Abstract
To assess the relationship of E2 gene disruption with viral gene expression and clinical outcome in human papillomavirus (HPV) positive head and neck squamous cell carcinoma, we evaluated 31 oropharyngeal and 17 non-oropharyngeal HPV16 positive carcinomas using two PCR-based methods to test for disruption of E2, followed by Sanger sequencing. Expression of HPV16 E6, E7 and E2 transcripts, along with cellular ARF and INK4A, were also assessed by RT-qPCR. Associations between E2 disruption, E2/E6/E7 expression, and clinical outcome were evaluated by Kaplan-Meier analysis for loco-regional recurrence and disease-specific survival. The majority (n = 21, 68%) of HPV16 positive oropharyngeal carcinomas had an intact E2 gene, whereas the majority of HPV16 positive non-oropharyngeal carcinomas (n = 10, 59%) had a disrupted E2 gene. Three of the oropharyngeal tumors and two of the non-oropharyngeal tumors had deletions within E2. Detection of an intact E2 gene was associated with a higher DNA viral load and increased E2/E6/E7, ARF and INK4A expression in oropharyngeal tumors. Oropharyngeal carcinomas with an intact E2 had a lower risk of loco-regional recurrence (log-rank p = 0.04) and improved disease-specific survival (p = 0.03) compared to tumors with disrupted E2. In addition, high E7 expression was associated with lower risk of loco-regional recurrence (p = 0.004) as was high E6 expression (p = 0.006). In summary, an intact E2 gene is more common in HPV16 positive oropharyngeal than non-oropharyngeal carcinomas; the presence of an intact E2 gene is associated with higher HPV viral load, higher viral oncogene expression, and improved clinical outcome compared to patients with a disrupted E2 gene in oropharyngeal cancer.
Introduction
Each year head and neck squamous cell carcinoma (HNSCC) accounts for 550,000 cancer cases worldwide, resulting in 300,000 deaths [1]. A subset of HNSCC is associated with human papillomavirus (HPV) infection, with most arising in the oropharynx. More than 150 alpha HPV types have been identified [2] of which a subset is considered oncogenic [3] in several human cancers including cervical, penile, vulvovaginal and anal carcinomas (Munger, 2004). HPV16 is one such oncogenic, or high-risk type, found in over 90% of HPV positive oropharyngeal squamous cell carcinomas (OPSCCs) [4]. HPV16 positive OPSCCs have been shown to have a better prognosis than HPV16 negative OPSCCs [5,6].
The HPV16 virus is a double-stranded circular DNA virus that is 8 kb in length, and encodes six early (E) genes, E1, E2, E4, E5, E6, E7 and two late (L) genes: L1 and L2 [7]. The L1 and L2 proteins form the viral capsid structure [8] and primers amplifying conserved regions of L1 are used to test clinical samples for the presence of HPV DNA [9]. Two of the early gene products, E6 and E7, are oncogenes that disrupt tumor suppressor pathways and are consistently expressed following HPV16 infection [10]. E6 promotes ubiquitination and degradation of the tumor suppressor p53, impairing the cellular response to DNA damage. E7 binds to and inactivates the retinoblastoma protein (pRb), which controls the G1-S phase entry into the cell cycle [7]. Inhibition of pRb activity by E7 is in turn associated with overexpression of the cell cycle regulator p16, and p16 immunohistochemistry has been used as a surrogate test for HPV infection in OPSCC [5,6,11–13]. The p16 transcript, p16INK4A, is transcribed from the CDKN2A locus, which also encodes an alternative transcript p14ARF. Both p16INK4A and p14ARF are tumor suppressors [14] and over expression of both transcripts has been associated with HPV status in OPSCC [15].
The HPV16 genome can exist in the cell as an episome, or it can integrate into the human genome [16]. HPV episomes can be detected in non-malignant and pre-malignant tissues, while integrated HPV is detected largely in malignancies, particular in the cervix [17]. Disruption of the E2 gene occurs frequently upon integration of the virus into the human genome [18], and has been correlated with increased expression of E6 and E7 in vitro [19,20]. However, correlations with HPV16 E2 disruption and increased E6 and E7 expression in cervical cancer have been inconsistent [21–24]. Although the status of E2 disruption in HNSCC was previously unknown, recent studies have analyzed integration of HPV in HNSCC, some with conflicting results [25]. A study on HNSCC cell lines did not find a correlation between HPV integration and viral expression, including E2 mRNA levels [26].Another recent study of HPV positive head and neck cancer cell lines found that there was viral integration in all cell lines and that E6 and E7 transcripts were expressed in all of these cell lines [27]. However, a study of OPSCC tumor samples found HPV16 integration in only 2 of 13 tumors, with expression of E6, E7 and E2 being concordant [28]. Alternatively a recent analysis of HPV16 positive HNSCC data from the TCGA by Nulton et al. found three categories of HPV genomic DNA including episomal, integrated and human-viral episomal hybrids; three quarters of samples retained viral episomes or human-viral hybrid episomes which replicated by an E1-E2 dependent manner [29].
While disruption of the E2 gene has been associated with poor prognosis in cervical cancer [30,31] this has been studied to a lesser extent in HNSCC [32,33]. In this study, we evaluated the status of the E2 gene by directly testing for E2 disruption in HPV16 positive OPSCC and non-OP HNSCC tumors to assess the relationship between E2 disruption to viral oncogene expression, host gene expression and clinical outcome.
Materials and methods
Patients and samples
Patients with primary HNSCC at Montefiore Medical Center (MMC) in the Bronx, NY were enrolled in an ongoing study approved by the Institutional Review Boards of MMC and Albert Einstein College of Medicine. All patients who enrolled provided written, informed consent. Tumor samples were collected prior to therapy and snap frozen in liquid nitrogen within 30 minutes of biopsy or surgical resection. 31 OPSCC samples were selected and 17 non-OP HNSCC samples were selected. Samples selected for this study were restricted to HPV16 positive samples only, and were identified as HPV positive prior to this study by the methods described under HPV DNA detection.
Cell culture
Two cervical cancer cell lines, CaSki and SiHa (American Type Culture Collection), and two oral cavity SCC cell lines, UPCI:SCC090 and UMSCC-47 (courtesy of Dr. Thomas Carey, University of MI)(48), were used as positive controls in this study. Cells were cultured in 10cm TC plates (Corning, Corning NY) and incubated at 37°C with 5% CO2. Caski cells were cultured in RPMI 1640 (Invitrogen, Grand Island NY) and SiHa cells were cultured in MEM with Earle's salts (Fisher, Hampton NH) with 0.01% 100mM (100X) sodium pyruvate (Gibco, Grand Island NY) and 0.01% non-essential amino acids (Hyclone). Cervical cancer cell lines were cultured in 10% FBS (Gibco) and 0.01% penicillin/streptomycin. Medium was changed every 2–3 days. UPCI: SCC090 cells were cultured in MEM with Earle's salts and UMSCC-47 cells were cultured in high glucose DMEM (Gibco). Cell lines were cultured in 0.01% L-glutamine (Hyclone, Logan UT), 0.01% penicillin/streptomycin, 0.01% non- essential amino acids and 10% FBS.
