See Rogaeva and Schmitt-Ulms (doi: 10.1093/aww201 ) for a scientific commentary on this article .
The brain-derived neurotrophic factor (BDNF) Val66Met polymorphism modulates Aβ+ related memory decline in sporadic Alzheimer’s disease. Yen Ying Lim et al. report that this polymorphism also affects outcomes in autosomal dominant Alzheimer’s disease. Met66 carriers show greater dysfunction in cognition, glucose metabolism and tau, with implications for clinical trial design.
Keywords: Alzheimer’s disease, amyloid-β, tau, dementia, genetics
See Rogaeva and Schmitt-Ulms (doi: 10.1093/aww201 ) for a scientific commentary on this article .
The brain-derived neurotrophic factor (BDNF) Val66Met polymorphism modulates Aβ+ related memory decline in sporadic Alzheimer’s disease. Yen Ying Lim et al. report that this polymorphism also affects outcomes in autosomal dominant Alzheimer’s disease. Met66 carriers show greater dysfunction in cognition, glucose metabolism and tau, with implications for clinical trial design.
Abstract
See Rogaeva and Schmitt-Ulms (doi: 10.1093/aww201 ) for a scientific commentary on this article .
The brain-derived neurotrophic factor ( BDNF ) Val66Met polymorphism is implicated in synaptic excitation and neuronal integrity, and has previously been shown to moderate amyloid-β-related memory decline and hippocampal atrophy in preclinical sporadic Alzheimer’s disease. However, the effect of BDNF in autosomal dominant Alzheimer’s disease is unknown. We aimed to determine the effect of BDNF Val66Met on cognitive function, hippocampal function, tau and amyloid-β in preclinical autosomal dominant Alzheimer’s disease. We explored effects of apolipoprotein E ( APOE ) ε4 on these relationships. The Dominantly Inherited Alzheimer Network conducted clinical, neuropsychological, genetic, biomarker and neuroimaging measures at baseline in 131 mutation non-carriers and 143 preclinical autosomal dominant Alzheimer’s disease mutation carriers on average 12 years before clinical symptom onset. BDNF genotype data were obtained for mutation carriers (95 Val 66 homozygotes, 48 Met 66 carriers). Among preclinical mutation carriers, Met 66 carriers had worse memory performance, lower hippocampal glucose metabolism and increased levels of cerebrospinal fluid tau and phosphorylated tau (p-tau) than Val 66 homozygotes. Cortical amyloid-β and cerebrospinal fluid amyloid-β 42 levels were significantly different from non-carriers but did not differ between preclinical mutation carrier Val 66 homozygotes and Met 66 carriers. There was an effect of APOE on amyloid-β levels, but not cognitive function, glucose metabolism or tau. As in sporadic Alzheimer’s disease, the deleterious effects of amyloid-β on memory, hippocampal function, and tau in preclinical autosomal dominant Alzheimer’s disease mutation carriers are greater in Met 66 carriers. To date, this is the only genetic factor found to moderate downstream effects of amyloid-β in autosomal dominant Alzheimer’s disease.
Introduction
Alzheimer’s disease begins with the aggregation of amyloid-β, the development and spread of hyperphosphorylated tau ( Ballatore et al. , 2007 ; Ittner and Götz, 2011 ), and ultimately neuronal and synaptic loss. This characteristic pathological process manifests initially as cognitive impairment, which increases progressively so eventually classification of dementia is warranted ( Hardy and Higgins, 1992 ; Ittner and Götz, 2011 ; Spires-Jones and Hyman, 2014 ). Clinical pathological relationships in Alzheimer’s disease are still not understood completely; however, recent in vitro ( Hariri et al. , 2003 ; Lee et al. , 2012 ), post-mortem ( Peng et al. , 2005 ; Garzon and Fahnestock, 2007 ; Buchman et al. , 2016 ) and animal ( Caccamo et al. , 2010 ; Lee et al. , 2012 ; Rosa and Fahnestock, 2015 ) studies suggest neurotrophic factors, such as brain-derived neurotrophic factor (BDNF) moderate neuronal and synaptic dysfunction and their behavioural expression in Alzheimer’s disease ( Fahnestock, 2011 ; Lu et al. , 2013 ).
Clinical studies of the role of BDNF in Alzheimer’s disease are limited by the absence of validated biomarkers for CNS BDNF ( Forlenza et al. , 2010 ; Kim et al. , 2015 ). However, the BDNF Val66Met (rs6265) polymorphism Met protein can result in reduced dendritic trafficking and synaptic localization of the protein and up to a 30% reduction in activity-dependent BDNF secretion ( Egan et al. , 2003 ; Chen et al. , 2006 ). In healthy young adults, memory-dependent hippocampal activity is reduced in Met 66 carriers ( Hariri et al. , 2003 ). In the preclinical and prodromal stages of sporadic Alzheimer’s disease, prospective studies show Met 66 carriers to have increased rates of decline in episodic memory and hippocampal atrophy relative to Val 66 homozygotes ( Feng et al. , 2013 ; Lim et al. , 2013 , 2014 b ). These same studies observe rates of cortical amyloid-β accumulation to be unaffected by the Met 66 allele ( Lim et al. , 2013 , 2014 b ), suggesting that BDNF Met 66 may accelerate neuronal dysfunction and memory decline by moderating pathological processes downstream of cortical amyloid-β accumulation, such as tau aggregation.
While the processes that give rise to cortical amyloid-β accumulation are likely to differ between sporadic and autosomal dominant Alzheimer’s disease, the effects of amyloid-β on neurodegeneration and cognition are similar, albeit occurring at markedly younger ages in autosomal dominant Alzheimer’s disease (ADAD) (mean age of onset is 45 years) ( Bateman et al. , 2012 ; Jack and Holtzman, 2013 ; Ryman et al. , 2014 ). Therefore the aim of this study was to investigate the effects of the BDNF Met 66 allele on episodic memory, hippocampal function, amyloid-β and tau in ADAD. The first hypothesis was that in preclinical ADAD mutation carriers, impairment in episodic memory and hippocampal function would be greater in individuals who carry at least one copy of the BDNF Met 66 allele compared to Val 66 homozygotes. The second hypothesis was that cortical amyloid-β levels would be unrelated to variation in BDNF Val66Met. The third hypothesis was that CSF tau levels would be greater in BDNF Met 66 carriers compared to Val 66 homozygotes. We also explored the extent to which carriage of the BDNF Met 66 allele was associated with domains of cognition beyond episodic memory, neuronal function in the precuneus and CSF biomarkers of amyloid-β 1-42 and phosphorylated tau (p-tau 181 ). Finally, while the apolipoprotein E ( APOE ) ε4 allele does not increase severity of clinical presentation in ADAD ( Ryman et al. , 2014 ), we observed previously additive effects of the BDNF Met 66 and APOE ε4 alleles on amyloid-β-related cognitive decline in preclinical sporadic Alzheimer’s disease ( Lim et al. , 2015 b ). Therefore, we also explored the extent to which APOE acts independently, or with BDNF , to impact disease processes in ADAD.
