Abstract
Background
There is an on-going debate whether 2- or 3-weekly administration of R-CHOP is the preferred first-line treatment for elderly patients with diffuse large B-cell lymphoma (DLBCL). The UK NCRI R-CHOP14v21 randomized phase 3 trial did not demonstrate a difference in outcomes between R-CHOP-14 and R-CHOP-21 in newly diagnosed DLBCL patients aged 19–88 years, but data on elderly patients have not been reported in detail so far. Here, we provide a subgroup analysis of patients ≥60 years treated on the R-CHOP14v21 trial with extended follow-up.
Patients and methods
Six hundred and four R-CHOP14v21 patients ≥60 years were included in this subgroup analysis, with a median follow-up of 77.7 months. To assess the impact of MYC rearrangements (MYC-R) and double-hit-lymphoma (DHL) on outcome in elderly patients, we performed a joint analysis of cases with available molecular data from the R-CHOP14v21 (N = 217) and RICOVER-60 (N = 204) trials.
Results
Elderly DLBCL patients received high dose intensities with median total doses of ≥98% for all agents. Toxicities were similar in both arms with the exception of more grade ≥3 neutropenia (P < 0.0001) and fewer grade ≥3 thrombocytopenia (P = 0.05) in R-CHOP-21 versus R-CHOP-14. The elderly patient population had a favorable 5-year overall survival (OS) of 69% (95% CI: 65–73). We did not identify any subgroup of patients that showed differential response to either regimen. In multivariable analysis including individual factors of the IPI, gender, bulk, B2M and albumin levels, only age and B2M were of independent prognostic significance for OS. Molecular analyses demonstrated a significant impact of MYC-R (HR = 1.96; 95% CI: 1.22–3.16; P = 0.01) and DHL (HR = 2.21; 95% CI: 1.18–4.11; P = 0.01) on OS in the combined trial cohorts, independent of other prognostic factors.
Conclusions
Our data support equivalence of both R-CHOP application forms in elderly DLBCL patients. Elderly MYC-R and DHL patients have inferior prognosis and should be considered for alternative treatment approaches.
Trial numbers
ISCRTN 16017947 (R-CHOP14v21); NCT00052936 (RICOVER-60).
Keywords: diffuse large B-cell lymphoma, elderly, R-CHOP, MYC, double-hit lymphoma
Introduction
Elderly patients with diffuse large B-cell lymphoma (DLBCL) have a worse prognosis compared to the younger patient population. This is partly explained by lower treatment tolerability in elderly patients with difficulties to administer adequate doses of chemotherapy. However, even when receiving comparable treatment intensities, elderly DLBCL patients have inferior outcome, potentially indicating more aggressive disease biology. Therefore, dose-intensified administration of R-CHOP immunochemotherapy might be of particular benefit for elderly DLBCL patients to overcome these high-risk factors. Treatment of patients >60 years (y) with 6× R-CHOP-14 plus 2× rituximab in the German RICOVER-60 trial has achieved the best long-term outcome in elderly DLBCL patients published to date [1]. However, superiority of dose-intensified R-CHOP-14 compared to the 3-weekly administration in elderly DLBCL patients could not be demonstrated in randomized trials.
The GELA LNH03-6B trial comparing R-CHOP-14 and R-CHOP-21 in DLBCL patients aged 60–80y showed no difference of either regimen [2], but results were criticized due to high treatment-related mortality and low dose intensities in the R-CHOP-14 arm. The UK NCRI R-CHOP14v21 trial compared the 2- and 3-weekly R-CHOP regimens in DLBCL patients aged 18–88y and similarly did not observe a difference in outcome across age groups [3]. However, outcomes of the elderly R-CHOP14v21 trial cohort have not been reported separately and it remained unclear whether particular subgroups of elderly patients benefit from intensified treatment.
