Summary
Self-reported disability among human immunodeficiency virus (HIV)-infected older adults is strongly associated with neurocognitive impairment and socioeconomic or other lifestyle factors. Potentially modifiable factors (smoking, low physical activity) were identified as targets for interventions designed to reduce disability.
Keywords: HIV, disability, neurocognitive impairment, physical activity, frailty
Abstract
Background
Older human immunodeficiency virus (HIV)-infected adults may experience higher rates of frailty and disability than the general population. Improved understanding of the prevalence, risk factors, and types of impairment can better inform providers and the healthcare system.
Methods
HIV-infected participants within the AIDS Clinical Trials Group A5322 HAILO study self-reported disability by the Lawton-Brody Instrumental Activities of Daily Living (IADL) Questionnaire. Frailty was measured by 4-m walk time, grip strength, self-reported weight loss, exhaustion, and low activity. Logistic regression models identified characteristics associated with any IADL impairment. Agreement between IADL impairment and frailty was assessed using the weighted kappa statistic.
Results
Of 1015 participants, the median age was 51 years, 15% were aged ≥60 years, 19% were female, 29% black, and 20% Hispanic. At least 1 IADL impairment was reported in 18% of participants, most commonly with housekeeping (48%) and transportation (36%) and least commonly with medication management (5%). In multivariable models, greater disability was significantly associated with neurocognitive impairment, lower education, Medicare/Medicaid insurance (vs private/other coverage), smoking, and low physical activity. Although a greater proportion of frail participants had IADL impairment (52%) compared to non-frail (11%) persons, agreement was poor (weighted kappa <0.18, 95% confidence interval, 0.13, 0.23).
Conclusion
IADL disability occurs frequently among middle-aged and older HIV-infected adults on effective antiretroviral therapy. Potentially modifiable risk factors (smoking, physical activity) provide targets for interventions to maintain independent living. Systematic recognition of persons at greater risk for disability can facilitate connection to resources that may help preserve independence.
The success of antiretroviral therapy (ART) has changed the characteristics of the human immunodeficiency virus (HIV)/AIDS epidemic, such that an estimated 70% of persons living with HIV may be aged >50 years by 2030 [1]. Within this aging HIV-infected population, an increased risk for and burden of age-associated comorbidities is seen, including cardiovascular disease, chronic kidney disease, osteoporosis, and neurocognitive disorders [2–7]. This growing burden of age-related comorbidities may predispose ART-treated HIV-infected persons to a particularly vulnerable or frail state with a heightened risk of disability [8].
The phenotype of frailty is characterized by vulnerability to adverse health outcomes and incorporates domains of weight loss, weakness, low physical activity, poor endurance, and slow gait [9]. In contrast, disability refers to difficulty in completing daily tasks and activities [10] and may include difficulties in higher-level tasks such as medication management and food preparation (instrumental activities of daily living [IADLs]) or basic needs such as bathing and feeding (activities of daily living [ADLs]). While frailty and other measures of physical, cognitive, and mental health impairment are strong predictors of disability, ultimately an individual’s own expectations of ability and the physical and social environment are key components of disability [10–12]. Thus, the experience of disability may vary widely among individuals with similar symptoms, depending on important socioeconomic factors that allow that individual to live independently. Although frailty and disability are commonly and interchangeably used to describe older adults in poor health, they are distinct concepts that describe aspects of physical and cognitive health related to aging. These distinctions are important when anticipating the needs of aging persons; changes in physical health, disability, and frailty may disproportionally impact health-related quality of life (QoL) or independence. Furthermore, coordination of local resources may mitigate disability, while interventions to reduce frailty may involve exercise, nutrition, and management of underlying comorbidities.
Multiple studies in the current ART treatment era indicate that older, HIV-infected adults are at increased risk of both frailty [13] and disability [14, 15]. Prevalence rates of disability vary widely in the population and by cultural contexts. For example, among HIV-infected adults aged ≥50 years in San Francisco, California, 39% reported difficulty in at least 1 IADL [16], and 18% of HIV-infected adults aged ≥50 years in Mexico City, Mexico, reported an IADL difficulty [17]. Among HIV-infected adults, disability, as measured by IADLs, ADLs, or other measures, has been associated with older age, HIV-related characteristics such as low CD4 count [15], depression [18], lower physical activity [19], and neurocognitive impairment [2, 20]. The identification of factors that contribute to disability, particularly if modifiable, can inform development of interventions to prevent or limit disability in the vulnerable population of older, HIV-infected adults and ultimately improve QoL.
Recently, we published the characteristics associated with frailty in the AIDS Clinical Trials Group (ACTG) Study A5322, or the HIV Infection, Aging, and Immune Function Long-Term Observational Study (HAILO) [21]. Our goals of this analysis were to examine the prevalence of and characteristics associated with disability among HIV-infected adults in HAILO and to explore the overlap between disability and frailty.
METHODS
Study Population
The ACTG Study A5322, or HAILO, is a prospective observational study of HIV-infected persons aged ≥40 years who received randomized assignment of their initial ART regimen through an ACTG interventional trial and were followed long term in the ACTG A5001 observational study after their randomized trial participation ended. HAILO enrollment occurred in 2013–2014; ongoing visits occur every 6 months. The current analysis reports on cross-sectional findings at HAILO entry (baseline).
Instrumental Activities of Daily Living
Disability was assessed at baseline with the Lawton-Brody Instrumental Activities of Daily Living (IADL) Questionnaire [22]. The questionnaire used self-reported limitations in performing the following 8 tasks: housekeeping, money management, cooking, transportation, telephone use, shopping, laundry, and medication management.
