Abstract
Background
Limited treatment options exist for patients with locoregional recurrences of head and neck squamous cell carcinoma (HNSCC). In the palliative setting, a single session, minimally invasive, and relatively safe therapy is desirable. This case series illustrates the feasibility of a direct intratumoral injection of radioactive holmium-166 microspheres (166HoMS) in patients as a palliative treatment for recurrent HNSCC.
Patients and methods
In this retrospective analysis, patients with already reirradiated irresectable recurrent HNSCC, for whom palliative chemotherapy was unsuccessful or impossible, were offered microbrachytherapy with 166HoMS. The intratumoral injection was administered manually under ultrasound guidance. Parameters scored were technical feasibility (i.e. administration, leakage, and distribution), clinical response (response evaluation criteria in solid tumors 1.1), and complications (Common Terminology Criteria for Adverse Events 4.3).
Results
From 2015 to 2017, three patients were treated. None of the patients experienced adverse events; however, therapeutic effects were minimal. Technical difficulties, including precipitating of microspheres and high intratumoral pressure, resulted in suboptimal distribution of the microspheres.
Conclusion
Intratumoral injections with 166HoMS are minimally invasive and relatively safe in palliation of HNSCC patients. Careful patient selection and improved administration techniques are required to provide a more effective treatment. Further investigation of this novel treatment modality should be carried out because of the absence of side effects and lack of other treatment options.
Keywords: brachytherapy, head and neck squamous cell carcinoma, holmium-166, intratumoral treatment, palliation
Introduction
Cancer of the head and neck accounts for ∼685 000 (4.9%) of all new cancer cases worldwide and over 375 000 deaths annually. The survival of patients with head and neck squamous cell carcinoma (HNSCC) mainly depends on the stage of disease at the time of diagnosis. For advanced (stage III–IV) carcinomas, the survival rate decreases to ∼35%. Over the last decades, only limited improvements in survival were achieved, which urges the need for new treatment modalities. Especially for patients with locoregional recurrences, limited options exist 1–3.
For previously irradiated, irresectable regional recurrences, only two options for palliative treatment remain: palliative reirradiation or chemotherapy. In most cases with recurrent HNSCC, palliative reirradiation can be administered only once. This reirradiation usually consists of accelerated hyper-fractionated schemes of 1.5 Gy twice daily, up to 60 Gy depending on the dose previously administered 4,5. With new developments such as intensity-modulated radiotherapy and stereotactic body radiation therapy, new schemes of 4–6 fractions of 6 Gy reduce treatment duration in patients with a limited life expectancy 6. Palliative chemotherapy may provide a meaningful response in some patients. However, many patients are medically unfit for a platinum compound with capecitabine/fluorouracil and/or cetuximab 7. There are currently some developments with monoclonal antibodies and tyrosine kinase inhibitors such as PI3K/AKT/mTOR that may prove beneficial in the near future 8. Furthermore, brachytherapy with a high or a pulsed dose rate 9 and bleomycin-electroporation therapy 10,11 are being studied in salvage patients, but these treatment modalities require a good performance status. As a result, only best supportive care remains for patients with severe comorbidities or a poor performance status.
Radioactive holmium-166 microspheres (166HoMS) and currently used in intra-arterial radioembolization of liver malignancies 12. These microspheres emit β radiation with a maximum penetration depth of 8.7 mm and are also used off-label in veterinary patients with unresectable oral squamous cell carcinomas and other tumors 13,14. Intratumoral injections of 166HoMs in veterinary patients showed a relevant response without severe morbidity 13. This article presents the first experience on the feasibility and safety of intratumoral injections of 166HoMS in human patients with recurrent cancer in the head and neck.
Patients and methods
Patient selection
In this retrospective analysis, patients with confirmed local or regional recurrence of HNSCC with or without distant metastasis, as evidenced by recent imaging, were discussed in the multidisciplinary head and neck oncology team for regular treatment options and/or ongoing trials. If no palliative treatment options were available, and nonetheless a strong wish for treatment existed, patients were amenable for direct intratumoral injections of 166HoMS, with the aim of improving the patients’ quality of life. Only tumors accessible for ultrasound (US)-guided manual injections with at least more than 5 mm distance to vital anatomical structures, such as the common carotid artery, were selected. Immediately after the injection procedure, a planar scintigraphy of the thorax and abdomen, as well as a single-photon emission computed tomography (SPECT)/computed tomography (CT) of the head and neck region were performed. Patients provided informed consent after receiving detailed information. The Ethical Committee of the Universty Medical Center Utrecht approved the study.