Materials and methods
Participants
Individuals at risk for carrying a mutation for ADAD [i.e. presenilin 1 ( PSEN1 ), presenilin 2 ( PSEN2 ), or amyloid precursor protein ( APP ) mutations] were enrolled in the Dominantly Inherited Alzheimer Network (DIAN) study. Participants from families with known pathogenic ADAD mutations were recruited from 197 families at six sites in the USA, one in the UK and three in Australia ( Morris et al. , 2012 ). The process of recruitment and enrolment has been described in detail previously ( Bateman et al. , 2012 ; Morris et al. , 2012 ). Baseline data from 274 participants (131 non-carriers, 143 preclinical mutation carriers) who were cognitively normal, as defined by a Clinical Dementia Rating (CDR) of 0, and who had completed assessments of cognitive function, neuroimaging and CSF sampling were included. APOE genotype was determined for all individuals as part of the DIAN study protocol. Additionally, for mutation carriers, only individuals whose BDNF Val66Met polymorphism was available were included. Table 1 shows the demographic characteristics of each participant group.
Table 1.
Demographic and clinical characteristics
| Mutation non-carriers | Mutation carrier Val 66 /Val 66 | Mutation carrier Met 66 | P -value | |
|---|---|---|---|---|
| ( n = 131) | ( n = 95) | ( n = 48) | ||
| n (%) Female | 58 (44.3%) | 37 (38.9%) | 25 (52.1%) | 0.325 |
| n (%) APOE ε4 carrier | 39 (29.8%) | 24 (25.3%) | 12 (25.0%) | 0.784 |
| Age | 38.37 (10.13) | 34.45 (8.54) | 35.12 (9.51) | 0.012 |
| Estimated year of onset | N/A | −12.44 (8.11) | −12.70 (7.60) | 0.855 |
| Years of education | 14.79 (2.64) | 14.72 (3.54) | 14.24 (2.56) | 0.328 |
| GDS | 1.24 (1.66) | 1.45 (1.83) | 1.47 (1.60) | 0.566 |
| CDR sum of boxes | 0.01 (0.06) | 0.02 (0.10) | 0.06 (0.17) | 0.005 |
| MMSE | 29.20 (1.17) | 28.97 (1.37) | 29.04 (0.99) | 0.340 |
CDR = Clinical Dementia Rating scale; GDS = Geriatric Depression Scale; HADS = Hospital Anxiety and Depression Scale; MACQ = Memory Complaints Questionnaire; MMSE = Mini-Mental State Examination.
Clinical assessment
Without reference to participants’ performance on the neuropsychological test battery, a clinician assessed each participant for the presence and severity of clinical symptoms of dementia at baseline. This was operationalized using the CDR scale, for which a CDR total score of 0 indicates cognitive normality ( Morris, 1983 ). Participants also completed the Mini-Mental State Examination (MMSE) and the Geriatric Depression Scale (GDS) at baseline.
Neuropsychological assessment
All participants were assessed using the DIAN neuropsychological test battery, which includes the Wechsler Memory Scale–Revised Logical Memory (Story A only, immediate and delayed recall) and Digit Span; Category Fluency (animals, vegetables); Trail Making Test A and B; Digit Symbol from the Wechsler Adult Intelligence Scale–Revised (WAIS-R); the Boston Naming Test (30 odd items), letter fluency for F, A, and S, and immediate and delayed recall of a single presentation of a 16-item word list ( Storandt et al. , 2014 ). These tasks have been described previously, and were administered according to standard protocols by trained research assistants ( Storandt et al. , 2014 ). The process of standardization and quality control of neuropsychological assessments across all DIAN sites have also been described previously ( Storandt et al. , 2014 ).
Outcome measures for each neuropsychological test were standardized against the baseline mean and standard deviation for the non-carriers group. Standardized scores were then averaged to form four cognitive domain-specific composite scores for episodic memory (Logical Memory delayed recall, word list learning delayed recall); executive function (Letter Fluency, Trail Making Test B); language (Category Fluency animals + vegetables, Boston Naming Test); attention (Digit Span Forwards, Digit Symbol); and global cognition (Logical Memory delayed recall, word list learning delayed recall, Digit Symbol, MMSE) ( Donohue et al. , 2014 ).
Genotyping
Genotyping for pathogenic mutations in the APP , PSEN1 , and PSEN2 genes were performed on DNA extracted from peripheral blood samples using methods described previously ( Talbot et al. , 1994 ). Samples were also genotyped with the Infinium HumanExomeCore V1.0 Beadchip (Illumina, Inc.). Genotyping was performed at The Genome Technology Access Center (GTAC; https://gtac.wustl.edu/ ) at Washington University. All samples and genotypes underwent stringent quality control (QC). Genotype data were cleaned by applying a minimum call rate for single nucleotide polymorphisms (SNPs) and individuals (98%). SNPs not in Hardy-Weinberg equilibrium ( P < 1 × 10 −6 ) were excluded. No SNPs were removed due to low minor allele frequency. Gender identification was verified by analysis of X-chromosome SNPs. We tested for unanticipated duplicates using pairwise genome-wide estimates of proportion identity-by-descent using PLINK v1.9. Genotype data for the BDNF Val66Met (rs6265) polymorphism were extracted from using PLINK. Clinicians were blinded to all genetic information and genetic polymorphisms were not used diagnostically. BDNF Val66Met genotyping was performed only in samples from individuals with a known ADAD mutation.
Neuroimaging
Images obtained through PET with the use of fluorodeoxyglucose (FDG) and Pittsburgh compound B (PiB) (FDG-PET and PiB-PET, respectively) were co-registered with individual MRI images for region of interest determination. Volumetric (3 T) T 1 -weighted MRI scans from DIAN participants were acquired and processed through FreeSurfer (Martinos Center, Boston, MA) as previously described ( Benzinger et al. , 2013 ). Amyloid imaging was performed with a bolus injection of ∼15 mCi of 11 C-PiB. Dynamic imaging acquisition started either at injection for 70 or 40 min post-injection for 30 min. For analysis, PiB-PET data between 40 to 70 min were used. For PiB-PET, total neocortical standardized uptake value ratio (SUVR) was used to determine levels of cortical amyloid-β deposition, using cerebellar grey matter as the reference region and applying partial volume correction using a regional point spread function as previously described ( Su et al. , 2015 ).
Metabolic imaging with 18 F-FDG-PET was performed with a 3D dynamic acquisition begun 40 min after a bolus injection of ∼5 mCi of FDG and lasted for 20 min. In accordance with previous reports ( Bateman et al. , 2012 ), the regions of interest selected for this study were the hippocampus and the precuneus, with decreased FDG SUVR indicating decreased glucose metabolism and therefore reduced neuronal function in that area. The reference region used was the cerebellar cortex.
Biochemical analysis
Fasted CSF was collected in the morning via lumbar puncture. Samples were shipped on dry ice to the DIAN biomarker core laboratory. CSF concentrations of amyloid-β 42 , total tau, and tau phosphorylated at threonine 181 (p-tau 181 ) were measured by immunoassay (INNOTEST β-Amyloid1-42, Innogenetics). All values had to meet quality-control standards, including a coefficient of variation of 25% or less, kit ‘controls’ within the expected range as defined by the manufacturer, and measurement consistency between plates of a common sample that was included in each run.
Estimated year of onset
The estimated year from expected symptom onset was calculated as the age of the participant at the time of the baseline assessment minus the mean age at onset of all other individuals with the same mutation type ( Ryman et al. , 2014 ).
