Abstract
Background
Prognostic scores have been developed to estimate the risk of recurrence and the probability of survival after nephrectomy for renal cell carcinoma (RCC). The use of these tools, despite being helpful to plan a customized schedule of follow-up, to the patient's tailored counselling and to select individuals who could potentially benefit from adjuvant treatment, currently is not routine, due to their relative complexity and to the lack of histological data (i.e. necrosis).
Patients and methods
We developed a simple score called GRade, Age, Nodes and Tumor (GRANT) based on four easily obtained parameters: Fuhrman grade, age, pathological nodal status and pathological tumor size. Patients with 0 or 1 factor are classified as favorable risk, whereas patients with two or more risk factors as unfavorable risk. The large population of RCC patients from the ASSURE adjuvant trial was used as independent dataset for this external validation, to investigate the prognostic value of the new score in terms of disease-free survival and overall survival and to evaluate its possible application as predictive tool. Statistical analyses were carried out by the Department of Biostatistics & Computational Biology, Dana-Farber Cancer Institute (Boston, USA) for the ASSURE trial patients’ population.
Results
The performance of the new model is similar to that of the already validated score systems, but its strength, compared with the others already available, is the ease and clarity of its calculation, with great speed of use during the clinical practice. Limitations are the use of the Fuhrman nuclear grade, not valid for rare histologies, and the TNM classification modifications over time.
Conclusion
The GRANT score demonstrated its potential usefulness for clinical practice.
ClinicalTrials.gov Identifier for the ASSURE trial
Keywords: adjuvant therapy, ASSURE trial, GRANT score, prognostic score, RCC, renal cancer
Introduction
The incidence of renal cell carcinoma (RCC) is increasing worldwide, reaching 2%–3% of all malignant cancers in adults. The main therapeutic approach in early stage RCC is radical or partial nephrectomy, but disease recurrence occurs in 30%–40% of patients [1]. TNM staging system and prognostic scores have been developed to estimate both the risk of recurrence and the probability of survival after nephrectomy. These tools can be helpful to plan customized schedules of follow-up based on the risk of recurrence, to the patient's tailored counseling and, in the light of recent evidence in favor of adjuvant therapy, to better select individuals who could potentially benefit from adjuvant treatment with targeted agents [1, 2].
European Society of Medical Oncology (ESMO) 2016 guidelines suggest the use of two models for the risk assessment after radical surgical treatment: the Mayo Clinic Stage, Size, Grade and Necrosis (SSIGN) score and the University of California Los Angeles Integrated Staging System (UISS) [3]. The SSIGN model was developed to predict cancer-specific survival in patients with clear cell RCC (ccRCC) only, whereas the UISS model was separately developed to predict overall survival (OS) regardless of histological subtype [4, 5]. The Mayo group further developed a distinct model, the Leibovich score, to predict disease-free survival (DFS) in patients with early ccRCC, using the same parameters but differentiating their scoring weight [6, 7]. Both these systems consider T and N pathological stage (according to 6th edition of TNM Staging [8]), tumor size, Fuhrman nuclear grading [9] and histological tumor necrosis [4, 6]. The UISS is instead based on Eastern Cooperative Oncology Group (ECOG) performance status (PS), Fuhrman nuclear grade and TNM pathological stage [5, 8]. Although less used, three further prognostic models have been reported by the literature, one of which was developed for the non-clear cell RCC population [10–12].
Despite their validation, with the demonstration of a high predictive accuracy combined with robustness across different populations, the use of these prognostic scores in clinical practice is not routine. This may be related to a certain complexity in the score calculation and to the lack of histological data (i.e. the presence of necrosis).
Recently, our group developed a simple score based on four easily obtained parameters: Fuhrman grade, age, pathological nodal status (pN) and pathological tumor size (pT). In this model, called the GRANT score (GRade, Age, Nodes and Tumor), the number of unfavorable risk factors is summed: patients with 0 or 1 factor are classified as favorable risk, whereas patients with two or more risk factors are classified as unfavorable risk (Table 1).
Table 1.