Holmium-166 microspheres preparation
166HoMS of 30 µm diameter were prepared in the Universty Medical Center Utrecht as described previously 15,16 and CE-approved (QuiremSpheres; Quirem Medical B.V., Deventer, The Netherlands). Briefly, nonradioactive 165Ho complexed with acetylacetonate is incorporated into poly(l-lactic acid) by solvent evaporation to form microspheres. Subsequently, the nonradioactive 165HoMS were made radioactive by neutron activation in a nuclear facility (RID Reactor, Delft University of Technology, Delft, The Netherlands) to form 166HoMS. After neutron activation, 166HoMS were suspended in a PBS with 2% weight per volume of polyoxyethylene–polyoxypropylene copolymer (Pluronic F-68; Sigma-Aldrich Chemie B.V., Zwijndrecht, The Netherlands). The 166HoMS were suspended for 10 min on a vortex, followed by repeatedly drawing up and down with a syringe.
The amount of radioactivity present in each syringe was measured in a dose calibrator (VDC-404; Veenstra Instruments B.V., Joure, The Netherlands). Each syringe was placed in an acrylic glass cylinder to limit β-radiation exposure of personnel, especially to the hands, during dose preparation and administration. The treatment was performed with a quantity of 100–250 mg of 166HoMS, divided over 2–10 syringes, with a volume of 0.2–0.5 ml. The required 166Ho activity was determined on the basis of tumor volume and aimed tumor-absorbed dose according to the following equation: D=A×15.87/W, where D is the tumor-absorbed dose [in Gy (J/kg)]; A is the 166Ho activity (MBq); 166Ho-specific tissue dose conversion coefficient=15.87 mJ/MBq; and W is the tumor weight (g). Assumed tumor tissue density was 1.0 g/cm3 and β radiation was assumed to absorb completely in the treated tissue 17. The required activity was obtained by varying the neutron activation time of the microspheres.
