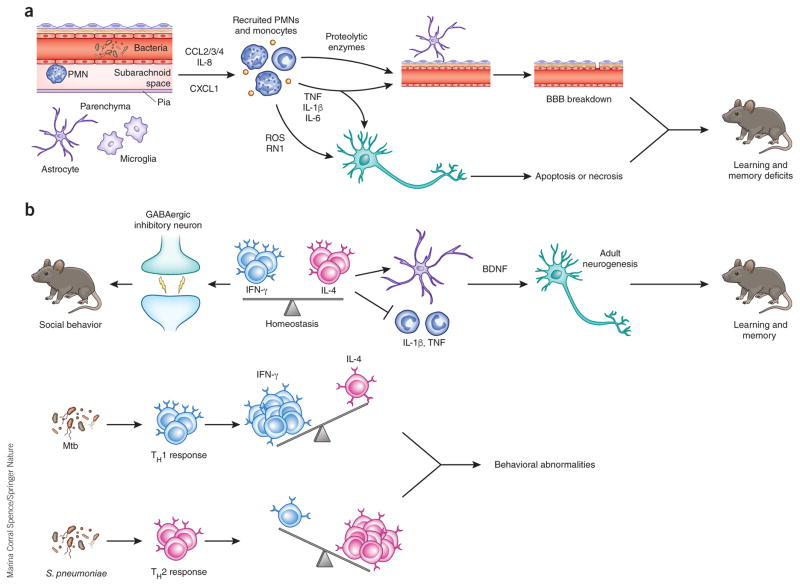
Potential mechanisms of neurological sequelae subsequent to bacterial and fungal meningoencephalitis. (a) Perivascular or meningeal macrophages (PVM) recognize invading pathogens during meningoencephalitis and release a variety of chemoattractants, including CCL2, CCL3, CCL4 (CCL2/3/4), IL-8 and CXCL1, to recruit neutrophils and monocytes to the CSF compartment. Recruited neutrophils release proteolytic enzymes, which contribute to BBB breakdown through loss of tight junctions and degradation of the basement membrane. Reactive oxygen (ROS) and nitrogen (RN1) species can initiate apoptosis or necrosis in neurons. TNF, IL-1β and IL-6 can participate in BBB breakdown and neuronal death. These proinflammatory cytokines are increased in the hippocampus and cortex during experimental meningitis in mouse models and can cause learning and memory deficits in surviving animals. (b) Balance between IFN-γ and IL-4 produced by meningeal T cells is necessary for normal behavior. IFN-γ acts on inhibitory neurons to increase GABAergic current and maintain normal social behavior. IL-4 maintains normal learning and memory, potentially through inhibition of proinflammatory cytokine production by meningeal myeloid cells and/or increasing brain-derived neurotrophic factor (BDNF) production by astrocytes, which is crucial for adult neurogenesis. During infection, the balance is disrupted, which may cause behavioral alteration. Chronic infection by Mtb results in TH1 cell recruitment to the CSF compartment, increasing IFN-γ production. Meningitis caused by S. pneumoniae and other acute bacterial pathogens results in the influx of IL-4 producing TH2 cells, shifting the balance toward an IL-4-dominated cytokine milieu.
Early clinical signs in patients with TBM, which are similar to those of acute bacterial meningitis, are consistent with an early neutrophilic response. However, CSF neutrophils are replaced early in the course of TBM by T and B cells82, and T cells may persist within the CSF compartment with or without bacterial clearance83. In a study that measured the numbers of Mtb antigens ESAT-6– and CFP-10–specific, IFN-γ–producing T cells within the CSF during 6 months of antibiotic treatment, patients whose T cell counts increased over time experienced a worsening of clinical symptoms, even with complete sterilization of CSF84. Similarly, TH1-polarizing immunity is critical for clearance of meningeal infection with C. neoformans and, although concentrations of CSF IFN-γ positively correlate with survival85, they are also associated with severe neurological disease. In addition, CNS biopsies from patients with tuberculosis or cryptococcal meningitis revealed extensive parenchymal infiltration of T cells and extensive activation of astrocytes and microglia86. These data suggest a role for IFN-γ–expressing T cells in ongoing neurological abnormalities in patients with chronic meningitis due to Mtb or C. neoformans via effects on non-neuronal cells.
Studies in animal models show that IFN-γ activation of the JAK–STAT pathway promotes microglial activation87, which alters the activity of neuronal networks via production of cytokines, reactive oxygen species and nitrogen species88. However, coactivation of Toll-like receptor TLR4 and IFN-γ receptors results in neuronal dysfunction and death caused mainly by enhanced microglial expression of inducible nitric oxide synthase (iNOS) and NO release. Taken together, these studies provide a mechanism for alterations in brain function and behavior in patients with infections of the CSF compartment. Of interest, experiments using positron emission tomography (PET) tracers to quantify 18-kDa translocator protein (TSPO), a hallmark of microglial activation, show higher abundance of TSPO in patients with schizophrenia and in people with subclinical symptoms who are at high risk of psychosis89. Although the etiology of psychotic disorders is multifactorial and may include genetic and noninfectious environmental causes, the similarities in symptomatology and inflammatory processes between such disorders and neuroinfectious diseases are interesting and suggest generalizable innate and adaptive immune mechanisms for many types of CNS diseases. Further studies evaluating the function and antigen specificity of persistent T cells within the CNS may provide new links between microbe-specific immunity and psychiatric diseases.
Inflammation-induced CNS dysfunction in parenchymal infection
DNA and RNA viruses of different families can infect the CNS, leading to the clinical syndromes of meningitis, encephalitis or meningoencephalitis. Many DNA viruses and a few RNA viruses replicate in the nucleus, establishing latency by integrating into the cellular DNA, whereas most RNA viruses replicate primarily in the cytosol and generally do not establish latency90. Herpes simplex encephalitis (HSE), caused by the DNA virus HSV-1, is the most common acute, sporadic encephalitis in the United States and worldwide91. Arthropod-born viruses, or arboviruses, are another important cause of encephalitis92. West Nile virus (WNV), a positive sense RNA flavivirus, is the most widely distributed arbovirus worldwide, with cases reported on every continent but Antarctica93. The pathophysiology, diagnosis and management of acute HSE and WNV have been reviewed7. Patients with viral encephalitis of any etiology typically present with fever, headache, confusion and altered mental status but may also present with seizures or focal neurological signs. Animal and human studies have demonstrated that both innate immune responses and lymphocyte trafficking to the CNS are important mechanisms of virologic control94,95. Neurological and neuropsychiatric sequelae can persist or develop after encephalitis due to a variety of neurotropic pathogens, which has become a prioritized area of research96. Patients surviving HSE or WNV encephalitis show high rates of neurological sequelae, including memory impairment (60%), speech disorders (35%) and cognitive impairment (29%)97. Of the 90% of patients who survive WNV, about 50% experience cognitive sequelae deficits, including depression, fatigue, memory impairment and changes in executive function that may persist for years98,99. Here we will focus on mechanisms underlying neurological sequelae in HSE and WNV encephalitis in individuals with intact immunity.
