Abstract
Aims
Cumulative coronary heart disease (CHD) events over 20 years were examined in men screened for, and in those randomized to, the West of Scotland Coronary Prevention Study.
Methods and results
Record linkage provided CHD-related events and days in hospital for the 80 230 screenees, including the randomized cohort of 6595 men. Risk factors were determined at baseline, and disease burden assessed for groups defined by cholesterol. Effects of cholesterol lowering were modelled from differences between groups, and from the treatment arms of the trial. Over 20 years, those without a history of CHD (n = 61 211) had 23.0 events per 100 subjects in the lowest cholesterol group (mean 4.0 mmol/L) and 65.1 per 100 in the highest (8.8 mmol/L). Corresponding days in hospital were 167.2–435.4 per 100 subjects. Analogous figures for men with a CHD history (n = 8570) were 77.3–141.7 events per 100 and 526.1–936.7 hospital days per 100. Lowering cholesterol by about 1.0 mmol/L in men with average cholesterol and no CHD was predicted to be associated with 8.9 fewer events and a saving of 56.0 hospital days per 100. In those with CHD this difference gave, depending on starting level, 26.8–36.5 fewer events and savings of 158.2–247.3 hospital days per 100 subjects. Comparison of cumulative events in 45–54 vs. 55–64 year olds in the trial revealed greater benefit from intervention in the younger decade.
Conclusion
Long-term, longitudinal data reveal the considerable CHD burden in middle-aged men and indicate substantial clinical benefits from both moderate and aggressive cholesterol lowering.
Keywords: Cholesterol, Data linkage, Myocardial infarction, Clinical trial
Introduction
LDL lowering is a cornerstone recommendation in coronary heart disease (CHD) prevention guidelines1,2 with meta-analyses3,4 and cost-effectiveness studies5–7 confirming its clinical and economic utility in secondary prevention and high-risk primary prevention. Population benefit assessments are based necessarily on models generated with tools such as the Framingham risk equation,8 and the outcome of clinical trials that are limited (often to 3–5 years) in duration relative to the decades-long course of the disease. Also, traditional indices of clinical utility such as number-needed-to-treat9 are probabilities based on the first major event and do not take into account the fact that patients often suffer multiple episodes of CHD over a number of years. Overall, as highlighted again in IMPROVE-IT10 and HOPE-3,11 these approaches can lead to a substantial underestimate of the full clinical and economic impact of therapy.
A number of trials have reported extended follow-up,12–15 and the present study takes advantage of the availability of comprehensive, longitudinal electronic health records not only for the subjects included in the West of Scotland Coronary Prevention Study (WOSCOPS) but also for the population of approximately 80 000 45–64-year-old men who were screened.16 Based on an evaluation of total CHD burden, we modelled the benefits of having lower LDL in a primary prevention setting which is still an area of contention,17–19 and since the screened population included those with a history of CHD, we assessed also the impact of reducing total cholesterol levels in a secondary prevention setting.
Methods
Recruitment for WOSCOPS16,20,21 involved inviting men aged 45–64 years from approximately 120 family practices to visit a screening clinic. Between September 1989 and July 1991, 80 230 attended Study Visit 1 at which risk factors were assessed including cholesterol, blood pressure, and smoking habit.16 Personal history of CHD was evaluated by questionnaire based on patient recall (specifically, whether a doctor had informed the subject that they suffered from chest pain due to angina or had had a heart attack). Those with total cholesterol >6.5 mmol/L were evaluated in three subsequent visits and 6595 men with LDL cholesterol (LDLc) 4.0–6.0 mmol/L but no history of myocardial infarction were randomized to pravastatin 40 mg/day or placebo. Follow-up was for a mean of 4.9 years with final trial visits held in May 1995. The outcome was a risk reduction of about one-third in a range of cardiovascular endpoints.21
Use of lipid-lowering therapy during the first 5 years after the trial ended was monitored by review of GP records. In the original pravastatin and placebo groups respectively, 28.6 and 24.3% at 1 year, 33.6 and 29.4% at 3 years, and 38.7 and 35.2% at 5 years post-trial were on statins.12 No further information on statin treatment was available for the trial participants after this point (i.e. 10 years post-randomization). For the screened population, no information on use of lipid-lowering treatment was available across the whole of the 20-year observation period.
Individual-level data were extracted from national electronic hospital discharge records and death registries22 to provide numbers of clinical events and length of hospitalizations for both the WOSCOPS randomized cohort, and the screenees who did not enter the trial. Events were classified using International Classification of Diseases (ICD) codes. Hospital duration for any single event was truncated at 182 days. No limit was placed on the number of events per subject.
The original trial was approved by the Ethics committees of the University of Glasgow and participating health boards and the long-term follow-up and associated record linkage by the Ethics committee of the Royal Infirmary, Glasgow and the Privacy Advisory Committee of the National Health Service for Scotland.