Frailty
Frailty was evaluated at baseline using the Fried’s frailty assessment, which includes the following 5 components: 4-meter walk speed, grip strength, and self-reported unintentional weight loss in the past 12 months, exhaustion, and low activity [9, 21]. Individuals meeting 3–5 components were categorized as frail, those with 1 or 2 as pre-frail, and those with no components as non-frail.
Demographics
Health insurance was categorized as no coverage, Medicare or Medicaid, private insurance, or other coverage. Education was categorized as high school education or less. Smoking was defined as current, prior, or never smoker. Self-reported alcohol use in the past 30 days was defined as abstainer (0 drinks), light drinker (men <7 drinks/week, women <3; no binge drinking), moderate drinker (men 7–14 drinks/week, women 3–7; no binging), or heavy drinker (men >14 drinks/week, women >7 or any binge drinking). Binge drinking (men ≥5 drinks, women ≥4 within a 2-hour period) was also included using the categories “no drinking,” “no binge drinking,” “binge drinking once a month,” or “binge drinking more than once a month.” Substance use was self-reported as current use of marijuana, cocaine, heroin, amphetamines, or nonprescribed, controlled medications.
HIV Characteristics
HIV-related characteristics included pre-ART CD4 T-lymphocyte count (cells/µL, CD4) and HIV-1 viral load (VL), and CD4, CD4/CD8 ratio, and VL at baseline. ART exposure included both initial (randomized) and baseline regimen. ART exposure was categorized as a protease inhibitor, nonnucleoside reverse transcriptase inhibitor (NNRTI), or integrase strand transfer inhibitor (INSTI), each with a nucleoside/nucleotide reverse transcriptase inhibitor backbone, or other combination therapy. Prior exposure to stavudine, didanosine, or zidovudine; years of cumulative NNRTI or INSTI use; and years of any ART therapy were also considered.
Comorbidities
Body mass index >30 kg/m2 was considered obese. Change in weight was calculated from entry in ACTG A5001 to baseline. Physical activity was self-reported using the International Physical Activity Questionnaire [23]; outcomes were dichotomized as low (≤2) or moderate/high (≥3) days per week of moderate or vigorous-intensity physical activity. Hepatitis C virus (HCV) infection was defined by a positive HCV serology. Hyperlipidemia was categorized as fasting low density lipoprotein cholesterol > or ≤130 mg/dL. Malignancy was defined as any malignancy (except nonmelanoma skin cancer) within 5 years of enrollment. Renal disease was defined as estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 at least 3 months apart with no intervening eGFR ≥60; eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. Diabetes was defined as 2 consecutive nonfasting glucose readings >200 mg/dL or 2 fasting glucose readings ≥126 mg/dL within 1 year, diagnosed diabetes, or hemoglobin A1C level ≥6.5%. Hypertension was defined as use of hypertensive medications, diagnosed hypertension, or systolic blood pressure ≥140 or diastolic blood pressure ≥90 mm Hg at 2 consecutive visits. Depression was defined as current use of an antidepressant medication, ongoing diagnosis of major depression, or greater than or equal to grade 3 major depression symptoms using Division of AIDS criteria [24]. Neurocognitive impairment was assessed using the ALLRT Neuroscreen [2], with sex, age, race/ethnicity, and education-adjusted scores for Trails-Making A, Trails-Making B, and Digit Symbol. Neurocognitive impairment was defined as having ≥1 test score ≥2 standard deviations (SDs) below the mean or ≥2 separate test scores ≥1 SD below the mean.
Statistical Analyses
The proportion of individuals with an IADL impairment, defined as difficulties in 1 and ≥2 IADL categories, overall and by age, and the frequency of difficulties in each IADL category were summarized. Demographic, behavioral, and clinical characteristics of individuals with and without IADL impairment were compared using χ2, Fisher exact, or Kruskal-Wallis tests. The agreement between IADL (0, 1, and ≥2 categories) and frailty (non-frail, pre-frail, and frail) was assessed using weighted kappa statistics. To identify independent predictors of IADL impairment, we defined the outcome as self-report of difficulties in ≥1 IADL category and used logistic regression modeling. Covariates with a P value < .10 in univariable models were retained in the multivariable model. When more than 1 definition of a covariate was evaluated, the definition with the largest effect estimate was retained.
RESULTS
Of 1015 participants at HAILO baseline, the median age was 51 years (interquartile range, 46–56); 15% were aged ≥60 years, 19% were female, 29% were black, and 20% were Hispanic. Eighty percent of participants had health insurance coverage, including 32% with Medicare or Medicaid and 41% with private health insurance. Most participants had well-controlled HIV infection; 67% had CD4 >500 cells/µL s and 94% had a VL <200 copies/mL. Additional baseline characteristics are shown in (Table 1).
Table 1.