RNA and DNA extraction
A standard TRIzol (Invitrogen, Carslbad, CA) protocol was used to extract total RNA. Tissue specimens were subdivided into two pieces. One piece was kept for RNA extraction and one piece for DNA extraction. For RNA extraction, tissue was homogenized in 1ml of TRIzol solution and cell lines were scraped from culture dishes into 1ml of TRIzol. DNA quality and quantity was assessed spectrophotometrically using a NanoDrop ND-1000 spectrophotometer (NanoDrop, Wilmington, DE). RNA quality was assayed on an Agilent Bioanalyzer and all RNA samples were stored at -80 C in ethanol [34]. DNA extraction from tissue specimens was done using the Qiagen protocol and reagents from the DNeasy Blood and Tissue extraction kit and handbook.
DNase treatment
To ensure there was no DNA contaminant in RNA samples, all RNA used for experiments was DNase treated. A standard DNase kit (Promega, Madison WI) and protocol were used to ensure the RNA samples used for experiments did not contain DNA contaminants.
DNA extraction from formalin-fixed and paraffin-embedded (FFPE) specimens
In order to confirm HPV16 status in fresh frozen samples, sections were taken from the formalin-fixed paraffin embedded blocks from the Pathology Department of Montefiore Medical Center. DNA was extracted from the FFPE blocks according to a Trizol-based method and ethanol precipitation described in Kotorashvili et al. 2012 [35]. After ethanol precipitation, one 24 hour incubation with 20ul of proteinase K was done followed by a second 48 hour incubation with 20ul of proteinase K. Afterwards samples were spun for one minute and FFPE-DNA was recovered from the lower phase of TRIzol using a Qiagen DNA FFPE kit (Valencia, CA) under standard protocol [36].
HPV DNA detection and viral load evaluation
HPV DNA status was determined by PCR amplification using MY09/MY11/HMB01-PCR system with Gold AmpliTaq that amplifies a conserved 450 base-pair segment in the L1-sequence of HPV under standard protocols [37]. In order to determine type-specific HPV infections, PCR reactions using type- specific biotin-labeled probes were done and products were detected by dot blot hybridization [9]. The signal intensity was evaluated as a qualitative measure of viral load, the scale being 1–5, where 1 is weakest and 5 is highest. The score is based on density and diameter of the PCR product on the autoradiogram [38,39]. For this study we analyzed the PCR signal strength index scores 1–5 separately, in order to compare samples with an intact E2 gene and samples with a disrupted E2 gene. We first analyzed all HNSCC samples together. Then, we analyzed OPSCC as one group and non-OP tumors as a separate group. All analyses on viral load were done by Mann-Whitney test. Samples were tested independently for DNA and RNA. If results were discordant, samples were tested by additional PCR. Discordant specimens were omitted from the study if HPV16 status was unable to be confirmed through additional PCR.
PCR detection of HPV16 E2 and E6 genes
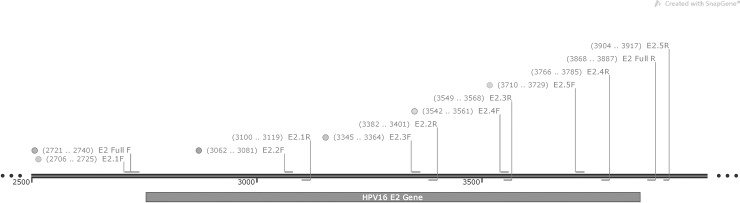
We used a previously published assay to determine the integrity of the E2 gene [40]. This included five primer sets which sequentially span the length of the gene and a single primer set that detects the full length E2 gene [22,40,41]. Primers for HPV16 E6 DNA were also used as an additional HPV16 control. Primer locations are shown in Fig 1 (figure designed with SnapGene software) and sequences as well as locations are given in S1 Table. DNA extracted from SiHa cells served as the E2 disrupted control. SiHa has one integrated HPV16 genome which is disrupted at nucleotides 3132 and 3384, therefore the second primer set of the five primer set for the E2 gene does not give a product for SiHa [10,40]. DNA from UPCI:SCC090 cells, which contain high copy numbers of HPV16 and was confirmed to harbor episomal virus, served as the E2 intact control [42,43].
Fig 1. Positions of HPV16 E2 primers along the HPV16 E2 gene.
The HPV16 genome encompassing the E2 gene region shown in gray. Primer locations of the E2 disruption assay and the E2 full gene primer set are shown above and numbered according to nucleotides on the whole genome. Overlapping primer design is illustrated below along with product size primer set.
In order to validate the results of the initial E2 disruption assays, we ran a PCR screen of all samples using primers designed to amplify the entire E2 gene. A full length PCR product confirmed E2 disruption assay results indicating presence of an intact E2 gene for all cases identified by the five primer assay. Samples generating no band indicated presence of only disrupted E2 gene products. A smaller product suggested a deletion. The presence of an intact E2 gene or deletion was further confirmed by Sanger sequencing. Examples of the gene products are presented in S1 Fig.
PCR conditions were optimized in a previous study and carried out under the same conditions for all E2 primers and E6 primers [40]. Briefly they were done with 12.5μl of 2X master mix (Thermoscientific, Waltham MA), 3μl of primer (2.5 pmol forward + reverse) and 20ng of DNA, brought up to a total reaction volume of 25μl. Cycling conditions were: 95°C for 5 min, followed by 60 cycles of 95°C for 30s, melting temperature of 55°C for 1 min and 72°C for 2 min, and a final extension of 72°C for 10 min. Products from the five primer sets of the E2 disruption assay were then electrophoresed on a 2% agarose gel. Products from the full length E2 PCR reaction were analyzed on a 1% agarose gel [40].
Quantitative Real-Time PCR
The DNase treated RNA and untreated RNA was diluted to 100ng/μl for reverse transcription. Expression of HPV16 genes in tissue samples was measured using TaqMan® RNA-to-CT™ 1-Step RT-PCR Master Mix Kits (Applied Biosystems, Carlsbad CA) with probes for E6, E7, E2-3’ & E2-5’ (primer and probe sequences are given in S2 Table). All gene expression was normalized to the control probe glyceraldehyde-3-phosphate dehydrogenase. Assays were performed in triplicate on the StepOnePlus™ Real-Time PCR apparatus (Applied Biosystems) [34,44].
Statistical analysis
We first tested for statistical differences in the proportions of HPV16 positive OPSCC and non-OPSCC tumors that had any intact vs. only disrupted E2 gene using the Fisher’s exact test. Box-and-Whisker plots and Mann-Whitney U tests were used to determine the statistical significance of observed differences in transcript levels for viral E6, E7 and E2 oncogenes, for CDKN2A INK4A and ARF, and for HPV16 DNA viral load, comparing OPSCC and non-OPSCC tumors with any intact vs. only disrupted E2. Transcript levels generated by RT-qPCR were estimated using 2maxΔCT- ΔCT, where maxΔCT was the lower limit threshold of detection, and ΔCT was the experimental value minus the endogenous control (GAPDH). HPV16 DNA viral load was derived from dot blot hybridization intensity scores. Statistical analyses were done separately for OPSCC and non-OPSCC tumor groups using GraphPad Prism software (San Diego, CA), and all tests were two-sided.
We used contingency tables to assess the associations between E2 status (i.e., comparing tumors any intact vs. only disrupted E2 gene) and clinicopathologic factors at diagnosis (e.g., age, gender, tumor stage), and tested for statistical differences using Fisher Exact tests. Associations between E2 status and clinical outcome (for local regional recurrence and cancer death) were assessed for OPSCC tumors only, using Kaplan-Meier plots and Log-rank (Mantel- Cox) tests. Associations with clinical outcome for OPSCC tumors were also assessed for HPV16 E7 or E6 expression grouping tumors into low vs. high expression based on median cut-point using derived (2maxΔCT- ΔCT) RT-qPCR results.