Increasing evidence suggests that developmental pathways regulating synaptic plasticity by microglia and astrocytes can be reactivated during disease states. Alterations, caused by aberrant activation of glia, in synapse homeostasis may contribute to the cognitive dysfunction in many neurocognitive disorders, including autism spectrum disorders and a variety of dementias including Alzheimer’s disease100,101. The complement system, part of innate immunity that initiates adaptive responses to clear virus peripherally102, influences synapse homeostasis during development103,104 and can promote synapse elimination during neurodegenerative and neuroinfectious diseases12,15. In a model of Alzheimer’s disease using J20 transgenic mice that harbor a familial mutant of human amyloid precursor protein105, C1q deposition preceded synapse elimination and plaque formation, and mice deficient in complement signaling had fewer phagocytic microglia and reduced synaptic elimination15. Loss of C1q also protected mice from synaptic elimination and downstream behavioral phenotypes in a model of dementia caused by deficiency of progranulin, a pleotropic protein that has a major role in genetic causes of frontotemporal dementia106. Progranulin-deficient microglia show increased lysosomal activity and complement production, leading to preferential elimination of inhibitory neurons107. In a model of WNV-induced memory impairment, phagocytic microglia persisted for weeks after viral clearance, and gene signatures associated with complement-mediated synapse remodeling were elevated in the hippocampus, a CNS region responsible for memory12. Delayed recovery from acute synapse loss correlates with poor spatial learning, and mice with fewer microglia (Il34−/−) or complement deficiency (C3−/− or C3ar−/−) were protected from virus-induced synaptic elimination12 (Fig. 3a), which suggests that aberrant microglial activation and complement-mediated deletion of neuronal communication after viral encephalitis may result in neurological sequelae seen in neurotropic virus-infected survivors.
Figure 3.
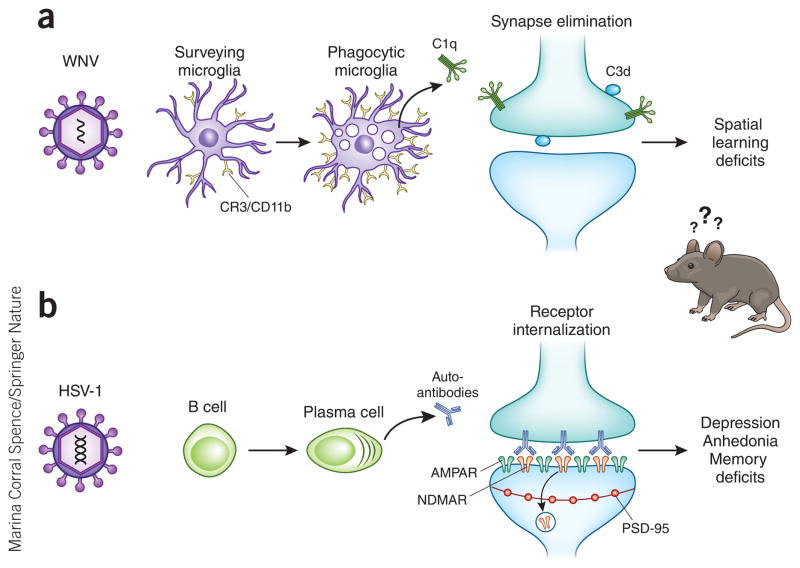
Mechanisms underlying neurological sequelae in a subset of viral encephalitis survivors. (a) Microglia are activated after WNV infection, leading to upregulation of complement receptor 3 (CR3) and expression of complement component C1qa. C1qa and the downstream complement cleavage protein C3d localize to presynaptic terminals in the hippocampus. Complement-mediated engulfment of tagged synapses by microglia leads to selective loss of presynaptic terminals in the CA3 region of the hippocampus and deficits in spatial learning. CD11b, cluster of differentiaton 11b. (b) B cells can produce autoantibodies to synaptic proteins, including NMDAR, after HSV-1 mediated encephalitis. Autoantibodies bind to NMDAR on the post-synaptic terminal, which leads to selective internalization of NMDAR, whereas other synaptic components, such as AMPAR and PSD-95, remain intact. Loss of NMDAR in the hippocampus leads to behavioral memory deficits and neuropsychiatric symptoms of depression and anhedonia.
Instituting intravenous acyclovir as the standard of care for HSE has greatly improved survival rates108, but patients who survive acute infection are at risk for clinical neurologic relapse and the development of new neuropsychiatric symptoms, including memory loss and epilepsy109. PCR analysis of the CSF during relapse at times reveals an absence of CNS viral replication, leading to the hypothesis that these relapses are immune mediated110. In recent years, numerous studies and case reports have linked clinical relapses to the development of auto-immune encephalitis, and in particular to anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis111 (Fig. 3b). Administration of CSF from patients with autoimmune encephalitis, but not healthy controls, to hippocampal cultures leads to a titer-dependent but reversible decrease in NMDAR density without altering the number of synapses or the density of other synaptic proteins, such as PSD-95, GluR1, GluR2 receptor clusters or GABA receptor112. Intracerebral infusion of patient CSF or purified IgG similarly increases in vivo NMDAR internalization and alteration in glutamate homeostasis and signaling112,113. Furthermore, these autoantibodies inhibited the development of long-term synaptic plasticity in vitro114. The recent development of a rodent model for anti-NMDAR encephalitis demonstrates that continuous infusion of patient autoantibodies can lead to behavioral deficits, providing a platform for preclinical testing113. Whether autoantibodies develop via molecular mimicry, secondarily to release of antigens following neuronal lysis or as a primary mechanism of neuroprotection by the immune system requires further study.
CNS infections with certain neurotropic parasites are also known to cause alterations in host behavior. Among the best known is Toxoplasma gondii, a common zoonotic parasite with a worldwide seroprevalance of 30–70%. T. gondii causes chronic CNS infection and neuroinflammation after ingestion of oocysts in contaminated food or water115. Ingested oocysts develop into fast-dividing tachyzoites that invade the gut epithelium and lamina propria, replicate asexually through a process called endodyogeny and then exit and infect myeloid cells, which allow dissemination to multiple tissue sites in the body (including the eye, heart, liver, lung, lymph nodes, muscles and CNS)116. The life cycle of T. gondii requires infection of feline prey intermediate hosts, whose ingestion leads to infection of feline definitive hosts117. Infection of the intermediate host CNS appears to be a critical stage of the T. gondii life cycle, ensuring successful predation via modification of the prey’s olfactory preferences to reduce its avoidance of predators118,119. Epidemiological studies of T. gondii infection in humans, which may have evolved when human ancestors were still under feline predation120, similarly link parasite seropositivity with alterations in olfactory preference and behavioral abnormalities in immunocompetent individuals, the latter of which include those associated with neurodegenerative and psychiatric diseases.
CNS invasion by T. gondii121 leads to infection of all neural cell types, most of which are promptly cleared of parasite via astrocyte and microglial expression of T cell chemoattractants CCL5, CXCL9 and CXCL10, that recruit IFN-γ-expressing CD4+ and CD8+ T cells directed at T. gondii antigens122. Neurons remain latently infected with slowly replicating bradyzoites throughout the life of the host. In murine studies, behavioral effects of T. gondii are associated with direct infection of cortical neurons and astrocytes and modification of their functions, including those that affect innate immunity and dopaminergic and glutamate signaling123,124. Direct effects of T. gondii on neuronal function—including derailment of neurotransmitter expression, modulation of calcium signaling and loss of myelinated fibers, MAP-2+ neurites and NeuN+ cells—are all suggestive of parasite-mediated impairment or injury125. These effects have been proposed to underlie a variety of behavioral alterations and psychiatric diseases and certain neurodegenerative disorders observed in T. gondii–infected hosts. However, aversion to cat urine is also observed in rodents after clearance of cysts126, and observations of low total cyst burden and lack of specific neuronal tropism observed in infected brain regions raise the important possibility that chronic T. gondii infection induces inflammatory-mediated dysfunction that does not require the persistence of parasite.