The International Prognostic Index (IPI) is widely used for prognostication of younger and elderly DLBCL patients. Due to differences in disease biology and outcomes it has been proposed to use separate prognostic scores for the elderly patient group [4, 5], but these have not yet been validated in large independent cohorts.
Several molecular high-risk markers have been identified in DLBCL that could potentially refine clinical prognostic models. Cell-of-origin (COO) assessment of DLBCL according to gene-expression-profiling separates the germinal center B-cell (GCB) and the poor prognostic activated B-cell (ABC) subtypes, but these analyses lack prospective validation and methodological problems currently limit their use in standard practice. The negative prognostic impact of MYC rearrangements (MYC-R) as well as MYC- and concomitant BCL2- or BCL6 rearrangements (double-hit lymphoma; DHL) has been shown in several DLBCL cohorts [6, 7]. The prognostic significance of MYC-R seems to be particularly high in older DLBCL patients [6]. Due to the low incidence of MYC-R and DHL and possibly due to their age-dependent relevance, an independent prognostic significance of these markers in multivariate models has not yet been demonstrated in prospective trial cohorts of R-CHOP-treated patients.
The aim of this subgroup analysis was to provide detailed outcomes and toxicity data on elderly patients treated within the R-CHOP14v21 trial and to investigate the impact of clinical and molecular factors on outcome in this age group.
Patients and methods
Patient characteristics in the R-CHOP14v21 trial have been published in detail [3]. A brief description of the trial is given in the Supplement (available at Annals of Oncology online).
Of 1080 R-CHOP14v21 patients, 604 were ≥60y and included in the current analysis. Details of statistical analyses are provided in the Supplement.
COO was assessed by the immunohistochemistry (IHC)-based Hans algorithm. Assessment of MYC-, BCL2- and BCL6-rearrangements was done with fluorescence insitu hybridization (FISH; N = 217). DHL was defined as presence of MYC- and either BCL2- or BCL6-rearrangements. In order to increase the sample size to assess the impact of MYC-R and DHL on outcome in elderly DLBCL patients, we performed a joint analysis with data from 204 elderly DLBCL patients treated on the RICOVER-60 trial who had molecular results available (supplementary Table S1, available at Annals of Oncology online). Details of the German high-grade non-Hodgkin lymphoma study group (DSHNHL) RICOVER-60 trial and methods of molecular analyses within the trial have been previously described [1, 7].
Results
We included 604 elderly patients from the R-CHOP14v21 trial in this subgroup analysis. Patients’ median age was 67y (range 60–88). Baseline characteristics were well balanced between treatment arms (Table 1). There was a trend towards a higher rate of BCL6 rearrangements and DHL in R-CHOP-14 (P = 0.10 and P = 0.06, respectively).
Table 1.
Baseline characteristics
| Characteristics | R-CHOP-21 | R-CHOP-14 |
|---|---|---|
| (N=301) | (N=303) | |
| n (%) | n (%) | |
| Age (years) | ||
| 60–69 | 192 (64) | 196 (65) |
| ≥70 | 109 (36) | 107 (35) |
| Sex | ||
| Female | 148 (49) | 150 (50) |
| Male | 153 (51) | 153 (50) |
| WHO performance status | ||
| 0 | 120 (40) | 143 (47) |
| 1 | 132 (44) | 118 (39) |
| 2 | 49 (16) | 42 (14) |
| Stage (N=596) | ||