Treatment
Before injection, the 166HoMS were resuspended in the syringe by gentle agitation. The injections were administered manually under US guidance to prevent accidental intravenous administration. On the basis of the veterinary experience and review of the available literature, the following assumptions were made. The average injection percentage of 166HoMS in the veterinary experience was ∼50%; therefore, the prepared activity was doubled. The injection volume ranged from ∼7 to 25% of the tumor volume. Per injection, a 1 cm3 distribution of microspheres was expected. A 1 ml syringe was used for optimal control during the injection of small volumes with a ‘luerlock’ tip to reduce the risk of needle dislodgement. 21 G×2′ and 23 G×11/4′ needles were used depending on the tumor location. The aimed absorbed dose was 70–100 Gy. Immediately after treatment, a SPECT/CT was performed. Follow-up was performed at 1, 2, and at 4 weeks combined with a PET/CT. The following parameters were scored: technical feasibility (i.e. administration, leakage, distribution), response according to the response evaluation criteria in solid tumors (RECIST 1.1) 18, and complications according to the Common Terminology Criteria for Adverse Events (CTCAE 4.3) 19.
Case series
Three consecutive patients with a locoregional recurrence of HNSCC, who presented to the University Medical Center Utrecht between November 2015 and June 2017, were treated with intratumoral 166HoMS (Tables 1 and 2).
Table 1.
Patient characteristics
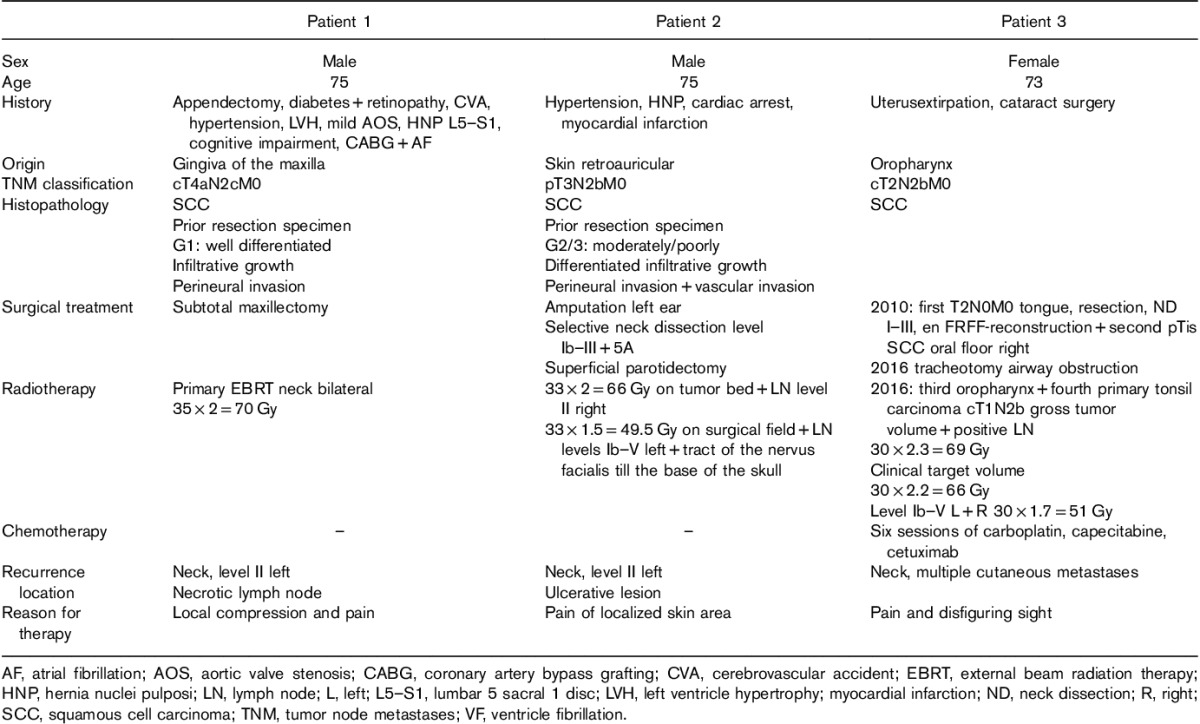
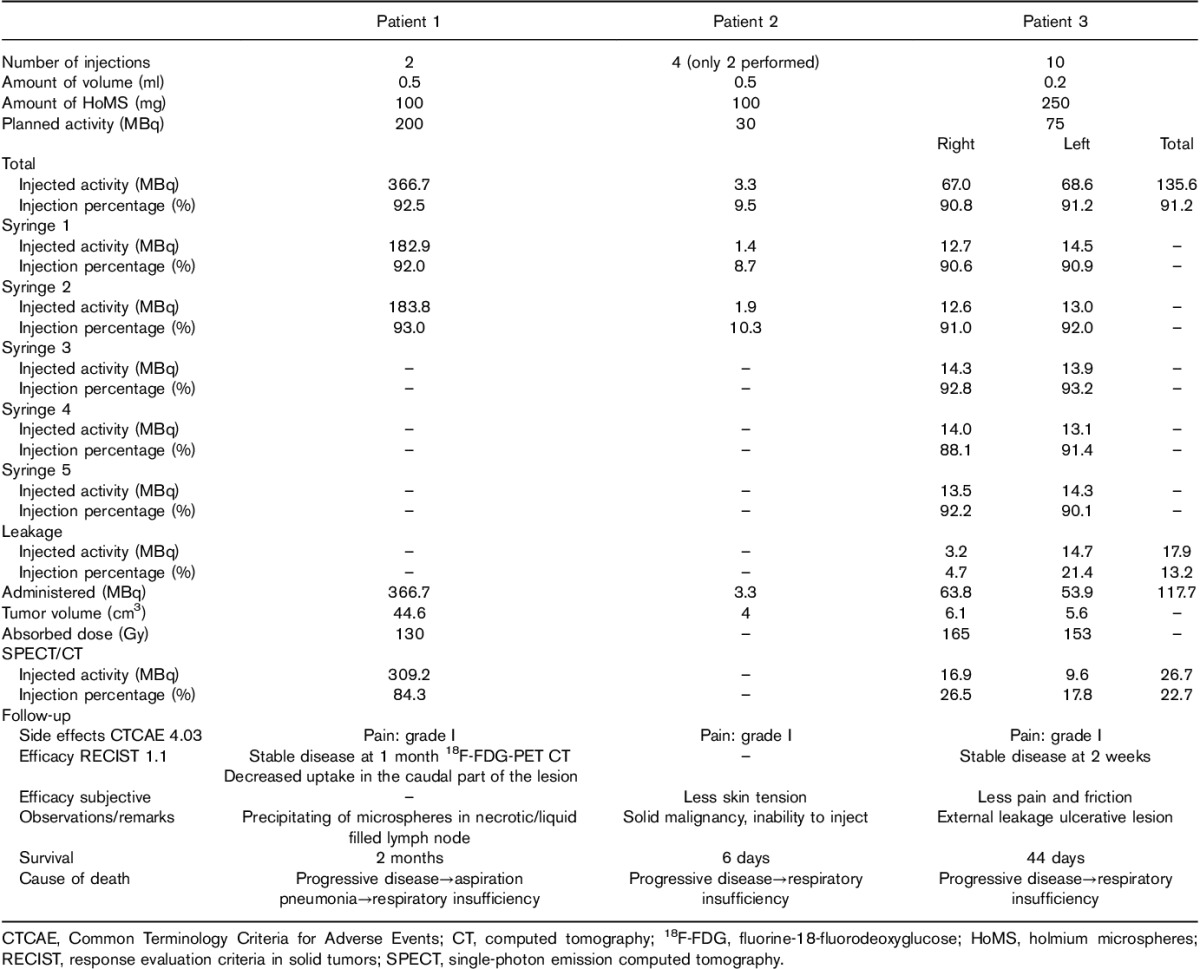
Table 2.