The GRANT score: the number of unfavorable risk factors is summed, and patients with 0 or 1 factor are classified in the favorable risk group, whereas patients with two or more risk factors are classified in the unfavorable risk group
| Variable | Score |
|---|---|
| Age | |
| >60 | 1 |
| ≤60 | 0 |
| pT (TNM 2002a) | |
| 1–2–3a | 0 |
| 3b–3c–4 | 1 |
| Pathologic nodal status | |
| 0–X | 0 |
| 1–2 | 1 |
| Fuhrman grade | |
| 1–2 | 0 |
| 3–4 | 1 |
| Favorable group | 0–1 |
| Unfavorable group | ≥2 |
TNM according to 2002 TNM Staging (American Joint Committee on Cancer 6th edition).
The original version of this score was developed in a population of 310 patients with completely resected RCC patients enrolled in a prospective, randomized, phase III trial comparing the efficacy of 5 years of adjuvant immunotherapy with low-dose interleukin-2 (IL-2) and interferon-α (IFN-α) versus observation [13]. Subgroup analysis of the original study showed benefit from adjuvant treatment of patients with tumor Fuhrman grade 1–2, age ≤60 years, pN0 and pT3a stage. Among patients with at least two of these factors, adjuvant immunotherapy had a positive effect on relapse-free survival (RFS) [hazard ratio (HR) = 0.44; 95% confidence intervals (95% CI) 0.24–0.82; P = 0.008], whereas patients with fewer than two factors in the treatment arm exhibited a significantly poorer OS (HR = 2.27; 95% CI 1.03–5.03, P = 0.037). Based on this evidence, the score seemed to have predictive value for relapse and a prognostic role in patients treated with adjuvant immunotherapy, albeit these results needs to be confirmed in a prospective adjuvant trial [13].
Currently, the potential usefulness of adjuvant treatment with tyrosine kinase inhibitors after radical resection of the primary tumor in early RCC is controversial, in the light of discordant or still ongoing studies [2, 14–17].
The aim of this study was to externally validate the GRANT score in the large population of patients from the ASSURE trial (E2805), to investigate its prognostic value and to evaluate its possible application as predictive tool.
Materials and methods
Statistical analyses were carried out by the Department of Biostatistics & Computational Biology, Dana-Farber Cancer Institute, Boston, USA, for E2805 patients’ population. The goal was the validation of the GRANT score in an independent dataset.
The parameters for the calculation of the score were the subsequent:
Grade 1–2 versus 3–4;
Age ≤60 years versus >60 years;
pN0–NX versus pN1–N2;
pT1–T3a versus all others (any pT3b, pT3c, pT4).
Patients were given one point for each of age >60, Fuhrman grade >2, pathologic T-stage of T3b, T3c or T4, and pathologic N-stage other than N0 or NX. The number of unfavorable risk factors was summed (Table 1), and patients with 0 or 1 point were classified as favorable risk (GRANT Group 0), whereas patients with two or more risk factors (≥2 points) were classified as unfavorable risk (GRANT Group 1).
The 6th edition of the UICC-AJCC TNM staging system was used.
Patients from all arms of E2805 were used to validate the index.
Considering the histologic heterogeneity of the ASSURE trial population, we carried out a secondary analysis in the clear cell subgroup of patients (1538 cases), excluding all non-clear cell histologies.
Descriptive statistics were used to show the distribution of the marker components among patients randomized to the study. The Kaplan–Meier method was used to plot DFS by risk groups. Greenwood's formula was used to estimate variance for construction of confidence intervals [18–20].
The C-statistic suggested by Pencina was used to describe concordance [21].
Results
There were 1943 patients with resected RCC with relatively high risk of recurrence enrolled on E2805: 17 were missing for the GRANT score (because of missing Fuhrman grade), leaving a total of 1926 cases included in this analysis. Of these, 639 were randomized to sunitinib, 647 to sorafenib and 640 to placebo. Characteristics of patients in the trial population are reported in the supplementary material (supplementary Table S1, available at Annals of Oncology online).
