Abstract
Background: There is no standard second-line treatment for advanced urothelial carcinoma (UC). Response rates to second-line chemotherapy for advanced UC are low and response duration is short. Bortezomib is a proteasome inhibitor with preclinical activity against UC.
Patients and methods: Treatment consisted of bortezomib 1.3 mg/m2 i.v. twice weekly for two consecutive weeks, followed by a 1-week break. The primary end point was objective response rate (complete response + partial response) by Reponse Evaluation Criteria in Solid Tumors criteria. Secondary end points included safety, toxicity, and progression-free and overall survival.
Results: In all, 25 patients with advanced UC previously treated with combination chemotherapy were enrolled in a multi-institutional single-arm trial from December 2003 through April 2005. Only 29% of patients had node-only metastases. Grade 3/4 drug-related toxic effects included thrombocytopenia (4%), anemia (8%), lymphopenia (8%), sensory neuropathy (6%), hyperglycemia (4%), hypernatremia (4%), fatigue (4%), neuropathic pain (6%), dehydration (4%), and vomiting (4%). No objective responses were observed [95% confidence interval (CI) = 0–12]. The median time to progression was 1.4 months (95% CI = 1.1–2.0 months), and the median survival time was 5.7 months (95% CI = 3.6–8.4 months). There were no treatment-related deaths.
Conclusion: Although bortezomib is well tolerated, it does not have antitumor activity as second-line therapy in UC.
Keywords: bladder cancer, bortezomib, proteasome, PS-341, salvage therapy, urothelial carcinoma
introduction
Urothelial carcinoma (UC) is the fifth most common new cancer reported in the United States, with an incidence estimated to be ∼61 000 new cases per year and 13 000 deaths per year [1]. Although most patients present with superficial disease, metastatic bladder cancer remains a significant problem. Cisplatin-containing regimens [methotrexate, vinblastine, doxoribucin and cisplatin (MVAC); combination chemotherapy with methotrexate, vinblastine and cisplatin; gemcitabine/cisplatin] are the most commonly used first-line regimens and produce responses in 30%–70% of patients. Despite improvements in therapy over the past 15 years, durable complete remissions in patients with advanced disease are rare, and median survival remains ∼14 months. In an updated report of the randomized trial comparing gemcitabine/cisplatin with MVAC, only 15% of patients were alive and continuously disease free [2].
While gemcitabine/cisplatin is considered by many to be the standard frontline regimen, there is no accepted second-line therapy. Although second-line chemotherapy (ifosfamide, paclitaxel, docetaxel, or other agents) is frequently undertaken in this setting, the response proportions are low and the duration of response tends to be short. In addition, cumulative toxic effects often reduce quality of life and prevent further treatment. Development of novel agents with more activity and less toxicity is necessary. Since no established standard of care exists for patients who have developed progressive disease after first-line therapy, novel agents can be tested in this setting. If activity is observed, strategies incorporating the new agent into frontline therapy can be considered.
The ubiquitin–proteasome pathway degrades intracellular proteins, including crucial proteins involved in cell cycle regulation, transcription factor activation, and apoptosis [3]. Bortezomib is a novel, specific, selective, and reversible inhibitor of the 26S proteasome complex that inhibits the degradation of such crucial proteins as cyclins, nuclear factor (NF)-kappa B, cyclin-dependent kinase inhibitors (i.e. p21 and p27), and tumor suppressor genes. Inhibitors of the 26S proteasome act through multiple mechanisms to suppress tumor survival pathways, arrest tumor growth, tumor spread, and angiogenesis. By inhibiting the proteasome, bortezomib affects a combination of cellular regulatory mechanisms, thereby providing a novel therapeutic approach to cancer treatment. The mechanisms of antitumor activity that have been established for bortezomib involve many pathways thought to be integral to cancer treatment strategies. Bortezomib directly induces apoptosis of tumor cells, inhibits activation of NF-kappa B in cells and in tumor microenvironment, blocks production and expression of proangiogenic mediators, and overcomes defects in apoptotic regulators, such as Bcl-2 overexpression and alterations (i.e. mutations) in the tumor suppressor p53 and loss of Apaf-1 [4–6]. Bortezomib has been demonstrated to have promising cytotoxic activity against a variety of cancers, including transitional cell carcinoma in the National Cancer Institute (NCI) hollow fiber screen as well as in human xenograft mouse models [7]. Phase I studies demonstrated that 1.3 mg/m2 twice weekly for 2 weeks every 21 days was the recommended phase II dose in solid tumor patients [8]. On the basis of these data, a phase II study of bortezomib in progressive advanced transitional cell carcinoma of the urothelium was undertaken in patients previously treated with multiagent chemotherapy.