Data analysis
The study hypotheses that in ADAD, mutation carrier BDNF Met 66 carriage would be associated with greater impairment in memory and hippocampal function, higher CSF tau but not cortical amyloid-β levels were tested by submitting the episodic memory composite, PiB-PET amyloid-β, CSF tau and glucose metabolism in the hippocampus (FDG-PET) to separate analyses of covariance (ANCOVA). In each ANCOVA, estimated year of onset was added as a covariate, and Group (non-carriers, Val 66 /Val 66 mutation carrier, Met 66 mutation carrier) as a fixed factor. Within each ANCOVA, two planned comparisons were constructed with the first comparing Val 66 homozygotes and Met 66 mutation carriers and the second comparing Val 66 homozygote mutation carriers to the non-carriers group. Exploratory analyses were conducted only if a statistically significant difference between the Val 66 homozygote and Met 66 mutation carrier groups was observed for at least one of the primary outcome measures. With this criterion met, the ANCOVAs were repeated for the remaining cognitive composite scores, CSF amyloid-β 42 , CSF p-tau 181 , and FDG-PET in the precuneus. The extent to which the presence of the APOE ε4 allele influenced the effect of BDNF on cognitive function, amyloid-β burden, tau and neuronal function was determined by repeating these analyses with ε4 status (carrier versus non-carriers) entered into all statistical models. Finally, to further understand the effect of BDNF Val66Met on cognitive and biomarker outcomes in ADAD, we expressed each cognitive and biomarker outcome variable as a function of estimated year of onset. For the primary outcomes, statistical significance was classified as P < 0.05. This was to balance the risk of false positive findings against the identification of important relationships because (i) this is an exploratory investigation in a relatively new area in which an important clinical issue has been identified; (ii) as all four primary outcome measures are recognized as part of the Alzheimer’s disease pathological process, changes in these will be correlated; and (iii) effect sizes (Cohen’s d ) were used to guide interpretation about the meaningfulness of statistical tests and comparisons with effect sizes <0.2 were classified as trivial and not interpreted regardless of statistical significance ( Cohen, 1988 ).
Results
Demographic and clinical characteristics
Mutation carriers were significantly younger than non-carriers, although the estimated year of onset between Val 66 homozygotes and Met 66 mutation carriers did not differ significantly. Non-carriers and mutation carrier groups did not differ on any other demographic characteristic. While the inclusion criteria required all individuals to have a CDR score of 0, the CDR sum of boxes score was significantly higher in mutation carrier Met 66 carriers than in mutation carrier Val 66 homozygotes and non-carriers ( Table 1 ). Groups did not differ in MMSE total scores or levels of depressive symptoms.
Effect of BDNF Val66Met on episodic memory, cortical amyloid-β, CSF tau and glucose metabolism in the hippocampus
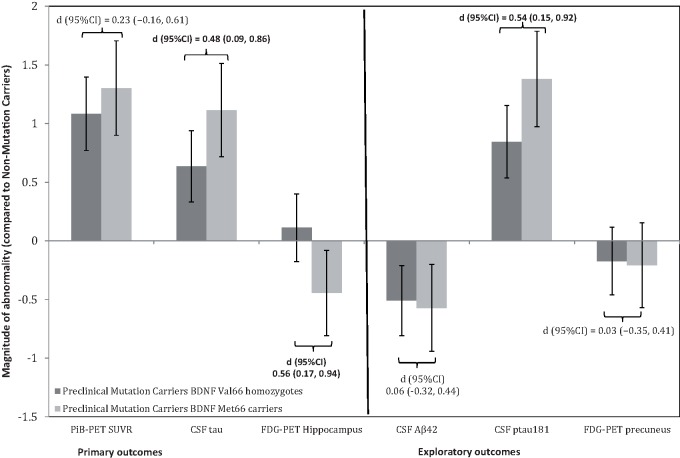
Group means and standard deviations for raw scores on each of the primary outcome cognitive and biomarker measures for each group are summarized on Table 2 . The outcomes of the primary analyses are summarized on Fig. 1 for episodic memory and Fig. 2 for the Alzheimer’s disease biomarkers. Statistically significant group differences between Val 66 homozygot- and Met 66 mutation carriers were observed for episodic memory ( Fig. 1 ), glucose metabolism in the hippocampus and CSF tau, but not cortical amyloid-β ( Fig. 2 ). Effect sizes for these comparisons were, by convention, moderate-to-large in magnitude for episodic memory, glucose metabolism in the hippocampus and CSF tau levels, but were trivial for levels of cortical amyloid-β. No statistically significant differences between non-carriers and Val 66 homozygote mutation carriers were observed for any of the primary outcome measures, with all differences small in magnitude.
Table 2.
Differences in each cognitive marker and biomarker between mutation non-carriers, mutation carriers who are BDNF Val 66 homozygotes, and mutation carriers who are BDNF Met 66 carriers
|
Estimated year of onset
|
Group
|
Mutation non-carriers | Mutation carrier Val 66 /Val 66 | Mutation carrier Met 66 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (df) F | P | (df) F | P | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | |
| Primary outcomes | ||||||||||
| Episodic memory | (1,268) 20.87 | 0.00 | (2,268) 5.35 | 0.00 | 0.03 (0.82) | 131 | −0.12 (0.83) | 95 | −0.43 (0.83) | 48 |
| PiB-PET SUVR | (1,223) 18.31 | 0.00 | (2,223) 38.85 | 0.00 | 1.04 (0.54) | 106 | 1.62 (0.53) | 82 | 1.74 (0.53) | 39 |
| CSF tau | (1,216) 16.20 | 0.00 | (2,216) 19.94 | 0.00 | 57.18 (40.42) | 101 | 82.83 (40.29) | 80 | 102.15 (40.29) | 39 |
| FDG-PET hippocampus | (1,225) 12.13 | 0.00 | (2,225) 3.91 | 0.02 | 1.25 (0.09) | 109 | 1.26 (0.09) | 80 | 1.21 (0.09) | 40 |
| Exploratory outcomes | ||||||||||
| Executive function | (1,268) 2.23 | 0.14 | (2,268) 2.10 | 0.12 | 0.02 (0.80) | 131 | −0.15 (0.81) | 95 | −0.22 (0.81) | 48 |
| Language | (1,267) 0.28 | 0.60 | (2,267) 2.90 | 0.06 | 0.03 (0.86) | 131 | −0.15 (0.86) | 95 | −0.29 (0.86) | 48 |
| Attention | (1,268) 2.20 | 0.14 | (2,268) 4.00 | 0.02 | 0.01 (0.81) | 131 | −0.15 (0.81) | 95 | −0.37 (0.81) | 48 |
| Global Cognition | (1,267) 8.36 | 0.00 | (2,267) 3.58 | 0.03 | 0.02 (0.65) | 131 | −0.12 (0.65) | 95 | −0.26 (0.66) | 48 |
| CSF amyloid-β 42 | (1,213) 8.03 | 0.01 | (2,213) 7.55 | 0.00 | 430.72 (147.29) | 99 | 355.78 (146.91) | 78 | 346.57 (146.79) | 40 |
| CSF p-tau 181 | (1,217) 8.12 | 0.01 | (2,217) 32.22 | 0.00 | 29.27 (22.75) | 101 | 48.48 (22.69) | 80 | 60.62 (22.67) | 40 |
| FDG-PET precuneus | (1,225) 4.62 | 0.03 | (2,225) 1.13 | 0.33 | 2.79 (0.29) | 109 | 2.74 (0.29) | 80 | 2.73 (0.29) | 40 |
Group = effect of group membership as non-carriers, mutation carrier Val 66 homozygote or mutation carrier Met 66 carrier; all models have been adjusted for estimated year of symptom onset; bolded values are significant at the P < 0.05 or P < 0.001 level.