Association of total cholesterol levels with hospital admissions for CHD causes (ICD9 codes 410-414 and ICD10 codes I20-I25) was assessed over the observation period (i.e. until October 2011) or until death. We estimated the benefit of cholesterol reduction by comparing differences in cumulative events between groups categorized by baseline total cholesterol level.
An analysis was also undertaken to evaluate the relative benefit of initiating cholesterol lowering at age 45–54 compared with 55–64 years in the WOSCOPS randomized cohort, and separately in screenee groups with total cholesterol levels similar to those seen in the treatment arms of the trial. The ‘QRISK’ tool5,23 (www.qrisk.org; accessed 3 June 2017) was used to estimate 10-year risk of a cardiovascular disease (CVD) event for males aged 50 and 60 years (i.e. the mean ages in the two age decades) with the same risk factor levels as the WOSCOPS randomized subjects at baseline21 (total cholesterol: HDL cholesterol ratio of 5.0, systolic blood pressure 137 mmHg, height 173 cm, weight 77.5 kg, non-smoker or moderate smoker).
Statistical methods
Total CHD burden for each individual was the cumulative number of CHD hospitalization events and the total number of days spent in hospital attributed to CHD causes. This was analysed separately for (i) the WOSCOPS randomized cohort with its placebo and pravastatin arms, (ii) WOSCOPS screenees without a history of CHD at baseline (‘primary prevention’ setting), and (iii) WOSCOPS screenees with a history of CHD (‘secondary prevention’ setting). The last two groups were divided into five categories (P1–P5 and S1–S5, respectively) based on total cholesterol at screening—≤4.5, 4.5–5.5, >5.5–6.5, >6.5–8.0, and >8.0 mmol/L. These intervals were chosen so that, where possible, mean cholesterol levels differed by increments of approximately 1.0 mmol/L, and hence our findings could be related to the commonly used metric of risk reduction per mmol/L change.3,4 Incidence of the first CHD event (death or hospitalization for a CHD related reason), the cumulative number of hospital admissions for CHD (events and length of stay in days) and the total number of deaths were obtained for each cholesterol group. Risk ratios were derived by quasi-Poisson regression (which allows for over-dispersion in the data) using the lowest total cholesterol category as referent and adjusting for other major risk factors (age, blood pressure, and smoking status).
Total event rates, expressed per 100 subjects over 20 years, were compared between categories of total cholesterol level to obtain relative risks and differences in number of CHD hospitalizations and hospital days attributed to CHD causes per nominal 1.0 mmol/L change. The effects of more aggressive cholesterol reductions were estimated by comparing groups that differed by 2.0 mmol/L or more. Groups were also characterized by the percentage of subjects experiencing CHD death, multiple CHD events, or a single CHD events (see Supplementary material online, Tables).
All statistical analyses were conducted using R version 3.1.2.
Results
A total of 80 230 men attended Study Visit 1 and individual-level record linkage-based follow-up was available for 76 376 (95.2%). Over the observation period (which ranged from 20 to 22 years), there were 35 690 hospitalizations for CHD causes in 15 398 screenees. Once those with incomplete Visit 1 information were excluded, total CHD burden data were available for 61 211 screenees with no prior history of CHD and 8570 screenees with a history of CHD. (Note that the analyses of screenees does not include subjects randomized to the trial).
Plasma cholesterol and total coronary heart disease burden
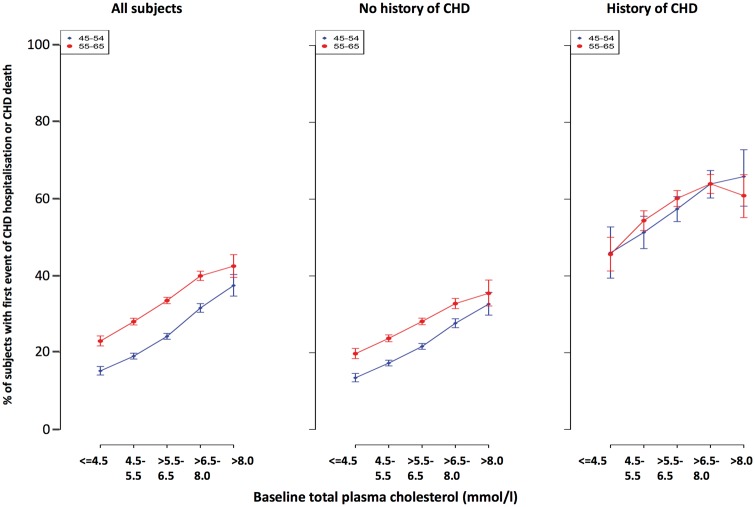
Over 20 years follow-up, for all screenees there was a positive relationship between total plasma cholesterol and incidence of a first event of CHD hospitalization or CHD death for both age decades (Figure 1A).
Figure 1.