Participant Characteristics at Baseline, Overall, and by Impairment in Activities of Daily Living Impairment Group
| Impairment in Activities of Daily Living at Baseline | ||||||
|---|---|---|---|---|---|---|
| Characteristic | Total (N = 1015) | Not Impaired (N = 837) | 1 Impaired Category (N = 115) | ≥2 Impaired Categories (N = 63) | P Value | |
| Age at baseline (y)a | ≤44 | 203 (20%) | 172 (21%) | 23 (20%) | 8 (13%) | .42 |
| 45–64 | 752 (74%) | 618 (74%) | 84 (73%) | 50 (79%) | ||
| 65–74 | 56 (6%) | 44 (5%) | 8 (7%) | 4 (6%) | ||
| ≥75 | 4 (0%) | 3 (0%) | 0 (0%) | 1 (2%) | ||
| Age at baseline (y) | <50 | 449 (44%) | 382 (46%) | 48 (42%) | 19 (30%) | .19 |
| 50–59 | 415 (41%) | 332 (40%) | 50 (43%) | 33 (52%) | ||
| ≥60 | 151 (15%) | 123 (15%) | 17 (15%) | 11 (17%) | ||
| Sex | Male | 818 (81%) | 688 (82%) | 90 (78%) | 40 (63%) | .001 |
| Female | 197 (19%) | 149 (18%) | 25 (22%) | 23 (37%) | ||
| Race/Ethnicity | White non-Hispanic | 488 (48%) | 430 (51%) | 42 (37%) | 16 (25%) | <.001 |
| Black non-Hispanic | 297 (29%) | 238 (28%) | 30 (26%) | 29 (46%) | ||
| Hispanic (regardless of race) | 203 (20%) | 148 (18%) | 39 (34%) | 16 (25%) | ||
| Otherb | 25 (2%) | 19 (2%) | 4 (3%) | 2 (4%) | ||
| Years of education | Less than high school | 151 (15%) | 96 (11%) | 32 (28%) | 23 (37%) | <.001 |
| High school | 225 (22%) | 172 (21%) | 38 (33%) | 15 (24%) | ||
| More than high school | 639 (63%) | 569 (68%) | 45 (39%) | 25 (40%) | ||
| Health insurance at baseline | No medical coverage | 199 (20%) | 149 (18%) | 35 (30%) | 15 (24%) | <.001 |
| Medicare/Medicaid | 323 (32%) | 234 (28%) | 48 (42%) | 41 (65%) | ||
| Private | 421 (41%) | 393 (47%) | 24 (21%) | 4 (6%) | ||
| Other | 63 (6%) | 53 (6%) | 8 (7%) | 2 (3%) | ||
| Unknown/missing | 9 (1%) | 8 (1%) | 0 (0%) | 1 (2%) | ||
| Frailty status at baseline | Non-frail | 560 (55%) | 498 (59%) | 48 (42%) | 14 (22%) | <.001 |
| Pre-frail | 377 (37%) | 297 (35%) | 56 (49%) | 24 (38%) | ||
| Frail | 62 (6%) | 30 (4%) | 11 (10%) | 21 (33%) | ||
| Missing | 16 (2%) | 12 (1%) | 0 (0%) | 4 (6%) | ||
| CD4 at baseline (cells/mm3) | <350 | 125 (12%) | 95 (11%) | 18 (16%) | 12 (19%) | .19 |
| 350–500 | 205 (20%) | 175 (21%) | 22 (19%) | 8 (13%) | ||
| >500 | 679 (67%) | 562 (67%) | 75 (65%) | 42 (67%) | ||
| Missing | 6 (1%) | 5 (1%) | 0 (0%) | 1 (2%) | ||
| CD4/CD8 ratio at baseline | >0.4 | 886 (87%) | 740 (88%) | 98 (85%) | 48 (76%) | .028 |
| ≤0.4 | 114 (11%) | 86 (10%) | 15 (13%) | 13 (21%) | ||
| Missing | 15 (1%) | 11 (1%) | 2 (2%) | 2 (3%) | ||
| Human immunodeficiency virus RNA at baseline (copies/mL) | <200 | 959 (94%) | 793 (95%) | 109 (95%) | 57 (90%) | .59 |
| Missing | 3 (0%) | 2 (0%) | 0 (0%) | 1 (2%) | ||
| Initial randomized ART regimen | NRTI-backbone + PI | 464 (46%) | 385 (46%) | 48 (42%) | 31 (49%) | .80 |
| NRTI-backbone + NNRTI | 241 (24%) | 199 (24%) | 28 (24%) | 14 (22%) | ||
| NRTI-backbone + INSTI | 107 (11%) | 83 (10%) | 16 (14%) | 8 (13%) | ||
| Other | 203 (20%) | 170 (20%) | 23 (20%) | 10 (16%) | ||
| ART regimens at baseline | NRTI-backbone + PI | 401 (40%) | 324 (39%) | 47 (41%) | 30 (48%) | .05 |
| NRTI-backbone + NNRTI | 389 (38%) | 332 (40%) | 45 (39%) | 12 (19%) | ||
| NRTI-backbone + INSTI | 192 (19%) | 154 (18%) | 19 (17%) | 19 (30%) | ||
| Other | 33 (3%) | 27 (3%) | 4 (3%) | 2 (3%) | ||
| Hepatitis C serology positivity | Positive | 121 (12%) | 86 (10%) | 22 (19%) | 13 (21%) | .002 |
| Smoking at baseline | Never smoker | 421 (41%) | 367 (44%) | 37 (32%) | 17 (27%) | <.001 |
| Prior smoker | 337 (33%) | 282 (34%) | 34 (30%) | 21 (33%) | ||
| Current smoker | 257 (25%) | 188 (22%) | 44 (38%) | 25 (40%) | ||
| Alcohol use | Abstainer | 376 (37%) | 295 (35%) | 54 (47%) | 27 (43%) | .11 |
| Light | 368 (36%) | 318 (38%) | 33 (29%) | 17 (27%) | ||
| Moderate | 62 (6%) | 52 (6%) | 7 (6%) | 3 (5%) | ||