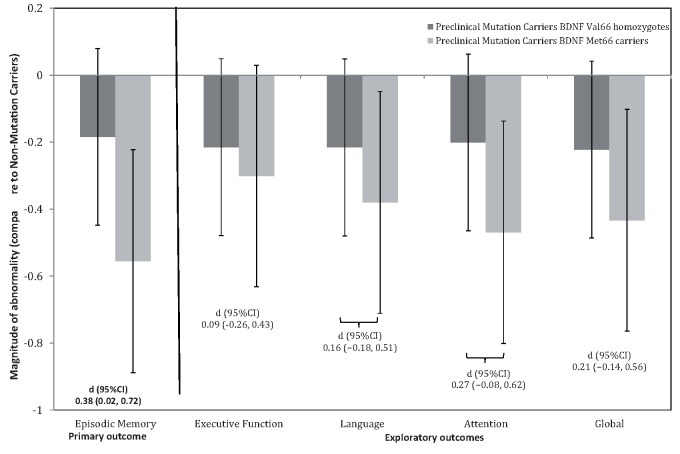
Figure 1.
Magnitude of cognitive impairment in preclinical mutation carrier Val 66 homozygotes and preclinical mutation carrier Met 66 carriers when compared to mutation non-carriers . Error bars represent 95% confidence intervals. Statistical significance occurs when 95% confidence intervals do not cross ‘0’ line.
Figure 2.
Magnitude of abnormality on markers of amyloid-β, tau and glucose metabolism in preclinical mutation carrier Val 66 homozygotes and preclinical mutation carrier Met 66 carriers when compared to mutation non-carriers . Error bars represent 95% confidence intervals. Statistical significance occurs when 95% confidence intervals do not cross ‘0’ line.
Effect of BDNF Val66Met on cognition, CSF amyloid-β 42 , CSF p-tau 181 and glucose metabolism in the precuneus
For each exploratory cognitive and biomarker outcome measure, raw group means and standard deviations are summarized on Table 2 . Figures 1 and 2 also summarize the outcomes of the exploratory analyses for cognitive measures and Alzheimer’s disease biomarkers, respectively. Statistically significant group differences of a moderate-to-large magnitude, were observed between Val 66 homozygotes and Met 66 mutation carriers for CSF p-tau 181 levels ( Fig. 2 ), but not for glucose metabolism in the precuneus or for the executive function, language, attention or global cognition composites ( Fig. 1 ). There were also no statistically significant differences between mutation carrier Val 66 homozygotes and mutation carrier Met 66 carriers on CSF amyloid-β 42 levels, with these differences small in magnitude ( Fig. 2 ).
When compared to non-carriers, Val 66 homozygote mutation carriers showed no statistically significant impairment in any domain of cognitive function ( Fig. 1 ) and did not differ significantly in the extent of glucose metabolism in the hippocampus or the precuneus ( Fig. 2 ). Compared to non-carriers, both Val 66 homozygote and Met 66 mutation carriers showed elevated levels of CSF tau and p-tau 181 , and increased PiB-PET SUVR and decreased CSF amyloid-β 42 levels ( Table 2 ).
Effect of APOE ε4 on cognitive function, neuronal dysfunction, amyloid-β and tau
Reanalyses of the primary hypotheses with the addition of APOE status indicated no significant main effect of APOE status and no significant interaction between APOE and BDNF status on any measure of cognitive function ( Table 3 ). Similarly, there was no significant main effect of APOE or interaction between APOE and BDNF for any outcome measure of glucose metabolism or tau ( Table 3 ). However, there was a significant main effect of APOE for both PiB-PET SUVR and CSF amyloid-β 42 , although there were no significant interactions between APOE and BDNF for either measure ( Table 3 ). Post hoc analyses showed that when compared to mutation carrier ε4 non-carriers, mutation carrier ε4 carriers had significantly increased PiB-PET SUVR { d [95%CI = 0.45 (0.05, 0.85), P = 0.03]} and decreased CSF amyloid-β 42 levels { d [95%CI = 0.76 (0.34, 1.17), P < 0.001]}.
Table 3.
Effect of estimated year of symptom onset, APOE ε4 status, BDNF Val66Met status, and the interaction between APOE and BDNF on each cognitive and biomarker outcome measure
|
Estimated year of onset
|
APOE
Group
|
BDNF
Group
|
APOE
×
BDNF
Group
|
|||||
|---|---|---|---|---|---|---|---|---|
| (df) F | P | (df) F | P | (df) F | P | (df) F | P | |
| Primary outcomes | ||||||||
| Episodic memory | (1,266) 19.32 | 0.00 | (1,266) 1.79 | 0.18 | (1,266) 3.84 | 0.05 | (1,266) 0.08 | 0.77 |
| PiB-PET SUVR | (1,221) 16.73 | 0.00 | (1,221) 7.21 | 0.01 | (1,221) 2.65 | 0.11 | (1,221) 1.18 | 0.28 |
| CSF tau | (1,214) 15.50 | 0.00 | (1,214) 0.24 | 0.63 | (1,214) 4.06 | 0.04 | (1,214) 0.05 | 0.82 |
| FDG-PET hippocampus | (1,223) 11.88 | 0.00 | (1,223) 0.05 | 0.83 | (1,223) 6.03 | 0.02 | (1,223) 0.09 | 0.77 |
| Exploratory outcomes | ||||||||
| Executive function | (1,266) 2.50 | 0.12 | (1,266) 0.93 | 0.34 | (1,266) 0.08 | 0.78 | (1,266) 0.08 | 0.78 |
| Language | (1,266) 0.20 | 0.65 | (1,266) 0.39 | 0.54 | (1,266) 0.99 | 0.32 | (1,266) 0.15 | 0.70 |
| Attention | (1,266) 2.40 | 0.12 | (1,266) 1.46 | 0.23 | (1,266) 2.78 | 0.10 | (1,266) 0.49 | 0.49 |
| DIAN Composite | (1,266) 8.14 | 0.01 | (1,266) 0.04 | 0.84 | (1,266) 0.84 | 0.36 | (1,266) 0.06 | 0. 81 |
| CSF amyloid-β 42 | (1,211) 6.94 | 0.01 | (1,211) 9.28 | 0.00 | (1,211) 0.02 | 0.90 | (1,211) 1.39 | 0.24 |
| CSF p-tau 181 | (1,215) 7.39 | 0.01 | (1,215) 2.00 | 0.16 | (1,215) 4.60 | 0.03 | (1,215) 0.34 | 0.56 |
| FDG-PET precuneus | (1,223) 4.82 | 0.03 | (1,223) 0.65 | 0.42 | (1,223) 0.001 | 0.98 | (1,223) 0.02 | 0.91 |
All models have been adjusted for estimated year of symptom onset; APOE Group indicates effect of group membership as non-carriers, APOE ε4 carrier or APOE ε4 non-carrier; BDNF Group indicates effect of group membership as non-carriers, mutation carrier Val 66 homozygote or mutation carrier Met 66 carrier; bold values are significant at the P < 0.05 level.