patients and methods
study design and eligibility
To be eligible for this study, patients had histologically confirmed transitional cell carcinoma of the urothelium. All patients were required to have received only one prior systemic chemotherapy regimen for advanced or metastatic disease, with progression documented during or after that treatment. This chemotherapy must have included at least one of the following agents: cisplatin, carboplatin, paclitaxel, docetaxel, or gemcitabine. For the purposes of this study, radiosensitizing single-agent chemotherapy was not considered before systemic therapy. Patients were not allowed to have received prior treatment with proteasome inhibitors or other single-agent investigational therapy. Measurable disease according to Reponse Evaluation Criteria in Solid Tumors (RECIST) criteria was required.
All patients were required to have a common toxicity criteria (CTC) performance status of zero to two and lesser than or equal to grade 1 neuropathy. Prior radiotherapy or chemotherapy must have been completed >4 weeks before trial enrollment. Patients with active brain metastases were excluded. Required laboratory values included creatinine clearance ≤2.5 × upper limits of normal (ULN) or calculated creatinine clearance >30 cm3/min; alanine aminotransferase and aspartate aminotransferase ≤2.5 × ULN; granulocytes ≥1500/mm3; platelets ≥100 000/mm3; total bilirubin level <1.8 mg/dl, and standard chemistry panel within normal limits. Pregnant or breast-feeding women were excluded from participation due to risk to the fetus or infant.
This clinical trial was sponsored by the Cancer Therapy Evaluation Program of the NCI and the Cancer and Leukemia Group B (CALGB). The institutional review boards of all participating institutions approved this clinical trial. All patients provided written informed consent before participation in this study.
treatment plan
Eligible patients received bortezomib at 1.3 mg/m2 i.v. on days 1, 4, 8, and 11 of a 21-day cycle. Bortezomib was administered as a rapid i.v. bolus over 3–5 s. Antiemetic premedication was administered at the discretion of the treating physician. Patients continued treatment if there was no evidence of disease progression and no more than or equal to grade 3 toxicity attributed to therapy lasting for >3 weeks. Dose reductions were made according to the degree of toxicity. Patients were able to undergo up to two dose reductions (1 and 0.7 mg/m2) for grade 4 hematologic toxicity, grade 3 or greater non-hematologic toxicity, and grade 2 neurologic toxicity. Patients requiring more than two dose reductions were removed from protocol therapy. Patients with evidence of disease progression, unacceptable adverse events, grade 3 or greater toxicity lasting >3 weeks were removed from protocol therapy.
response and toxicity evaluation
Patients were evaluated with cross-sectional imaging (computed tomography or magnetic resonance imaging) of the chest, abdomen, and pelvis every two cycles to evaluate tumor response according to RECIST guidelines. Toxicity was graded using the NCI Common Terminology Criteria for Adverse Events.
statistical considerations
The primary objective of this multicenter phase II study was to determine the objective response rate to bortezomib in patients with measurable advanced UC previously treated with one prior chemotherapy regimen. Response proportions [complete response (CR) + partial response (PR)] were determined according to the RECIST criteria. Secondary end points included duration of objective response, toxicity, progression-free survival (PFS), and overall survival (OS) in this patient population treated with bortezomib. Duration of response was defined as the date of the first CR or PR to the date that the patient had disease progression or death. OS was measured from the date of initiation of treatment to the date of death due to any cause. PFS was measured from the date of initiation of treatment to the date of progression or death due to any cause, whichever occurs first.
The target accrual for this trial was 40 patients using a Simon two-stage design [9]. The study was designed to test the null hypothesis that the objective response rate was ≤10% versus the alternative hypothesis that the response rate was at least 30%. In the first stage, 15 patients were to be enrolled and the decision rule was to halt accrual and declare the treatment ineffective if two or fewer patients demonstrated a PR or CR to therapy. Accrual to the study was not suspended while assessing response. This study had a type I error rate of 0.10 and a power of 0.88.