The distribution of the classical prognostic score elements, the number of risk factors and the scores obtained according to the GRANT algorithm are reported in Table 2. The distribution for the ECOG PS and for the UISS score [22] is also shown for comparative analysis. Overall 1117 patients had a favorable score (group 0) whilst 809 patients had an unfavorable score (group 1).
Table 2.
Distribution of prognostic factors in the patients’ population
| Total |
N (%) |
|
|---|---|---|
| 1926 (100) | ||
| T-stage | 1 | 196 (10.2) |
| 2 | 512 (26.6) | |
| 3 | 1195 (62) | |
| 4 | 23 (1.2) | |
| N-stage | 0 | 728 (37.8) |
| 1 | 89 (4.6) | |
| 2 | 74 (3.8) | |
| X | 1035 (53.7) | |
| Fuhrman grade | 1 | 48 (2.5) |
| 2 | 605 (31.4) | |
| 3 | 897 (46.6) | |
| 4 | 376 (19.5) | |
| UISS score | Intermediate high | 962 (49.9) |
| Very high | 964 (50.1) | |
| PS | 0 | 1518 (78.8) |
| 1 | 408 (21.2) | |
| Number of factors | 0 | 307 (15.9) |
| 1 | 810 (42.1) | |
| 2 | 618 (32.1) | |
| 3 | 178 (9.2) | |
| 4 | 13 (0.7) | |
| GRANT risk group | 0 (favorable) | 1117 (58) |
| 1 (unfavorable) | 809 (42) | |
According to GRANT score, group 1 represents the poor risk patients with two or more poor prognostic elements and group 0 represents the good risk patients’ with 0–1 negative factors.
For both DFS and OS there was a significant difference between favorable and unfavorable risk groups (P < 0.001, P < 0.001).
The also positive results of the secondary analysis carried out in the clear cell subset of patients (1538 cases) are reported in the supplementary materials (supplementary Tables S2–S4 and Figures S1 and S2, available at Annals of Oncology online).
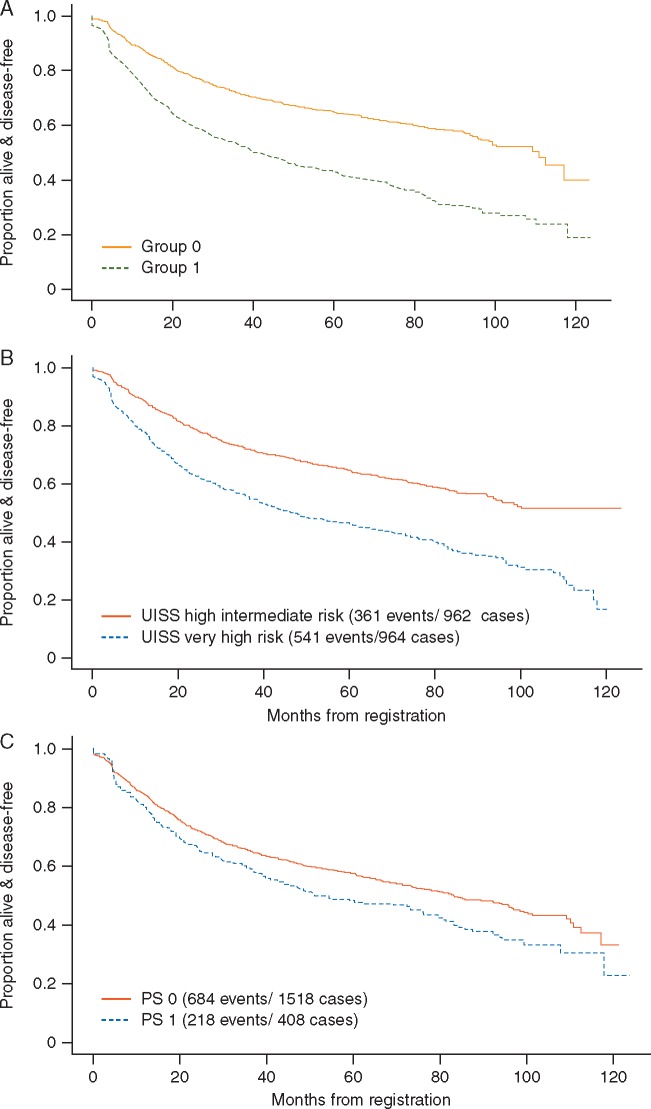
Disease-free survival
DFS by risk group as determined by the new proposed scoring system is shown in Figure 1A. For comparison, the figure also shows DFS by UISS group (Figure 1B) and by ECOG PS (Figure 1C).