Effect of BDNF Val66Met on the relationship between estimated year of onset and markers of cognitive and neuronal function, amyloid-β and tau
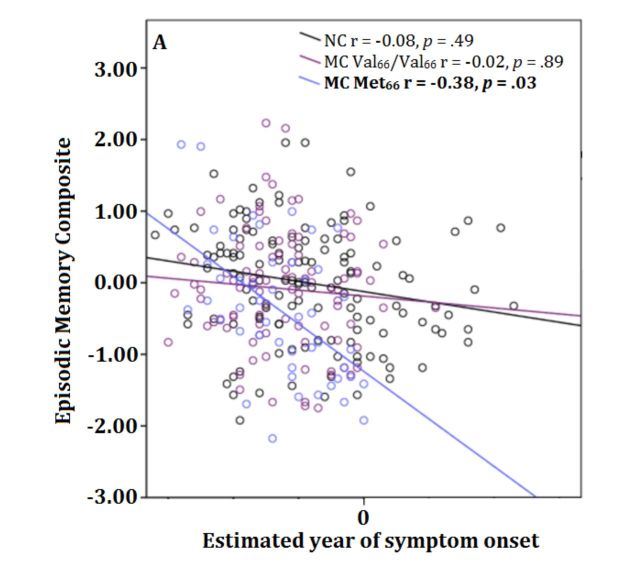
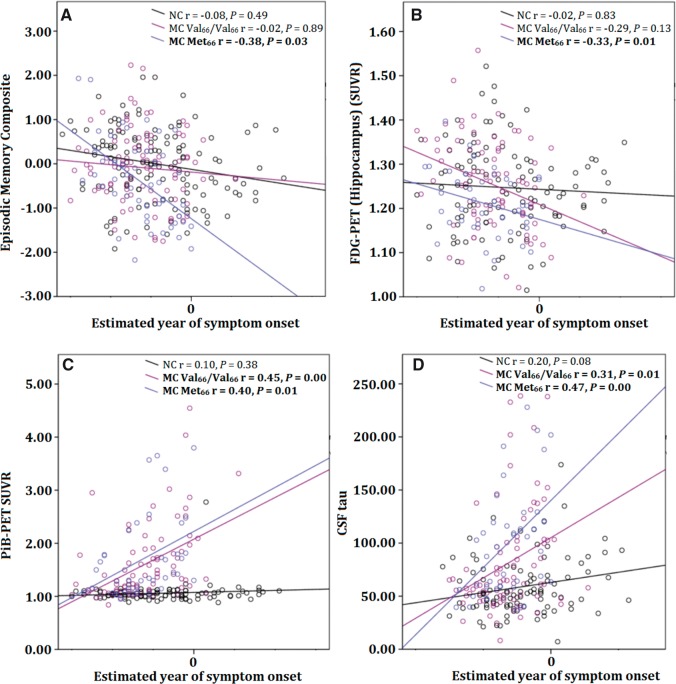
There were no statistically significant relationships between level of cognitive function and estimated year of onset in non-carriers or in Val 66 homozygote mutation carriers. However, the relationship between estimated year of onset and episodic memory was statistically significant and moderate in magnitude for Met 66 mutation carriers ( Fig. 3 A). Similarly, there were no statistically significant relationships between glucose metabolism in the hippocampus and estimated year of onset in non-carriers or in mutation carrier Val 66 homozygotes. However, the relationship between glucose metabolism in the hippocampus and estimated year of onset was moderate in magnitude and statistically significant for mutation carrier Met 66 carriers ( Fig. 3 B).
Figure 3.
Relationship between estimated year of clinical symptom onset and episodic memory performance (A), glucose metabolism in the hippocampus (B), cortical amyloid-β levels (C), and CSF p-tau 181 levels (D), in mutation non-carriers, preclinical mutation carrier Val 66 homozygotes, and preclinical mutation carrier Met 66 carriers .
There was no relationship between levels of cortical amyloid-β and estimated year of onset in non-carriers, but there was a significant moderate association between cortical amyloid-β levels and estimated year of onset in mutation carriers irrespective of BDNF Val66Met polymorphism ( Fig. 3 C). Similarly, while there was no association between CSF tau and estimated year of onset in non-carriers, there was a significant moderate association between CSF tau and estimated year of onset in mutation carriers, irrespective of BDNF Val66Met genotype ( Fig. 3 D), with Met 66 mutation carriers showing systematically higher levels of CSF tau relative to their estimated year of onset than Val 66 homozygote mutation carriers ( Fig. 3 D).
Discussion
The results show that the presence of one copy of the BDNF Met 66 allele increased the severity of impairment in episodic memory and hippocampal function in preclinical ADAD. This effect is clinically important as the magnitude of memory impairment related to Met 66 mutation carriers was approximately double that observed in Val 66 homozygote mutation carriers. These findings in the DIAN cohort are consistent with the greater memory decline and hippocampal volume loss observed in older adults with preclinical or prodromal sporadic Alzheimer’s disease from the AIBL and ADNI studies ( Feng et al. , 2013 ; Lim et al. , 2013 , 2014 b ). The results confirm therefore, in an independent sample, that BDNF is important to the preclinical presentation of Alzheimer’s disease.
The current data support the first hypothesis that in preclinical mutation carriers, impairment in memory and hippocampal function would be greater in Met 66 carriers compared to Val 66 homozygotes. Compared to Val 66 homozygote mutation carriers, Met 66 mutation carriers had worse episodic memory function ( Fig. 1 ). In contrast, no memory impairment was observed in Val 66 homozygote mutation carriers compared to non-carriers. Similarly, hippocampal function, determined by cerebral glucose metabolism, was also reduced in Met 66 mutation carriers compared to Val 66 homozygote mutation carriers. However, Val 66 homozygote mutation carriers did not show lower glucose metabolism compared to non-carriers. As increased oxidative stress has been previously observed in females ( Keaney et al. , 2003 ), it is possible that the sex of participants may better account for the memory impairment in Met 66 mutation carriers. However, reanalysis of all primary outcome measures suggest that even when the sex of participants was considered, the effect of BDNF Val66Met on memory impairment, hippocampal function and tau remains ( Table 4 ). Finally, mutation carrier Met 66 carriers who were estimated to be nearer to their expected year of clinical symptom onset showed increased memory impairment and lower glucose metabolism in the hippocampus ( Fig. 3 ). In contrast, estimated year of onset was not associated with memory impairment or glucose metabolism in non-carriers or Val 66 homozygote mutation carriers.
Table 4.