Treatment characteristics
Patient 1
A 75-year-old man with a history of diabetes, hypertension, a cerebrovascular ischemic accident, coronary artery bypass grafting, and atrial fibrillation was diagnosed with a squamous cell carcinoma of the maxillary gum, staged cT4aN2cM0. A partial maxillectomy was performed, with primary bilateral radiotherapy (70 Gy) of the neck because of his poor general condition. After 1 year, the patient developed a bilateral regional recurrence. US and CT indicated neck nodes with a diameter of 27 and 44 mm on the right and left side, respectively. Because of his poor medical condition, the patient was not amendable for salvage surgery or chemotherapy. The patient refused palliative reirradiation. In consultation with the patient, the largest left sided neck node was treated because of complaints of local compression and pain.
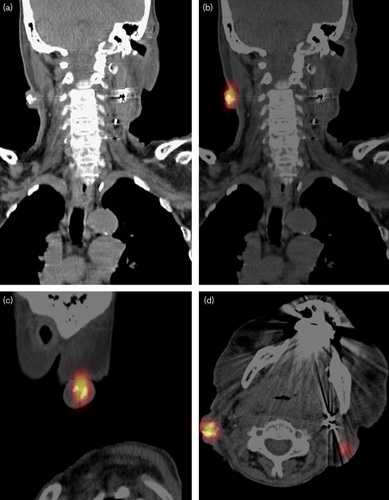
The treatment plan was to inject 200 MBq in 100 mg of 166HoMS divided over two syringes with a volume of 0.5 ml and a 21 G×2′ needle, which would result in an average absorbed dose of 70 Gy. Syringes were filled with 396.3 MBq and 204.1 mg of 166HoMS to correct for the expected injection percentage of 50%. The injection procedure was not painful (maximum grade 1) and particle-reflections could be observed on US during administration (Fig. 1). In this patient, 366.8 MBq (92.5%) of the prescribed activity could be injected, equivalent to an absorbed dose of 130 Gy. SPECT/CT imaging showed some precipitation of the microspheres, especially in the dorsocaudal part of the tumor. Estimated activity on SPECT/CT was 309.2 MBq or 84.3% of the injected activity (Fig. 2).
Fig. 1.
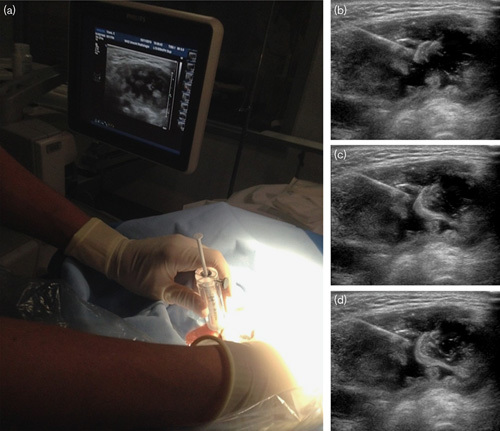
(a) Setup of the ultrasound-guided injection with the syringe with holmium-166 microspheres shielded with acrylic glass. (b–d) Ultrasound images of an injection in a large necrotic fluid-filled lymph node metastasis, clearly visible flow of microspheres inside the tumor.
Fig. 2.
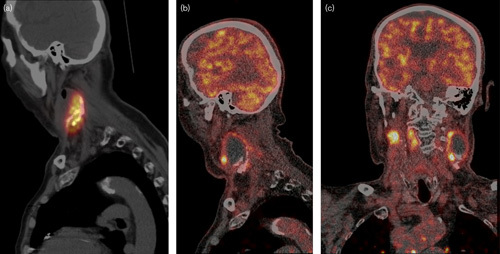
Patient 1 with a large necrotic lymph node metastasis with clearly visible precipitation of the holmium-166 microspheres. (a) Single-photon emission computed tomography immediately after injection in the supine position. (b, c) Sagittal and coronal slices of fluorine-18-fluorodeoxyglucose PET 1 month after injection.