Relationship between plasma cholesterol and first CHD event (death or hospitalization) in (A) the whole group of screenees, (B) men with no self-reported history of CHD at screening, and (C) men who reported a history of CHD at screening.
In screenees with no history of CHD (Figure 1B) the rate in the older compared with the younger decade was about 29% higher, and when these subjects, using the entire age range, were grouped by total cholesterol (P1–P5) there was a 2.7-fold increase in adjusted risk comparing the lowest to highest levels (Table 1). The cumulative number of events per 100 subjects rose from 23.0 to 65.1, and the corresponding number of days of hospitalization for CHD causes increased from 167.2 to 435.4 per 100 subjects.
Table 1.
Cumulative coronary heart disease events and hazard ratios for screened population by cholesterol level
| Cholesterol group (limits in mmol/L) | Mean total cholesterol [LDL] (mmol/L) | Mean age (years) | Smokers (current %/ former %) | Mean systolic/ diastolic BP (mmHg) | No. of subjects (% died) | Observed CHD hospitalization events per 100 subjects over 20 years | Observed CHD hospital days per 100 subjects over 20 years | Adjusted hazard ratioa (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|
| No CHD history—‘primary prevention’ | |||||||||
| P5 (>8.0)b | 8.77 [6.8]c | 54.1 | 46.0%/29.3%d | 140.4/86.6 | 1790 (42.0%) | 65.1 | 435.4 | 2.7 (2.4–3.1) | |
| P4 (>6.5–8.0) | 7.05 [5.0] | 54.5 | 42.9%/27.4% | 139.6/86.2 | 10767 (39.7%) | 52.7 | 349.6 | 2.2 (2.0–2.5) | |
| P3 (>5.5–6.5) | 5.98 [4.0] | 54.7 | 42.0%/26.4% | 137.7/85.1 | 22288 (37.3%) | 42.4 | 288.3 | 1.8 (1.7–2.0) | |
| P2 (>4.5–5.5) | 5.06 [3.1] | 54.6 | 41.7%/25.8% | 136.3/84.3 | 18952 (36.9%) | 33.6 | 232.2 | 1.5 (1.3–1.6) | |
| P1 (≤4.5) | 4.00 [2.0] | 54.6 | 43.7%/23.9% | 135.2/83.4 | 7414 (41.2%) | 23.0 | 167.2 | 1.0 (referent) | |
| With CHD history—‘secondary prevention’ | |||||||||
| S5 (>8.0) | 8.81 [6.8] | 56.8 | 47.0%/36.1% | 138.0/84.8 | 477 (64.8%) | 141.7 | 936.7 | 1.8 (1.4–2.3) | |
| S4 (>6.5–8.0) | 7.10 [5.1] | 57.3 | 40.8%/40.6% | 137.7/84.8 | 2210 (64.3%) | 152.9 | 1089.9 | 1.9 (1.6–2.4) | |
| S3 (>5.5–6.5) | 6.02 [4.0] | 57.6 | 40.2%/39.1% | 138.1/84.7 | 3105 (61.3%) | 140.6 | 931.7 | 1.8 (1.5–2.2) | |
| S2 (>4.5–5.5) | 5.08 [3.1] | 57.7 | 43.5%/36.1% | 137.2/83.9 | 2037 (61.8%) | 113.8 | 773.4 | 1.5 (1.2–1.8) | |
| S1 (≤4.5) | 3.98 [2.0] | 57.7 | 50.6%/30.4% | 135.9/83.1 | 741 (67.9%) | 77.3 | 526.1 | 1.0 (referent) | |
CHD, coronary heart disease.
Hazard ratio from quasi-Poisson regression adjusted for age, smoking and blood pressure.
Subjects were categorized into groups according to cholesterol level and CHD history at screening; S1–S5 had a history of CHD and P1–P5 did not.
LDL cholesterol values were assumed to be 2.0 mmol/L less that the total cholesterol.16
Percentages are given for current and former smokers, never smokers comprised the remainder.
In screenees with a history of CHD there was a positive association between first incidence of a subsequent event and total cholesterol but little difference between the two age decades (Figure 1C). In Table 1, the adjusted risk ratio virtually doubled across the cholesterol categories S1 to S4 but did not appear to increase further in the S5 group. In general, cumulative event rates were two- to three-fold higher compared with those seen in screenees without a CHD history. Across the cholesterol range total CHD burden was 77.3–141.7 events per 100 subjects, and the number of days in hospital for CHD causes was 526 to about 1000 per 100 subjects.
Benefit of cholesterol lowering in a ‘primary prevention’ setting
Differences in cumulative events and hospital days attributable to CHD for groups of interest are presented in Table 2. In taking this approach to modelling the impact of lowering cholesterol, it is assumed that any difference is attributable solely to variation in LDLc, and that the result is a reasonable estimate of the benefit of using pharmacological intervention.
Table 2.