Any patient who enrolled and received at least one treatment was assessed for response and toxicity. Response rate (CR + PR), toxicity rates, and 95% confidence intervals (CIs) for the response and toxicity rates were computed using the binomial distribution. Toxicity was reported by type, frequency, and severity. The Kaplan–Meier product-limit method was used to estimate OS and PFS distributions.
As part of the quality assurance program of the CALGB, members of the Data Audit Committee visit all participating institutions at least once every 3 years to review source documents. The auditors verify compliance with federal regulations and protocol requirements, including those pertaining to eligibility, treatment, adverse events, tumor response, and outcome in a sample of protocols at each institution. Such on-site review of medical records was carried out for a subgroup of 6 (24%) of the 25 patients under this study.
results
patient characteristics
From 15 October 2003 to 8 April 2005, 25 patients were enrolled. One patient withdrew consent before receiving any study therapy. Twenty-four patients were treated and assessable for toxicity, response, and clinical outcomes (OS and PFS). Patient and disease characteristics are shown in Table 1. Sixty-seven percent of patients had the bladder as their primary site of disease. Seventy-one percent of patients had extranodal (visceral) metastases. Prior chemotherapy regimens were all gemcitabine/platinum-based combinations. All patients had received this chemotherapy for metastatic disease.
Table 1.
Baseline characteristics patients treated on this trial (N = 24)
| Demographics | ||
| Median age (years, IQR) | 63.8 | (56.7–72.4) |
| Race, n (%) | 19 | (79) |
| Caucasian | ||
| Gender, n (%) | ||
| Male | 18 | (75) |
| Female | 6 | (25) |
| Location of primary tumor, n (%) | ||
| Bladder | 16 | (67) |
| Renal pelvis | 9 | (38) |
| Ureter | 7 | (29) |
| Urethra | 1 | (4) |
| Hemoglobin (g/dl) (median, IQR) | 11.3 | (10.6–12.3) |
| Location of metastasisa, n (%) | ||
| Nodal/soft tissue | 16 | (67) |
| Lung/pleura | 9 | (38) |
| Liver | 9 | (38) |
| Bone | 6 | (25) |
| Other sites | 4 | (17) |
| Nodal versus other metastasis, n (%) | ||
| Nodal metastases only | 7 | (29) |
| Nonnodal metastases | 17 | (71) |
| Prior chemotherapy, n (%) | ||
| Gemcitabine/cisplatin | 13 | (54) |
| Gemcitabine/cisplatin/paclitaxel | 1 | (4) |
| Gemcitabine/cisplatin/gefitinib | 1 | (4) |
| Gemcitabine/carboplatin | 6 | (25) |
| Gemcitabine/carboplatin/paclitaxel | 3 | (13) |
May add up to >100% due to multiple organ sites of disease.
IQR, interquartile range.
clinical outcomes
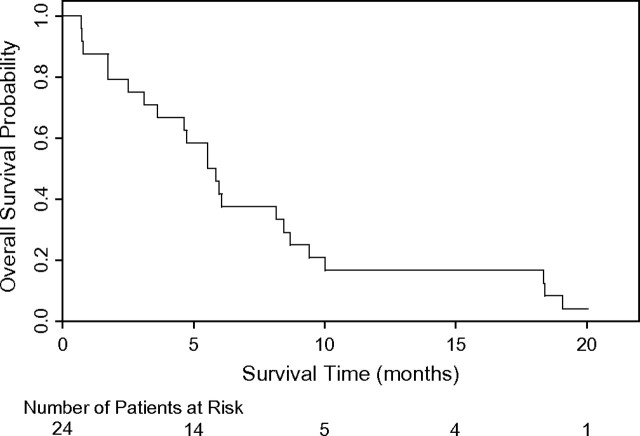
The median number of cycles of study treatment was 2 (range 1–12). No patient experienced an objective response (95% CI = 0–12). The study was closed after the interim analysis for lack of activity of the study therapy. The median time to progression was 1.4 months (95% CI = 1.1–2.0 months) and the median survival time was 5.7 months (95% CI = 3.6–8.4 months). Twenty-three patients have died and one patient is alive at 20.0 months of follow-up (Figure 1).
Figure 1.