| Heavy | 169 (17%) | 143 (17%) | 14 (12%) | 12 (19%) | ||
| Missing | 40 (4%) | 29 (3%) | 7 (6%) | 4 (6%) | ||
| Binge drinking | Abstainer | 376 (37%) | 295 (35%) | 54 (47%) | 27 (43%) | .13 |
| No binging | 445 (44%) | 381 (46%) | 42 (37%) | 22 (35%) | ||
| Binge once/month | 77 (8%) | 65 (8%) | 6 (5%) | 6 (10%) | ||
| Binge >1/month | 81 (8%) | 70 (8%) | 6 (5%) | 5 (8%) | ||
| Missing | 36 (4%) | 26 (3%) | 7 (6%) | 3 (5%) | ||
| Active substance use | Yes | 209 (21%) | 167 (20%) | 29 (25%) | 13 (21%) | .30 |
| Missing | 48 (5%) | 34 (4%) | 9 (8%) | 5 (8%) | ||
| Days of vigorous/moderate activities/week | ≥3 days of physical activity | 507 (50%) | 447 (53%) | 45 (39%) | 15 (24%) | <.001 |
| Missing | 57 (6%) | 40 (5%) | 11 (10%) | 6 (10%) | ||
| Body mass index | Underweight | 6 (1%) | 3 (0%) | 2 (2%) | 1 (2%) | .32 |
| Normal weight | 321 (32%) | 258 (31%) | 43 (37%) | 20 (32%) | ||
| Overweight | 388 (38%) | 324 (39%) | 43 (37%) | 21 (33%) | ||
| Obese | 284 (28%) | 240 (29%) | 27 (23%) | 17 (27%) | ||
| Missing | 16 (2%) | 12 (1%) | 0 (0%) | 4 (6%) | ||
| Cardiovascular disease | 60 (6%) | 43 (5%) | 13 (1%) | 4 (6%) | .031 | |
| Renal disease | 102 (10%) | 82 (10%) | 10 (9%) | 10 (16%) | .27 | |
| Liver disease | 8 (1%) | 4 (0%) | 3 (3%) | 1 (2%) | .040 | |
| Diabetes | 142 (14%) | 101 (12%) | 24 (21%) | 17 (27%) | <.001 | |
| Hypertension | 757 (75% | 619 (74% | 88 (77%) | 50 (79%) | .56 | |
| Low-density lipoprotein cholesterol | >130 mg/dL | 219 (22%) | 183 (22%) | 22 (19%) | 14 (22%) | .84 |
| Depression | 89 (9%) | 68 (8%) | 11 (10%) | 10 (16%) | .11 | |
| Cancer (within 5 years) | 32 (3%) | 25 (3%) | 4 (3%) | 1 (2%) | .72 | |
Baseline is entry into HIV Infection, Aging, and Immune Function Long-Term Observational Study (A5322).
Abbreviations: ART, antiretroviral therapy; INSTI, integrase strand transfer inhibitor; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside/nucleotide reverse-transcriptase inhibitor; PI, protease inhibitor.
aAge categories used for comparison with NHANES data.
bOther race is defined as Asian/Pacific Islander, American Indian/Alaskan Native, or more than 1 race.
Sixty-three (6%) participants had 2 or more impairments in IADLs and 115 (11%) had 1 impairment. By age, the percentage of participants with 1 or more impairments was as follows: 15%, aged <45 years; 18%, 45–64 years; 21%, 65–74 years; and 25%, aged ≥75 years. The most common difficulties were with housekeeping (48%), transportation (36%), and shopping (28%); the least common difficulties were with medication management (5%) and use of the telephone (12%) (Table 2).
Table 2.
Type of Impairment Present Among Participants With at Least 1 Impairment in Activities of Daily Living
| Impairment in Activities of Daily Living at Baseline | |||
|---|---|---|---|
| Type of Impairment | Total (N = 178) | 1 Impaired Category (N = 115) | ≥2 Impaired Categories (N = 63) |
| Housekeeping difficulty | 48% | 39% | 63% |
| Transportation difficulty | 36% | 25% | 56% |
| Shopping difficulty | 28% | 10% | 59% |
| Laundry difficulty | 20% | 4% | 48% |
| Finance management difficulty | 14% | 10% | 21% |
| Cooking difficulty | 15% | 7% | 29% |
| Difficulty in using the phone | 12% | 2% | 30% |
| Difficulty with medications | 5% | 2% | 11% |
Findings from univariable and multivariable models are summarized in (Table 3). In the multivariable model, lower education, Medicare or Medicaid insurance (vs private insurance), current smoking, and lower physical activity were associated with IADL impairment. Neurocognitive impairment at baseline was the only comorbidity significantly associated with IADL impairment (Table 3). Notably, no HIV characteristics (CD4 count, CD4/CD8 ratio) were associated with IADL impairment in the multivariable model.
Table 3.