| IA | 9 (3) | 9 (3) |
| IB | 6 (2) | 7 (2) |
| II | 90 (30) | 83 (28) |
| III | 91 (31) | 104 (35) |
| IV | 102 (34) | 95 (32) |
| Bulk (N=601) | 139 (47) | 126 (42) |
| B symptoms | 121 (40) | 134 (44) |
| Elevated LDH | 200 (66) | 197 (65) |
| >1 extranodal sites | 94 (31) | 82 (27) |
| IPI score | ||
| 1 | 48 (16) | 44 (15) |
| 2 | 75 (25) | 90 (30) |
| 3 | 98 (33) | 104 (34) |
| 4 | 66 (22) | 56 (18) |
| 5 | 14 (5) | 9 (3) |
| Subtype (N=317) | ||
| GCB | 76 (50) | 82 (50) |
| Non-GCB | 77 (50) | 82 (50) |
| β2-microglobulin ≥3mg/L (N=371) | 88 (51) | 102 (52) |
| Albumin ≤35g/L (N=598) | 100 (34) | 86 (29) |
| MYC rearrangement (N=217) | 9 (9) | 14 (12) |
| BCL2 translocation (N=220) | 26 (25) | 33 (28) |
| BCL6 rearrangement (N=218) | 17 (16) | 30 (26) |
| Double-hit abnormality (N=215) | 5 (5) | 9 (8) |
Dose intensities were high in both trial arms. Median total doses of cyclosphosphamide, doxorubicin, vincristine, prednisolone and rituximab received were 98% versus 99%, 98% versus 99%, 100% versus 100%, 98% versus 100%, and 98% versus 98% in R-CHOP-21 and R-CHOP-14, respectively. Seventy-one (24%) patients on R-CHOP-21 and 46 (15%) patients on R-CHOP-14 did not complete all treatment cycles (P = 0.01). Reasons for early treatment termination are listed in supplementary Table S2, available at Annals of Oncology online, with treatment-related toxicity being the most common cause. Frequency of dose reductions was similar in both arms (15% for R-CHOP-21 versus 16% for R-CHOP-14; P = 0.73).
Treatment toxicities are given in Table 2. There was evidence of more grade ≥3 neutropenia (62% versus 36%; P < 0.0001) and less grade ≥3 thrombocytopenia (7% versus 12%; P = 0.05) in R-CHOP-21 compared to R-CHOP-14. Patients on R-CHOP-21 had lower incidence of anemia (20% versus 31%; P = 0.001), with a similar trend for grade ≥3 anemia (2% versus 5%; P = 0.11). No significant difference in the incidence of fever and infections or any other toxicity was observed. The incidence of treatment-related deaths, fatal cardiac events and secondary malignancies were similar in both arms (supplementary Table S3, available at Annals of Oncology online).
Table 2.
Most common grade ≥3 toxicities and cause of treatment-related deaths
| R-CHOP-21 (N=301) |
R-CHOP-14 (N=303) |
|||
|---|---|---|---|---|
| Any grade | Grade ≥3 | Any grade | Grade ≥3 | |
| All toxicities | 292 (97%) | 216 (72%) | 299 (99%) | 182 (60%) |
| Neutropenia | 224 (74%) | 185 (61%) | 138 (46%) | 109 (36%) |
| Thrombocytopenia | 73 (24%) | 22 (7%) | 112 (37%) | 37 (12%) |
| Anemia | 60 (20%) | 6 (2%) | 95 (31%) | 14 (5%) |
| Infection | 145 (48%) | 71 (24%) | 146 (48%) | 71 (23%) |
| Fever | 70 (23%) | 16 (5%) | 56 (18%) | 16 (5%) |
| Mucositis | 143 (48%) | 4 (1%) | 167 (55%) | 8 (3%) |
| Nausea | 188 (62%) | 7 (2%) | 151 (50%) | 12 (4%) |
| Vomiting | 98 (33%) | 7 (2%) | 82 (27%) | 9 (3%) |
| Diarrhoea | 109 (36%) | 12 (4%) | 113 (37%) | 16 (5%) |
| Constipation | 185 (61%) | 7 (2%) | 160 (53%) | 8 (3%) |
| Neurological | 167 (55%) | 23 (8%) | 183 (60%) | 36 (12%) |
| Fatigue | 240 (80%) | 31 (10%) | 252 (83%) | 40 (13%) |
| Bone pain | 68 (23%) | 7 (2%) | 102 (34%) | 6 (2%) |
| Cardiac | 29 (10%) | 2 (1%) | 29 (10%) | 9 (3%) |
Treatment-related deaths: 3 in R-CHOP-21: 2 nonneutropenic sepsis and 1 neutropenic sepsis; and 7 in R-CHOP-14: 2 nonneutropenic sepsis, 1 neutropenic sepsis, 1 renal failure and 3 not specified.