The patient was admitted for observation of unexpected side effects before discharge 24 h after treatment. Clinical follow-up was performed at 1 and 2 weeks and did not indicate any toxicity. His complaints of tension in his neck diminished. At 4 weeks, an fluorine-18-fluorodeoxyglucose PET was performed to evaluate the response. This showed a 37% volume increase of the nontreated right lesion and a 30% volume increase of the treated left lesion. The treated lesion showed a lower maximum standardized uptake value on the fluorine-18-fluorodeoxyglucose PET/CT of 8.7 compared with 9.5 of the nontreated lesion, respectively. Especially, the uptake of the dorsal and caudal tumor parts was lower. Two months after 166HoMS treatment, the patient died of respiratory insufficiency caused by aspiration pneumonia and progressive disease.
Patient 2
A 75-year-old man with a history of hypertension, myocardial infarction, and an out of hospital cardiac arrest was referred with an irradical excision of a left-sided retroauricular cutaneous squamous cell carcinoma. At the initial consultation, a 3×3 cm skin defect with nodules in the parotid gland was found. After surgical resection, which included a left ear amputation, selective neck dissection, and a superficial parotidectomy, the tumor was staged as pT3N2b with extracapsular extension. Subsequently, the patient received 49.5 Gy on the entire surgical field and neck with a simultaneous integrated boost technique: 66 Gy on the tumor bed and lymph nodes in level II. After 8 months, the patient presented with a regional recurrence in the left neck with carotid encasement up to the base of the skull, involvement of the vagal nerve, and tumor infiltration in the skin.
At clinical examination, a massive tumor, infiltrating and ulcerating the skin, was observed. The patient’s main complaint was localized pain of the skin just below the mandible, poorly responding to oral morphine. In consultation with the patient, it was decided, despite the poor prognosis, to treat only this superficial area of the recurrence, avoiding the risk of traumatizing the carotid artery, and with the aim to palliate symptoms.
Four syringes with an activity of 13.5–16.5 MBq in 0.3 ml were prepared, which would result in an absorbed dose of 100 Gy for 4 cm3. However, during the first two injections, an unexplained obstruction of the syringe occurred almost immediately. As a result, the injection procedure was terminated. Post-treatment measurements showed that only 3.3 MBq (9.5%) of the 166HoMS was injected. The injection procedure was minimally uncomfortable (i.e. pain: maximum grade 1). Unfortunately, the patient was unable to undergo the SPECT/CT imaging because of pre-existing dyspnea. The patient was discharged, but readmitted 2 days later, because of progressive dyspnea. No adverse effects of the 166HoMS injections were noted and a subjective decline in skin tension and pain was experienced, but no objective response was observed. The clinical condition declined rapidly, and the patient died of respiratory insufficiency 6 days after the treatment.
Patient 3
A 73-year-old woman with a history of alcohol and tobacco abuse was referred with a cT2N0M0 tongue carcinoma. A partial glossectomy, resection of the oral floor, a level I–III neck dissection, and reconstruction with a free radial forearm flap were performed. Simultaneously, a synchronous primary pTis of the floor of the mouth was resected. Six years later, she presented with a third primary T2N2bM0 oropharyngeal carcinoma with a synchronous primary contralateral cT1N2b tonsillar fossa carcinoma. Both tumors were treated with radiotherapy: 69 Gy on both gross tumor volumes and the positive lymph nodes. Level Ib–V left and right received 51 Gy. After 6 months, cutaneous metastases developed on both sides of the neck, causing dyspnea. Additional staging indicated multiple pulmonary nodules suspicious for metastatic disease. The patient received a tracheostomy and palliative carboplatin/capecitabine/cetuximab weekly, which showed a substantial response initially. However, after six cycles of systemic therapy, growth of the cutaneous metastases progressed. The patient’s complaints were friction, pain, and the disfiguring sight of the cutaneous metastases.
It was planned to treat multiple nodules; however, in the 3 weeks between consent and treatment, the metastases progressed rapidly (Fig. 4). Therefore, only two large lateral lesions were treated with five injections of 0.2 ml, with an average of 25 mg and 15 MBq 166HoMS, respectively. Of the total prescribed activity of 148.7 MBq, 91.2% was injected. Because of the superficial lesions, some backflow of microspheres was observed with needle retraction. In addition, some leakage of 166HoMS was observed during the injection of the ulcerative tumor on the left side. The cumulative leakage was 3.2 MBq (4.5%) on the right side and 14.7 MBq (21.5%) on the left side, resulting in a total administered activity of 117.7 MBq (79.2%) (Fig. 3).