Reanalysis of the effect of BDNF Val66Met on each primary outcome variable, covarying for the potential confounding effect of sex
|
Estimated year of onset
|
Sex
|
BDNF
group
|
||||
|---|---|---|---|---|---|---|
| (df) F | P | (df) F | P | (df) F | P | |
| Episodic memory | (1,267) 13.96 | 0.00 | (1,267) 8.24 | 0.00 | (2,267) 5.17 | 0.00 |
| PiB-PET SUVR | (1,222) 18.43 | 0.00 | (1,222) 0.01 | 0.92 | (2,222) 40.59 | 0.00 |
| CSF tau | (1,215) 17.02 | 0.00 | (1,215) 0.56 | 0.46 | (2,215) 20.98 | 0.00 |
| FDG-PET hippocampus | (1,224) 5.60 | 0.02 | (1,224) 1.35 | 0.25 | (2,224) 3.95 | 0.02 |
The second hypothesis that cortical amyloid-β and CSF amyloid-β 42 levels would be unrelated to allelic variation in BDNF Val66Met was also supported. Preclinical Met 66 and Val 66 homozygote mutation carriers had equivalent levels of higher cortical amyloid-β and CSF amyloid-β 42 . Furthermore, these group differences were, by convention, small (i.e. d < 0.2; Fig. 2 ) in magnitude indicating that absence of statistically significant differences was not due to insufficient statistical power. Compared to non-carriers, both Met 66 carriers and Val 66 homozygotes showed increased levels of cortical amyloid-β deposition and decreased levels of CSF amyloid-β 42 . Similarly, cortical amyloid-β burden was higher in preclinical mutation carriers who were nearer to their estimated year of onset; although this relationship was not moderated by the BDNF Val66Met polymorphism ( Fig. 3 C). Increased cortical amyloid-β and lower CSF amyloid-β 42 levels have been observed previously in preclinical ADAD ( Bateman et al. , 2012 ; Ryman et al. , 2014 ). The absence of any effect of Met 66 carriage on amyloid-β burden in preclinical ADAD is also consistent with observations that Met carriage was unrelated to rates of cortical amyloid-β accumulation over 3 years in preclinical and prodromal sporadic Alzheimer’s disease ( Feng et al. , 2013 ; Lim et al. , 2013 , 2014 b ). Together, these findings suggest that the effect of the BDNF Met 66 allele is independent of the effect of amyloid-β on risk for, and progression of, Alzheimer’s disease.
The results also support the third hypothesis that CSF levels of tau would be greater in Met 66 mutation carriers compared to Val 66 homozygote mutation carriers. Levels of both CSF tau and p-tau 181 were increased substantially in preclinical Met 66 mutation carriers compared to preclinical Val 66 homozygote mutation carriers ( Fig. 2 ). Compared to non-carriers, preclinical mutation carrier Val 66 homozygotes also showed increased levels of CSF tau and p-tau 181 , although not to the same extent as Met 66 mutation carriers. Despite the overall increase in these biochemical markers, strong relationships between estimated year of onset and CSF tau were observed in both Val 66 homozygotes and Met 66 mutation carriers, and the magnitude of these relationships were equivalent ( Fig. 3 D). Thus, while the Met 66 allele hastens memory dysfunction in preclinical ADAD, it does not necessarily affect the rate at which p-tau 181 accumulates in CSF. Instead, substantial differences in CSF p-tau 181 levels between Met 66 mutation carriers and Val 66 homozygote mutation carriers ( Fig. 2 ) suggest that Val 66 homozygotes may have an increased level of resilience to the neurotoxic effects of tau and amyloid-β.
Finally, we explored the extent to which APOE acts independently or with BDNF to impact disease processes in ADAD. There were no independent effects of APOE ε4, or combined effects of APOE and BDNF , on cognition, neuronal function or CSF tau ( Table 3 ). However, compared to mutation carrier ε4 non-carriers, mutation carrier ε4 carriers showed increased cortical amyloid-β and decreased CSF amyloid-β 42. This indicates that in preclinical ADAD, the abnormal accumulation of cortical amyloid-β resulting from pathogenic mutations is increased further by the APOE ε4 allele, although this increased amyloid-β was not associated with any greater impairment in cognition or neuronal function. Importantly, the increase in cortical amyloid-β in mutation carrier ε4 carriers was not affected by the BDNF Met allele. Thus, allelic variation in BDNF and APOE may affect different Alzheimer’s disease processes with ε4 increasing cortical amyloid-β accumulation and BDNF Met 66 moderating amyloid-β-related impairment in cognition and neuronal function through its effects on tau.
Neuronal and synaptic loss characteristic of both sporadic and autosomal dominant Alzheimer’s disease is due to the combined accumulation of amyloid-β plaques and tau aggregation ( Ballatore et al. , 2007 ; Ittner and Götz, 2011 ; Spires-Jones and Hyman, 2014 ). Neuropathological and CSF biomarker studies show that in Alzheimer’s disease, cognitive impairment and synaptic loss are associated more strongly with the presence and number of neurofibrillary tangles than amyloid-β plaques ( Giannakopoulos et al. , 2003 ; Bennett et al. , 2004 ; Ingelsson et al. , 2004 ). However, neuroimaging studies in preclinical Alzheimer’s disease report that higher cortical amyloid-β load is associated with greater rates of cognitive decline and progression to MCI ( Rowe et al. , 2013 ; Lim et al. , 2014 a ), with these effects mediated by the effect of amyloid-β on neurodegeneration ( Jack and Holtzman, 2013 ; Lim et al. , 2015 a ). In this context, dissociation of the effects of BDNF on amyloid-β and tau associated cognitive impairment observed here are important because they provide evidence that BDNF Met 66 influences disease progression through effects on neuronal dysfunction and cognitive impairment associated with tau.
The current observation that BDNF Met 66 in preclinical ADAD was associated with increased tau, hippocampal dysfunction and memory impairment is consistent with the role that CNS BDNF plays in synaptic excitation, long-term potentiation and neuronal plasticity ( Hariri et al. , 2003 ; Peng et al. , 2005 ; Garzon and Fahnestock, 2007 ; Forlenza et al. , 2010 ; Fahnestock, 2011 ; Lee et al. , 2012 ; Lu et al. , 2013 ). Evidence of a mechanistic relationship between BDNF and tau has been shown in cellular studies that demonstrate that BDNF can induce rapid dephosphorylation of tau through TrkB activation ( Elliott et al. , 2005 ) and that BDNF loss in Alzheimer’s disease is specific to tangle-bearing neurons ( Ferrer et al. , 1999 ). This has prompted the hypothesis that there may be a direct relationship between CNS BDNF levels and tau ( Belrose et al. , 2013 ), although this remains under investigation. Even in the absence of a direct mechanistic link, the large and clinically important effects of BDNF Met 66 on memory, hippocampal function and tau, observed in the current ADAD sample, indicate that studying allelic variation in BDNF Val66Met may help clarify pathological models of Alzheimer’s disease and may even provide a reference for the investigation of the effects and clinical consequences of other neurotrophic factors in Alzheimer’s disease.
As we have noted ( Lim et al. , 2013 ), genome-wide association studies (GWAS) of Alzheimer’s disease do not identify the BDNF Val66Met polymorphism as increasing the risk for Alzheimer’s disease ( Lambert et al. , 2013 ). One possible explanation for this is that GWAS typically use a clinical classification of dementia as the target phenotype. Consequently, they may overlook the contribution of BDNF because the effects of this gene manifest only in the earliest stages of the disease ( Feng et al. , 2013 ; Lim et al. , 2013 , 2014 b ). This hypothesis is supported by GWAS of cognitive ageing in non-demented older adults, where BDNF Val66Met has been associated with memory impairment and decline ( Harris and Deary, 2011 ; Papenberg et al. , 2015 ). Thus, the hypothesis arising from the current and previous studies ( Lim et al. , 2013 , 2014 b ) is that in studies of cognitive ageing, memory decline associated with BDNF Met 66 may reflect occult Alzheimer’s disease as opposed to the effects of normal ageing. In contrast to BDNF , GWAS of Alzheimer’s disease identify carriage of APOE ε4 as increasing risk for Alzheimer’s disease ( Lambert et al. , 2013 ). We have also reported that in preclinical sporadic Alzheimer’s disease, the APOE ε4 allele increases the rate of memory decline and brain volume loss associated with high amyloid-β ( Dore et al. , 2013 ; Lim et al. , 2014 a , 2015 c ). We have also observed that amyloid-β+ older adults who carry both the APOE ε4 and BDNF Met 66 allele show greater memory decline than those who carry either one by itself ( Lim et al. , 2015 b ). Reanalysis of the current data taking into account APOE ε4 did not indicate any effect of APOE or any interaction between APOE and BDNF on cognition ( Table 3 ). The absence of any effect of APOE on cognition in this study is consistent with the results of a detailed meta-analysis of three ADAD cohorts which showed that APOE did not moderate age of clinical symptom onset ( Ryman et al. , 2014 ). However, despite having no effect on cognitive function or clinical symptom onset, APOE ε4 was associated with increasing cortical amyloid-β levels in preclinical mutation carriers. Consequently, one hypothesis for the absence of any APOE effect on cognitive and clinical outcomes in ADAD is that these outcomes are related more strongly to neuronal dysfunction and tau than to amyloid-β accumulation.