Modelling prevention scenarios using cumulative coronary heart disease event and hospitalization rates in the West of Scotland Coronary Prevention Study screened population
| Cholesterol difference | Relative risk reductiona | Total CVD events difference over 20 years per 100 subjects | Total CVD hospital days difference over 20 years per 100 subjects |
|---|---|---|---|
| No CHD history (primary prevention setting) | |||
| Severe hypercholesterolaemia—P5; starting total cholesterol of 8.8 mmo/L (LDL approx 6.8 mmol/L)b | |||
| 1.7 mmol/L (25% LDL)c difference (change to level seen in P4) | 19.1% | 12.4 | 85.8 |
| 3.7 mmol/L (54% LDL) difference (change to level seen in P2) | 48.4% | 31.5 | 203.2 |
| Moderate hypercholesterolaemia—P4; starting total cholesterol of 7.0 mmol/L (LDL approx. 5.0 mmol/L) WOSCOPS equivalent | |||
| 1.0 mmol/L (20% LDL) difference (change to level seen in P3) | 19.4% | 10.2 | 61.3 |
| Population average—P3; starting total cholesterol of 6.0 mmol/L (LDL approx 4.0 mmol/L) | |||
| 0.9 mmol/L (23% LDL) difference (change to level seen in P2) | 20.9% | 8.9 | 56.0 |
| 2.0 mmol/L (50% LDL) difference (change to level seen in P1) | 45.7% | 19.4 | 121.1 |
| Previous CHD history (secondary prevention setting) | |||
| Population average—S3; starting cholesterol of 6.0 mmol/L (LDL approx 4.0 mmol/L) | |||
| 1.0 mmol/L (23% LDL) difference (change to level seen in S2) | 19.1% | 26.8 | 158.2 |
| 2.0 mmol/L (50% LDL) difference (change to level seen in S1) | 45.0% | 63.2 | 405.5 |
| Low—S2; starting total cholesterol of 5.0 mmol/L (LDL approx 3.0 mmol/L) | |||
| 1.1 mmol/L (35% LDL) difference (change to level seen in S1) | 32.0% | 36.5 | 247.3 |
CHD, coronary heart disease; CVD, cardiovascular disease.
Cholesterol lowering scenarios were tested for a range of starting total cholesterol levels using the groups defined in Table 1. Relative risk reduction compared the CHD event rate in the lower vs. higher cholesterol group.
Estimated LDL cholesterol was 2.0 mmol/L less than the total plasma cholesterol level.16
The per cent difference in parentheses refers to the predicted difference in LDL cholesterol between the two groups examined. Since they are based on estimates the % change is only an approximate guide.
Reduction in total CHD burden was predicted to be substantial in men with the highest cholesterol levels (P5) who were subject to moderate (25%) or intensive (54%) LDL lowering (Table 2). Group P4 was moderately hypercholesterolaemic with a mean cholesterol similar to that in the placebo arm of WOSCOPS (i.e.7.0 mmol/L21) Comparing event rates in P4 with those for P3 (with its mean cholesterol of 6.0 mmol/L) it can be seen that a nominal 1.0 mmol/L difference was associated with a 19.4% lower risk of CHD, 10.2 fewer events, and 61.3 fewer days in hospital per 100 subjects. The analogous data from the placebo and pravastatin arms in the trial (where there was a 1.1 mmol/L difference in LDLc during the formal 5-year intervention) were a relative risk reduction of 21% over 20 years of follow-up (Table2 in Ref. 14) and a difference in cumulative CHD events of 10.8 per 100 subjects (for non-day case admissions; table 3 in Ref. 14) The compatibility, for approximately the same degree of cholesterol lowering, of the trial results with those from modelling screenees lends support to our general approach.
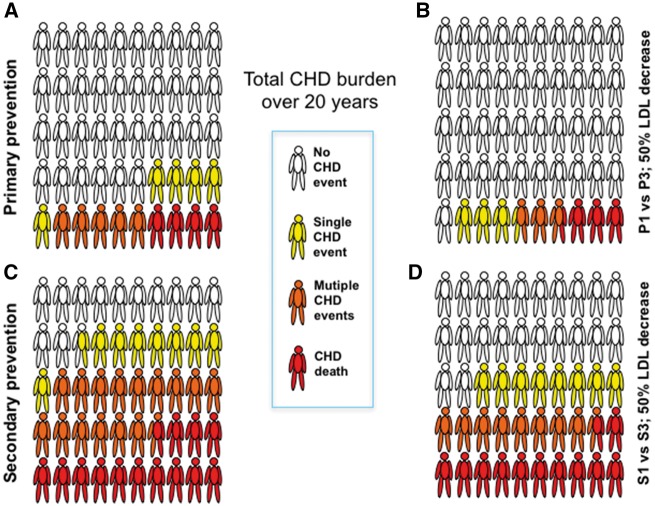
In other scenarios presented in Table 2, it can be seen that even at the population average total cholesterol (in group P3), over 20 years, achievable decreases in cholesterol of the order of 0.9–2.0 mmol/L were predicted to prevent respectively 8.9–19.4 events and 56.0–121.1 days in hospital per 100 subjects. The impact of having lower cholesterol expressed per subject rather than total number of CHD events is depicted schematically in Figure 2A and B. Here is can be seen that comparing groups P1 with P3 there was a notable reduction in the numbers experiencing single and multiple CHD events over the 20 years.