Associations of Demographics, Human Immunodeficiency Virus, and Comorbidities With Impairment in Activities of Daily Living
| Univariable Model (N = 1015) | Multivariable Model (N = 873) | ||||
|---|---|---|---|---|---|
| Variable | Category | OR (95% CI) | P Value | OR (95% CI) | P Value |
| Female (vs male) | 1.70 (1.17, 2.48) | .005 | 1.25 (0.75, 2.09) | .39 | |
| Race (vs white, non-Hispanic) | Black, non-Hispanic | 1.84 (1.24,2.73) | .003 | 0.94 (0.56,1.58) | .82 |
| Hispanic + othera | 2.68 (1.79,4.00) | <.001 | 1.46 (0.8,2.67) | .21 | |
| Baseline age (vs 40–49 y) | 50–59 years | 1.43 (1.00,2.03) | .05 | 1.41 (0.9,2.2) | .13 |
| ≥60 years | 1.30 (0.80,2.11) | .29 | 0.92 (0.47,1.77) | .8 | |
| High school education or less (vs > high school) | 3.28 (2.35,4.57) | <.001 | 2.16 (1.38,3.4) | <.001 | |
| Health insurance (vs Medicare/Medicaid) | No coverage | 0.88 (0.59,1.32) | .54 | 0.97 (0.57,1.66) | >.90 |
| Private + Other | 0.22 (0.15,0.34) | <.001 | 0.45 (0.27,0.75) | .002 | |
| Baseline CD4 cell counts (vs >500) cells/mm3 | <350 cells/mm3 | 1.52 (0.96,2.39) | .07 | 0.78 (0.34,1.8) | .56 |
| 350–500 cells/mm3 | 0.82 (0.53,1.27) | .38 | 0.81 (0.46,1.41) | .45 | |
| CD4/CD8 ratio ≤0.4 at baseline (vs >0.4) | 1.65 (1.04,2.62) | .03 | 1.38 (0.63,3.04) | .43 | |
| Baseline ART regimen (vs NRTI-backbone + PI)b | NRTI-backbone + NNRTI | 0.72 (0.50,1.05) | .09 | ||
| NRTI-backbone + INSTI | 1.04 (0.67,1.60) | .87 | |||
| Other | 0.94 (0.37,2.34) | .89 | |||
| NNRTI in ART regimens at baseline | 0.72 (0.51,1.01) | .06 | 1.24 (0.78,1.99) | .37 | |
| Integrase inhibitor in ART regimens at baseline | 1.42 (0.98,2.05) | .06 | 1.27 (0.77,2.12) | .35 | |
| Positive hepatitis C serology (vs never) | 2.14 (1.39,3.29) | <.001 | 1.41 (0.8,2.49) | .24 | |
| History of smoking (vs never) | Prior smoker | 1.33 (0.88,1.99) | .17 | 1.16 (0.68,1.96) | .59 |
| Current smoker | 2.49 (1.68,3.71) | <.001 | 2.55 (1.54,4.24) | <.001 | |
| Current alcohol use at baseline (vs no alcohol use) | Light Drinker | 0.57 (0.39,0.84) | .005 | 0.82 (0.51,1.33) | .43 |
| Moderate/Heavy Drinker | 0.67 (0.44,1.04) | .07 | 0.97 (0.56,1.68) | >.90 | |
| <3 days of vigorous/moderate activities/week (vs ≥3 days) | 2.15 (1.52,3.05) | <.001 | 1.95 (1.28,2.97) | .002 | |
| Neurocognitive impairment at baseline | 3.48 (2.37,5.12) | <.001 | 2.29 (1.4,3.76) | .001 | |
| Cardiovascular disease | 1.95 (1.08,3.51) | .03 | 1.8 (0.87,3.71) | .11 | |
| Liver disease | 4.79 (1.19,19.33) | .03 | 3.36 (0.39,28.9) | .27 | |
| Diabetes | 2.18 (1.45,3.27) | <.001 | 1.46 (0.84,2.56) | .18 | |
Outcome modeled is difficulties in ≥1 category of IADL. Other covariates considered but not associated (P ≥ .10) with IADL impairment in univariable models included the following: nadir CD4 count; pre-ART CD4 count and human immunodeficiency virus type 1 viral load; initial ART regimen; prior exposure to didanosine, stavudine, or zidovudine on or before baseline; years since ART initiation; cumulative years of NNRTI or integrase inhibitor use; substance use; any alcohol use; weekly amount of alcohol use; obesity at ART initiation or baseline; weight change in first year of ART initiation and between A5001 entry and A5322 baseline; baseline low-density lipoprotein cholesterol, depression; history of renal disease; hypertension; and history of cancer within 5 years.
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; IADL, impairment in activities of daily living; INSTI, integrase strand transfer inhibitor; NRTI, nucleoside/nucleotide reverse-transcriptase inhibitor; NNRTI, nonnucleoside reverse-transcriptase inhibitor; OR, odds ratio.
aOther race is defined as Asian/Pacific Islander, American Indian/Alaskan Native, or more than 1 race.
bBaseline ART regimen was not included in the multivariable model as it was correlated with NNRTI in baseline regimen and integrase inhibitor in baseline regimen variables.
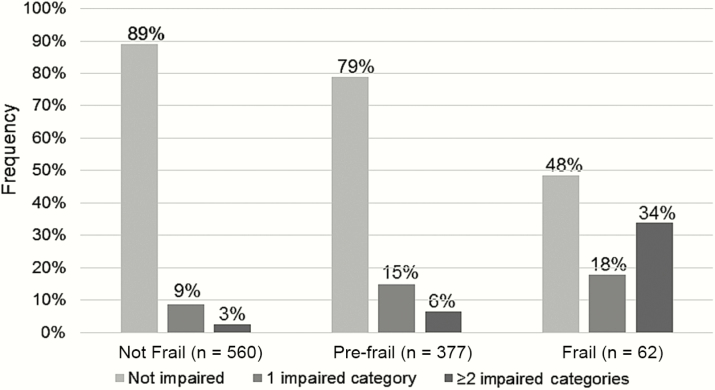
Last, we assessed the agreement between categorizations of disability and frailty. As previously reported [21], 62 (6%) participants were identified as frail and 377 (37%) as pre-frail. Of the participants who underwent both IADL and frailty assessments (98%), 52% (n = 32) of frail participants reported having at least 1 IADL impairment, 21% (n = 80) of pre-frail participants had at least 1 impairment, and only 11% (n = 62) of non-frail persons had an IADL impairment (Figure 1). A weighted kappa score indicated limited agreement between frailty and disability (weighted kappa <0.18, 95% confidence interval [CI], 0.13, 0.23). Among non-frail participants (n = 508 in multivariable model), IADL impairment was associated with lower education (odds ratio [OR], 3.86; 95% CI, 1.95, 7.64; P < .001), current smoking (OR, 2.38; 95% CI, 1.09, 5.22; P = .03), and Medicare or Medicaid insurance (vs private insurance; OR, 2.22; 95% CI, 0.99, 5.0; P = .53). Among pre-frail/frail participants (n = 381), IADL impairment was associated with neurocognitive impairment (OR, 2.10; 95% CI, 1.16, 3.78; P = .014), Medicare or Medicaid insurance (vs private insurance; OR, 2.38; 95% CI, 1.22, 4.64; P = .01), and lower physical activity (OR, 2.28; 95% CI, 1.28, 4.03; P = .005).