This study demonstrates that the deleterious effects of amyloid-β in ADAD were increased in preclinical individuals who carried the BDNF Met 66 allele. Therefore, the results of this study also confirm the similarity between the development of dementia in ADAD and sporadic Alzheimer’s disease. However, as the current findings are based on cross-sectional data, it will be necessary to replicate these results prospectively. Nonetheless, the strength and consistency of our results with that in sporadic Alzheimer’s disease is important because they suggest that strategies designed to increase CNS BDNF levels may be a viable therapeutic alternative or addition to those which seek to reduce the neurotoxic effects of amyloid-β. Our results also suggest strongly that the BDNF Val66Met polymorphism should be considered as a potential moderator of clinical trial outcomes in current treatment and prevention trials in ADAD and sporadic Alzheimer’s disease ( Mills et al. , 2013 ; Donohue et al. , 2014 ).
Acknowledgements
We thank all participants and their families for their commitment and dedication to helping advance research into the early detection and causation of Alzheimer’s disease; and the DIAN research and support staff at each of the participating sites for their contributions to this study.
Glossary
Abbreviations
- ADAD
autosomal dominant Alzheimer’s disease
- DIAN
Dominantly Inherited Alzheimer Network
- FDG
fluorodeoxyglucose
- PiB
Pittsburgh compound B
- SUVR
standardized uptake value ratio
Funding
Data collection and sharing for this project was supported by The Dominantly Inherited Alzheimer’s Network (DIAN, U19AG032438) funded by the National Institute on Aging (NIA) and the German Center for Neurodegenerative Diseases (DZNE). This work was also supported by the NIHR Queen Square Dementia Biomedical Research Unit and the MRC Dementias Platform UK (MR/L023784/1 and MR/009076/1). We thank the Genome Technology Access Center in the Department of Genetics at Washington University School of Medicine for help with genomic analysis. The Center is partially supported by NCI Cancer Center Support Grant #P30 CA91842 to the Siteman Cancer Center and by ICTS/CTSA Grant# UL1TR000448 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Data and/or tissue generated from DIAN fibroblasts (or IPSCs) and/or exome chip sequencing was supported by the DIAN-TU Pharma Consortium, (the DIAN-TU Pharma Consortium, http://dian-tu.wustl.edu/en/pharma-consortium-members/ ). This publication is solely the responsibility of the authors and does not necessarily represent the official view of NCRR or NIH. JP Chhatwal is supported by K23-AG049087. YY Lim is supported by the Alzheimer’s Australia Dementia Research Fellowship, the Yulgilbar Alzheimer's Research Program, and the NHMRC-ARC Dementia Research Development Fellowship.
References
- Ballatore C, Lee VMY, Trojanowski JQ . Tau-mediated neurodegeneration in Alzheimer's disease and related disorders . Nat Rev Neurosci 2007. ; 8 : 663 – 72 . [DOI] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TLS, Fagan AM, Goate A, Fox NC, et al. . Clinical and biomarker changes in dominantly inherited Alzheimer's disease . N Engl J Med 2012. ; 367 : 795 – 804 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belrose JC, Masoudi R, Michalski B, Fahnestock M . Increased pro–nerve growth factor and decreased brain-derived neurotrophic factor in non–Alzheimer's disease tauopathies . Neurobiol Aging 2013. ; 35 : 926 – 33 . [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE . Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function . Arch Neurol 2004. ; 61 : 378 – 84 . [DOI] [PubMed] [Google Scholar]
- Benzinger TL, Blazey T, Jack CR, Koeppe RA, Su Y, Xiong C, et al. . Regional variability of imaging biomarkers in autosomal dominant Alzheimer's disease . Proc Natl Acad Sci USA 2013. ; 110 : E4502 – 9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman AS, Yu L, Boyle PA, Schneider JA, De Jager PL, Bennett DA . Higher brain BDNF gene expression is associated with slower cognitive decline in older adults . Neurology 2016. ; 86 : 735 – 41 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo A, Maldonado MA, Bokov AF, Majumder S, Oddo S . CBP gene transfer increases BDNF levels and ameliorates learning and memory deficits in a mouse model of Alzheimer's disease . Proc Natl Acad Sci USA 2010. ; 107 : 22687 – 92 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, et al. . Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior . Science 2006. ; 314 : 140 – 3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J . Statistical power analysis for the behavioral sciences . 2nd edn. Hillsdale, NJ: : Lawrence Erlbaum Associates; ; 1988. . [Google Scholar]
- Donohue MC, Sperling RA, Salmon DP, Rentz DM, Raman R, Thomas RG, et al. . The preclinical Alzheimer cognitive composite: measuring amyloid-related decline . JAMA Neurol 2014. ; 71 : 961 – 70 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore V, Villemagne VL, Bourgeat P, Fripp J, Acosta O, Chetelat G, et al. . Cross-sectional and longitudinal analysis of the relationship between Aβ deposition, cortical thickness, and memory in cognitively unimpaired individuals and in Alzheimer's disease . JAMA Neurol 2013. ; 70 : 903 – 11 . [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. . The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function . Cell 2003. ; 112 : 257 – 69 . [DOI] [PubMed] [Google Scholar]
- Elliott E, Atlas R, Lange A, Ginzburg I . Brain-derived neurotrophic factor induces a rapid dephosphorylation of tau protein through a PI-3 kinase signalling mechanism . Eur J Neurosci 2005. ; 22 : 1081 – 9 . [DOI] [PubMed] [Google Scholar]
- Fahnestock M . Brain-derived neurotrophic factor: the link between amyloid-b and memory loss . Future Neurol 2011. ; 6 : 627 – 39 . [Google Scholar]
- Feng S, Sevigny J, Verma A, Bennett D, Lim YY, Maruff P . Genetic and image biomarkers associated with decline in cognitive measures and brain glucose metabolism in populations of early Alzheimer's disease . Alzheimers Dement 2013. ; 9 : 178 . [Google Scholar]
- Ferrer I, Marín C, Rey MJ, Ribalta T, Goutan E, Blanco RT, et al. . BDNF and full-length and truncated TrkB expression in Alzheimer disease: implications in therapeutic strategies . J Neuropathol Exp Neurol 1999. ; 58 : 729 – 39 . [DOI] [PubMed] [Google Scholar]
- Forlenza OV, Diniz BS, Teixeira AL, Ojopi EB, Talib LL, Mendonça VA, et al. . Effect of brain-derived neurotrophic factor Val66Met polymorphism and serum levels on the progression of mild cognitive impairment . World J Biol Psychiatry 2010. ; 11 : 774 – 80 . [DOI] [PubMed] [Google Scholar]