Fig. 4.
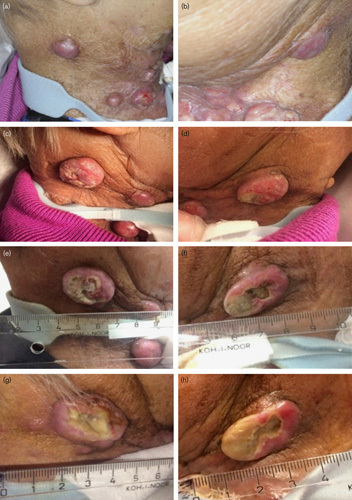
Patient 3, (a, b) tumor 3 weeks before treatment, (c, d) tumor 2 days after treatment, (e, f) tumor 8 days after treatment, (g, h) tumor 15 days after treatment.
Fig. 3.
Patient 3, (a) clearly visible accumulations of holmium-166 microspheres as white dots on computed tomography. (b–d) Single-photon emission computed tomography reconstructions in coronal, sagittal, and axial directions.
After the procedure, the patient was discharged. No discomfort was experienced during or after the treatment. The progression of her complaints of friction and the disfiguring sight was halted. Tumor inspection after 1 week showed marked central necrosis of both injected tumors. This remained visible at 2 weeks (Fig. 4). This was interpreted as treatment effect. The right lesion measured 28×24 mm before treatment and 30×23 mm after 2 weeks, and the left lesion 24×15 and 25×14 mm, respectively. The tumor edge seemed still partially vital 2 weeks after treatment. Progressive dyspnea and growth of nontarget lesions resulted in severe discomfort. The patient asked for and received euthanasia 6 weeks later.
Discussion
In this case series, the first experience with a direct intratumoral free-hand injection of 166HoMS in patients with recurrent HNSCC for whom no other palliative retreatment options were available was described. The preparation of syringes with the desired activity and amount of 166HoMS was accurate, and the resuspension before injection consisted of some gentle agitation of the syringe. The injection procedure went smoothly in patients 1 and 3, with some leakage in superficial and ulcerative lesions. This leakage was easily absorbed in a compress and did not result in contamination of personnel or equipment.
In the palliative setting, a single-session, minimally invasive, and relatively safe treatment is desirable. Intratumoral injections with 166HoMS are minimally invasive and seem to be safe. Only slight discomfort during needle insertion was experienced. No discomfort related to radiation was experienced during follow-up. In addition, no systemic side effects were observed, or expected, as post-treatment imaging did not show activity outside the tumor.
There were some subjective improvements in tension and pain in the tumor region of all patients; however, objective efficacy (i.e. reduction in the size of the targeted metastasis) was not observed. The tissue penetration of the β radiation of 166Ho is limited and 90% of the dose is absorbed in the first 3 mm 20. Subsequently, the efficacy is probably strongly related to an appropriate dose distribution. The microspheres distribution therefore needs to be homogeneous for antitumor efficacy and the absorbed radiation dose should sufficiently cover all areas of vital and proliferating tumor tissue. In contrast, however, post-treatment SPECT/CT imaging showed a nonhomogenous distribution of 166HoMS after the injections (Fig. 2). This could explain tumor progression in patient 1, albeit decreased metabolic activity on PET/CT dorsocaudally in the tumor, consistent with the area of higher microsphere density on SPECT/CT. In patient 3, the absorbed dose was high enough and the distribution seemed sufficient for inhibition of tumor growth, although size reduction of targeted metastases was not observed.
An accurate selection of patients for 166HoMS treatment seems important. Treatment of a large, cyst-like lymph node metastasis with central necrosis resulted in precipitation of microspheres inside the tumor of patient 1. In patient 2, the injection of 166HoMS proved difficult because of obstruction of the syringes. It was initially hypothesized that precipitation and agglomeration of 166HoMS could have resulted in obstruction of (smaller) needles. However, in patient 2 also the larger 21 G needles seemed obstructed/occluded. With the multisizer (Multisizer 3; Beckman Coulter Life Sciences, Indianapolis, Indiana, USA) (data not shown) and under a microscope, no agglomerations of 166HoMS were detected in the suspension. Therefore, difficulty in injecting the 166HoMS suspension was probably caused by a high intratumoral pressure in the firm consistency of the tumor in patient 2. As an increased pressure force can result in needle dislodgement, the injection position was sometimes changed in case of high resistance in the veterinary experience 13. In patient 3, with soft cutaneous metastases without large ulceration, the injection procedure was feasible. However, in the case of a skin ulcer, some precaution is necessary to prevent a potential spill of activity through the skin defect.