Results
The HPV16 E2 gene is intact in OPSCC and disrupted in non-OP HNSCC tumors
We tested a total of 31 oropharyngeal and 17 non-oropharyngeal HPV16 positive tumors for disruption of the HPV16 E2 gene by PCR analysis and found that of the 31 HPV16 positive OPSCCs tested, 21 (68%) contained intact or possibly mixed (both intact and disrupted) E2 gene products and 7 (22%) contained only disrupted E2. Five (29%) of the 17 HPV16 positive non-OPSCC tumors were found to have an intact E2, while 10 (59%) had a disrupted E2 (Table 1). In addition, there were five tumors with deletions in the E2 gene (3 OP and 2 non-OP), which are described in the following section. There was a significant difference between the oropharyngeal and non-oropharyngeal tumors with respect to the presence of intact E2 or disrupted E2 gene products (Fisher’s exact test, p = 0.01) (Table 1).
Table 1. Study population characteristics stratified by HPV16 E2 gene status and tumor site.
| Oropharynx3, n = 31 | |||||||
| Intact E2 | Disrupted E2 | p value* | Deletions | ||||
| n = 21 (68%) | n = 7 (22%) | 0.011 | n = 3 (10%) | ||||
| Age at diagnosis in years | |||||||
| <60 | 12 | 57% | 3 | 43% | 0.4 | 0 | 0% |
| ≥60 | 9 | 43% | 4 | 57% | 3 | 100% | |
| Sex | |||||||
| Men | 18 | 86% | 6 | 86% | 1 | 1 | 33% |
| Women | 3 | 14% | 1 | 14% | 2 | 67% | |
| Smoking Status2 | |||||||
| Current Smoker | 5 | 24% | 3 | 43% | 0.42 | 1 | 33% |
| Ex-smoker | 12 | 57% | 2 | 29% | 1 | 33% | |
| Never Smoker | 4 | 19% | 2 | 29% | 1 | 33% | |
| Overall Stage | |||||||
| I–II | 3 | 14% | 1 | 14% | 1 | 1 | 33% |
| III–IV | 18 | 86% | 6 | 86% | 2 | 67% | |
| Nodal Stage n (%) | |||||||
| N0 | 3 | 14% | 1 | 14% | 0.8 | 1 | 33% |
| N1 | 1 | 5% | 0 | 0% | 0 | 0% | |
| N2 | 14 | 67% | 4 | 57% | 2 | 67% | |
| N3 | 3 | 14% | 2 | 29% | 0 | 0% | |
| Tumor Size n (%) | |||||||
| T1–T2 | 17 | 81% | 4 | 57% | 0.32 | 2 | 67% |
| T3–T4 | 4 | 19% | 3 | 43% | 1 | 33% |
|
| Anatomical Site3, n (%) | |||||||
| Tonsil | 14 | 67% | 2 | 29% | 0.05 | 2 | 67% |
| Base of Tongue | 6 | 28% | 2 | 29% | 1 | 33% |
|
| Oropharyngeal Wall | 0 | 0% | 2 | 29% | |||
| Not Specified | 1 | 5% | 1 | 14% | |||
| Non-oropharynx, n = 17 | |||||||
| Intact E2 | Disrupted E2 | p value* | Deletions | ||||
| Age at diagnosis in years | n = 5 (29%) | n = 10 (59%) | 0.011 | n = 2 (12%) | |||
| <60 | 3 | 60% | 6 | 60% | 0.61 | 1 | 50% |
| ≥60 | 2 | 40% | 4 | 40% | 1 | 50% | |
| Sex, n (%) | |||||||
| Men | 4 | 80% | 7 | 70% | 1 | 2 | 100% |
| Women | 1 | 20% | 3 | 30% | 0 | 0% | |
| Smoking Status2 | |||||||
| Current Smoker | 1 | 20% | 5 | 50% | 0.47 | 0 | 0% |
| Ex-smoker | 3 | 60% | 3 | 30% | 2 | 100% | |
| Never Smoker | 1 | 20% | 2 | 20% | 0 | 0% | |
| Overall Stage | |||||||
| I–II | 1 | 20% | 2 | 20% | 1 | 0 | 0% |
| III–IV | 4 | 80% | 8 | 80% | 2 | 100% | |
| Nodal Stage n (%) | |||||||
| N0 | 2 | 40% | 4 | 40% | 0.49 | 0 | 0% |
| N1 | 1 | 20% | 2 | 20% | 0 | 0% | |
| N2 | 1 | 20% | 4 | 40% | 2 | 100% | |
| N3 | 1 | 20% | 0 | 0% | 0 | 0% | |
| Tumor Size n (%) | |||||||
| T1–T2 | 3 | 60% | 4 | 40% | 0.61 | 0 | 0% |
| T3–T4 | 2 | 40% | 6 | 60% | 2 | 100% | |
| Anatomical Site3, n (%) | |||||||
| Oral Cavity | 1 | 20% | 4 | 40% | 74 | 0 | 0% |
| Larynx | 2 | 40% | 3 | 30% | 1 | 50% | |
| Hypopharynx | 2 | 40% | 3 | 30% | 0 | 0% | |
| Nasopharynx | 0 | 0% | 0 | 0% | 1 | 50% | |
*p-Value for 2-sided Fisher exact test. Row numbers may not sum to column totals due to missing data.
1p-value determined by Fisher exact or Chi-square test on results from intact and disrupted E2 status for oropharynx and non-oropharynx.
2Smoking status was defined as never smoked, exsmoker (at time of diagnosis) and current smoker
3Oropharynx includes: base of tongue, tonsil, soft palate, oropharyngeal wall, uvula and oropharynx-NOS. Hypopharynx includes: posterior pharyngeal wall, pyriform sinus and hypopharynx-NOS. Larynx includes glottis, supraglottis-aryepiglottic fold and epiglottis. Oral cavity includes buccal, alveolar ridge, anterior tongue, floor of mouth, hard palate, inner lip mucosa and retromolar trigone.
As noted in Table 1, five samples demonstrated a deletion in the E2 gene. Deletions in all three of the OPSCC samples were found in the hinge region between nucleotides 3394–3474, 3573–3596 and 3577–3594. Deletions found in the two non-OP tumor samples occurred in the transactivation domain and hinge regions (nucleotides 3293–3306 and 2766–2816, respectively). We ran the E2 sequences from the OPSCC samples with deletions through Geneious 10.2.2 and based on the sequence, these are complete ORFs that can produce transcripts and do not result in a frame shift.
The HPV16 E6, E7 and E2 are expressed at higher levels in OPSCCs with an intact E2 gene
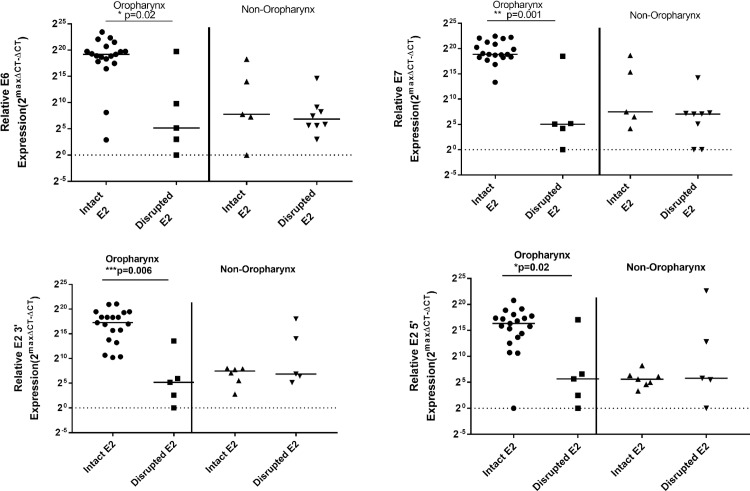
We next determined the prevalence of E2 disruption in HPV16 positive HNSCC and the correlation between E2 disruption and E6/E7 viral oncogene expression in our HPV16 positive HNSCC samples (Fig 2). We evaluated RNA levels for both oncogenes in 19 of the intact and 5 of the disrupted OPSCCs. Of the non-OP tumors we evaluated viral expression in 5 of the tumors with an intact E2 gene and 8 of the tumors with a disrupted E2 gene. Due to limited availability of RNA from clinical samples, the full sample set could not be tested. We found that E2 disruption was significantly associated with lower expression of both E6 (2A; Mann-Whitney test p = 0.02) and E7 (2B; p = 0.001) in OPSCCs. An association between intact E2 and E6 / E7 expression was not observed in the non-OPSCC samples.