Figure 1.
(A) Disease-free survival by GRANT score risk category: group 1 represents the poor risk patients, with two or more poor prognostic elements, and group 0 represents the good risk patients with 0–1 negative factors. (B) Disease-free survival by UISS risk category. (C) Disease-free survival by ECOG performance status.
Concordance scores and corresponding 95% CIs for DFS according to GRANT score, UISS and ECOG PS were, respectively: 0.589 (95% CI 0.571–0.6074); 0.581 (95% CI 0.56–0.599); 0.521 (95% CI 0.506–0.636).
Hazard ratios (HR) from proportional hazards models of the risk groups were similar for GRANT score (HR = 2.04, 95% CI 1.79–2.32, P < 0.001), UISS score (HR = 1.84, 95% CI 1.61–2.10, P < 0.001) and ECOG PS (HR = 1.29, 95% CI 1.11–1.50, P = 0.001).
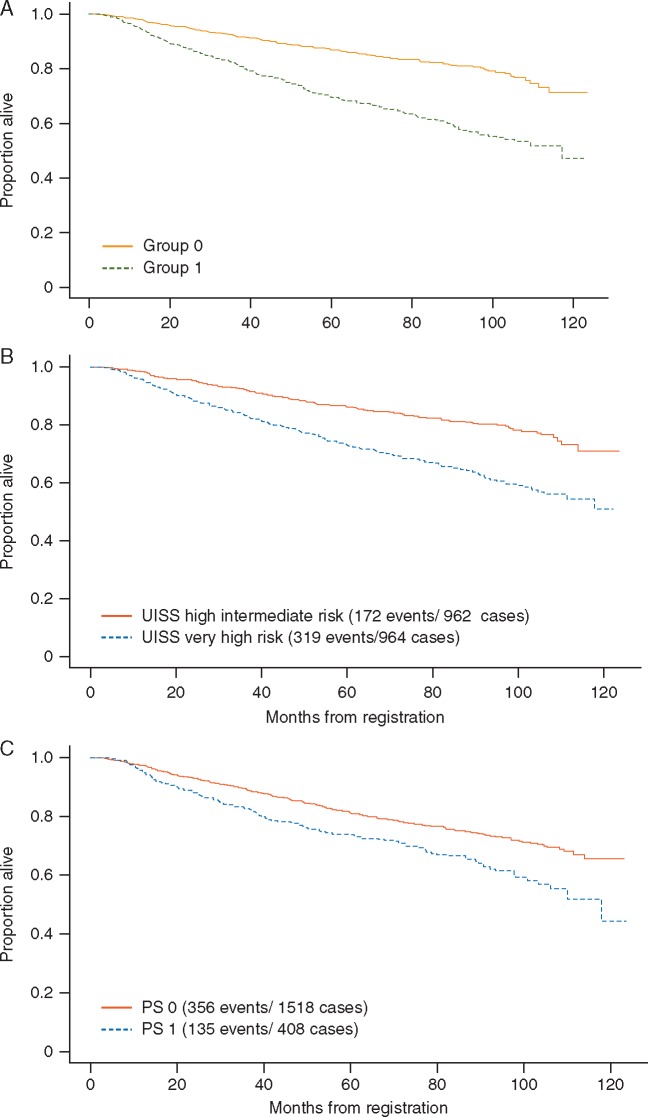
Overall survival
OS by risk group as determined by the GRANT algorithm is shown in Figure 2A, which also shows OS by UISS group (Figure 2B) and by ECOG PS (Figure 2C). Concordance scores and corresponding 95% CIs for OS according to GRANT score, UISS and ECOG PS were, respectively: 0.613 (95% CI 0.589–0.636); 0.590 (95% CI 0.567–0.613); 0.536 (95% CI 0.515–0.557).