Figure 1.
Frequency of any disability in instrumental activities of daily living among categories of non-frail, pre-frail, and frail participants in the Human Immunodeficiency Virus Infection, Aging, and Immune Function Long-Term Observational Study.
DISCUSSION
In this well-characterized cohort of middle-aged and older adults with well-controlled HIV infection, we found a higher-than-expected prevalence of disability by IADLs. A recent publication using National Health and Nutrition Examination Survey (NHANES) data found a markedly lower (6%–8%) prevalence of IADL disability among participants aged 65–74 years compared to nearly 20% in our HIV-infected population aged ≥50 years [25]. The prevalence of disability in HAILO is similar or slightly lower than what was found in 2 recent publications that reported on HIV-infected persons aged ≥50 years [16, 17]. One of these 2 studies, from Mexico City, Mexico, showed overall rates of disability similar to those seen in our cohort, with IADL impairment in 18% of participants aged ≥50 years. In contrast, the second study reported on a San Francisco, California cohort with IADL impairment of 39%, but included a higher proportion of participants aged >60 years. The San Francisco study population was drawn largely from a safety-net clinic where nearly all patients were publicly insured, while 41% of our population had private insurance. We postulate that the different disability rates among cohorts reflect differing perceptions of disability, differences in the family unit, and variable access to senior resources. Our findings complement these other studies of older HIV-infected persons and highlight the 2 to 3 times higher rate of IADL impairment observed across cohorts compared to the general population, especially among middle-aged adults.
As in prior studies [18–20, 26], neurocognitive impairment was a strong predictor of IADL; in our study it was the comorbidity most strongly associated with risk of IADL disability. Although earlier initiation of ART decreases the development of neurocognitive impairment among HIV-infected persons [27], several factors including ART toxicity, prior opportunistic infections, cerebrovascular disease, and age-associated cognitive decline may further adversely impact neurocognitive function among older, HIV-infected adults in the current ART era [28]. Regardless of the causes, even mild cognitive impairment may result in a loss of function that impairs daily living [20] and quality of life [29]. Given these factors, neurocognitive function should be considered routinely when evaluating disability in older HIV-infected persons.
Consistent with the concept of disability as a social phenomenon, we found a strong association between disability and socioeconomic (education, health insurance) and lifestyle factors (smoking, physical activity). While these socioeconomic variables may merely serve as a proxy for other key factors not readily ascertainable in our cohort, including financial resources, employment, and housing access, the prominence of social and economic factors as important factors in an individual’s experience of disability is notable. The effects of socioeconomic factors on prevalent disability may be explained by the inability to mobilize resources. Aging HIV-infected adults may face unique challenges with use of services, including ageism at HIV-specific resource centers and HIV or gender identity/sexual identity stigma from other resource centers that cater to older adults in the general population, such as senior centers or churches [30]. Many older, HIV-infected adults have tenuous relationships with family and therefore rely on other, often inconsistent, forms of social support when it comes to day-to-day needs [31]. Economic challenges may be exacerbated by periods of temporary disability or participation restrictions, which in turn have an effect on the ability to return to work [32–34]. Unhealthy lifestyle behaviors, including smoking and limited physical activity, as identified in this analysis, are more frequently observed among populations with lower socioeconomic status, regardless of HIV serostatus [35]. Last, in the general older adult population there is evidence that socioeconomic disadvantages may exert cumulative adverse effects over an individual’s lifetime [36]. Thus, when considering the social and environmental aspects of disability, it is perhaps not surprising that these socioeconomic and lifestyle factors were more significant than other health or physical function–related measures.
We did not observe statistically significant associations between disability and age, HIV-related characteristics, and comorbidities other than neurocognitive impairment, although the number of older participants in our population may have limited associations between disability and age. Early in the AIDS era, CD4 count, viral load, AIDS wasting syndrome, and HIV-associated neurocognitive disorder were commonly associated with disability [37]. Studies in the current era suggest that CD4 count and depression are still contributing factors [17, 18, 26]. The impact of specific ART regimens on disability has not been well described, and no association was detected in our study. Nevertheless, the introduction of effective ART has decreased the incidence of severe neurocognitive impairment and reduced IADL disability, even after accounting for HIV disease severity and status and demographic variables [38]. The lack of apparent associations between disability and many evaluated comorbidities stands in contrast to limited prior study findings where comorbidity was associated with greater IADL disability [39], impaired physical function [40], and frailty [13]. Overall, these findings highlight the importance of evaluating disability outside of the context of other correlates of health status.