Fig 2. HPV16 E6, E7 and E2 are overexpressed in OPSCCs with an intact E2.
HPV16 E6 (A) and HPV16 E7 (B) expression with HPV16 E2 gene disruption. Expression is defined as Y = 2maxΔCT- ΔCT where maxΔCT is the lower limit threshold of detection which equals 20 or 1. ΔCT is the experimental value minus the endogenous control (GAPDH). Statistical significance for difference in HPVE6 and E7 expression by site and between samples with an intact or disrupted HPV16 E2 gene was assessed by Mann-Whitney test.
In the 28 oropharyngeal tumors, those cases with an intact E2 gene had significantly higher E6 and E7 expression than the oropharyngeal cases with a disrupted E2 gene (Mann Whitney test, p = 0.02 and p = 0.001). HPV16 E2 3’ (C) and 5’ (D) expression is also significantly higher in oropharyngeal cancers cases with an intact E2 gene than the oropharyngeal cases with a disrupted E2 gene (Mann Whitney test, p = 0.0006 and p = 0.02). There was no significant difference in expression of E2 in non-oropharyngeal HNSCC tumors. With the E2 protein containing a DNA-binding domain, located at the 3’ end of the E2 gene, and a transactivation domain at the 5’ end, we designed primers specific to the 3’ and 5’ regions of the E2 transcript to test for expression of the full length transcript (Fig 2C and 2D). RNA levels of the E2 transcript corresponding to DNA-binding and transactivation domain were high in OPSCC samples with an intact E2 gene compared to those with disrupted E2 (Mann-Whitney test p = 0.0006 for E2 3’and p = 0.02 for E2 5’), indicating that the full length transcript is present in the cell. No association was observed between E2 disruption and 3’ or 5’ domain transcript levels in non-OPSCCs.
HPV16 viral load is higher in HNSCCs with an intact E2 gene regardless of anatomic site
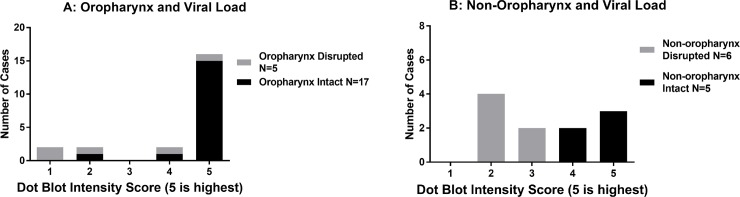
Since our findings that expression of E6 and E7 was high in OPSCCs with an intact E2 gene, which is counter to findings of studies in cervical cancer, we hypothesized that viral load might explain in part the increased oncogene expression observed in tumors with an intact E2 gene (Fig 3). Tumors with intact E2 genes were significantly more likely to have higher viral loads than those with disrupted E2 for both OPSCC (3A; Mann Whitney test p = 0.002) and non-OPSCC (4B; p = 0.003) patients.
Fig 3.
Low HPV16 DNA viral load correlates with E2 gene disruption in HPV16 positive OPSCC (A) and non-OP tumors (B). Viral load was determined by dot-blot intensity and scored from 1–5, 5 being highest. Scores are graphed separately across the x-axis. Samples with an intact HPV16 E2 gene are represented by the grey bars and samples with a disrupted HPV16 E2 gene are represented by the black bars.
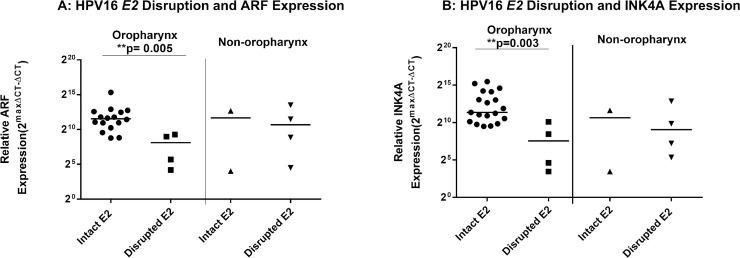
Tumors with intact E2 genes were significantly more likely to have higher viral loads than those with disrupted E2 for both the oropharynx (3A) (p = 0.002) and non-oropharynx (3B) (p = 0.003). Tumor suppressor p14ARF and p16INK4A expression is higher in OPSCCs with an intact E2 gene. Our previous research revealed that HPV16 positive OPSCC have higher expression of CDKN2A locus transcripts than HPV16 negative OPSCC. In order to determine if CDKN2A expression also correlated with E2 gene status, we measured p14ARF and p16INK4A RNA levels in our tumor samples (Fig 4A and 4B). Sixteen OPSCC samples with an intact E2 gene were tested for ARF expression and 17 samples were tested for p16INK4A. Four disrupted OPSCCs were tested for both. Two E2 intact and 4 disrupted non-OP tumors were tested. Sample size for these experiments was limited due to availability of RNA. Similar to our findings with E6 and E7 expression, p14ARF and p16INK4A were expressed at significantly higher levels in OPSCC with intact vs. disrupted E2 gene products (Mann-Whitney test p = 0.005 and p = 0.004, respectively). There were no significant differences observed with p14ARF or p16INK4A expression in non-OPSCC.
Fig 4. ARF and INK4A are overexpressed in OPSCCs with an intact E2.
ARF (A) and INK4A (B) expression with HPV16 E2 gene disruption. Expression is defined as Y = 2maxΔCT- ΔCT where maxΔCT is the lower limit threshold of detection which equals 20 or 1. ΔCT is the experimental value minus the endogenous control (GAPDH). Statistical significance for difference in HPVE6 and E7 expression by site and between samples with an intact or disrupted HPV16 E2 gene was assessed by Mann-Whitney test.
Similar to our results for E6 and E7 expression, in oropharyngeal tumors, those cases with an intact E2 gene had significantly higher ARF and INK4A expression then the oropharyngeal cases with a disrupted E2 gene (Mann Whitney test, p = 0.005 and p = 0.004). There was no significant difference in expression of ARF and INK4A between non-oropharyngeal HNSCC tumors with an intact and disrupted E2.