The T. gondii–infected CNS shows increased astrocyte expression of TNF, IL-1β and IL-6 (ref. 127), each of which has been implicated in the regulation of neural correlates of memory including adult neurogenesis, synaptic plasticity and modulation of long-term potentiation128–130. Molecular interactions between dopaminergic and inflammatory cascades within neurons may underlie behavioral alterations during chronic infection with T. gondii. The NR4A transcription factors NR4A1, NR4A2 and NR4A3 (also known as Nur77, Nurr1 and Nor1, respectively) share similar DNA-binding properties and have been implicated in regulation of genes involved in dopamine neurotransmission131. Nurr1 induces tyrosine hydroxylase expression during differentiation of dopaminergic neurons and has a key role in the maintenance of the adult brain dopaminergic system. Consistent with this, NR4A2 polymorphisms are associated with a variety of neurological and psychiatric disorders, including Parkinson’s disease, Lewy body dementia, addiction and attention deficit disorder132,133. Nurr1 and its coregulating factor, glycogen synthase kinase 3, recruits CoREST, a complex of several proteins that assembles chromatin-modifying enzymes, also interacts with the transcription factor complex NF-κB–p65, protecting dopaminergic neurons during LPS-induced inflammation by reducing expression of, for example, Tnf, NO and Il1b in microglia and astrocytes134. Nurr1+/− mice show more exploratory behavior and lower anxiety than wild-type mice, and these changes are exacerbated by chronic infection with T. gondii compared with the behaviors of similarly infected wild-type animals135. There are no studies examining NR4A2 polymorphisms in psychiatric patients chronically infected with T. gondii, but cognitive deterioration among T. gondii–infected patients with bipolar disorder has been reported136. Genome-wide analyses may be useful to identify susceptibility genes that predispose individuals to development or worsening of affective disorders after infection with T. gondii.
Summary and future perspectives
The intersecting mechanisms of CNS damage discovered for infectious, psychiatric and neurodegenerative diseases are leading to new hypotheses about the roles of immune system molecules in normal brain function and in the etiologies of neurological diseases. Type I interferons, for example, are now known to be critical for normal neuronal homeostasis as a regulator of autophagy-mediated protein degradation137, and T cell cytokines IFN-γ and IL-4 are involved in social and cognitive behavior138,139.
Genetic studies have further identified putative roles for aberrantly expressed innate immune molecules in psychiatric and neurodegenerative diseases140, supporting the idea that immune function is crucial in maintaining the flow of information in the normal CNS. Although inflammation is critical for CNS pathogen clearance, lasting effects of immune molecules and pathogen by-products may represent an underlying mechanism of neurologic dysfunction. During infectious and noninfectious neurological diseases, innate immune molecules such as complement proteins and cytokines regulate synaptic plasticity and neurogenesis, whereas amyloid-β and α-synuclein, biomarkers of neurodegenerative diseases, show antimicrobial roles141,142. These findings suggest that, depending on the pathogen, host genotype or environmental factors, both canonical and noncanonical anti-pathogen pathways may affect interneuronal communication, leading to adaptive or maladaptive effects on brain function. Future efforts to elucidate the molecular mechanisms of neurological illnesses will lead to a more integrated view of how the immune and nervous systems’ combined activities contribute to the physiology of each. Understanding the interplay between immunity and neurological function after CNS infection has the potential to shed light on pathway intersection and novel drug targets.
Acknowledgments
We thank J. Williams for critical reading of the manuscript. Funding for this research was provided by the US National Institutes of Health grants T32_HL007317 (N.H.), R01 NS052632, P01 NS059560, R01 AI126909, R21AI114549 and U19 AI083019 (R.S.K.) and a grant from the National Multiple Sclerosis society (R.S.K.).
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Aliberti J. Host persistence: exploitation of anti-inflammatory pathways by Toxoplasma gondii. Nat Rev Immunol. 2005;5:162–170. doi: 10.1038/nri1547. [DOI] [PubMed] [Google Scholar]
- 2.Ronca SE, Dineley KT, Paessler S. Neurological sequelae resulting from encephalitic alphavirus infection. Front Microbiol. 2016;7:959. doi: 10.3389/fmicb.2016.00959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barichello T, et al. Interleukin-1β receptor antagonism prevents cognitive impairment following experimental bacterial meningitis. Curr Neurovasc Res. 2015;12:253–261. doi: 10.2174/1567202612666150605122200. [DOI] [PubMed] [Google Scholar]
- 4.Chandran A, Herbert H, Misurski D, Santosham M. Long-term sequelae of childhood bacterial meningitis: an underappreciated problem. Pediatr Infect Dis J. 2011;30:3–6. doi: 10.1097/INF.0b013e3181ef25f7. [DOI] [PubMed] [Google Scholar]
- 5.Harden LM, Kent S, Pittman QJ, Roth J. Fever and sickness behavior: friend or foe? Brain Behav Immun. 2015;50:322–333. doi: 10.1016/j.bbi.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Lauritsen A, Oberg B. Adjunctive corticosteroid therapy in bacterial meningitis. Scand J Infect Dis. 1995;27:431–434. doi: 10.3109/00365549509047040. [DOI] [PubMed] [Google Scholar]
- 7.Bradshaw MJ, Venkatesan A. Herpes simplex virus-1 encephalitis in adults: pathophysiology, diagnosis, and management. Neurotherapeutics. 2016;13:493–508. doi: 10.1007/s13311-016-0433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke P, et al. Death receptor-mediated apoptotic signaling is activated in the brain following infection with West Nile virus in the absence of a peripheral immune response. J Virol. 2014;88:1080–1089. doi: 10.1128/JVI.02944-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta K, Banerjee A, Saggar K, Ahluwalia A, Saggar K. A prospective study of magnetic resonance imaging patterns of central nervous system infections in pediatric age group and young adults and their clinico-biochemical correlation. J Pediatr Neurosci. 2016;11:46–51. doi: 10.4103/1817-1745.181244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali M, Safriel Y, Sohi J, Llave A, Weathers S. West Nile virus infection: MR imaging findings in the nervous system. AJNR Am J Neuroradiol. 2005;26:289–297. [PMC free article] [PubMed] [Google Scholar]
- 11.Szretter KJ, et al. 2′-O methylation of the viral mRNA cap by West Nile virus evades ifit1-dependent and -independent mechanisms of host restriction in vivo. PLoS Pathog. 2012;8:e1002698. doi: 10.1371/journal.ppat.1002698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasek MJ, et al. A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature. 2016;534:538–543. doi: 10.1038/nature18283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leypoldt F, et al. Herpes simplex virus-1 encephalitis can trigger anti-NMDA receptor encephalitis: case report. Neurology. 2013;81:1637–1639. doi: 10.1212/WNL.0b013e3182a9f531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kashyap RS, et al. Changes in cerebrospinal fluid cytokine expression in tuberculous meningitis patients with treatment. Neuroimmunomodulation. 2010;17:333–339. doi: 10.1159/000292023. [DOI] [PubMed] [Google Scholar]