On the basis of the current experience, the absence of significant side effects, and lack of other treatment options, an intratumoral injection with 166HoMS deserves further investigation. Therefore, the following considerations are suggested to improve the efficacy of future 166HoMS treatments. Patients with relatively small, soft, and superficial tumors seem more amenable for 166HoMS treatment in comparison with patients with large necrotic or indurated tumors. In addition, the focus should be directed toward the vital tumor edge, and peritumoral injections of the tumor bed should be considered. Furthermore, the main advantage of 166Ho over other high-energy β-emitting radionuclides for local treatment is its visibility on SPECT, CT, and MRI. This should be used for imaging of the 166HoMS distribution and dosimetry 21–23. The distribution of 166HoMS must be a priority in future studies, and the effect of injection volume, the amount of microspheres, and injection locations should be investigated. When image-guided feedback is used, the dose distribution can be improved and treatment efficacy will likely improve. In addition, the aid of robotic administration systems 24 could allow for quicker and more accurate needle placement.
Conclusion
Intratumoral injections with 166HoMS seem to be feasible as a single-session, minimally invasive, and relatively safe treatment in the palliative setting for heavily comorbid HNSCC patients. Improving patient selection, administration techniques, and use of real-time high-resolution imaging is necessary to optimize the dose distribution. Considering the suggested improvements and the absence of side effects, this palliative microbrachy treatment may be of additional value in a specific group of HNSCC patients.
Acknowledgements
R.C. Bakker is funded by the Dutch Cancer Society research grant: 2014-7075.
Conflicts of interest
J.F.W. Nijsen is co-founder and scientific director of Quirem Medical B.V., and has a minority share in the company Quirem Medical B.V. Furthermore, Nijsen is inventor on the patents related to the 166Ho-PLLA-microspheres, which are assigned to University Medical Center Utrecht Holding B.V. (patent numbers: O2012060707 A1 and US 2005/0201940 A1). The Department of Radiology and Nuclear Medicine of the Universty Medical Center Utrecht receives royalties from Quirem Medical B.V. M.G.E.H. Lam is consultant for Sirtex, BTG, Mirada and Bayer Healthcare. For the remaining authors there are no conflicts of interest.
References
- 1.Bourhis J, Le Maître A, Baujat B, Audry H, Pignon JP. Individual patients’ data meta-analyses in head and neck cancer. Curr Opin Oncol 2007; 19:188–194. [DOI] [PubMed] [Google Scholar]
- 2.Brockstein B, Haraf DJ, Rademaker AW, Kies MS, Stenson KM, Rosen F, et al. Patterns of failure, prognostic factors and survival in locoregionally advanced head and neck cancer treated with concomitant chemoradiotherapy: a 9-year, 337-patient, multi-institutional experience. Ann Oncol 2004; 15:1179–1186. [DOI] [PubMed] [Google Scholar]
- 3.Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med 2007; 357:1705–1715. [DOI] [PubMed] [Google Scholar]
- 4.Spencer S, Harris J, Wheeler R, Machtay M, Schultz C, Spanos WM, et al. Final report of RTOG 9610, a multi-institutional trial of reirradiation and chemotherapy for unresectable recurrent squamous cell carcinoma of the head and neck. Head Neck 2008; 30:281. [DOI] [PubMed] [Google Scholar]
- 5.Tortochaux J, Tao Y, Tournay E, Lapeyre M, Lesaunier F, Bardet E, et al. Randomized phase III trial (GORTEC 98-03) comparing re-irradiation plus chemotherapy versus methotrexate in patients with recurrent or a second primary head and neck squamous cell carcinoma, treated with a palliative intent. Radiother Oncol 2011; 100:70–75. [DOI] [PubMed] [Google Scholar]
- 6.Rwigema JC, Heron DE, Ferris RL, Gibson MK, Quinn A, Yang Y, et al. Fractionated stereotactic body radiation therapy in the treatment of previously-irradiated recurrent head and neck carcinoma: updated report of the University of Pittsburgh experience. Am J Clin Oncol 2010; 33:286–293. [DOI] [PubMed] [Google Scholar]