Response was assessable in 274 patients in each arm. There was no evidence of a difference in response rates between R-CHOP-21 and R-CHOP-14 [complete response (CR)/unconfirmed CR (CRu): 67% versus 62%, P = 0.21; overall response rate (ORR) both 91%; Table 3]. CR/CRu rates after four cycles of therapy were 39% and 33%, respectively (P = 0.15). 61% and 60% of patients are still alive without progression (supplementary Table S3, available at Annals of Oncology online). Four patients on R-CHOP-21 and seven on R-CHOP-14 presented with central nervous system relapse (P = 0.55).
Table 3.
Response to treatment
| End of treatment response | R-CHOP-21 | R-CHOP-14 |
|---|---|---|
| (N=274) | (N=274) | |
| n (%) | n (%) | |
| Complete response (CR) | 145 (53) | 119 (43) |
| Unconfirmed complete response (CRu) | 39 (14) | 50 (18) |
| Partial response | 64 (23) | 80 (29) |
| Stable disease | 16 (6) | 16 (6) |
| Progressive disease or relapse | 10 (4) | 9 (3) |
| CR/Cru | 184 (67) | 169 (62) |
| Overall response rate | 248 (91) | 249 (91) |
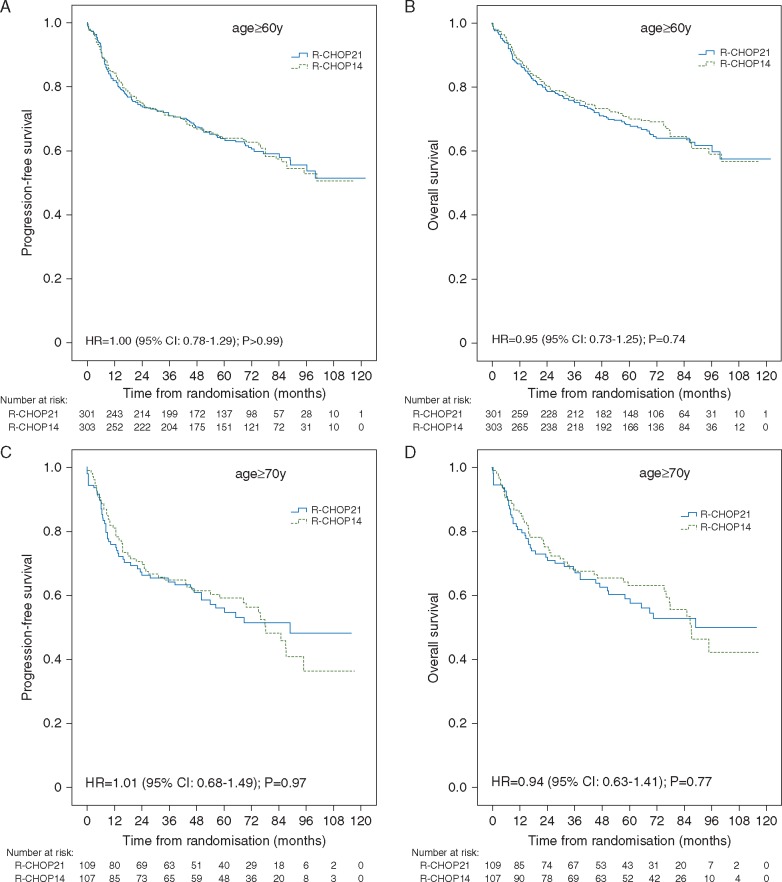
After a median follow-up of 77.7 months, there was no evidence of a difference in progression-free survival (PFS) and overall survival (OS) between treatment arms in patients ≥60y or ≥70y (Figure 1A-D). No difference in survival between R-CHOP-21 and R-CHOP-14 was observed in patients who only achieved partial response (PR) after four cycles (P = 0.79 for PFS; P = 0.68 for OS). There was also no difference between treatment arms with respect to gender (P = 0.54 for PFS; P = 0.67 for OS) or IPI (P = 0.64 for PFS; P = 0.50 for OS). 5y-PFS was 64% (95% CI: 60-68) in patients ≥60y and 58% (95% CI: 51-65) in patients ≥70y. 5y-OS was 69% (95% CI: 66–73) and 61% (95% CI: 54–68), respectively.