As geriatric syndromes, both frailty and disability are key considerations in the care of older adults, with or without HIV infection. Although the terms are often used interchangeably, the concepts of frailty and disability are distinct, each with unique contributing factors and interventions for treatment and prevention [9]. As such, we have demonstrated that although IADL impairment was more common in persons with increasing frailty, nearly 50% of frail individuals did not have IADL impairments while IADL impairments were seen in 11% of non-frail participants. Although similar characteristics predicted both frailty and disability (eg, insurance, education, physical activity, neurocognitive impairment), other characteristics that had predicted frailty (ie, obesity) [13] were unrelated to disability. Furthermore, different characteristics were associated with disability among those identified as frail (physical activity, neurocognitive impairment) than those who were not frail (education). A recent emphasis on use of the International Classification of Function to define disability across international clinical and research settings provides a similar distinction between concepts of “activity limitation” (as might be seen with grip and gait components of the frailty definition) and “participation restriction” (including limitations in IADLs), where restrictions are moderated by an individual’s ability to respond to those deficits within their environment [10–12].
The strengths of our study include use of a well-characterized cohort with detailed ART use data, use of a validated instrument for measuring neurocognitive function, and identification of a number of factors that may be related to disability. The study population includes participants from more than 30 US sites, thus incorporating a diverse range of participants who vary by age, gender, race, ethnicity, risk factors for HIV acquisition, rural vs urban, and geographic characteristics that may influence one’s self-perception and experience of disability.
The study is not without limitations. Due to its cross-sectional nature, a causal relationship between IADL impairment and participant characteristics cannot be assumed. Our population was predominantly male, and perceived difficulty in certain IADL tasks could differ between men and women, particularly with roles traditionally assigned to a specific gender. The participants in this study had been enrolled in ART treatment trials and observational studies for a median of 7.8 years and are likely not fully representative of the broader HIV-infected population. As an individual’s own support and environment determine his or her perceived and real ability to function independently, conclusions from this study may not be broadly applicable to all HIV-infected persons.
In summary, we found that self-reported disability among HIV-infected older adults is strongly associated with neurocognitive impairment and socioeconomic or other lifestyle factors but not with other comorbidities or HIV-related factors. Our findings identified modifiable factors (smoking, low physical activity) as potential targets for interventions designed to reduce IADL impairment and to maintain independent living. Interventions aimed at improving socioeconomic status and support networks of those aging with HIV would likely have a significant beneficial impact on health but would be increasingly challenging to implement at a systemic level. Lastly, by identifying persons at highest risk for disability, health providers can ensure that daily needs are met and that these individuals are linked to appropriate resources.
Notes
Author contributions. K. M. E., K. W., and K. T. developed the analysis and interpretation. K. W. performed the data analysis under the supervision of K.T. N. J. and K. M. E. prepared the first draft of the manuscript. All authors contributed to the study design, implementation, and interpretation of data; reviewed and revised the manuscript; and approved the final draft.
Acknowledgments. We thank the study volunteers who participated in ALLRT/A5001 and HAILO/A5322, the ACTG clinical units who enroll and follow participants, and the ACTG.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Financial support. This work was supported by the National Institute of Aging of the National Institutes of Health (NIH; K23AG050260 to K. M. E.), the National Institute of Allergy and Infectious Diseases of the NIH (AI 036219, U01AI068636), the National Institute of Mental Health, and the National Institute of Dental and Craniofacial Research. This research was also supported by the Veterans Administration, Geriatric Research Educational and Clinical Centers, Louis Stokes Cleveland Veterans Administration Medical Center (VISN10 to R. C. K).
Potential Conflicts of Interest. R. C. K. receives grant support from Gilead Sciences and has consulted for Gilead Sciences and Theratechnologies. F. J. P. is a consultant and/or on the speakers bureau for Gilead Sciences, Janssen Pharmaceuticals, Merck and Co., and Bristol Myers Squibb. B. T. has received honoraria and/or grant support to Northwestern University from ViiV Healthcare, Gilead Sciences, Glaxo Smith Kline, and Janssen. K. M. E. has received grant support from Gilead Sciences and has served as a consultant for Theratechnologies and Gilead Sciences. All other authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Smit M, Brinkman K, Geerlings S, et al. ; ATHENA observational cohort Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis 2015; 15:810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Robertson KR, Smurzynski M, Parsons TD, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS 2007; 21:1915–21. [DOI] [PubMed] [Google Scholar]
- 3. Esser S, Gelbrich G, Brockmeyer N, et al. Prevalence of cardiovascular diseases in HIV-infected outpatients: results from a prospective, multicenter cohort study. Clin Res Cardiol 2013; 102:203–13. [DOI] [PubMed] [Google Scholar]
- 4. Schouten J, Wit FW, Stolte IG, et al. ; AGEhIV Cohort Study Group Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis 2014; 59:1787–97. [DOI] [PubMed] [Google Scholar]
- 5. Wyatt CM, Winston JA, Malvestutto CD, et al. Chronic kidney disease in HIV infection: an urban epidemic. AIDS 2007; 21:2101–3. [DOI] [PubMed] [Google Scholar]
- 6. Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS 2006; 20:2165–74. [DOI] [PubMed] [Google Scholar]
- 7. Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med 2008; 148:728–36. [DOI] [PubMed] [Google Scholar]