- Garzon DJ, Fahnestock M . Oligomeric amyloid decreases basal levels of brain-derived neurotrophic factor (BDNF) mRNA via specific downregulation of BDNF transcripts IV and V in differentiated human neuroblastoma cells . J Neurosci 2007. ; 27 : 2628 – 35 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakopoulos P, Herrmann FR, Bussière T, Bouras C, Kövari E, Perl DP, et al. . Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer's disease . Neurology 2003. ; 60 : 1495 – 500 . [DOI] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA . Alzheimer's disease: the amyloid cascade hypothesis . Science 1992. ; 256 : 184 – 5 . [DOI] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, et al. . Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance . J Neurosci 2003. ; 23 : 6690 – 4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SE, Deary IJ . The genetics of cognitive ability and cognitive ageing in healthy older people . Trends Cogn Sci 2011. ; 15 : 388 – 94 . [DOI] [PubMed] [Google Scholar]
- Ingelsson M, Fukumoto H, Newell KL, Growdon JH, Hedley-Whyte ET, Frosch MP, et al. . Early abeta accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain . Neurology 2004. ; 62 : 925 – 31 . [DOI] [PubMed] [Google Scholar]
- Ittner LM, Götz J . Amyloid-β and tau - a toxic pas de deux in Alzheimer's disease . Nat Rev Neurosci 2011. ; 12 : 65 – 72 . [DOI] [PubMed] [Google Scholar]
- Jack CR, Holtzman DM . Biomarker modeling of Alzheimer's disease . Neuron 2013. ; 80 : 1347 – 58 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keaney JFJ, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, et al. . Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham study . Arterioscler Thromb Vasc Biol 2003. ; 23 : 434 – 9 . [DOI] [PubMed] [Google Scholar]
- Kim A, Fagan AM, Goate AM, Benzinger TL, Morris JC, Head D, et al. . Lack of an association of BDNF Val66Met polymorphism and plasma BDNF with hippocampal volume and memory . Cogn Affect Behav Neurosci 2015. ; 15 : 625 – 43 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, et al. . Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease . Nat Genet 2013. ; 45 : 1452 – 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ST, Chu K, Jung KH, Kim JH, Huh JY, Yoon H, et al. . miR-206 regulates brain-derived neurotrophic factor in Alzheimer disease model . Ann Neurol 2012. ; 72 : 269 – 77 . [DOI] [PubMed] [Google Scholar]
- Lim YY, Maruff P, Pietrzak RH, Ames D, Ellis KA, Harrington K, et al. . Effect of amyloid on memory and non-memory decline from preclinical to clinical Alzheimer's disease . Brain 2014a. ; 137 : 221 – 31 . [DOI] [PubMed] [Google Scholar]
- Lim YY, Pietrzak RH, Bourgeat P, Ames D, Ellis KA, Rembach A, et al. . Relationships between performance on the Cogstate Brief Battery, neurodegeneration, and Aβ accumulation in cognitively normal older adults and adults with MCI . Arch Clin Neuropsychol 2015a. ; 30 : 49 – 58 . [DOI] [PubMed] [Google Scholar]
- Lim YY, Villemagne VL, Laws SM, Ames D, Pietrzak RH, Ellis KA, et al. . BDNF Val66Met moderates Aβ-related memory decline and hippocampal atrophy in prodromal Alzheimer's disease: a preliminary study . PLoS One 2014b. ; 9 : e86498 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YY, Villemagne VL, Laws SM, Ames D, Pietrzak RH, Ellis KA, et al. . BDNF Val66Met, Aβ amyloid and cognitive decline in preclinical Alzheimer’s disease . Neurobiol Aging 2013. ; 34 : 2457 – 64 . [DOI] [PubMed] [Google Scholar]
- Lim YY, Villemagne VL, Laws SM, Pietrzak RH, Snyder PJ, Ames D, et al. . APOE and BDNF polymorphisms moderate amyloid β-related cognitive decline in preclinical Alzheimer's disease . Mol Psychiatry 2015b. ; 20 : 1322 – 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YY, Villemagne VL, Pietrzak RH, Ames D, Ellis KA, Harrington K, et al. . APOE ε4 moderates amyloid-related memory decline in preclinical Alzheimer's disease . Neurobiol Aging 2015c. ; 36 : 1239 – 44 . [DOI] [PubMed] [Google Scholar]
- Lu B, Nagappan G, Guan X, Nathan PJ, Wren P . BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases . Nature Reviews, Neuroscience 2013. ; 14 : 401 – 16 . [DOI] [PubMed] [Google Scholar]
- Mills SM, Mallmann J, Santacruz AM, Fuqua A, Carril M, Aisen PS, et al. . Preclinical trials in autosomal dominant AD: implementation of the DIAN-TU trial . Rev Neurol 2013. ; 169 : 737 – 43 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC . The Clinical Dementia Rating (CDR): current version and scoring rules . Neurology 1983. ; 43 : 2412 – 14 . [DOI] [PubMed] [Google Scholar]
- Morris JC, Aisen PS, Bateman RJ, Benzinger T, Cairns NJ, Fagan AM, et al. . Developing an international network for Alzheimer’s research: the dominantly inherited Alzheimer etwork . Clin Invest 2012. ; 2 : 975 – 84 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papenberg G, Salami A, Persson J, Lindenberger U, Bäckman L . Genetics and functional imaging: effects of APOE, BDNF, COMT, and KIBRA in aging . Neuropsychol Rev 2015. ; 25 : 47 – 62 . [DOI] [PubMed] [Google Scholar]
- Peng S, Wuu J, Mufson EJ, Fahnestock M . Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the preclinical stages of Alzheimer's disease . J Neurochem 2005. ; 93 : 1412 – 21 . [DOI] [PubMed] [Google Scholar]
- Rosa E, Fahnestock M . CREB expression mediates amyloid β-induced basal BDNF downregulation . Neurobiol Aging 2015. ; 36 : 2406 – 13 . [DOI] [PubMed] [Google Scholar]
- Rowe CC, Bourgeat P, Ellis KA, Brown B, Lim YY, Mulligan R, et al. . Predicting Alzheimer disease with β-amyloid imaging: results from the Australian imaging, biomarkers, and lifestyle study of ageing . Ann Neurol 2013. ; 74 : 905 – 13 . [DOI] [PubMed] [Google Scholar]
- Ryman DC, Acosta-Baena N, Aisen PS, Bird T, Danek A, Fox NC, et al. . Symptom onset in autosomal dominant Alzheimer disease: a systematic review and meta-analysis . Neurology 2014. ; 85 : 253 – 60 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires-Jones T, Hyman BT . The intersection of amyloid beta and tau at synapses in Alzheimer's disease . Neuron 2014. ; 82 : 756 – 71 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storandt M, Balota DA, Aschenbrenner AJ, Morris JC . Clinical and psychological characteristics of the initial cohort of the Dominantly Inherited Alzheimer Network (DIAN) . Neuropsychology 2014. ; 28 : 19 – 29 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Blazey TM, Snyder AZ, Raichle ME, Marcus DS, Ances BM, et al. . Partial volume correction in quantitative amyloid imaging . NeuroImage 2015. ; 107 : 55 – 64 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot C, Lendon C, Craddock N, Shears S, Morris JC, Goate A . Protection against Alzheimer's disease with apoE epsilon 2 . Lancet 1994. ; 343 : 1432 – 3 . [DOI] [PubMed] [Google Scholar]