Figure 1.
Kaplan–Meier curves of PFS and OS in (A-B) patients over 60 years and (C-D) patients over 70 years.
63/280 (23%) patients with available data received consolidation radiotherapy. Of those, 36 had initial bulk, 20 extranodal disease, and 10 had both. Disease status before radiotherapy was available for 61 patients: 23 (37%) CR/CRu, 31 (51%) PR and 7 (12%) SD. In patients with PR or SD who are supposed to benefit most from radiotherapy, the use of radiotherapy was not associated with OS (supplementary Figure S3, available at Annals of Oncology online).
In multivariable analysis, only age and B2M levels were of independent prognostic significance for OS (supplementary Table S4, available at Annals of Oncology online). There was no significant impact of COO subtypes on outcomes (supplementary Figure S1, available at Annals of Oncology online). When comparing prognostic scores IPI, R-IPI, E-IPI and ABE4 (supplementary Table S5 and Figure S2, available at Annals of Oncology online), ABE4 achieved the best fit and discrimination for predicting OS, followed by the IPI. Similar results were obtained for PFS (data not shown).
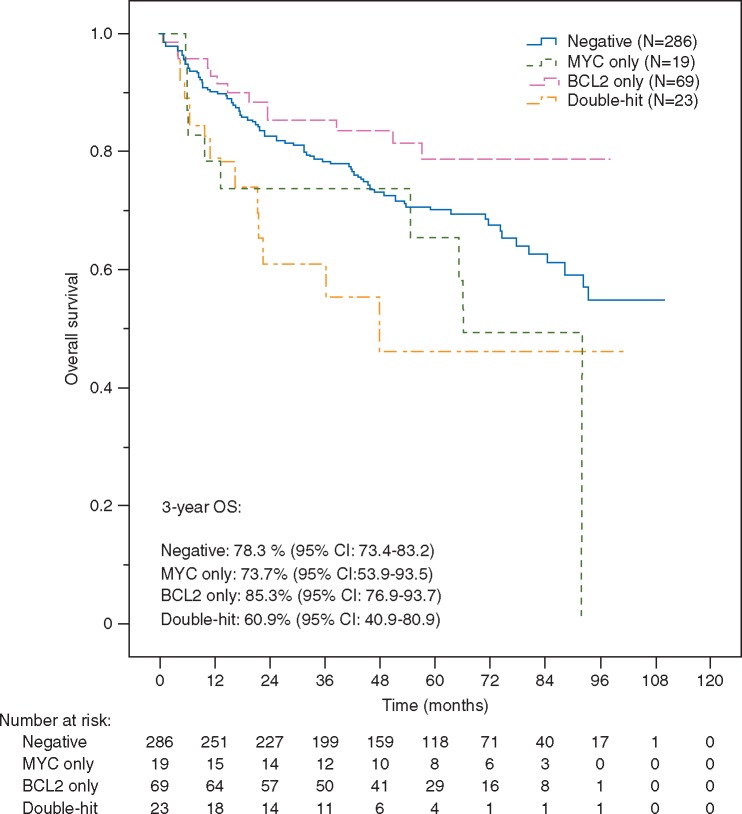
To assess the impact of MYC-R and DHL on outcome we performed a joint analysis with cases from RICOVER-60. 23/217 (11%) patients from our cohort and 19/204 (9%) patients from RICOVER-60 had MYC-R as determined by FISH. 14/215 (7%) and 9/182 (5%) had DHL, respectively. MYC-R and DHL cases had significantly worse OS compared to cases without these abnormalities [HR = 1.96 (95% CI: 1.22–3.16); P = 0.01 and HR = 2.21 (95% CI: 1.18–4.11); P = 0.01, respectively); Figure 2]. Similar effect sizes were observed after adjusting for individual IPI factors and trial arms [HR = 1.76 (95% CI: 1.09–2.85); P = 0.02 and HR = 2.08 (95% CI: 1.11–3.90); P = 0.02, respectively)]. The difference in OS between DHL and MYC-R was not significant (HR = 1.38 (95% CI: 0.55–3.43; P = 0.49). There was no significant impact of BCL2- or BCL6-rearrangements on OS (P = 0.34 and P = 0.99, respectively).