- 8. F Onen N, Turner Overton E. A review of premature frailty in HIV-infected persons; another manifestation of HIV-related accelerated aging. Curr Aging Sci 2011; 4:33–41. [PubMed] [Google Scholar]
- 9. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–56. [DOI] [PubMed] [Google Scholar]
- 10. Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci 2004; 59:M255–M63. [DOI] [PubMed] [Google Scholar]
- 11. Jette AM. Toward a common language of disablement. J Gerontol A Biol Sci Med Sci 2009; 64:1165–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. World Health Organization. International Classification of Functioning, Disability and Health: ICF. World Health Organization, 2001. Accessed at http://www.who.int/classifications/icf/en/, 1 November 2016. [Google Scholar]
- 13. Piggott DA, Erlandson KM, Yarasheski KE. Frailty in HIV: epidemiology, biology, measurement, interventions, and research needs. Curr HIV/AIDS Rep 2016; 13:340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Solomon P, O’Brien K, Wilkins S, Gervais N. Aging with HIV: a model of disability. J Int Assoc Provid AIDS Care 2014; 13:519–25. [DOI] [PubMed] [Google Scholar]
- 15. Erlandson KM, Schrack JA, Jankowski CM, Brown TT, Campbell TB. Functional impairment, disability, and frailty in adults aging with HIV-infection. Curr HIV/AIDS Rep 2014; 11:279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. John MD, Greene M, Hessol NA, et al. Geriatric assessments and association with VACS index among HIV-infected older adults in San Francisco. J Acquir Immune Defic Syndr 2016; 72:534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ávila-Funes JA, Belaunzarán-Zamudio PF, Tamez-Rivera O, et al. Correlates of prevalent disability among HIV-infected elderly patients. AIDS Res Hum Retroviruses 2016; 32:155–62. [DOI] [PubMed] [Google Scholar]
- 18. Morgan EE, Iudicello JE, Weber E, et al. ; HIV Neurobehavioral Research Program Group Synergistic effects of HIV infection and older age on daily functioning. J Acquir Immune Defic Syndr 2012; 61:341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fazeli PL, Marquine MJ, Dufour C, et al. ; Neurobehavioral Research Program Group Physical activity is associated with better neurocognitive and everyday functioning among older adults with HIV disease. AIDS Behav 2015; 19:1470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sheppard DP, Iudicello JE, Bondi MW, et al. Elevated rates of mild cognitive impairment in HIV disease. J Neurovirol 2015; 21:576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Erlandson KM, Wu K, Koletar SL, et al. Frailty and components of the frailty phenotype are associated with modifiable risk factors and antiretroviral therapy. J Infect Dis 2017;[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lawton M, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Nurs Res 1970; 19:278. [PubMed] [Google Scholar]
- 23. Booth M. Assessment of physical activity: an international perspective. Res Q Exerc Sport 2000; 71Suppl 2:114–20. [DOI] [PubMed] [Google Scholar]
- 24. US Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS. Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 2.0. http://rcc.tech-res.com/docs/default-source/safety/daids_ae_grading_table_v2_nov2014.pdf?sfvrsn=8, November 2014 [Google Scholar]
- 25. MMWR. QuickStats: Percentage of Adults with Activity Limitations, by Age Group and Type of Limitation—National Health Interview Survey. Vol. 65;14. MMWR: CDC, 2016. [DOI] [PubMed] [Google Scholar]
- 26. Heaton RK, Marcotte TD, Mindt MR, et al. ; Neurobehavioral Research Program Group The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc 2004; 10:317–31. [DOI] [PubMed] [Google Scholar]
- 27. Heaton RK, Franklin DR, Jr, Deutsch R, et al. ; CHARTER Group Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clin Infect Dis 2015; 60:473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Underwood J, Robertson KR, Winston A. Could antiretroviral neurotoxicity play a role in the pathogenesis of cognitive impairment in treated HIV disease? AIDS 2015; 29:253–61. [DOI] [PubMed] [Google Scholar]
- 29. Moore RC, Fazeli PL, Jeste DV, Moore DJ, Grant I, Woods SP; HIV Neurobehavioral Research Program Group Successful cognitive aging and health-related quality of life in younger and older adults infected with HIV. AIDS Behav 2014; 18:1186–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brennan-Ing M, Seidel L, London AS, Cahill S, Karpiak SE. Service utilization among older adults with HIV: the joint association of sexual identity and gender. J Homosex 2014; 61:166–96. [DOI] [PubMed] [Google Scholar]
- 31. Emlet CA. An examination of the social networks and social isolation in older and younger adults living with HIV/AIDS. Health Soc Work 2006; 31:299–308. [DOI] [PubMed] [Google Scholar]
- 32. Elzi L, Conen A, Patzen A, et al. Ability to work and employment rates in human immunodeficiency virus (HIV)-1-infected individuals receiving combination antiretroviral therapy: the Swiss HIV Cohort Study. Open Forum Infect Dis 2016; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Solomon P, Wilkins S. Participation among women living with HIV: a rehabilitation perspective. AIDS Care 2008; 20:292–6. [DOI] [PubMed] [Google Scholar]
- 34. Gallagher S, Biro S, Creamer E, et al. “It’s a Hidden Issue”: exploring the experiences of women with HIV-associated neurocognitive challenges using a disability framework. Disabil Rehabil 2013; 35:36–46. [DOI] [PubMed] [Google Scholar]
- 35. Pampel FC, Krueger PM, Denney JT. Socioeconomic disparities in health behaviors. Annu Rev Sociol 2010; 36:349–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim J, Richardson V. The impact of socioeconomic inequalities and lack of health insurance on physical functioning among middle-aged and older adults in the United States. Health Soc Care Community 2012; 20:42–51. [DOI] [PubMed] [Google Scholar]
- 37. Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007; 69:1789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Heaton RK, Franklin DR, Ellis RJ, et al. ; CHARTER Group; Neurobehavioral Research Program Group HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 2011; 17:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wilson IB, Cleary PD. Clinical predictors of declines in physical functioning in persons with AIDS: results of a longitudinal study. J Acquir Immune Defic Syndr Hum Retrovirol 1997; 16:343–9. [DOI] [PubMed] [Google Scholar]
- 40. Oursler KK, Goulet JL, Crystal S, et al. Association of age and comorbidity with physical function in HIV-infected and uninfected patients: results from the Veterans Aging Cohort Study. AIDS Patient Care STDS 2011; 25: 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]