Figure 2.
CHD impact over 20 years for groups P1 vs. P3 and S1 vs. S3 expressed as percentages of subjects experiencing a fatal CHD event, multiple non-fatal CHD events or a single CHD event. These are depicted schematically per 50 subjects for the purposes of clarity. Note, if a subject has both a non-fatal and then a fatal event, he is counted for both in order to show the total CHD burden. Complete data are provided in Supplementary material online, Tables S1 and S2.
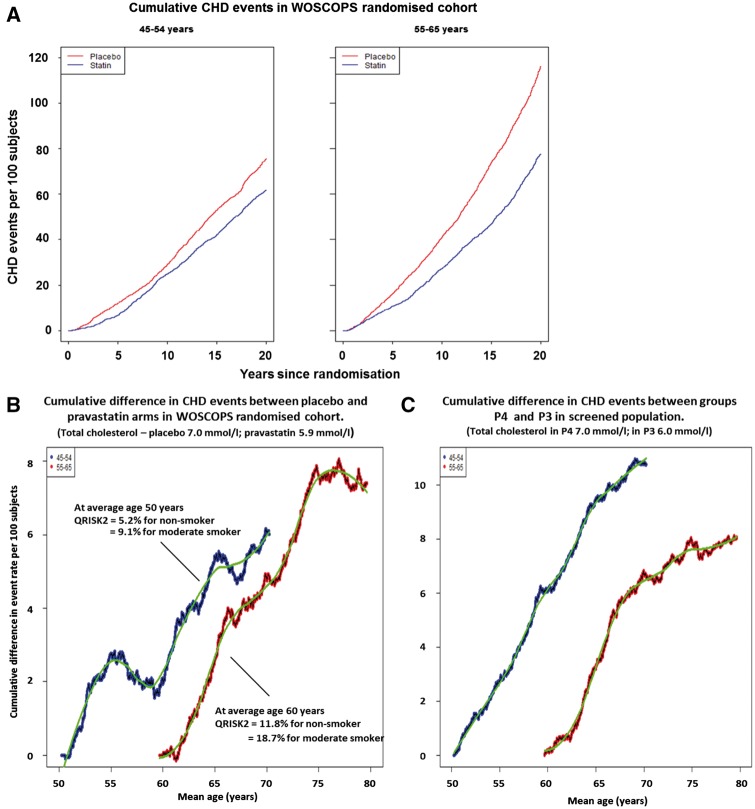
Figure 3A shows the cumulative incidence for CHD hospitalizations in 45 to 54-year-old and 55 to 64-year-old men within the WOSCOPS randomized cohort (n = 6544; 51 men with incomplete Visit 1 information were not included in this analysis), and Figure 3B the difference plot with time. It is clear from consideration of Figure 3B that the cumulative benefit of cholesterol lowering in primary prevention is greater if intervention is started in the first compared with the second age decade. The former group (average age 50 years) in the placebo arm of the trial experienced about 75 events per 100 subjects by the end of the 20-year observation period (Figure 3A). The observed accrued benefit at age 65 and 70 years respectively was a decrease of about 5.1 to 6.2 events per 100 subjects (Figure 3B). On the other hand, waiting until men were 55–64 years (average age 60 years) before intervention led to a lower accrued benefit of 2.7 events prevented per 100 subjects by age 65, and 4.7 events prevented by age 70.
Figure 3.
Cumulative events over 20 years for WOSCOPS randomized cohort divided by age decade and treatment allocation (A). Panel B is the difference plot (placebo minus pravastatin) for each age decade for cumulative events in the two treatment arms. (C) The difference plot by age decade for cumulative CHD events in groups P4 and P3 in Table 1. Groups P4 and P3 comprise men who were not included in the WOSCOPS randomized trial cohort but had the same cholesterol levels as the two treatment arms. In (B) 10-year risk estimates obtained using the QRISK-2 algorithm are provided for the average WOSCOPS trial participant for non-smokers and moderate smokers. WOSCOPS, West of Scotland Coronary Prevention Study.
The potential impact of starting cholesterol lowering earlier is even clearer if the screenee data are used to model the outcome for each age decade (Figure 3C). Cumulative differences between the P4 and P3 groups in Table 1 were determined, and for 45–54 year old men by age 70 years the number of events prevented by being at a 1.0 mmol/L lower total cholesterol was over 10 per 100 subjects, whereas for 55–64 year olds by age 70 the same difference in cholesterol saved only 6 events per 100 subjects.