Figure 2.
Kaplan–Meier curves of OS according to MYC- and BCL2 rearrangements and double-hit abnormality in R-CHOP treated elderly patients from R-CHOP14v21 (N = 215) and RICOVER-60 (N = 182).
Discussion
With a median follow-up of 6.5y, we provide a detailed analysis of outcome and toxicities from patients with newly diagnosed DLBCL aged ≥60y treated on the phase 3 R-CHOP14v21 trial.
Elderly DLBCL patients in our cohort had an excellent long-term outcome with 5y-OS of 69% (3y-PFS 71%; 3y-OS 76%). These results are similar to data from elderly DLBCL patients treated with 6× R-CHOP-14 on RICOVER-60 (3y-PFS 73%; 3y-OS 78%) and better than outcomes in the GELA LNH03-6B trial (3y-PFS 61%; 3y-OS 73%) [1, 2]. Of note, patients’ median age was higher in LNH03-6B (70y) compared to our cohort (67y) and RICOVER-60 (68y). In addition, there were more cases presenting with high IPI (3–5) in the LNH03-6B trial (75% versus 57% in our subgroup versus 43% in RICOVER-60), which might have contributed to inferior outcome seen in this trial population.
Toxicity profiles in our cohort of elderly DLBCL patients were favorable in both treatment arms. As expected, patients on R-CHOP-21 had a higher incidence of neutropenia probably due to reduced use of G-CSF, but less thrombocytopenia. Importantly, there was no difference in infectious complications or treatment-related deaths. The incidence of deaths during chemotherapy was very low at 1.7%, suggesting adequate management of elderly patients in participating centers. In the LNH03-6B trial, a high treatment-related mortality of 9% was observed in the initial recruitment period, which improved towards the end of the study, indicating gain of clinical experience with dose-intensified treatment in elderly patients. With 6.5y median follow-up, there was no difference in long-term toxicity, specifically cardiac events and secondary malignancies, between arms.
Dose intensities were high in both arms and as seen in the entire R-CHOP14v21 trial cohort [3]. The low dose intensity of 88% for R-CHOP-14 in the LNH03-6B trial could have potentially underestimated efficacy of the 2-weekly regimen. Our results support equivalence of both regimens in elderly DLBCL patients when adequate doses are achieved. However, the study was not powered for this posthoc subgroup analysis in elderly patients.
We did not identify any subgroup of elderly DLBCL patients that showed differential response to either regimen, including gender and IPI groups. No difference between treatment arms could be seen in patients ≥70y. An analysis of patients ≥80y was not feasible due to low numbers (N = 20). Moreover, there was no benefit of dose-intense treatment in late responders who had not achieved CR/Cru after four cycles.