Figure 2.
(A) Overall survival by GRANT score risk category: group 1 represents the poor risk patients, with two or more poor prognostic elements, and group 0 represents the good risk patients with 0–1 negative factors. (B) Overall survival by UISS risk category. (C) Overall survival by ECOG performance status.
HR from proportional hazards models of the risk groups with OS as the end point were similar for GRANT score, UISS nomogram and ECOG PS, respectively, HR = 2.49, 95% CI 2.07–2.98; HR = 2.10, 95% CI 1.75–2.53; HR = 1.53, 95% CI 1.25–1.87; P < 0.001 in all cases. Again, the GRANT algorithm carried out similarly to the UISS staging criteria in this population.
Predicting benefit
In the stratified Cox models in the interaction term for the group and treatment was tested. This was done for both sunitinib and sorafenib versus placebo for DFS and OS. At 0.05 level none of the interaction terms were significant (supplementary Table S5, available at Annals of Oncology online).
Supplementary Figures S3 and S4, available at Annals of Oncology online, show DFS by GRANT risk group for patients treated on each experimental arm and patients treated on the placebo arm.
Supplementary Figures S5 and S6, available at Annals of Oncology online, show OS by GRANT risk group for patients treated on each experimental arm and patients treated on the placebo arm.
Although the main effect of the GRANT group was significant in all models, it did not appear to be predictive of benefit in terms of DFS and OS from adjuvant treatments administered in this study.
Discussion
An accurate system for predicting prognosis in cancer, such as the widely used TNM staging [23], is useful for counseling patients, individualizing follow-up, choosing treatment options, tailoring decisions and interpreting results of clinical trials.
In the development of predictive algorithms there is often a compromise between predictive accuracy and ease of use. Although adding more variables and data can increase the accuracy of a model, it also increases the complexity and may not significantly improve its predictive ability or clinical utility.
The UISS score was developed using the kidney cancer database from the UCLA Kidney Cancer Program, with the goal of providing a clinically simple and accurate algorithm for predicting survival, using a few variables readily available in medical practice [7]. An impediment to the widespread clinical adoption and external validation of the SSIGN is its reliance on tumor necrosis, a pathological variable that lacks a standardized definition and reporting method and is not quickly available at most centers, especially in private practice [24].
A 16-gene assay to predict recurrence after surgery in localized RCC was recently developed, but it is not currently accessible in clinical practice in terms of cost and complexity [25].
The present study had the purpose of improve and externally validate a new prognostic tool, originally developed in the context of a clinical trial with immunotherapy in the adjuvant setting [13]. Our original score system demonstrated and utilized pT3a (versus any other stages) as a positive prognostic factor among those treated with adjuvant immunotherapy, given that its immunomodulatory effect on prognosis might be optimized in moderately advanced tumors [13]. This ‘paradoxical’ phenomenon has recently been externally confirmed with a large Japanese cohort (n = 436) [26]. Meanwhile, to establish a more versatile model, we have perfected the original nomogram in the current version, namely the GRANT score, in which pT1–T3a (versus pT3b–T4) is served as an alternative good factor instead of the solely T3a, with the other three factors unchanged.
The validation cohort included a large population of patients underwent radical surgery for early stage renal cancer, enrolled in a big randomized phase III trial to receive tyrosine-kinase inhibitors versus placebo as adjuvant treatment [15]. In this population, the strength of the score was significant both in terms of OS and DFS prediction.
Despite a concordance score quite low for both the GRANT score and the already validated UISS, the almost identical results demonstrate that the GRANT algorithm performs very similarly to the UISS staging criteria in this population.
The strength of the new model, compared with the others already available, is the ease and clarity of its calculation, with great speed of use during the clinical practice. This simplicity could make the new score preferable to others, as it utilizes easily available parameters, fostering the communication with the patient and favoring the follow-up planning. Based on these results and considerations, the GRANT score demonstrated its potential usefulness for clinical practice.
The fact that it works just as well (even better) for the clear cell RCC patients’ subgroup, compared with the entire population, confirms the versatility of the model.