- 15.Hong S, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352:712–716. doi: 10.1126/science.aad8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams JL, Holman DW, Klein RS. Chemokines in the balance: maintenance of homeostasis and protection at CNS barriers. Front Cell Neurosci. 2014;8:154. doi: 10.3389/fncel.2014.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louveau A, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iliff JJ, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid-β. Sci Transl Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Detje CN, et al. Upon intranasal vesicular stomatitis virus infection, astrocytes in the olfactory bulb are important interferon-β producers that protect from lethal encephalitis. J Virol. 2015;89:2731–2738. doi: 10.1128/JVI.02044-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malone KE, Stohlman SA, Ramakrishna C, Macklin W, Bergmann CC. Induction of class I antigen processing components in oligodendroglia and microglia during viral encephalomyelitis. Glia. 2008;56:426–435. doi: 10.1002/glia.20625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engelhardt B, et al. Nat. Immunol. 2017;18:123–131. doi: 10.1038/ni.3666. [DOI] [PubMed] [Google Scholar]
- 22.Schwerk C, Tenenbaum T, Kim KS, Schroten H. The choroid plexus-a multi-role player during infectious diseases of the CNS. Front Cell Neurosci. 2015;9:80. doi: 10.3389/fncel.2015.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinemann A, Galm I, Chip S, Nitsch C, Maly IP. Claudin-1, -2 and -3 are selectively expressed in the epithelia of the choroid plexus of the mouse from early development and into adulthood while claudin-5 is restricted to endothelial cells. Front Neuroanat. 2016;10:16. doi: 10.3389/fnana.2016.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oda Y, Nakanishi I. Ultrastructure of the caudal portion of the fourth ventricular roof in the mouse. J Comp Neurol. 1987;256:299–307. doi: 10.1002/cne.902560209. [DOI] [PubMed] [Google Scholar]
- 25.Mildner A, et al. Ly-6G+CCR2– myeloid cells rather than Ly-6ChighCCR2+ monocytes are required for the control of bacterial infection in the central nervous system. J Immunol. 2008;181:2713–2722. doi: 10.4049/jimmunol.181.4.2713. [DOI] [PubMed] [Google Scholar]
- 26.Robinson K, Taraktsoglou M, Rowe KS, Wooldridge KG, Ala’Aldeen DA. Secreted proteins from Neisseria meningitidis mediate differential human gene expression and immune activation. Cell Microbiol. 2004;6:927–938. doi: 10.1111/j.1462-5822.2004.00410.x. [DOI] [PubMed] [Google Scholar]
- 27.Banerjee A, et al. Activation of brain endothelium by pneumococcal neuraminidase NanA promotes bacterial internalization. Cell Microbiol. 2010;12:1576–1588. doi: 10.1111/j.1462-5822.2010.01490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ernst JD, Hartiala KT, Goldstein IM, Sande MA. Complement (C5)-derived chemotactic activity accounts for accumulation of polymorphonuclear leukocytes in cerebrospinal fluid of rabbits with pneumococcal meningitis. Infect Immun. 1984;46:81–86. doi: 10.1128/iai.46.1.81-86.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flierl MA, et al. Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature. 2007;449:721–725. doi: 10.1038/nature06185. [DOI] [PubMed] [Google Scholar]
- 30.Aydin N, et al. An experimental study of the neurophysical mechanisms of photophobia induced by subarachnoid hemorrhage. Neurosci Lett. 2016;630:93–100. doi: 10.1016/j.neulet.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Quintana E, et al. DNGR-1+ dendritic cells are located in meningeal membrane and choroid plexus of the noninjured brain. Glia. 2015;63:2231–2248. doi: 10.1002/glia.22889. [DOI] [PubMed] [Google Scholar]
- 32.Durrant DM, Daniels BP, Klein RS. IL-1R1 signaling regulates CXCL12-mediated T cell localization and fate within the central nervous system during West Nile Virus encephalitis. J Immunol. 2014;193:4095–4106. doi: 10.4049/jimmunol.1401192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clarkson BD, et al. CCR2-dependent dendritic cell accumulation in the central nervous system during early effector experimental autoimmune encephalomyelitis is essential for effector T cell restimulation in situ and disease progression. J Immunol. 2015;194:531–541. doi: 10.4049/jimmunol.1401320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hikita N, et al. Relationship between severity of aseptic meningitis and cerebrospinal fluid cytokine levels. Osaka City Med J. 2015;61:63–71. [PubMed] [Google Scholar]
- 35.Jarvis JN, et al. Cerebrospinal fluid cytokine profiles predict risk of early mortality and immune reconstitution inflammatory syndrome in HIV-associated cryptococcal meningitis. PLoS Pathog. 2015;11:e1004754. doi: 10.1371/journal.ppat.1004754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Netea MG, et al. Two patients with cryptococcal meningitis and idiopathic CD4 lymphopenia: defective cytokine production and reversal by recombinant interferon-γ therapy. Clin Infect Dis. 2004;39:e83–e87. doi: 10.1086/425121. [DOI] [PubMed] [Google Scholar]
- 37.Schläger C, et al. Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid. Nature. 2016;530:349–353. doi: 10.1038/nature16939. [DOI] [PubMed] [Google Scholar]
- 38.Gadani SP, Cronk JC, Norris GT, Kipnis J. IL-4 in the brain: a cytokine to remember. J Immunol. 2012;189:4213–4219. doi: 10.4049/jimmunol.1202246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmidt AK, et al. Adjuvant granulocyte colony-stimulating factor therapy results in improved spatial learning and stimulates hippocampal neurogenesis in a mouse model of pneumococcal meningitis. J Neuropathol Exp Neurol. 2015;74:85–94. doi: 10.1097/NEN.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 40.Wippel C, et al. Bacterial cytolysin during meningitis disrupts the regulation of glutamate in the brain, leading to synaptic damage. PLoS Pathog. 2013;9:e1003380. doi: 10.1371/journal.ppat.1003380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kreutzfeldt M, et al. Neuroprotective intervention by interferon-γ blockade prevents CD8+ T cell-mediated dendrite and synapse loss. J Exp Med. 2013;210:2087–2103. doi: 10.1084/jem.20122143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7:a020412. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim BJ, et al. Bacterial induction of Snail1 contributes to blood-brain barrier disruption. J Clin Invest. 2015;125:2473–2483. doi: 10.1172/JCI74159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daniels BP, et al. Viral pathogen-associated molecular patterns regulate blood-brain barrier integrity via competing innate cytokine signals. MBio. 2014;5:e01476–e14. doi: 10.1128/mBio.01476-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lazear HM, et al. Interferon-λ restricts West Nile virus neuroinvasion by tightening the blood-brain barrier. Sci Transl Med. 2015;7:284ra59. doi: 10.1126/scitranslmed.aaa4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhattacharyya S, et al. Enveloped viruses disable innate immune responses in den-dritic cells by direct activation of TAM receptors. Cell Host Microbe. 2013;14:136–147. doi: 10.1016/j.chom.