Consolidation radiotherapy was at the discretion of the investigators and performed in 23% of elderly patients with available data. The main indication for radiotherapy was initial bulky or extranodal disease. The benefit of radiotherapy to initial bulk in elderly DLBCL patients is reported to be greatest for patients who are not in CR/CRu after induction therapy [8]. Accordingly, most patients in our analysis received radiotherapy to PR or SD at the end of treatment, without evidence of a survival benefit for this strategy. However, these data have significant limitations (nonrandomized approach, small numbers). In addition, no PET-CT data were recorded. The on-going DSHNHL OPTIMAL > 60 trial will investigate whether consolidation radiotherapy can be safely omitted in elderly DLBCL patients who are PET-negative at the end of treatment.
Remarkably, PFS of elderly patients was only 8 percentage points worse at 5y compared to younger patients (5y-PFS 64% versus 72%), supporting the concept of treating elderly patients with full doses of chemotherapy whenever possible. Toxicities were also similar between elderly and younger patients (data not shown), besides a significantly higher rate of grade ≥3 neutropenia in elderly (P≤0.001).
Differences between DLBCL of elderly and younger patients have been described on the molecular level, with higher frequencies of ABC subtypes, BCL6 rearrangements, gains in 1q21, 18q21, and 7q21, and a higher genetic complexity associated with increasing age [9]. We did not observe material differences in the frequency of MYC-R, BCL6- and BCL2-rearrangements between age groups, nor in the incidence of IHC-based cell-of-origin subtypes (data not shown). We found lower frequency of bulky disease and higher B2M levels in elderly compared to younger patients, implying differences in disease biology between both groups.
Age-specific clinical and molecular features suggest the need for a separate prognostic scoring system for elderly patients. We compared performances of two recently proposed prognostic scores for elderly DLBCL (ABE4 [5] and E-IPI [4]) with the standard IPI and R-IPI in our cohort. Both scores use an age cut-off of 70y. ABE4 further incorporates bulky disease and separates PS ≥ 1 instead of ≥ 2. The ABE4 performed best in our cohort, despite bulky disease not being significantly associated with patient outcomes. Therefore, separating patients with PS 0 from those with PS ≥ 1 could be a more appropriate cut-off in an elderly patient group. Both ABE4 and IPI distinguished meaningful prognostic groups for PFS and OS. However, clinical utility of the ABE4 score might be limited by the fact that only 9% of patients from our cohort were in the high-risk group compared with 14% in the original Czech Lymphoma Registry [5]. As discussed by Ziepert et al. [10], introduction of new scores have to be seen with caution and should only be considered if properly validated and if changing patients’ management. The main use of the IPI has been in the context of clinical trials, allowing risk-stratification of patients and facilitating comparison of results across trials. A NCCN-IPI has recently been proposed which separates three different age groups as risk factors [11]. The great disadvantage of this score is that it cannot be used for elderly and young patient groups separately and is therefore unsuitable for age-specific DLBCL trials. In contrast, the IPI as age-adjusted IPI has been validated in both young and elderly DLBCL.
In line with previous findings, IHC-based cell-of-origin classification did not impact on outcomes, further underscoring limitations of this method. However, final analyses of the REMoDL-B trial will reveal if the concept of cell-of-origin classification as prognostic marker holds true when assessed prospectively [12]. Our combined analysis of FISH data from R-CHOP14v21 and RICOVER-60 demonstrates for the first time independent prognostic significance of both MYC-R and DHL in patients treated with R-CHOP within prospective cohorts. A negative prognostic impact of MYC-R and DHL has been reported in several heterogeneous DLBCL populations, but did not reach independent significance in trial cohorts due to small numbers [3, 7]. On-going prospective trials will reveal if these patients benefit from upfront treatment intensification.
In conclusion, our data demonstrate excellent short and long-term results with both R-CHOP-14 and R-CHOP-21 in elderly DLBCL patients. This analysis contributes important information to the longstanding discussion about optimal management of the elderly DLBCL patient population and provides a detailed analysis of molecular and clinical prognostic factors in this age group.