Applying the QRISK2 calculation to the younger decade of the WOSCOPS randomized cohort (Figure 3B) gave a 10-year cardiovascular disease risk of 5.2–9.1% depending on smoking habit which does not meet the threshold of 10% required before drug treatment is recommended.5 Finally, it was reassuring again that estimated rates of accrued benefit per 100 subjects were similar in the modelled outcome to those seen in the trial cohort.
Benefit of cholesterol lowering in a ‘secondary prevention’ setting
The predicted benefit of lowering cholesterol in the screenees with a history of CHD (Table 1), as expected, exceeded that seen in the primary prevention setting. Men in group S3 with mean total cholesterol levels close to that seen in the whole screened population (5.9 mmol/L 16) had on average 140.6 CHD events per 100 over 20 years (Table 1) and utilized 931.7 hospital days for CHD causes. In this ‘secondary prevention’ setting it was estimated that 26.8 events per 100 men would be prevented by lowering cholesterol by approximately 1.0 mmol/L from the mean level, and about 63.2 events per 100 by reducing cholesterol by 2.0 mmol/L (Table 2). The impact of this magnitude of cholesterol reduction on a per subject basis is depicted in Figure 2C and D. This shows the substantial number of subjects experiencing CHD death and multiple CHD events and the predicted reduction when LDLc is 50% lower. The number of hospital days saved amounted to 158.2–405.5 per 100 subjects, respectively (Table 2). Even comparing the two groups with the lowest cholesterol levels (S1 and S2) a 1.1 mmol/L difference was associated potentially with 36.5 fewer events and a saving of 247.3 hospital days per 100 subjects.
Discussion
For many subjects the 20-year observation period was an estimate of ‘lifelong’ benefit since the mean age rose from 55 to 75 years and life expectancy for men in Scotland is 76 years (see Registrar General’s report at www.nrscotland.gov.uk; accessed 3 June 2017). Our main findings were that total CHD burden over this time was considerable and strongly related to plasma cholesterol levels both in men without (‘primary prevention’ setting) or with (‘secondary prevention’) a history of CHD at screening. Predicted benefits of intervention were large in that a decrease of around 1.0 mmol/L would lead 8.9 fewer CHD events per 100 subjects in primary prevention at starting total cholesterol levels of 6.0 mmol/L (close to the population average), and 26.8–36.5 fewer events per 100 in secondary prevention when the initial total cholesterol was 6.0 and 5.0 mmol/L, respectively. If the estimated LDLc was reduced by about 50% then the potential decrease in CHD events was 19.4–31.5 per 100 subjects in primary prevention and 63.2 CHD events per 100 subjects in secondary prevention (Table 2). The corresponding savings in hospital days were also substantial.
The need to take a long-term view in a disease with a decades-long trajectory is recognized increasingly. In the IMPROVE-IT10,24 and HOPE-3 studies11 LDL lowering decreased risk of a second or third cardiovascular event to a similar extent to that seen for the first event. Comparing findings in these trials with the total CHD burden modelled here it can be seen that in HOPE-3 the number of co-primary endpoints prevented by a 1.0 mmol/L decrease in a cohort with population average LDLc (3.31 mmol/L) was 1.1 per 100 subjects over 5.6 years (the study duration)11 whereas the cumulative events over 20 years predicted to be prevented in a similar group by an equivalent cholesterol reduction was 8.9 per 100 with a saving of 56 days in hospital per 100 subjects. Likewise, in IMPROVE-IT24 the difference in LDLc between the two treatment arms was 0.4 mmol/L and the number of primary endpoint events prevented was 1.87 per 100 subjects. The same cholesterol decrease (taken as a proportion of the difference between the levels seen in groups S2 and S1 in Table 2) would give 13.3 fewer CHD events and save 95.4 hospital days per 100 subjects over 20 years. With respect to European guidelines,2 patients at very high risk have a recommended LDLc goal of <1.8 mmol/L and a desired reduction of ≥50% if the starting LDLc is 1.8–3.5 mmol/L. The present study predicts that the long-term (lifelong) benefit of LDL lowering of this magnitude would be considerable as can be seen by comparing groups S3 and S1 for total events in Table 2, and on a per-subject basis in Figure 2.