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miner JJ, et al. The TAM receptor Mertk protects against neuroinvasive viral infection by maintaining blood-brain barrier integrity. Nat Med. 2015;21:1464–1472. doi: 10.1038/nm.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Terrando N, et al. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol. 2011;70:986–995. doi: 10.1002/ana.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Espinosa-Oliva AM, et al. Role of dopamine in the recruitment of immune cells to the nigro-striatal dopaminergic structures. Neurotoxicology. 2014;41:89–101. doi: 10.1016/j.neuro.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 50.Wen J, et al. TNF-like weak inducer of apoptosis promotes blood brain barrier disruption and increases neuronal cell death in MRL/lpr mice. J Autoimmun. 2015;60:40–50. doi: 10.1016/j.jaut.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blank T, et al. Brain endothelial- and epithelial-specific interferon receptor chain 1 drives virus-induced sickness behavior and cognitive impairment. Immunity. 2016;44:901–912. doi: 10.1016/j.immuni.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 52.Nair S, Diamond MS. Innate immune interactions within the central nervous system modulate pathogenesis of viral infections. Curr Opin Immunol. 2015;36:47–53. doi: 10.1016/j.coi.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nave KA. Myelination and support of axonal integrity by glia. Nature. 2010;468:244–252. doi: 10.1038/nature09614. [DOI] [PubMed] [Google Scholar]
- 54.Eroglu C, Barres BA. Regulation of synaptic connectivity by glia. Nature. 2010;468:223–231. doi: 10.1038/nature09612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schitine C, Nogaroli L, Costa MR, Hedin-Pereira C. Astrocyte heterogeneity in the brain: from development to disease. Front Cell Neurosci. 2015;9:76. doi: 10.3389/fncel.2015.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bardehle S, et al. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat Neurosci. 2013;16:580–586. doi: 10.1038/nn.3371. [DOI] [PubMed] [Google Scholar]
- 57.Kipp M, et al. Brain-region-specific astroglial responses in vitro after LPS exposure. J Mol Neurosci. 2008;35:235–243. doi: 10.1007/s12031-008-9057-7. [DOI] [PubMed] [Google Scholar]
- 58.Morga E, Faber C, Heuschling P. Cultured astrocytes express regional heterogeneity of the immunoreactive phenotype under basal conditions and after γ-IFN induction. J Neuroimmunol. 1998;87:179–184. doi: 10.1016/s0165-5728(98)00099-x. [DOI] [PubMed] [Google Scholar]
- 59.McKimmie CS, Graham GJ. Astrocytes modulate the chemokine network in a pathogen-specific manner. Biochem Biophys Res Commun. 2010;394:1006–1011. doi: 10.1016/j.bbrc.2010.03.111. [DOI] [PubMed] [Google Scholar]
- 60.Vesce S, Rossi D, Brambilla L, Volterra A. Glutamate release from astrocytes in physiological conditions and in neurodegenerative disorders characterized by neuroinflammation. Int Rev Neurobiol. 2007;82:57–71. doi: 10.1016/S0074-7742(07)82003-4. [DOI] [PubMed] [Google Scholar]
- 61.Rossi D, Volterra A. Astrocytic dysfunction: insights on the role in neurodegeneration. Brain Res Bull. 2009;80:224–232. doi: 10.1016/j.brainresbull.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 62.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 63.De Lucia C, et al. Microglia regulate hippocampal neurogenesis during chronic neurodegeneration. Brain Behav Immun. 2016;55:179–190. doi: 10.1016/j.bbi.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Djukic M, et al. Circulating monocytes engraft in the brain, differentiate into microglia and contribute to the pathology following meningitis in mice. Brain. 2006;129:2394–2403. doi: 10.1093/brain/awl206. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y, et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol. 2012;13:753–760. doi: 10.1038/ni.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Michell-Robinson MA, et al. Roles of microglia in brain development, tissue maintenance and repair. Brain. 2015;138:1138–1159. doi: 10.1093/brain/awv066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lai AY, Dhami KS, Dibal CD, Todd KG. Neonatal rat microglia derived from different brain regions have distinct activation responses. Neuron Glia Biol. 2011;7:5–16. doi: 10.1017/S1740925X12000154. [DOI] [PubMed] [Google Scholar]
- 68.Carter JA, Neville BG, Newton CR. Neuro-cognitive impairment following acquired central nervous system infections in childhood: a systematic review. Brain Res Brain Res Rev. 2003;43:57–69. doi: 10.1016/s0165-0173(03)00192-9. [DOI] [PubMed] [Google Scholar]
- 69.Lehnardt S, et al. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4–dependent pathway. Proc Natl Acad Sci USA. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scheld WM, Koedel U, Nathan B, Pfister HW. Pathophysiology of bacterial meningitis: mechanism(s) of neuronal injury. J Infect Dis. 2002;186(suppl 2):S225–S233. doi: 10.1086/344939. [DOI] [PubMed] [Google Scholar]
- 71.Grindborg Ö, Naucler P, Sjölin J, Glimåker M. Adult bacterial meningitis—a quality registry study: earlier treatment and favourable outcome if initial management by infectious diseases physicians. Clin Microbiol Infect. 2015;21:560–566. doi: 10.1016/j.cmi.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 72.Polfliet MM, et al. Meningeal and perivascular macrophages of the central nervous system play a protective role during bacterial meningitis. J Immunol. 2001;167:4644–4650. doi: 10.4049/jimmunol.167.8.4644. [DOI] [PubMed] [Google Scholar]
- 73.Gerber J, Nau R. Mechanisms of injury in bacterial meningitis. Curr Opin Neurol. 2010;23:312–318. doi: 10.1097/WCO.0b013e32833950dd. [DOI] [PubMed] [Google Scholar]
- 74.Braun JS, et al. Neuroprotection by a caspase inhibitor in acute bacterial meningitis. Nat Med. 1999;5:298–302. doi: 10.1038/6514. [DOI] [PubMed] [Google Scholar]
- 75.Geldhoff M, et al. Inflammasome activation mediates inflammation and outcome in humans and mice with pneumococcal meningitis. BMC Infect Dis. 2013;13:358. doi: 10.1186/1471-2334-13-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aurangzeb S, Badshah M, Khan RS. Chest radiographic findings in neurotuber-culosis without pulmonary signs and symptoms. J Coll Physici. 2008;18:27–30. [PubMed] [Google Scholar]
- 77.Cherian A, Thomas SV. Central nervous system tuberculosis. Afr Health Sci. 2011;11:116–127. [PMC free article] [PubMed] [Google Scholar]
- 78.Pyrgos V, Seitz AE, Steiner CA, Prevots DR, Williamson PR. Epidemiology of cryptococcal meningitis in the US: 1997–2009. PLoS One. 2013;8:e56269. doi: 10.1371/journal.pone.0056269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lu CH, et al. Assessing the chronic neuropsychologic sequelae of human immuno-deficiency virus-negative cryptococcal meningitis by using diffusion tensor imaging. Am J Neuroradiol. 2011;32:1333–1339. doi: 10.3174/ajnr.A2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hoffmann M, Muniz J, Carroll E, De Villasante J. Cryptococcal meningitis mis-diagnosed as Alzheimer’s disease: complete neurological and cognitive recovery with treatment. J Alzheimers Dis. 2009;16:517–520. doi: 10.3233/JAD-2009-0985. [DOI] [PubMed] [Google Scholar]