Disruption of the E2 gene and expression of the E7 oncogene are associated with local-regional recurrence and disease-specific survival
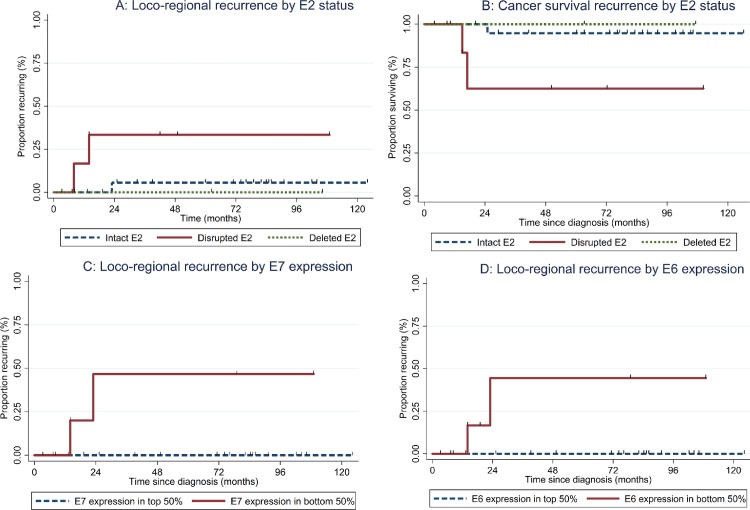
Studies in both cervical and oropharyngeal cancers have reported E2 disruption to be associated with poor prognosis. We further explored the relationship between E2 disruption, oncogene expression and clinical outcome in our cohort of HPV16 positive OPSCC patients. Our clinical analysis was done on data collected from patients followed prospectively after diagnosis and treatment. Patients are followed until time of death or withdrawal from the study. We found that tumors derived from the oropharynx containing only a disrupted E2 gene had higher risk of local-regional recurrence (Fig 5A; Mantel-Cox test, p = 0.04) and poorer disease-specific survival (Fig 5B;p = 0.03) compared to HPV16 positive OPSCC tumors with an intact E2 gene status.
Fig 5. Association of local-regional recurrence and disease specific survival with HPV16 E2 disruption in the oropharynx.
(A) Samples with an intact E2 gene (broken black line) have a lower incidence of local regional recurrence than oropharyngeal cases with a disrupted E2 gene (solid black line) (Mantel-Cox test p = 0.04). (B) Samples with an intact E2 gene (broken black line) have better disease specific survival than oropharyngeal cases with a disrupted E2 gene (solid black line) (Mantel-Cox test p = 0.03). (C and D) Samples were separated into high (broken black) or low (solid black) expression of viral genes based on median cutoff using derived (2maxΔCT- ΔCT) qRT-PCR results compared to GAPDH for: (C) HPV16 E7 (median = 1.69 2maxΔCT- ΔCT; Mantel-Cox test p = 0.004), and (D) HPV16 E6 (median = 1.23 2maxΔCT- ΔCT; Mantel-Cox test p = 0.006).
Based on our data showing that the presence of an intact E2 gene was associated with high E6 and E7 expression in OPSCC, and that HPV16 positive OPSCC with disrupted E2 also had poorer clinical outcomes compared to HPV16 positive OPSCC with intact (or mixed) E2, we then evaluated if expression of the E6 and E7 oncogenes was associated with clinical outcome. We analyzed all OPSCC samples with available clinical data regardless of E2 disruption results ranked by level of expression (Fig 5C and 5D). We found that tumors derived from the oropharynx which had E7 expression levels below the median cutoff had a higher risk of local-regional recurrence (6C; median = 1.69 2maxΔCT- ΔCT; Mantel-Cox test p = 0.004). Tumors derived from the oropharynx with E6 expression levels below the median cutoff had a higher risk of local-regional recurrence as well (6D; median = 1.23 2maxΔCT- ΔCT; Mantel-Cox test p = 0.006).
Discussion
HPV16 detection in HNSCC has been associated with improved response to treatment and clinical outcome [5,6]. However, 10–20% of HPV16 positive HNSCC fail to respond to treatment [5]. The HPV16 E2 gene plays a role in cervical cancer progression and has been shown to regulate expression of the E6 and E7 oncogenes [45,46]. However, the relationship between E2 disruption and E6/E7 expression has been largely unstudied in HNSCC.
This study evaluates the physical state of the E2 gene in HPV16 positive HNSCC tumors and assesses the relationship between E2 disruption and viral oncogene expression as well as clinical outcome. Evaluating 48 HPV16 positive tumors from a cohort of HNSCC patients followed prospectively at a large urban health center, we found that the majority of OPSCC samples had intact or possibly mixed (intact and disrupted) E2 gene products compared to HPV16 positive non-OPSCC tumors. While seemingly in contrast to what has been reported in cervical cancers, this was in line with recent studies of OPSCC in which episomal or mixed HPV16 (samples containing both episomal and integrated HPV) were detected in the majority of tumors [25,47,48].
The E2 gene itself is comprised of three domains: a transactivation domain, a DNA-binding domain, and a flexible hinge region which connects the two domains [49]. We independently measured expression of transcripts containing both the transactivation domain and DNA-binding domain of the E2 transcript and found that both measurements showed high levels of transcripts expressed in OPSCCs with an intact E2 gene compared to samples with a disrupted E2 gene. This indicates it is likely the full length E2 transcript is present in OPSCCs with an intact gene.
In addition to our findings that the majority of HPV16 positive OPSCC have an intact E2, we found that five HNSCC samples harbored E2 genes with deletions and mutations. Three of these were OPSCCs and two were non-OPSCC. Of the three OPSCC samples, all three involved deletions within the hinge region, which has been reported to harbor deletions in cervical cancer [50–52]. Deletions within the hinge region may hinder nuclear localization of E2 and E2-dependent replication. However, it has also been reported that deletions within the hinge region may not totally abolish the activity of the E2 protein, indicating there may still be a functional E2 protein in these samples [53]. Among the two non-OPSCC tumors, one harbored a deletion in the hinge region and the other a deletion in the transactivation domain. However these E2 sequences were identified as being non-functional due to mutations within the E2 gene (Genebank IDs HM162463.1 and HM162476.1) [52].
In addition to viral transcripts, we also assessed the expression of two host transcripts from the CDKN2A locus, p16INK4A and p14ARF, that are affected by E6 and E7 deregulation of the p53 and Rb tumor suppressor pathways [54–57]. Expression of these transcripts has been shown to be associated with HPV16 infection in OPSCC [15] and improved clinical outcome in HPV16 negative non-OPSCC [58]. In this study, p16INK4A and p14ARF expression was increased in OPSCC samples with an intact E2 gene.
Studies in cervical cancer have shown that the presence of a disrupted E2 gene is associated with poor prognosis [30,31,59] and radioinsensitivity [33]. A similar association between E2 disruption, local treatment failure and poor survival has also been reported for HPV16 positive OPSCC [60]. We found that OPSCC patients with HPV16 positive tumors harboring a disrupted E2 had increased local-regional recurrence (p = 0.04) and shortened disease-specific survival (p = 0.03). This was in accordance with a 2001 study of HPV16 positive OPSCC, which found that only those tumors with E2 disruption had local treatment failure [60]. A separate study found a positive association between E6 and E7 expression levels and improved risk of local-regional recurrence (p = 0.04), but no association with viral integration or detection of extrachromasomal and/or mixed forms of HPV16 [32].