Even with the demonstration that primary prevention subjects have a useful risk reduction19,25 in an era when the costs of intervention are modest, there is a perceived reluctance to begin therapy, possibly because the benefits are difficult to quantify in terms understood by patients and occur in the future.17–19 Presentation of the total CHD burden that an individual may experience, and the considerable advantages of treatment, may help deliver better strategies and compliance. Further, our findings in the two age decades add to conclusion from genetic studies26 that earlier intervention is better. Greater emphasis on benefit rather than risk (as exemplified here in the QRISK2 results in the younger decade) may help further refine implementation strategies. Often, adverse effects are cited as a reason not to use statins more widely in the population.17–19 This concern, including the increased risk of developing type 2 diabetes, has been addressed in meta-analyses of trials,19 and also in the previously published long-term follow-up of the WOSCOPS trial cohort.12,14
Advantages of the present study were the size of the screened population and the longitudinal nature of the data sets. Also, it was notable that the modelled scenarios for an approximate 1.0 mmol/L difference in cholesterol were associated with about a 20% lower relative risk, a value in line with the 22% seen in meta-analysis of outcome trials.3,4 Likewise, the approximately three-fold higher event rates seen in the ‘secondary’ vs. ‘primary’ groups were in accord with the relative rates for similar CHD endpoints in 4S (a trial in secondary prevention) and WOSCOPS, contemporaneous studies in subjects with similar high LDLc levels.21,27 Limitations were that events were not adjudicated, no women were included, the modelling approach estimated treatment benefit from a difference in cholesterol levels in an epidemiological survey, and we had no information on which screenees were prescribed lipid-lowering medication during follow-up. Arguably, since statins once they were introduced would be used mainly in those with higher cholesterol levels, this would have the effect of compressing observed differences in event rates between the groups, and so the data presented may be a conservative estimate of benefit. On the other hand, population rates of CHD in Scotland fell substantially over the period of follow-up, so the impact of lowering cholesterol today may be quantitatively less than the values quoted in the current study (although the majority of events reported for our subjects occurred in the latter 10 years of observation).
In conclusion, this study used electronic health records to create a disease trajectory for each subject and permitted the modelling of clinical benefit of an intervention based on long-term observational data. Our findings support the view that primary CHD prevention is more effective if initiated in early mid-adult life and that the benefits of secondary prevention, given the very high total burden of disease, are substantial.
Supplementary material
Supplementary material is available at European Heart Journal – Quality of Care and Clinical Outcomes online.
Authors’ contributions
All authors from the academic institution contributed to the study design, analysis plan, interpretation of the data, and preparation of the manuscript. Baishali Ambegaonkar and Philippe Brudi (employees of the funder) contributed to the conceptual design and analytical plan, but had no role in the conduct of the study or in the analysis of the data. The ‘stick-man’ schematic in Figure 2 is borrowed from a diagram generated originally by Prof. Ulrich Laufs.
Supplementary Material
Acknowledgements
I.F. and R.Y. had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
Merck, Sharp & Dohme, Kenilworth, New Jersey as part of an Investigator Initiated Programme. The funder was provided with a draft for comment but editorial control remained with Drs Packard and Ford.
We would also acknowledge the support from The Farr Institute @ Scotland. The Farr Institute @ Scotland is supported by a 10-funder consortium: Arthritis Research UK, the British Heart Foundation, Cancer Research UK, the Economic and Social Research Council, the Engineering and Physical Sciences Research Council, the Medical Research Council, the National Institute of Health Research, the National Institute for Social Care and Health Research (Welsh Assembly Government), the Chief Scientist Office (Scottish Government Health Directorates), the Wellcome Trust, (MRC Grant No: MR/K007017/1).
Conflict of interest: I.F., R.Y. and C.M. have no conflict of interest to disclose other than research funding for this project from Merck, Sharpe and Dohme, as declared above. C.P. has research grants from Roche and honoraria from Merck, Sharpe and Dohme, Pfizer, and Sanofi. B.A. and P.B. are employees of Merck, Sharpe and Dohme, Kenilworth, NJ, USA.