- 81.Bratton EW, et al. Comparison and temporal trends of three groups with cryptococcosis: HIV-infected, solid organ transplant, and HIV-negative/non-transplant. PLoS One. 2012;7:e43582. doi: 10.1371/journal.pone.0043582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Garg RK. Tuberculosis of the central nervous system. Postgrad Med J. 1999;75:133–140. doi: 10.1136/pgmj.75.881.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pelc S, De Maertelaere E. CSF cells in tuberculous meningitis. Humoral and cellular immune response. J Neurol Sci. 1981;49:223–228. doi: 10.1016/0022-510x(81)90080-0. [DOI] [PubMed] [Google Scholar]
- 84.Park KH, et al. Kinetics of T-cell-based assays on cerebrospinal fluid and peripheral blood mononuclear cells in patients with tuberculous meningitis. Korean J Intern Med. 2014;29:793–799. doi: 10.3904/kjim.2014.29.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jarvis JN, et al. The phenotype of the Cryptococcus-specific CD4+ memory T-cell response is associated with disease severity and outcome in HIV-associated cryptococcal meningitis. J Infect Dis. 2013;207:1817–1828. doi: 10.1093/infdis/jit099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Panackal AA, et al. Paradoxical immune responses in non-HIV cryptococcal meningitis. PLoS Pathog. 2015;11:e1004884. doi: 10.1371/journal.ppat.1004884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Browne TC, et al. IFN-γ Production by amyloid β-specific Th1 cells promotes microglial activation and increases plaque burden in a mouse model of Alzheimer’s disease. J Immunol. 2013;190:2241–2251. doi: 10.4049/jimmunol.1200947. [DOI] [PubMed] [Google Scholar]
- 88.Papageorgiou IE, et al. TLR4-activated microglia require IFN-γ to induce severe neuronal dysfunction and death in situ. Proc Natl Acad Sci USA. 2016;113:212–217. doi: 10.1073/pnas.1513853113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bloomfield PS, et al. Microglial activity in people at ultra high risk of psychosis and in schizophrenia: an [11C]PBR28 PET brain imaging study. Am J Psychiatry. 2016;173:44–52. doi: 10.1176/appi.ajp.2015.14101358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mettenleiter TC. Breaching the barrier—the nuclear envelope in virus infection. J Mol Biol. 2016;428:1949–1961. doi: 10.1016/j.jmb.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 91.Granerod J, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10:835–844. doi: 10.1016/S1473-3099(10)70222-X. [DOI] [PubMed] [Google Scholar]
- 92.Salimi H, Cain MD, Klein RS. Encephalitic arboviruses: emergence, clinical presentation, and neuropathogenesis. Neurotherapeutics. 2016;13:514–534. doi: 10.1007/s13311-016-0443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kramer LD, Styer LM, Ebel GD. A global perspective on the epidemiology of West Nile virus. Annu Rev Entomol. 2008;53:61–81. doi: 10.1146/annurev.ento.53.103106.093258. [DOI] [PubMed] [Google Scholar]
- 94.Altfeld M, Gale M., Jr Innate immunity against HIV-1 infection. Nat Immunol. 2015;16:554–562. doi: 10.1038/ni.3157. [DOI] [PubMed] [Google Scholar]
- 95.Armangue T, et al. Autoimmune post-herpes simplex encephalitis of adults and teenagers. Neurology. 2015;85:1736–1743. doi: 10.1212/WNL.0000000000002125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.John CC, et al. Global research priorities for infections that affect the nervous system. Nature. 2015;527:S178–S186. doi: 10.1038/nature16033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stahl JP, Mailles A, De Broucker T Steering Committee and Investigators Group. Herpes simplex encephalitis and management of acyclovir in encephalitis patients in France. Epidemiol Infect. 2012;140:372–381. doi: 10.1017/S0950268811000483. [DOI] [PubMed] [Google Scholar]
- 98.Garcia MN, et al. Evaluation of prolonged fatigue post-West Nile virus infection and association of fatigue with elevated antiviral and proinflammatory cytokines. Viral Immunol. 2014;27:327–333. doi: 10.1089/vim.2014.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sadek JR, et al. Persistent neuropsychological impairment associated with West Nile virus infection. J Clin Exp Neuropsychol. 2010;32:81–87. doi: 10.1080/13803390902881918. [DOI] [PubMed] [Google Scholar]
- 100.Guerreiro R, et al. TREM2 variants in Alzheimer’s disease. N Engl J Med. 2013;368:117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chung WS, Welsh CA, Barres BA, Stevens B. Do glia drive synaptic and cognitive impairment in disease? Nat Neurosci. 2015;18:1539–1545. doi: 10.1038/nn.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mehlhop E, Diamond MS. Protective immune responses against West Nile virus are primed by distinct complement activation pathways. J Exp Med. 2006;203:1371–1381. doi: 10.1084/jem.20052388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schafer DP, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mucke L, et al. High-level neuronal expression of Aβ 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Woollacott IO, Rohrer JD. The clinical spectrum of sporadic and familial forms of frontotemporal dementia. J Neurochem. 2016;138(suppl 1):6–31. doi: 10.1111/jnc.13654. [DOI] [PubMed] [Google Scholar]
- 107.Lui H, et al. Progranulin deficiency promotes circuit-specific synaptic pruning by microglia via complement activation. Cell. 2016;165:921–935. doi: 10.1016/j.cell.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sköldenberg B, et al. Acyclovir versus vidarabine in herpes simplex encephalitis. Randomised multicentre study in consecutive Swedish patients. Lancet. 1984;2:707–711. doi: 10.1016/s0140-6736(84)92623-0. [DOI] [PubMed] [Google Scholar]
- 109.Sili U, Kaya A, Mert A H.S.V.E.S. Group. Herpes simplex virus encephalitis: clinical manifestations, diagnosis and outcome in 106 adult patients. J Clin Virol. 2014;60:112–118. doi: 10.1016/j.jcv.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 110.De Tiège X, et al. Herpes simplex encephalitis relapses in children: differentiation of two neurologic entities. Neurology. 2003;61:241–243. doi: 10.1212/01.wnl.0000073985.71759.7c. [DOI] [PubMed] [Google Scholar]
- 111.Leypoldt F, Armangue T, Dalmau J. Autoimmune encephalopathies. Ann NY Acad Sci. 2015;1338:94–114. doi: 10.1111/nyas.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hughes EG, et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci. 2010;30:5866–5875. doi: 10.1523/JNEUROSCI.0167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Planagumà J, et al. Human N-methyl D-aspartate receptor antibodies alter memory and behaviour in mice. Brain. 2015;138:94–109. doi: 10.1093/brain/awu310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mikasova L, et al. Disrupted surface cross-talk between NMDA and Ephrin-B2 receptors in anti-NMDA encephalitis. Brain. 2012;135:1606–1621. doi: 10.1093/brain/aws092. [DOI] [PubMed] [Google Scholar]
- 115.Guo M, et al. A Systematic meta-analysis of Toxoplasma gondii prevalence in food animals in the united states. Foodborne Pathog Dis. 2016;13:109–118. doi: 10.1089/fpd.2015.2070. [DOI] [PubMed] [Google Scholar]
- 116.Ueno N, Lodoen MB. From the blood to the brain: avenues of eukaryotic pathogen dissemination to the central nervous system. Curr Opin Microbiol. 2015;26:53–59. doi: 10.1016/j.mib.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.White MW, Radke JR, Radke JB. Toxoplasma development —turn the switch on or off? Cell Microbiol. 2014;16:466–472. doi: 10.1111/cmi.12267. [DOI] [PubMed] [Google Scholar]