- 7.Colevas AD. Chemotherapy options for patients with metastatic or recurrent squamous cell carcinoma of the head and neck. J Clin Oncol 2006; 24:2644–2652. [DOI] [PubMed] [Google Scholar]
- 8.Sacco AG, Cohen EE. Current treatment options for recurrent or metastatic head and neck squamous cell carcinoma. J Clin Oncol 2015; 33:3305–3315. [DOI] [PubMed] [Google Scholar]
- 9.Bartochowska A, Skowronek J, Wierzbicka M, Leszczynska M, Szyfter W. Is there a place for brachytherapy in the salvage treatment of cervical lymph node metastases of head and neck cancers? Brachytherapy 2015; 14:933–938. [DOI] [PubMed] [Google Scholar]
- 10.Bertino G, Sersa G, De Terlizzi F, Occhini A, Plaschke CC, Groselj A, et al. European Research on Electrochemotherapy in Head and Neck Cancer (EURECA) project: results of the treatment of skin cancer. Eur J Cancer 2016; 63:41–52. [DOI] [PubMed] [Google Scholar]
- 11.Tijink BM, De Bree R, van Dongen GA, Leemans CR. How we do it: chemo-electroporation in the head and neck for otherwise untreatable patients. Clin Otolaryngol 2006; 31:447–451. [DOI] [PubMed] [Google Scholar]
- 12.Smits ML, Nijsen JF, van den Bosch MA, Lam MG, Vente MA, Mali WP, et al. Holmium-166 radioembolisation in patients with unresectable, chemorefractory liver metastases (HEPAR trial): a phase 1, dose-escalation study. Lancet Oncol 2012; 13:1025–1034. [DOI] [PubMed] [Google Scholar]
- 13.Van Nimwegen SA, Bakker RC, Kirpensteijn J, van Es RJJ, Koole R, Lam MGEH, et al. Intratumoral injection of radioactive holmium ( 166 Ho) microspheres for treatment of oral squamous cell carcinoma in cats. Vet Comp Oncol 2017; 0:1–11. [DOI] [PubMed] [Google Scholar]
- 14.Van Es RJ, Nijsen JF, van het Schip AD, Dullens HF, Slootweg PJ, Koole R. Intra-arterial embolization of head-and-neck cancer with radioactive holmium-166 poly(l-lactic acid) microspheres: an experimental study in rabbits. Int J Oral Maxillofac Surg 2001; 30:407–413. [DOI] [PubMed] [Google Scholar]
- 15.Zielhuis SW, Nijsen JF, De Roos R, Krijger GC, van Rijk PP, Hennink WE, van Het Schip AD. Production of GMP-grade radioactive holmium loaded poly(l-lactic acid) microspheres for clinical application. Int J Pharm 2006; 311:69–74. [DOI] [PubMed] [Google Scholar]
- 16.Bult W, de Leeuw H, Steinebach OM, van Der Bom MJ, Wolterbeek HT, Heeren RMA, et al. Radioactive holmium acetylacetonate microspheres for interstitial microbrachytherapy: an in vitro and in vivo stability study. Pharm Res 2012; 29:827–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vente MA, Nijsen JF, de Wit TC, Seppenwoolde JH, Krijger GC, Seevinck PR, et al. Clinical effects of transcatheter hepatic arterial embolization with holmium-166 poly(l-lactic acid) microspheres in healthy pigs. Eur J Nucl Med Mol Imaging 2008; 35:1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45:228–247. [DOI] [PubMed] [Google Scholar]
- 19.National Cancer Institute. Common Terminology Criteria for Adverse Events v4.0 NCI, NIH, DHHS. 29 May 2009 NIH publication #09-7473.
- 20.Nijsen JF, Krijger GC, van Het Schip AD. The bright future of radionuclides for cancer therapy. Anticancer Agents Med Chem 2007; 7:271–290. [DOI] [PubMed] [Google Scholar]
- 21.Van De Maat GH, Seevinck PR, Elschot M, Smits ML, de Leeuw H, van het Schip AD, et al. MRI-based biodistribution assessment of holmium-166 poly(l-lactic acid) microspheres after radioembolisation. Eur Radiol 2013; 23:827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bult W, Kroeze SG, Elschot M, Seevinck PR, Beekman FJ, de Jong HW, et al. Intratumoral administration of holmium-166 acetylacetonate microspheres: antitumor efficacy and feasibility of multimodality imaging in renal cancer. PLoS One 2013; 8:e52178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smits ML, Elschot M, van den Bosch MA, van de Maat GH, van het Schip AD, Zonnenberg BA, et al. In vivo dosimetry based on SPECT and MR imaging of 166Ho-microspheres for treatment of liver malignancies. J Nucl Med 2013; 54:2093–2100. [DOI] [PubMed] [Google Scholar]
- 24.Arnolli MM, Hanumara NC, Franken M, Brouwer DM, Broeders IAMJ. An overview of systems for CT- and MRI-guided percutaneous needle placement in the thorax and abdomen. Int J Med Robot 2015; 11:458–475. [DOI] [PubMed] [Google Scholar]