Taken together, these results indicate that detection of disrupted HPV16 E2 or low levels of E7 expression may be useful in identifying the subset of HPV16 positive OPSCC tumors that are more likely to fail treatment. In this study we evaluated HPV16 positive OPSCC and non-OPSCC tumors. This study has a number of limitations that should be noted. Our stringent criteria for classifying a sample HPV16 positive included multiple HPV16 DNA and RNA tests. This approach was adopted as it was deemed to produce less false positives. However, it is possible HPV16 positive cases were excluded. This approach also reduced our study sample size, which precluded the possibility of conducting multivariable analysis. In addition, the lack of material, particularly for RNA, resulted in smaller sample sizes for correlative analyses between DNA and RNA measures. Our assay for detection of E2 status did not differentiate between samples with intact only or mixed (intact and disrupted) forms of the gene, nor did we assess for other (e.g., epigenetic) mechanisms that might explain our observed associations between E2 disruption and E6/E7 expression. We also did not test for the presence of tandemly integrated viral genomes which has been reported in cervical tumors with integrated HPV [61]. Another scenario we did not test for is the existence of viral-human hybrid episomes as described by Nulton et al. [29,61]. These conditions can result in viral genomes with an intact E2 gene suggesting that the genomes replicate in an E1-E2 dependent manner.
Another recent study by Koneva et al. on HNSCC tumors also found that tumors without HPV integration had improved survival compared to tumors that did have integration or were HPV negative. This study also found that in integration negative samples, immune related genes were the most over expressed group of genes, suggesting that improved survival in this group may be due to increased immunogenicity [62]. An earlier, study by the same group found that there were two distinct subtypes of HPV positive HNSCC tumors based on gene expression. These subtypes were identified as being high expressers of genes related to immune response or keratinocyte differentiation. The subtype associated with immune response was also reported to have less viral integration, higher E2 expression and higher full-length E6 activity compared to the tumors expressing genes for keratinocyte differentiation [63]. Taken together, these two studies indicate that HPV positive HNSCC cases can be stratified and that there appear to be two major carcinogenic pathways. This is similar to results reported in our study which suggest OPSCC cases may be stratified by viral expression or E2 gene status.
Despite these limitations, our findings indicate that E2 disruption is uncommon in OPSCC and that the presence of an intact E2 gene is associated with increased E6 and E7 expression. This is contrary to in vitro models of HPV16 negative induced transformation as well as what is reported in many cervical cancer studies, where E2 disruption is prevalent. However, others have shown that E6 and E7 can be expressed at high levels in the presence of an intact E2 gene [24,64], which has been attributed to methylation of the E2 gene binding sites [23,24]. The majority of HPV16 positive non-OP tumors in this study had a disrupted E2 gene, and E2 disruption showed no association with viral expression. In contrast, most OPSCC tumors had intact forms of the E2 gene, which correlated with high viral expression. These tumors also had high expression of host CDKN2A (p16INK4A and p14ARF) gene expression. We also found that HPV16 positive OPSCCs with disrupted E2 had poorer cancer prognosis (i.e., shorter disease-specific survival and increased local or regional recurrence), as did OPSCCs with low HPV16 E7 expression. This is in line with previous studies that have shown disruption of the HPV16 E2 gene to be associated with shorter disease-specific survival in cervical cancer, and with local treatment failure in OPSCC [30,31,60]. With current considerations for dose de-escalation for HPV16 negative associated OPSCC, an assay specific to detection of disruption of the E2 gene or E6/E7 oncogene expression among HPV16 DNA positive (or p16 protein positive) OPSCC may help identify HPV16 positive OPSCC patients at higher risk of treatment failure.
Supporting information
(DOCX)
(DOCX)
SiHa and UPCI:SCC090 are used as E2 disrupted and E2 intact controls. Column 5, 6, and 7 are results from three different patient samples: column 5 represents a sample which had E2 disruption, column 6 (red arrow) represents a sample which had a deletion in the E2 gene and was observed to have a smaller product. The deletion was confirmed by Sanger sequencing. Column 7 represents a sample which had an intact E2 gene.
(TIF)
Including RNA expression data and viral load data as well as site and E2 status.
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by Albert Einstein College of Medicine, Inc, https://www.einstein.yu.edu/administration/grant-support/, funding number 3A7078, recipient Dr. Michael Prystowsky; and Medical Research Council grant, https://www.mrc.ac.uk/funding/how-we-fund-research/research-grant/, fund number MR/N023498/1, recipient JLP.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA: A Cancer Journal for Clinicians 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 2.Doorbar J (2007) Papillomavirus Life Cycle Organization and Biomarker Selection. Disease Markers 23: 297–313. doi: 10.1155/2007/613150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F (2005) Carcinogenicity of human papillomaviruses. Lancet Oncol 6: 204 [DOI] [PubMed] [Google Scholar]
- 4.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, et al. (2000) Evidence for a causal association between human papillomavirus and a subset of head and neck cancers Journal of the National Cancer Institute: Department of Medical Oncology, The Johns Hopkins University School of Medicine, Baltimore, MD, USA: pp. 709–720. [DOI] [PubMed] [Google Scholar]
- 5.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. (2008) Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 100: 261–269. doi: 10.1093/jnci/djn011 [DOI] [PubMed] [Google Scholar]
- 6.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. (2010) Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363: 24–35. doi: 10.1056/NEJMoa0912217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.zur Hausen H (2002) Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer 2: 342–350. [DOI] [PubMed] [Google Scholar]
- 8.Doorbar J (2005) The papillomavirus life cycle. J Clin Virol 32 Suppl 1: S7–15. [DOI] [PubMed] [Google Scholar]
- 9.Qu W, Jiang G, Cruz Y, Chang CJ, Ho GY, Klein RS, et al. (1997) PCR detection of human papillomavirus: comparison between MY09/MY11 and GP5+/GP6+ primer systems. Journal of Clinical Microbiology 35: 1304–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker CC, Phelps WC, Lindgren V, Braun MJ, Gonda MA, Howley PM (1987) Structural and transcriptional analysis of human papillomavirus type 16 sequences in cervical carcinoma cell lines. J Virol 61: 962–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smeets SJ, Hesselink AT, Speel EJ, Haesevoets A, Snijders PJ, Pawlita M, et al. (2007) A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer 121: 2465–2472. doi: 10.1002/ijc.22980 [DOI] [PubMed] [Google Scholar]