- 118.Flegr J, Lenochová P, Hodný Z, Vondrová M. Fatal attraction phenomenon in humans: cat odour attractiveness increased for toxoplasma-infected men while decreased for infected women. PLoS Negl Trop Dis. 2011;5:e1389. doi: 10.1371/journal.pntd.0001389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vyas A, Kim SK, Giacomini N, Boothroyd JC, Sapolsky RM. Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. Proc Natl Acad Sci USA. 2007;104:6442–6447. doi: 10.1073/pnas.0608310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Poirotte C, et al. Morbid attraction to leopard urine in Toxoplasma-infected chimpanzees. Curr Biol. 2016;26:R98–R99. doi: 10.1016/j.cub.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 121.Blanchard N, Dunay IR, Schlüter D. Persistence of Toxoplasma gondii in the central nervous system: a fine-tuned balance between the parasite, the brain and the immune system. Parasite Immunol. 2015;37:150–158. doi: 10.1111/pim.12173. [DOI] [PubMed] [Google Scholar]
- 122.Landrith TA, Harris TH, Wilson EH. Characteristics and critical function of CD8+ T cells in the Toxoplasma-infected brain. Semin Immunopathol. 2015;37:261–270. doi: 10.1007/s00281-015-0487-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cabral CM, et al. Neurons are the primary target cell for the brain-tropic intracellular parasite Toxoplasma gondii. PLoS Pathog. 2016;12:e1005447. doi: 10.1371/journal.ppat.1005447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.David CN, et al. GLT-1-dependent disruption of CNS glutamate homeostasis and neuronal function by the protozoan parasite Toxoplasma gondii. PLoS Pathog. 2016;12:e1005643. doi: 10.1371/journal.ppat.1005643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Parlog A, Schlüter D, Dunay IR. Toxoplasma gondii-induced neuronal alterations. Parasite Immunol. 2015;37:159–170. doi: 10.1111/pim.12157. [DOI] [PubMed] [Google Scholar]
- 126.Ingram WM, Goodrich LM, Robey EA, Eisen MB. Mice infected with low-virulence strains of Toxoplasma gondii lose their innate aversion to cat urine, even after extensive parasite clearance. PLoS One. 2013;8:e75246. doi: 10.1371/journal.pone.0075246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mahmoudvand H, et al. Toxoplasma gondii infection promotes neuroinflammation through cytokine networks and induced hyperalgesia in BALB/c mice. Inflammation. 2016;39:405–412. doi: 10.1007/s10753-015-0262-6. [DOI] [PubMed] [Google Scholar]
- 128.Riazi K, et al. Microglia-dependent alteration of glutamatergic synaptic transmission and plasticity in the hippocampus during peripheral inflammation. J Neurosci. 2015;35:4942–4952. doi: 10.1523/JNEUROSCI.4485-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wu MD, Montgomery SL, Rivera-Escalera F, Olschowka JA, O’Banion MK. Sustained IL-1β expression impairs adult hippocampal neurogenesis independent of IL-1 signaling in nestin+ neural precursor cells. Brain Behav Immun. 2013;32:9–18. doi: 10.1016/j.bbi.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.del Rey A, Balschun D, Wetzel W, Randolf A, Besedovsky HO. A cytokine network involving brain-borne IL-1β, IL-1ra, IL-18, IL-6, and TNFα operates during long-term potentiation and learning. Brain Behav Immun. 2013;33:15–23. doi: 10.1016/j.bbi.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 131.Eells JB, Wilcots J, Sisk S, Guo-Ross SX. NR4A gene expression is dynamically regulated in the ventral tegmental area dopamine neurons and is related to expression of dopamine neurotransmission genes. J Mol Neurosci. 2012;46:545–553. doi: 10.1007/s12031-011-9642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Grimes DA, et al. Translated mutation in the Nurr1 gene as a cause for Parkinson’s disease. Mov Disord. 2006;21:906–909. doi: 10.1002/mds.20820. [DOI] [PubMed] [Google Scholar]
- 133.Wei YM, Du YL, Nie YQ, Li YY, Wan YJ. Nur-related receptor 1 gene polymorphisms and alcohol dependence in Mexican Americans. World J Gastroenterol. 2012;18:5276–5282. doi: 10.3748/wjg.v18.i37.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lallier SW, Graf AE, Waidyarante GR, Rogers LK. Nurr1 expression is modified by inflammation in microglia. Neuroreport. 2016;27:1120–1127. doi: 10.1097/WNR.0000000000000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Eells JB, et al. Chronic Toxoplasma gondii in Nurr1-null heterozygous mice exacerbates elevated open field activity. PLoS One. 2015;10:e0119280. doi: 10.1371/journal.pone.0119280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hamdani N, et al. Cognitive deterioration among bipolar disorder patients infected by Toxoplasma gondii is correlated to interleukin 6 levels. J Affect Disord. 2015;179:161–166. doi: 10.1016/j.jad.2015.03.038. [DOI] [PubMed] [Google Scholar]
- 137.Ejlerskov P, et al. Lack of neuronal IFN-β-IFNAR causes Lewy body- and Parkinson’s disease-like dementia. Cell. 2015;163:324–339. doi: 10.1016/j.cell.2015.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Filiano AJ, et al. Unexpected role of interferon-γ in regulating neuronal connectivity and social behaviour. Nature. 2016;535:425–429. doi: 10.1038/nature18626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Derecki NC, et al. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010;207:1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jones L, et al. Genetic evidence implicates the immune system and cholesterol metabolism in the aetiology of Alzheimer’s disease. PLoS One. 2010;5:e13950. doi: 10.1371/journal.pone.0013950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kumar DK, et al. Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci Transl Med. 2016;8:340ra72. doi: 10.1126/scitranslmed.aaf1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Beatman EL, et al. Alpha-synuclein expression restricts RNA viral infections in the brain. J Virol. 2015;90:2767–2782. doi: 10.1128/JVI.02949-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Stojakovic A, et al. Role of the IL-1 pathway in dopaminergic neurodegeneration and decreased voluntary movement. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-9988-x. http://dx.doi.org/10.1007/s12035-016-9988-x. [DOI] [PMC free article] [PubMed]
- 144.Prieto GA, et al. Synapse-specific IL-1 receptor subunit reconfiguration augments vulnerability to IL-1β in the aged hippocampus. Proc Natl Acad Sci USA. 2015;112:E5078–E5087. doi: 10.1073/pnas.1514486112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cutando L, et al. Microglial activation underlies cerebellar deficits produced by repeated cannabis exposure. J Clin Invest. 2013;123:2816–2831. doi: 10.1172/JCI67569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Prajapati P, et al. TNF-α regulates miRNA targeting mitochondrial complex-I and induces cell death in dopaminergic cells. Biochim Biophys Acta. 2015;1852:451–461. doi: 10.1016/j.bbadis.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 147.Wu Y, et al. Upregulation of tumor necrosis factor-α in nucleus accumbens attenuates morphine-induced rewarding in a neuropathic pain model. Biochem Biophys Res Commun. 2014;449:502–507. doi: 10.1016/j.bbrc.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 148.Pettigrew LC, Kryscio RJ, Norris CM. The TNFα-transgenic rat: hippocampal synaptic integrity, cognition, function, and post-ischemic cell loss. PLoS One. 2016;11:e0154721. doi: 10.1371/journal.pone.0154721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-α. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 150.Wall AM, Mukandala G, Greig NH, O’Connor JJ. Tumor necrosis factor-α potentiates long-term potentiation in the rat dentate gyrus after acute hypoxia. J Neurosci Res. 2015;93:815–829. doi: 10.1002/jnr.23540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen Z, Palmer TD. Differential roles of TNFR1 and TNFR2 signaling in adult hippocampal neurogenesis. Brain Behav Immun. 2013;30:45–53. doi: 10.1016/j.bbi.2013.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Habbas S, et al. Neuroinflammatory TNFα impairs memory via astrocyte signaling. Cell. 2015;163:1730–1741. doi: 10.1016/j.cell.2015.11.023. [DOI] [PubMed] [Google Scholar]