Overall survival (N = 24).
toxicity
Treatment with bortezomib was reasonably well tolerated. Grade 3/4 treatment-related hematologic toxic effects included anemia, thrombocytopenia, and lymphopenia. Grade 3 or 4 treatment-related non-hematologic toxic effects included fatigue, constipation, dehydration, vomiting, hyperglycermia, hypernatremia, sensory neuropathy, neuropathic pain, and hiccoughs. No grade 3 or 4 toxicity was seen in >10% of patients (Tables 2 and 3). One patient required dose modification due to neurotoxicity. Two patients ended treatment early due to toxicity, including one for neurotoxicity and another for gastrointestinal toxicity.
Table 2.
Treatment-related hematologic toxicity (N = 24)
| 1–2 | 3 | 4 | |
| n (%) | n (%) | n (%) | |
| Hematologic adverse events | |||
| Blood/bone marrow | |||
| Hemoglobin | 12 (50) | 2 (8) | 0 (0) |
| Leukocytes (total WBC) | 4 (17) | 0 (0) | 0 (0) |
| Lymphopenia | 2 (8) | 2 (8) | 0 (0) |
| Neutrophils/granulocytes (ANC/AGC) | 2 (8) | 0 (0) | 0 (0) |
| Platelets | 6 (25) | 1 (4) | 0 (0) |
| Summary | |||
| Maximum hematologic adverse event | 11 (46) | 5 (21) | 0 (0) |
WBC, white blood cell; ANC, absolute neutrophil count; AGC, absolute granulocyte count.
Table 3.
Treatment-related non-hematologic adverse events
| Grades 1 to 2 (%) | Grade 3 (%) | Grade 4 (%) | |
| Cardiovascular (general) | |||
| Hypotension | 1 (4) | 0 (0) | 0 (0) |
| Constitutional symptoms | |||
| Fatigue (asthenia, lethargy, and malaise) | 15 (63) | 1 (4) | 0 (0) |
| Gastrointestinal | |||
| Anorexia | 5 (21) | 0 (0) | 0 (0) |
| Constipation | 3 (13) | 0 (0) | 1 (4) |
| Dehydration | 0 (0) | 1 (4) | 0 (0) |
| Diarrhea | 4 (17) | 0 (0) | 0 (0) |
| Abdominal distension/bloating | 1 (4) | 0 (0) | 0 (0) |
| Dysphagia, esophagitis, and odynophagia | 1 (4) | 0 (0) | 0 (0) |
| Mucositis/stomatitis | 1 (4) | 0 (0) | 0 (0) |
| Nausea | 4 (17) | 0 (0) | 0 (0) |
| Vomiting | 3 (13) | 0 (0) | 1 (4) |
| Infection | |||
| Infection (nonneutropenic) | 1 (4) | 0 (0) | 0 (0) |
| Infection/febrile neutropenia | 1 (4) | 0 (0) | 0 (0) |
| Metabolic/laboratory | |||
| Alkaline phosphatase | 1 (4) | 0 (0) | 0 (0) |
| Alanine aminotransferase | 1 (4) | 0 (0) | 0 (0) |
| Aspartate aminotransferase | 1 (4) | 0 (0) | 0 (0) |
| Hypoalbuminemia | 4 (17) | 0 (0) | 0 (0) |
| Hypocalcemia | 1 (4) | 0 (0) | 0 (0) |
| Creatinine | 3 (13) | 0 (0) | 0 (0) |
| Hyperglycemia | 2 (8) | 1 (4) | 0 (0) |
| Hyperkalemia | 2 (8) | 0 (0) | 0 (0) |
| Hypernatremia | 0 (0) | 1 (4) | 0 (0) |
| Hypertriglyceridemia | 1 (4) | 0 (0) | 0 (0) |
| Hypokalemia | 1 (4) | 0 (0) | 0 (0) |
| Hyponatremia | 5 (21) | 0 (0) | 0 (0) |
| Musculoskeletal/soft tissue | |||
| Edema | 1 (4) | 0 (0) | 0 (0) |
| Muscle weakness | 0 (0) | 1 (4) | 0 (0) |
| Musculoskeletal/soft issue | 0 (0) | 1 (4) | 0 (0) |
| Neurology | |||
| Dizziness | 1 (4) | 0 (0) | 0 (0) |
| Mood alteration | 1 (4) | 0 (0) | 0 (0) |
| Sensory neuropathy | 5 (21) | 1 (4) | 0 (0) |
| Pain | |||
| Arthralgia | 1 (4) | 0 (0) | 0 (0) |
| Neuropathic pain | 1 (4) | 1 (4) | 0 (0) |
| Pain | 3 (13) | 0 (0) | 0 (0) |
| Pulmonary | |||
| Dyspnea | 1 (4) | 0 (0) | 0 (0) |
| Hiccoughs | 0 (0) | 1 (4) | 0 (0) |
discussion
While combination chemotherapy for advanced urothelial cancer has led to improved outcomes, the overwhelming majority of patients with metastatic disease will relapse and die of their disease. There is no standard second-line therapy for patients with advanced urothelial cancer. As many patients with advanced bladder cancer have multiple comorbid illnesses, testing of novel agents that may have less toxicity is imperative.