Supplementary Material
Acknowledgements
We would like to thank participating centers of the R-CHOP14v21 and RICOVER-60 trials, as well as patients and their families involved.
Funding
Cancer Research UK provided endorsement and funding of the R-CHOP14v21 trial (CRUKE/03/019). The National Health Service (NHS) provided funding to the National Institute for Health Research (NIHR) Biomedical Research centers at both University College London and the Royal Marsden Hospital/Institute of Cancer Research, London, UK (no grant numbers apply). Chugai Pharmaceuticals provided an educational grant and lenograstim within the R-CHOP14v21 trial (no grant numbers apply).
Key Message
We provide a detailed outcome analysis of elderly patients with DLBCL treated with 2- or 3-weekly R-CHOP within the UK R-CHOP14v21 trial, indicating equivalence of both regimens in the elderly patient population. In a joint analysis of R-CHOP14v21 and RICOVER-60 molecular data, we demonstrate that MYC rearrangements and double-hit-lymphoma are independent poor prognostic factors in elderly DLBCL.
Disclosure
DC has received research funding from Amgen, Astra Zeneca, Bayer, Celgene, Medimmune, Merrimack, Merck Serono and Sanofi. EAH has received travel expenses from Takeda and Bristol-Myers Squibb. CP has received travel expenses from Gilead and Speaker fees from Janssen. KMA has received research funding, conference expenses and honoraria for attending or chairing advisory boards from Roche. GO was supported by the Robert-Bosch-Stiftung, Stuttgart, Germany. All other authors have no conflicts of interest to disclose.
References
- 1. Pfreundschuh M. et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol 2008; 9: 105–116. [DOI] [PubMed] [Google Scholar]
- 2. Delarue R. et al. Dose-dense rituximab-CHOP compared with standard rituximab-CHOP in elderly patients with diffuse large B-cell lymphoma (the LNH03-6B study): a randomised phase 3 trial. Lancet Oncol 2013; 14: 525–533. [DOI] [PubMed] [Google Scholar]
- 3. Cunningham D. et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet 2013; 381: 1817–1826. [DOI] [PubMed] [Google Scholar]
- 4. Advani RH. et al. Comparison of conventional prognostic indices in patients older than 60 years with diffuse large B-cell lymphoma treated with R-CHOP in the US Intergroup Study (ECOG 4494, CALGB 9793). Br J Haematol 2010; 151: 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prochazka V. et al. A new prognostic score for elderly patients with diffuse large B-cell lymphoma treated with R-CHOP: the prognostic role of blood monocyte and lymphocyte counts is absent. PLoS One 2014; 9: e102594.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barrans S. et al. Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B-cell lymphoma treated in the era of rituximab. J Clin Oncol 2010; 28: 3360–3365. [DOI] [PubMed] [Google Scholar]
- 7. Horn H. et al. MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood 2013; 121: 2253–2263. [DOI] [PubMed] [Google Scholar]
- 8. Held G. et al. Role of radiotherapy to bulky disease in elderly patients with aggressive B-cell lymphoma. J Clin Oncol 2014; 32: 1112–1118. [DOI] [PubMed] [Google Scholar]
- 9. Klapper W. et al. Patient age at diagnosis is associated with the molecular characteristics of diffuse large B-cell lymphoma. Blood 2012; 119: 1882–1887. [DOI] [PubMed] [Google Scholar]
- 10. Ziepert M. et al. Standard international prognostic index remains a valid predictor of outcome for patients with aggressive CD20+ B-cell lymphoma in the rituximab era. J Clin Oncol 2010; 28: 2373–2380. [DOI] [PubMed] [Google Scholar]
- 11. Zhou Z. et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood 2014; 123: 837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davies A. et al. A prospective randomised trial of targeted therapy for diffuse large B-cell lymphoma (DLBCL) based upon real-time gene expression profiling: the Remodl-B study of the UK NCRI and SAKK lymphoma groups. Abstract 812, ASH Annual Meeting, 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.