- 12.Weinberger PM, Yu Z, Haffty BG, Kowalski D, Harigopal M, Sasaki C, et al. (2004) Prognostic significance of p16 protein levels in oropharyngeal squamous cell cancer. Clin Cancer Res 10: 5684–5691. doi: 10.1158/1078-0432.CCR-04-0448 [DOI] [PubMed] [Google Scholar]
- 13.Salazar CR, Anayannis N, Smith RV, Wang Y, Haigentz M Jr., Garg M, et al. (2014) Combined P16 and human papillomavirus testing predicts head and neck cancer survival. Int J Cancer 135: 2404–2412. doi: 10.1002/ijc.28876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinmann K, Sandner A, Schagdarsurengin U, Dammann RH (2009) Frequent promoter hypermethylation of tumor-related genes in head and neck squamous cell carcinoma. Oncol Rep 22: 1519–1526. [DOI] [PubMed] [Google Scholar]
- 15.Schlecht NF, Ben-Dayan M, Anayannis N, Lleras RA, Thomas C, Wang Y, et al. (2015) Epigenetic changes in the CDKN2A locus are associated with differential expression of P16INK4A and P14ARF in HPV-positive oropharyngeal squamous cell carcinoma. Cancer Medicine 4: 342–353. doi: 10.1002/cam4.374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeon S, Allen-Hoffmann BL, Lambert PF (1995) Integration of human papillomavirus type 16 into the human genome correlates with a selective growth advantage of cells. Journal of Virology 69: 2989–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vinokurova S, Wentzensen N, Kraus I, Klaes R, Driesch C, Melsheimer P, et al. (2008) Type-Dependent Integration Frequency of Human Papillomavirus Genomes in Cervical Lesions. Cancer Research 68: 307–313. doi: 10.1158/0008-5472.CAN-07-2754 [DOI] [PubMed] [Google Scholar]
- 18.Xu F, Cao M, Shi Q, Chen H, Wang Y, Li X (2015) Integration of the full-length HPV16 genome in cervical cancer and Caski and Siha cell lines and the possible ways of HPV integration. Virus Genes 50: 210–220. doi: 10.1007/s11262-014-1164-7 [DOI] [PubMed] [Google Scholar]
- 19.Schwarz E, Freese UK, Gissmann L, Mayer W, Roggenbuck B, Stremlau A, et al. (1985) Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature 314: 111–114. [DOI] [PubMed] [Google Scholar]
- 20.Smotkin D, Wettstein FO (1986) Transcription of human papillomavirus type 16 early genes in a cervical cancer and a cancer-derived cell line and identification of the E7 protein. Proc Natl Acad Sci U S A 83: 4680–4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith EM, Pawlita M, Rubenstein LM, Haugen TH, Hamsikova E, Turek LP (2010) Risk factors and survival by HPV-16 E6 and E7 antibody status in human papillomavirus positive head and neck cancer. Int J Cancer 127: 111–117. doi: 10.1002/ijc.25015 [DOI] [PubMed] [Google Scholar]
- 22.Smith JA, Haberstroh FS, White EA, Livingston DM, DeCaprio JA, Howley PM (2014) SMCX and components of the TIP60 complex contribute to E2 regulation of the HPV E6/E7 promoter. Virology 468–470: 311–321. doi: 10.1016/j.virol.2014.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reuschenbach M, Huebbers CU, Prigge ES, Bermejo JL, Kalteis MS, Preuss SF, et al. (2015) Methylation status of HPV16 E2-binding sites classifies subtypes of HPV-associated oropharyngeal cancers. Cancer 121: 1966–1976. doi: 10.1002/cncr.29315 [DOI] [PubMed] [Google Scholar]
- 24.Cheung JL, Cheung TH, Yu MY, Chan PK (2013) Virological characteristics of cervical cancers carrying pure episomal form of HPV16 genome. Gynecol Oncol 131: 374–379. doi: 10.1016/j.ygyno.2013.08.026 [DOI] [PubMed] [Google Scholar]
- 25.Olthof NC, Speel EJ, Kolligs J, Haesevoets A, Henfling M, Ramaekers FC, et al. (2014) Comprehensive analysis of HPV16 integration in OSCC reveals no significant impact of physical status on viral oncogene and virally disrupted human gene expression. PLoS One 9: e88718 doi: 10.1371/journal.pone.0088718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olthof NC, Huebbers CU, Kolligs J, Henfling M, Ramaekers FC, Cornet I, et al. (2015) Viral load, gene expression and mapping of viral integration sites in HPV16-associated HNSCC cell lines. Int J Cancer 136: E207–218. doi: 10.1002/ijc.29112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walline HM, Goudsmit CM, McHugh JB, Tang AL, Owen JH, Teh BT, et al. (2017) Integration of high-risk human papillomavirus into cellular cancer-related genes in head and neck cancer cell lines. Head Neck 39: 840–852. doi: 10.1002/hed.24729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao G, Johnson SH, Kasperbauer JL, Eckloff BW, Tombers NM, Vasmatzis G, et al. (2014) Mate pair sequencing of oropharyngeal squamous cell carcinomas reveals that HPV integration occurs much less frequently than in cervical cancer. J Clin Virol 59: 195–200. doi: 10.1016/j.jcv.2013.12.006 [DOI] [PubMed] [Google Scholar]
- 29.Nulton TJ, Olex AL, Dozmorov M, Morgan IM, Windle B (2017) Analysis of The Cancer Genome Atlas sequencing data reveals novel properties of the human papillomavirus 16 genome in head and neck squamous cell carcinoma. Oncotarget 8: 17684–17699. doi: 10.18632/oncotarget.15179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vernon SD, Unger ER, Miller DL, Lee DR, Reeves WC (1997) Association of human papillomavirus type 16 integration in the E2 gene with poor disease-free survival from cervical cancer. Int J Cancer 74: 50–56. [DOI] [PubMed] [Google Scholar]
- 31.Lindel K, de Villiers EM, Burri P, Studer U, Altermatt HJ, Greiner RH, et al. (2006) Impact of viral E2-gene status on outcome after radiotherapy for patients with human papillomavirus 16-positive cancer of the uterine cervix. Int J Radiat Oncol Biol Phys 65: 760–765. doi: 10.1016/j.ijrobp.2006.01.023 [DOI] [PubMed] [Google Scholar]
- 32.Vojtechova Z, Sabol I, Salakova M, Turek L, Grega M, Smahelova J, et al. (2016) Analysis of the integration of human papillomaviruses in head and neck tumours in relation to patients' prognosis. Int J Cancer 138: 386–395. doi: 10.1002/ijc.29712 [DOI] [PubMed] [Google Scholar]
- 33.Lindel K, Daffinger S, Weber K, de Villiers E, Beard P, Debus J (2008) Integration of Human Papillomavirus (HPV) 16 into the Host Genome Influences the Radiosensitivity via the Disruption of the Viral Transcriptional Regulator Gene E2. International Journal of Radiation Oncology*Biology*Physics 72: S686. [Google Scholar]
- 34.Lleras Ra, Adrien LR, Smith RV, Brown B, Jivraj N, Keller C, et al. (2011) Hypermethylation of a cluster of Krüppel-type zinc finger protein genes on chromosome 19q13 in oropharyngeal squamous cell carcinoma. The American journal of pathology 178: 1965–1974. doi: 10.1016/j.ajpath.2011.01.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kotorashvili A, Ramnauth A, Liu C, Lin J, Ye K, Kim R, et al. (2012) Effective DNA/RNA co-extraction for analysis of microRNAs, mRNAs, and genomic DNA from formalin-fixed paraffin-embedded specimens. PloS one 7: e34683 doi: 10.1371/journal.pone.0034683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loudig O, Milova E, Brandwein-Gensler M, Massimi A, Belbin TJ, Childs G, et al. (2007) Molecular restoration of archived transcriptional profiles by complementary-template reverse-transcription (CT-RT). Nucleic Acids Res 35: e94 doi: 10.1093/nar/gkm510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castle PE, Schiffman M, Gravitt PE, Kendall H, Fishman S, Dong H, et al. (2002) Comparisons of HPV DNA detection by MY09/11 PCR methods. J Med Virol 68: 417–423. [DOI] [PubMed] [Google Scholar]
- 38.Gravitt PE, Burk RD, Lorincz A, Herrero R, Hildesheim A, Sherman ME, et al. (2003) A comparison between real-time polymerase chain reaction and hybrid capture 2 for human papillomavirus DNA quantitation. Cancer Epidemiol Biomarkers Prev 12: 477–484. [PubMed] [Google Scholar]