References
- 1. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF.. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation 2014;129(Suppl 2):S1–S45. [DOI] [PubMed] [Google Scholar]
- 2. Catapano AL, Graham I,D, Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Ž, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WM, Vlachopoulos C, Wood DA, Zamorano JL.. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias: the Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2999–3058.27567407 [Google Scholar]
- 3. Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R.. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, Braunwald E, Sabatine MS.. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA 2016;316:1289–1297. [DOI] [PubMed] [Google Scholar]
- 5. National Institute for Health and Care Excellence. Lipid Modification: Cardiovascular Risk Assessment and the Modification of Blood Lipids for the Primary and Secondary Prevention of Cardiovascular Disease. London, England: National Institute for Health and Care Excellence; 2014. [PubMed] [Google Scholar]
- 6. Bleakley C, Pumb R, Harbinson M, McVeigh GE.. A reappraisal of the safety and cost-effectiveness of statin therapy in primary prevention. Can J Cardiol 2015;31:1411–1414. [DOI] [PubMed] [Google Scholar]
- 7. Pandya A, Sy S, Cho S, Weinstein MC, Gaziano TA.. Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA 2015; 314:142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anderson KM, Odell PM, Wilson PW, Kannel WB.. Cardiovascular disease risk profiles. Am Heart J 1991;121:293–298. [DOI] [PubMed] [Google Scholar]
- 9. Soran H, Schofield JD, Durrington PN.. Cholesterol, not just cardiovascular risk, is important in deciding who should receive statin treatment. Eur Heart J 2015;36:2975–2983. [DOI] [PubMed] [Google Scholar]
- 10. Murphy SA, Cannon CP, Blazing MA, Giugliano RP, White JA, Lokhnygina Y, Reist C, Im K, Bohula EA, Isaza D, Lopez-Sendon J, Dellborg M, Kher U, Tershakovec AM, Braunwald E.. Reduction in total cardiovascular events with ezetimibe/simvastatin post-acute coronary syndrome: the IMPROVE-IT Trial. J Am Coll Cardiol 2016;67:353–361. [DOI] [PubMed] [Google Scholar]
- 11. Yusuf S, Lonn E, Pais P, Bosch J, López-Jaramillo P, Zhu J, Xavier D, Avezum A, Leiter LA, Piegas LS, Parkhomenko A, Keltai M, Keltai K, Sliwa K, Chazova I, Peters RJ, Held C, Yusoff K, Lewis BS, Jansky P, Khunti K, Toff WD, Reid CM, Varigos J, Accini JL, McKelvie R, Pogue J, Jung H, Liu L, Diaz R, Dans A, Dagenais G; HOPE-3 Investigators. Blood pressure and cholesterol lowering in persons without cardiovascular disease. N Engl J Med 2016;374:2032–2043. [DOI] [PubMed] [Google Scholar]
- 12. Ford I, Murray H, Packard CJ, Shepherd J, Macfarlane PW, Cobbe SM.. Long-term follow up of the West of Scotland Coronary Prevention Study. N Engl J Med 2007;357:1477–1486. [DOI] [PubMed] [Google Scholar]
- 13. Strandberg TE, Pyörälä K, Cook TJ, Wilhelmsen L, Faergeman O, Thorgeirsson G, Pedersen TR, Kjekshus J; 4S Group. Mortality and incidence of cancer during 10-year follow up of the Scandinavian Simvastatin Survival Study (4S). Lancet 2004;364:771–777. [DOI] [PubMed] [Google Scholar]
- 14. Ford I, Murray H, McCowan C, Packard CJ.. Long-term follow-up of the West of Scotland Coronary Prevention Study. Circulation 2016;133:1073–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Packard C, Ford I.. Long term follow up of lipid lowering trials. Curr Opin Lipidol 2015;26:572–579. [DOI] [PubMed] [Google Scholar]
- 16. The WOSCOPS Study Group. Screening experience and baseline characteristics in the West of Scotland Coronary Prevention Study. Am J Cardiol 1995;76:485–491. [DOI] [PubMed] [Google Scholar]
- 17. D'Agostino RB. The guidelines battle on starting statins. New Engl J Med 2014;370:1652–1658. [DOI] [PubMed] [Google Scholar]
- 18. Redberg RF, Katz MH.. Statins for primary prevention: the debate is intense but the data are weak. JAMA 2016;316:1979–1981. [DOI] [PubMed] [Google Scholar]
- 19. Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, Blumenthal R, Danesh J, Smith GD, DeMets D, Evans S, Law M, MacMahon S, Martin S, Neal B, Poulter N, Preiss D, Ridker P, Roberts I, Rodgers A, Sandercock P, Schulz K, Sever P, Simes J, Smeeth L, Wald N, Yusuf S, Peto R.. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016;doi:10.1016/S0140-6736(16)31357-5. [DOI] [PubMed] [Google Scholar]
- 20. West of Scotland Coronary Prevention Study Group. A coronary primary prevention study of Scottish men aged 45-64 years: trial design. J Clin Epidemiol 1992;45:849–860. [DOI] [PubMed] [Google Scholar]
- 21. Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, Macfarlane PW, McKillop JH, Packard CJ.. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med 1995;333:1301–1307. [DOI] [PubMed] [Google Scholar]
- 22. The West of Scotland Coronary Prevention Study Group. Computerised record linkage: comparison with traditional patient follow-up methods in clinical trials and illustrated in a prospective epidemiological study. J Clin Epidemiol 1995;48:1441–1452. [DOI] [PubMed] [Google Scholar]
- 23. Hippisley-Cox J, Coupland C, Robson J, Brindle P.. Derivation, validation, and evaluation of a new QRISK model to estimate lifetime risk of cardiovascular disease: cohort study using QResearch database. BMJ 2010;341:c6624. doi:10.1136/bmj.c6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Eng J Med 2015;372:2387–2397. [DOI] [PubMed] [Google Scholar]
- 25. Cholesterol Treatment Trialists Collaborators. The effects of lowering LDL cholesterol in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 2012;380:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ference BA. Mendelian randomization studies: using naturally randomized genetic data to fill evidence gaps. Curr Opin Lipidol 2015;26:566–571. [DOI] [PubMed] [Google Scholar]
- 27. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study. Lancet 1994;344:1883–1889. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.