Moreover, considering its original development in the setting of an immune-based-therapy, in the light of recent therapeutic advances with the new check-point inhibitors such as nivolumab, an anti-PD-1 antibody approved for the treatment of advanced RCC, the GRANT score could be better applied to a population treated with the new immunotherapy or planned to be selected for adjuvant immunotherapy.
Future validation of a predictive value for the GRANT score, beyond the prognostic significance, could represent a landmark for planning adjuvant trials in high-risk selected populations of patients with completely resected RCC, providing the key element to select a cohort more liable to benefit from adjuvant treatment.
The advantage to test the score in adjuvant trials, despite with higher risk patients compared with the whole population of radically resected RCC, is the prospective feature of such populations, virtually free from bias when compared with retrospective urologic cohorts. Anyway, the ASSURE trial enrolled a wider risk population, also inclusive of pT1bN0 and pT2N0 tumors. This study had several limitations. First, the Fuhrman nuclear grade, not valid for rare histologies (such as chromophobe RCC) [8], was employed instead of the newest grading system indicated by the International Society of Urological Pathology [27]. Second, the TNM classification for pT3a has been modified over time: in 2002, it was defined as the extension to ‘perinephric tissue, renal sinus or contiguous into adrenal gland’ (T3b for renal vein involvement) while in 2010 it included ‘perinephric tissue, renal sinus or renal vein’ (the adrenal gland involvement was attributed to T4) [8, 23]. Third, the investigation of the predictive value of the score acquired a limited significance in the light of the negative results of the trial. Lastly, the c-index of the GRANT score is not very high, but anyway it is slightly better than those of the UISS score, already validated and herein compared with our model.
In conclusion, the GRANT score has been validated as prognostic model in a wide prospective population of patients with resected early stage RCC, it can be useful in clinical practice and for planning future trials with adjuvant immunotherapy.
Further validation of the score is currently underway in other large populations to confirm its value as a new prognostic and possibly predictive clinical tool.
Funding
This study was coordinated by the ECOG-ACRIN Cancer Research Group (Peter J. O’Dwyer, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported by the National Cancer Institute of the National Institutes of Health under the following award numbers: CA180820 and CA180794. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Disclosure
NBH is the study chair of the ASSURE trial. All remaining authors have declared no conflicts of interest.
Supplementary Material
References
- 1. Buti S, Bersanelli M, Donini M. et al. Systemic adjuvant therapies in renal cell carcinoma. Oncol Rev 2012; 6(2): e18.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ravaud A, Motzer RJ, Pandha HS. et al. Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N Engl J Med 2016; 375(23): 2246–2254. [DOI] [PubMed] [Google Scholar]
- 3. Escudier B, Porta C, Schmidinger M. et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016; 27(Suppl 5): v58–v68. [DOI] [PubMed] [Google Scholar]
- 4. Frank I, Blute ML, Cheville JC. et al. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol 2002; 168: 2395–2400. [DOI] [PubMed] [Google Scholar]
- 5. Zisman A, Pantuck AJ, Dorey F. et al. Mathematical model to predict individual survival for patients with renal cell carcinoma. J Clin Oncol 2002; 20: 1368–1374. [DOI] [PubMed] [Google Scholar]
- 6. Leibovich BC, Blute ML, Cheville JC. et al. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer 2003; 97: 1663–1671. [DOI] [PubMed] [Google Scholar]
- 7. Tan MH, Kanesvaran R, Li H. et al. Comparison of the UCLA Integrated Staging System and the Leibovich score in survival prediction for patients with nonmetastatic clear cell renal cell carcinoma. Urology 2010; 75(6): 1365–1370.e1–3. [DOI] [PubMed] [Google Scholar]