- 153.Chien CH, Lee MJ, Liou HC, Liou HH, Fu WM. Microglia-derived cytokines/chemokines are involved in the enhancement of LPS-induced loss of nigrostriatal dopaminergic neurons in DJ-1 knockout mice. PLoS One. 2016;11:e0151569. doi: 10.1371/journal.pone.0151569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Vlkolinský R, Siggins GR, Campbell IL, Krucker T. Acute exposure to CXC chemokine ligand 10, but not its chronic astroglial production, alters synaptic plasticity in mouse hippocampal slices. J Neuroimmunol. 2004;150:37–47. doi: 10.1016/j.jneuroim.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 155.Li L, Walker TL, Zhang Y, Mackay EW, Bartlett PF. Endogenous interferon-γ directly regulates neural precursors in the noninflammatory brain. J Neurosci. 2010;30:9038–9050. doi: 10.1523/JNEUROSCI.5691-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Corbin JG, et al. Targeted CNS expression of interferon-γ in transgenic mice leads to hypomyelination, reactive gliosis, and abnormal cerebellar development. Mol Cell Neurosci. 1996;7:354–370. doi: 10.1006/mcne.1996.0026. [DOI] [PubMed] [Google Scholar]
- 157.Hoyo-Becerra C, Schlaak JF, Hermann DM. Insights from interferon-α-related depression for the pathogenesis of depression associated with inflammation. Brain Behav Immun. 2014;42:222–231. doi: 10.1016/j.bbi.2014.06.200. [DOI] [PubMed] [Google Scholar]
- 158.Zheng LS, et al. Mechanisms for interferon-α-induced depression and neural stem cell dysfunction. Stem Cell Reports. 2014;3:73–84. doi: 10.1016/j.stemcr.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yang CS, et al. Reactive oxygen species and p47phox activation are essential for the Mycobacterium tuberculosis-induced pro-inflammatory response in murine microglia. J Neuroinflammation. 2007;4:27. doi: 10.1186/1742-2094-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Koedel U, et al. Experimental pneumococcal meningitis: cerebrovascular alterations, brain edema, and meningeal inflammation are linked to the production of nitric oxide. Ann Neurol. 1995;37:313–323. doi: 10.1002/ana.410370307. [DOI] [PubMed] [Google Scholar]
- 161.Winkler F, Koedel U, Kastenbauer S, Pfister HW. Differential expression of nitric oxide synthases in bacterial meningitis: role of the inducible isoform for blood-brain barrier breakdown. J Infect Dis. 2001;183:1749–1759. doi: 10.1086/320730. [DOI] [PubMed] [Google Scholar]
- 162.Liu X, Chauhan VS, Young AB, Marriott I. NOD2 mediates inflammatory responses of primary murine glia to Streptococcus pneumoniae. Glia. 2010;58:839–847. doi: 10.1002/glia.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sokolova O, et al. Interaction of Neisseria meningitidis with human brain microvascular endothelial cells: role of MAP- and tyrosine kinases in invasion and inflammatory cytokine release. Cell Microbiol. 2004;6:1153–1166. doi: 10.1111/j.1462-5822.2004.00422.x. [DOI] [PubMed] [Google Scholar]
- 164.Schubert-Unkmeir A, Sokolova O, Panzner U, Eigenthaler M, Frosch M. Gene expression pattern in human brain endothelial cells in response to Neisseria meningitidis. Infect Immun. 2007;75:899–914. doi: 10.1128/IAI.01508-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Steinmann U, et al. Transmigration of polymorphnuclear neutrophils and monocytes through the human blood-cerebrospinal fluid barrier after bacterial infection in vitro. J Neuroinflammation. 2013;10:31. doi: 10.1186/1742-2094-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cooley ID, Chauhan VS, Donneyz MA, Marriott I. Astrocytes produce IL-19 in response to bacterial challenge and are sensitive to the immunosuppressive effects of this IL-10 family member. Glia. 2014;62:818–828. doi: 10.1002/glia.22644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Waage A, et al. Local production of tumor necrosis factor alpha, interleukin 1, and interleukin 6 in meningococcal meningitis. Relation to the inflammatory response. J Exp Med. 1989;170:1859–1867. doi: 10.1084/jem.170.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Vu K, Eigenheer RA, Phinney BS, Gelli A. Cryptococcus neoformans promotes its transmigration into the central nervous system by inducing molecular and cellular changes in brain endothelial cells. Infect Immun. 2013;81:3139–3147. doi: 10.1128/IAI.00554-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lee SC, Dickson DW, Brosnan CF, Casadevall A. Human astrocytes inhibit Cryptococcus neoformans growth by a nitric oxide-mediated mechanism. J Exp Med. 1994;180:365–369. doi: 10.1084/jem.180.1.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liang CC, et al. Human endothelial cell activation and apoptosis induced by enterovirus 71 infection. J Med Virol. 2004;74:597–603. doi: 10.1002/jmv.20216. [DOI] [PubMed] [Google Scholar]
- 171.Schneider H, et al. Chemotaxis of T-cells after infection of human choroid plexus papilloma cells with Echovirus 30 in an in vitro model of the blood-cerebrospinal fluid barrier. Virus Res. 2012;170:66–74. doi: 10.1016/j.virusres.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 172.Wang C, et al. Intrinsic apoptosis and proinflammatory cytokines regulated in human astrocytes infected with enterovirus 71. J Gen Virol. 2015;96:3010–3022. doi: 10.1099/jgv.0.000235. [DOI] [PubMed] [Google Scholar]
- 173.Reinert LS, et al. TLR3 deficiency renders astrocytes permissive to herpes simplex virus infection and facilitates establishment of CNS infection in mice. J Clin Invest. 2012;122:1368–1376. doi: 10.1172/JCI60893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Liu Z, et al. HSV-1 activates NF-κB in mouse astrocytes and increases TNF-α and IL-6 expression via Toll-like receptor 3. Neurol Res. 2013;35:755–762. doi: 10.1179/016164113X13703372991516. [DOI] [PubMed] [Google Scholar]
- 175.Fitting S, et al. Regional heterogeneity and diversity in cytokine and chemokine production by astroglia: differential responses to HIV-1 Tat, gp120, and morphine revealed by multiplex analysis. J Proteome Res. 2010;9:1795–1804. doi: 10.1021/pr900926n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wacher C, et al. Coordinated regulation and widespread cellular expression of interferon-stimulated genes (ISG) ISG-49, ISG-54, and ISG-56 in the central nervous system after infection with distinct viruses. J Virol. 2007;81:860–871. doi: 10.1128/JVI.01167-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cho H, et al. Differential innate immune response programs in neuronal subtypes determine susceptibility to infection in the brain by positive-stranded RNA viruses. Nat Med. 2013;19:458–464. doi: 10.1038/nm.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]