- 39.Gravitt PE, Kovacic MB, Herrero R, Schiffman M, Bratti C, Hildesheim A, et al. (2007) High load for most high risk human papillomavirus genotypes is associated with prevalent cervical cancer precursors but only HPV16 load predicts the development of incident disease. Int J Cancer 121: 2787–2793. doi: 10.1002/ijc.23012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collins SI, Constandinou-Williams C, Wen K, Young LS, Roberts S, Murray PG, et al. (2009) Disruption of the E2 gene is a common and early event in the natural history of cervical human papillomavirus infection: a longitudinal cohort study. Cancer Res 69: 3828–3832. doi: 10.1158/0008-5472.CAN-08-3099 [DOI] [PubMed] [Google Scholar]
- 41.Graham DA, Herrington CS (2000) HPV-16 E2 gene disruption and sequence variation in CIN 3 lesions and invasive squamous cell carcinomas of the cervix: relation to numerical chromosome abnormalities. Mol Pathol 53: 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ragin CC, Reshmi SC, Gollin SM (2004) Mapping and analysis of HPV16 integration sites in a head and neck cancer cell line. Int J Cancer 110: 701–709. doi: 10.1002/ijc.20193 [DOI] [PubMed] [Google Scholar]
- 43.Ferris RL, Martinez I, Sirianni N, Wang J, Lopez-Albaitero A, Gollin SM, et al. (2005) Human papillomavirus-16 associated squamous cell carcinoma of the head and neck (SCCHN): a natural disease model provides insights into viral carcinogenesis. Eur J Cancer 41: 807–815. doi: 10.1016/j.ejca.2004.11.023 [DOI] [PubMed] [Google Scholar]
- 44.Harris T, Jimenez L, Kawachi N, Fan JB, Chen J, Belbin T, et al. (2012) Low-level expression of miR-375 correlates with poor outcome and metastasis while altering the invasive properties of head and neck squamous cell carcinomas. Am J Pathol 180: 917–928. doi: 10.1016/j.ajpath.2011.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lace MJ, Anson JR, Klussmann JP, Wang DH, Smith EM, Haugen TH, et al. (2011) Human Papillomavirus Type 16 (HPV-16) Genomes Integrated in Head and Neck Cancers and in HPV-16-Immortalized Human Keratinocyte Clones Express Chimeric Virus-Cell mRNAs Similar to Those Found in Cervical Cancers. Journal of Virology 85: 1645–1654. doi: 10.1128/JVI.02093-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hafner N, Driesch C, Gajda M, Jansen L, Kirchmayr R, Runnebaum IB, et al. (2007) Integration of the HPV16 genome does not invariably result in high levels of viral oncogene transcripts. Oncogene 27: 1610–1617. doi: 10.1038/sj.onc.1210791 [DOI] [PubMed] [Google Scholar]
- 47.Faust H, Eldenhed Alwan E, Roslin A, Wennerberg J, Forslund O (2016) Prevalence of HPV types, viral load and physical status of HPV16 in head and neck squamous cell carcinoma from the South Swedish Health Care Region. J Gen Virol. [DOI] [PubMed] [Google Scholar]
- 48.Deng Z, Hasegawa M, Kiyuna A, Matayoshi S, Uehara T, Agena S, et al. (2013) Viral load, physical status, and E6/E7 mRNA expression of human papillomavirus in head and neck squamous cell carcinoma. Head Neck 35: 800–808. doi: 10.1002/hed.23034 [DOI] [PubMed] [Google Scholar]
- 49.Giri I, Yaniv M (1988) Structural and mutational analysis of E2 trans-activating proteins of papillomaviruses reveals three distinct functional domains. EMBO J 7: 2823–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arias-Pulido H, Peyton CL, Joste NE, Vargas H, Wheeler CM (2006) Human papillomavirus type 16 integration in cervical carcinoma in situ and in invasive cervical cancer. J Clin Microbiol 44: 1755–1762. doi: 10.1128/JCM.44.5.1755-1762.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsakogiannis D, Gortsilas P, Kyriakopoulou Z, Ruether IG, Dimitriou TG, Orfanoudakis G, et al. (2015) Sites of disruption within E1 and E2 genes of HPV16 and association with cervical dysplasia. J Med Virol 87: 1973–1980. doi: 10.1002/jmv.24256 [DOI] [PubMed] [Google Scholar]
- 52.Tsakogiannis D, Ruether IGA, Kyriakopoulou Z, Pliaka V, Theoharopoulou A, Skordas V, et al. (2012) Sequence variation analysis of the E2 gene of human papilloma virus type 16 in cervical lesions from women in Greece. Archives of Virology 157: 825–832. doi: 10.1007/s00705-012-1236-8 [DOI] [PubMed] [Google Scholar]
- 53.Zou N, Lin BY, Duan F, Lee KY, Jin G, Guan R, et al. (2000) The hinge of the human papillomavirus type 11 E2 protein contains major determinants for nuclear localization and nuclear matrix association. J Virol 74: 3761–3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM (1990) The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63: 1129–1136. [DOI] [PubMed] [Google Scholar]
- 55.Werness BA, Levine AJ, Howley PM (1990) Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 248: 76 [DOI] [PubMed] [Google Scholar]
- 56.Dyson N, Howley PM, Munger K, Harlow E (1989) The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 243: 934 [DOI] [PubMed] [Google Scholar]
- 57.Münger K, Werness BA, Dyson N, Phelps WC, Harlow E, Howley PM (1989) Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. The EMBO Journal 8: 4099–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ben-Dayan MM, Ow TJ, Belbin TJ, Wetzler J, Smith RV, Childs G, et al. (2017) Nonpromoter methylation of the CDKN2A gene with active transcription is associated with improved locoregional control in laryngeal squamous cell carcinoma. Cancer Med 6: 397–407. doi: 10.1002/cam4.961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kalantari M, Karlsen F, Kristensen G, Holm R, Hagmar B, Johansson B (1998) Disruption of the E1 and E2 reading frames of HPV 16 in cervical carcinoma is associated with poor prognosis. Int J Gynecol Pathol 17: 146–153. [DOI] [PubMed] [Google Scholar]
- 60.Lindel K, Beer KT, Laissue J, Greiner RH, Aebersold DM (2001) Human papillomavirus positive squamous cell carcinoma of the oropharynx: a radiosensitive subgroup of head and neck carcinoma. Cancer 92: 805–813. [DOI] [PubMed] [Google Scholar]
- 61.Shin H-J, Joo J, Yoon JH, Yoo CW, Kim J-Y (2014) Physical Status of Human Papillomavirus Integration in Cervical Cancer Is Associated with Treatment Outcome of the Patients Treated with Radiotherapy. PLoS ONE 9: e78995 doi: 10.1371/journal.pone.0078995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koneva LA, Zhang Y, Virani S, Hall PB, McHugh JB, Chepeha DB, et al. (2017) HPV Integration in HNSCC Correlates with Survival Outcomes, Immune Response Signatures, and Candidate Drivers. Mol Cancer Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y, Koneva LA, Virani S, Arthur AE, Virani A, Hall PB, et al. (2016) Subtypes of HPV-Positive Head and Neck Cancers Are Associated with HPV Characteristics, Copy Number Alterations, PIK3CA Mutation, and Pathway Signatures. Clin Cancer Res 22: 4735–4745. doi: 10.1158/1078-0432.CCR-16-0323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sathish N, Abraham P, Peedicayil A, Sridharan G, John S, Chandy G (2004) Human Papillomavirus 16 E6/E7 Transcript and E2 Gene Status in Patients with Cervical Neoplasia. Molecular Diagnosis 8: 57–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
SiHa and UPCI:SCC090 are used as E2 disrupted and E2 intact controls. Column 5, 6, and 7 are results from three different patient samples: column 5 represents a sample which had E2 disruption, column 6 (red arrow) represents a sample which had a deletion in the E2 gene and was observed to have a smaller product. The deletion was confirmed by Sanger sequencing. Column 7 represents a sample which had an intact E2 gene.
(TIF)
Including RNA expression data and viral load data as well as site and E2 status.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.