Although preclinical testing indicated that proteasome inhibition may hold promise in urothelial cancer, this study was closed to accrual at the interim analysis on the basis of the lack of objective responses observed with bortezomib in this study. Median time to progression was low at 1.4 months, and median survival was 5.7 months. The poor time to progression and OS observed in this study may, in part, reflect the poor risk nature of the patient population treated, as the majority of patients treated on this trial had poor risk disease, as demonstrated by the high prevalence of visceral metastases in this patient population [10]. Treatment-related toxicity observed in this study was modest, and no single toxicity predominated.
Bortezomib has activity in multiple myeloma and is Food and Drug Administration approved for treatment of refractory disease. Bortezomib, however, has yet to show significant activity in solid tumors. Testing bortezomib as a single-agent in patients with metastatic breast cancer did not yield any objective responses [11, 12]. Other tumor types in which bortezomib has been tested as a single agent include neuroendocrine carcinoma, malignant melanoma, renal cell carcinoma, and small-cell lung cancer [13–17]. No significant antitumor activity was observed in any of these studies with single-agent therapy. A similar phase II study of bortezomib in UC failed to demonstrate any objective responses [18]. From the accumulated data, it is clear that single-agent bortezomib has little activity in solid tumors. These results imply that interference with ubiquitin-mediated protein degradation by single-agent bortezomib in solid tumors does not play a critical role in the maintenance of the malignant phenotype in many solid tumor types. Multiple redundant pathways that contribute to tumor growth and progression are likely responsible for the lack of response to bortezomib seen in this and other studies. While no correlative end points were incorporated in this trial, other trials using this dose and schedule have demonstrated significant proteasome inhibition [8, 19]. Combination studies with chemotherapy and other novel agents are ongoing in multiple tumor types. On the basis of the lack of activity seen in this study, there is no role for further testing of single-agent bortezomib in advanced UC.
funding
NCI (CA31946) to the CALGB (Richard L. Schilsky); CALGB Statistical Center (CA33601 to Stephen George); (CA45808 to J.N.A. and Southeast Cancer Control Consortium Inc. CCOP); (CA77651 to D.F.B. and Memorial Sloan-Kettering Cancer Center).
Acknowledgments
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NCI.
The following institutions participated in this study:
Christiana Care Health Services, Inc. CCOP, Wilmington, DE, Stephen Grubbs, supported by CA45418; Georgetown University Medical Center, Washington, DC, Edward Gelmann, supported by CA77597; Syracuse Hematology-Oncology Assoc. CCOP, Syracuse, NY, Jeffrey Kirshner, supported by CA45389; Kansas City Community Clinical Oncology Program CCOP, Kansas City, MO, Jorge C. Paradelo; Southeast Cancer Control Consortium Inc. CCOP, Goldsboro, NC, JNA, supported by CA45808; The Ohio State University Medical Center, Columbus, OH, Clara D. Bloomfield, supported by CA77658; University of California at San Diego, San Diego, CA, Joanne Mortimer, supported by CA11789; University of California at San Francisco, San Francisco, CA, Alan P. Venook, supported by CA60138; University of Iowa, Iowa City, IA, Gerald Clamon, supported by CA47642; University of Nebraska Medical Center, Omaha, NE, Anne Kessinger, supported by CA77298; Walter Reed Army Medical Center, Washington, DC, Thomas Reid, supported by CA26806; Washington University School of Medicine, St Louis, MO, Nancy Bartlett, supported by CA77440.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–4608. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 3.Mani A, Gelmann EP. The ubiquitin-proteasome pathway and its role in cancer. J Clin Oncol. 2005;23:4776–4789. doi: 10.1200/JCO.2005.05.081. [DOI] [PubMed] [Google Scholar]