- 8. Greene FL, Page D, Fleming ID. et al. AJCC Cancer Staging Manual, 6th edition New York: Springer-Verlag, 2002. [Google Scholar]
- 9. Fuhrman SA, Lasky LC, Limas C.. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol 1982; 6: 655–663. [DOI] [PubMed] [Google Scholar]
- 10. Karakiewicz PI, Briganti A, Chun FK. et al. Multi-institutional validation of a new renal cancer-specific survival nomogram. J Clin Oncol 2007; 25: 1316–1322. [DOI] [PubMed] [Google Scholar]
- 11. Kattan MW, Reuter V, Motzer RJ. et al. A postoperative prognostic nomogram for renal cell carcinoma. J Urol 2001; 166(1): 63–67. [PubMed] [Google Scholar]
- 12. Sorbellini M, Kattan MW, Snyder ME. et al. A postoperative prognostic nomogram predicting recurrence for patients with conventional clear cell renal cell carcinoma. J Urol 2005; 173(1): 48–51. [DOI] [PubMed] [Google Scholar]
- 13. Passalacqua R, Caminiti C, Buti S. et al. Adjuvant low-dose interleukin-2 (IL-2) plus interferon-α (IFN-α) in operable renal cell carcinoma (RCC): a phase III, randomized, multicentre trial of the Italian Oncology Group for Clinical Research (GOIRC). J Immunother 2014; 37(9): 440–447. [DOI] [PubMed] [Google Scholar]
- 14. Pal SK, Haas NB.. Adjuvant therapy for renal cell carcinoma: past, present, and future. Oncologist 2014; 19(8): 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haas NB, Manola J, Uzzo RG. et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet 2016; 387(10032): 2008–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. SORCE: A Phase III Randomised Double-Blind Study Comparing Sorafenib With Placebo in Patients With Resected Primary Renal Cell Carcinoma at High or Intermediate Risk of Relapse. NCT00492258. https://www.clinicaltrials.gov/ct2/show/NCT00492258 (6 September 2017, date last accessed).
- 17. A Randomized, Double-blind, Placebo-controlled Phase III Study to Evaluate the Efficacy and Safety of Pazopanib as Adjuvant Therapy for Subjects With Localized or Locally Advanced RCC Following Nephrectomy. NCT01235962. https://www.clinicaltrials.gov/ct2/show/NCT01235962 (6 September 2017, date last accessed).
- 18. Kaplan EL, Meier P.. Nonparametric estimation from incomplete observations. J Am Stat Assn 1958; 53: 457–481. [Google Scholar]
- 19. Cox DR. Regression models and life tables. J Royal Stat Soc B 1972; 34: 181–220. [Google Scholar]
- 20. Collett D, Modelling Survival Data in Medical Research. London: Chapman and Hall, 1994. [Google Scholar]
- 21. Pencina MJ, D'Agostino RB.. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Statist Med 2004; 23: 2109–2123. [DOI] [PubMed] [Google Scholar]
- 22. Lam JS, Shvarts O, Leppert JT. et al. Postoperative surveillance protocol for patients with localized and locally advanced renal cell carcinoma based on a validated prognostic nomogram and risk group stratification system. J Urol 2005; 174(2): 466–472. [DOI] [PubMed] [Google Scholar]
- 23. Eggener S. TNM staging for renal cell carcinoma: time for a new method. Eur Urol 2010; 58(4): 517–519. [DOI] [PubMed] [Google Scholar]
- 24. Ficarra V, Novara G, Galfano A. et al. The ′Stage, Size, Grade and Necrosis′ score is more accurate than the University of California Los Angeles Integrated Staging System for predicting cancer-specific survival in patients with clear cell renal cell carcinoma. BJU Int 2009; 103(2): 165–170. [DOI] [PubMed] [Google Scholar]
- 25. Rini B, Goddard A, Knezevic D. et al. A 16-gene assay to predict recurrence after surgery in localised renal cell carcinoma: development and validation studies. Lancet Oncol 2015; 16(6): 676–685. [DOI] [PubMed] [Google Scholar]
- 26. Taguchi S, Buti S, Fukuhara H. et al. Benefit of adjuvant immunotherapy in renal cell carcinoma: a myth or a reality? PLoS One 2017; 12(2): e0172341.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Delahunt B, Cheville JC, Martignoni G. et al. Members of the ISUP Renal Tumor Panel. The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am J Surg Pathol 2013; 37(10): 1490–1504. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.