- 4.Ling YH, Liebes L, Ng B, et al. PS-341, a novel proteasome inhibitor, induces Bcl-2 phosphorylation and cleavage in association with G2-M phase arrest and apoptosis. Mol Cancer Ther. 2002;1:841–849. [PubMed] [Google Scholar]
- 5.Pei XY, Dai Y, Grant S. The proteasome inhibitor bortezomib promotes mitochondrial injury and apoptosis induced by the small molecule Bcl-2 inhibitor HA14-1 in multiple myeloma cells. Leukemia. 2003;17:2036–2045. doi: 10.1038/sj.leu.2403109. [DOI] [PubMed] [Google Scholar]
- 6.Voorhees PM, Dees EC, O'Neil B, Orlowski RZ. The proteasome as a target for cancer therapy. Clin Cancer Res. 2003;9:6316–6325. [PubMed] [Google Scholar]
- 7.Adams J. Proteasome Inhibitors in Cancer Therapy. Totowa, NJ: Humana Press 2004. [Google Scholar]
- 8.Aghajanian C, Soignet S, Dizon DS, et al. A phase I trial of the novel proteasome inhibitor PS341 in advanced solid tumor malignancies. Clin Cancer Res. 2002;8:2505–2511. [PubMed] [Google Scholar]
- 9.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 10.Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol. 1999;17:3173–3181. doi: 10.1200/JCO.1999.17.10.3173. [DOI] [PubMed] [Google Scholar]
- 11.Brown J, Von Roenn J, O'Regan R, et al. A phase II study of the proteasome inhibitor PS-341 in patients (pts) with metastatic breast cancer (MBC) J Clin Oncol. 2004;22 (Abstr 546) [Google Scholar]
- 12.Yang CH, Gonzalez-Angulo AM, Reuben JM, et al. Bortezomib (VELCADE(R)) in metastatic breast cancer: pharmacodynamics, biological effects, and prediction of clinical benefits. Ann Oncol. 2006;17:813–817. doi: 10.1093/annonc/mdj131. [DOI] [PubMed] [Google Scholar]
- 13.Shah MH, Young D, Kindler HL, et al. Phase II study of the proteasome inhibitor bortezomib (PS-341) in patients with metastatic neuroendocrine tumors. Clin Cancer Res. 2004;10:6111–6118. doi: 10.1158/1078-0432.CCR-04-0422. [DOI] [PubMed] [Google Scholar]
- 14.Svetomir N, Geyer SM, Dawkins F, et al. A phase II study of bortezomib in the treatment of metastatic malignant melanoma. Cancer. 2005;103:2584–2589. doi: 10.1002/cncr.21108. [DOI] [PubMed] [Google Scholar]
- 15.Davis NB, Taber DA, Ansari RH, et al. Phase II trial of PS-341 in patients with renal cell cancer: a University of Chicago phase II consortium study. J Clin Oncol. 2004;22:115–119. doi: 10.1200/JCO.2004.07.165. [DOI] [PubMed] [Google Scholar]
- 16.Kondagunta GV, Drucker B, Schwartz L, et al. Phase II trial of bortezomib for patients with advanced renal cell carcinoma. J Clin Oncol. 2004;22:3720–3725. doi: 10.1200/JCO.2004.10.155. [DOI] [PubMed] [Google Scholar]
- 17.Johl J, Chansky K, Lara PN, et al. The proteasome inhibitor PS-341 (bortezomib) in platinum (plat)-treated extensive-stage small cell lung cancer (E-SCLC): a SWOG (0327) phase II trial. J Clin Oncol. 2005;23 (Abstr 7047) [Google Scholar]
- 18.Gomez-Abuin G, Winquist E, Stadler WM, et al. A phase II study of PS-341 (bortezomib) in advanced or metastatic urothelial cancer: a trial of the Princess Margaret Hospital and University of Chicago phase II consortia. Invest New Drugs. 2007;25:181–185. doi: 10.1007/s10637-006-9009-4. [DOI] [PubMed] [Google Scholar]
- 19.Orlowski RZ, Stinchcombe TE, Mitchell BS, et al. Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. J Clin Oncol. 2002;20:4420–4427. doi: 10.1200/JCO.2002.01.133. [DOI] [PubMed] [Google Scholar]