Abstract
Vascular dementia (VaD) is one of the most common forms of dementia, and second only to Alzheimer's disease. The purpose of this study was to evaluate the potential diagnostic value of Framingham risk score (FRS) in VaD by investigating the relationship among cardiovascular risks, FRS, and VaD.
Data were collected from patients (n = 130) at Tongji Hospital in Wuhan, China. They were divided into 2 groups, including the control group (n = 70) and the VaD group (n = 60). Statistical methods including t-test, logistic regression model, multiple linear regression model, and receiver-operating characteristic (ROC) curve were adopted for the assessment.
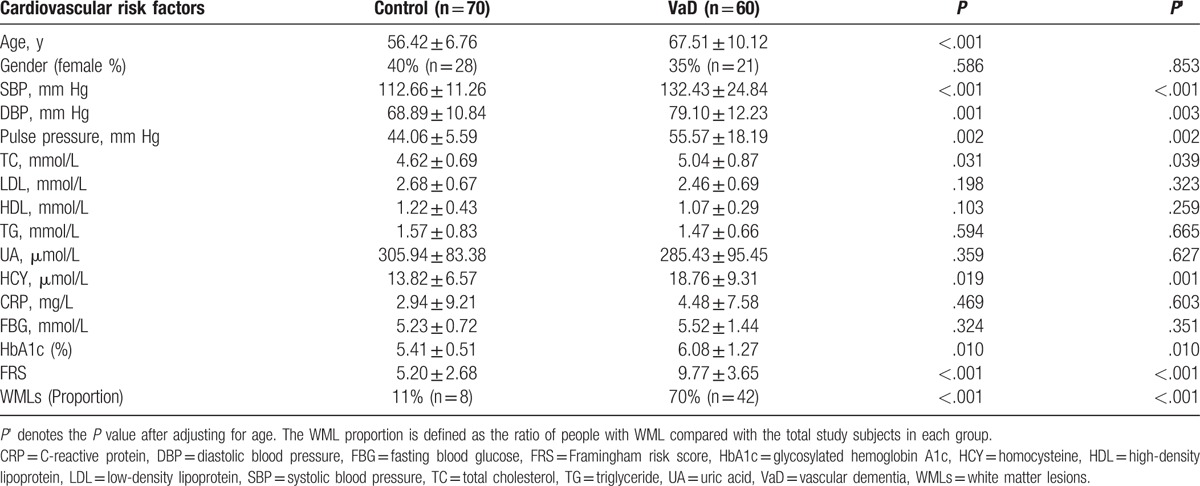
A significant difference (all P < .05) was observed in systolic blood pressure (SBP), diastolic blood pressure (DBP), pulse pressure, total cholesterol (TC), homosysteine (HCY), glycosylated hemoglobin A1c (HbA1c), FRS, and cerebral white matter lesions (WMLs) between the 2 groups, even after adjusting for age (both P < .05). Age [odds ratio (OR) = 1.20; P = .002], FRS (OR = 1.55; P = .006), and WMLs (OR = 10.17; P = .011) were independent prognostic factors for VaD. The area under the ROC curve (AUC) of FRS for VaD diagnosis prediction was 0.830 (95% confidence interval, 95% CI: 0.730∼ 0.929). There was a significant difference in the AUC between WMLs and WMLs combined with FRS (0.788 (95% CI: 0.667 ∼ 0.880) versus 0.863 (95% CI: 0.754 ∼ 0.936, P = .049). Age, HbA1c, and FRS were negatively correlated with the mini-mental state examination (MMSE) scores (all P < .05) in the VaD group. Moreover, multiple stepwise linear regression analysis showed that the age and FRS were independent predictors of MMSE scores.
FRS has a moderate predictive value for the VaD diagnosis, and also increases the risk of cognitive decline.
Keywords: cerebral white matter lesions, Framingham risk score, mini-mental state examination score, receiver-operating characteristic curve, vascular dementia
1. Introduction
Vascular dementia (VaD) is one of the most common forms of dementia, and second only to Alzheimer's disease.[1–3] Statistics have found that the incidence rate of VaD in China is between 1.1% and 3.0% and it is increasing in tendency.[4] Previous studies have suggested that VaD might be the primary cause of senile dementia in Asian countries.[5] Although the pathogenesis of VaD remains unknown, brain ischemia-hypoxia seems to be the leading cause of VaD. Vascular diseases can influence cognition in a number of ways, including decreased cerebral perfusion, cerebral micro-thrombus, and lacunar infarction. White matter lesions (WMLs), referred to as leukoaraiosis, have been indicated as a risk factors for incident dementia.[6] Recent clinical-pathological studies have suggested that the risk factors for VaD are almost identical as factors for cardiovascular disease (CVD) and stroke.[7] The most important nonmodifiable risk factor is age. Hebert and Brayne[8] reported that 4% of people over 65 years suffered from VaD. The modifiable risk factors for VaD should be regarded as therapy targets, including hypertension, diabetes, hyperlipidemia, smoking.[9] Targeting the risk factors could afford the best chance of minimizing dementia.[10] Some epidemiological data showed that antihypertensive treatment could have protective effect against dementia,[11] although the evidence for the conclusion was conflicting. People with VaD have a higher incidence of CVD than people without VaD. Framingham risk score (FRS), a typical indicator used in cardiovascular risk assessment, was performed to predict the risk of coronary events (angina pectoris, myocardial infarction) within 10 years.[12] Clerici et al[13] have found that the joint effect of WMLs and high FRS nearly doubled the risk of dementia. We suppose that FRS is important to predict VaD in general population. By reducing vascular risk factors and using rational treatment of CVDs, it may be possible to prevent or delay the progression of VaD. This paper aims to evaluate the predictive value of FRS for VaD through an analysis of the correlation between FRS and VaD in the Chinese population.
2. Subjects and methods
2.1. Cases and groups
Between July 2012 and December 2014, a total of 130 patients from department of Neurology of Tongji Hospital (including the health examination population) were admitted to this study (Fig. 1), including 81 males and 49 females, and ranging from 45 to 83 years (average age 61.54 ± 10.09 years). All observed cases were divided into 2 groups: the control group (n = 70) and the VaD group (n = 60). Written informed consent was obtained from each participant.
Figure 1.
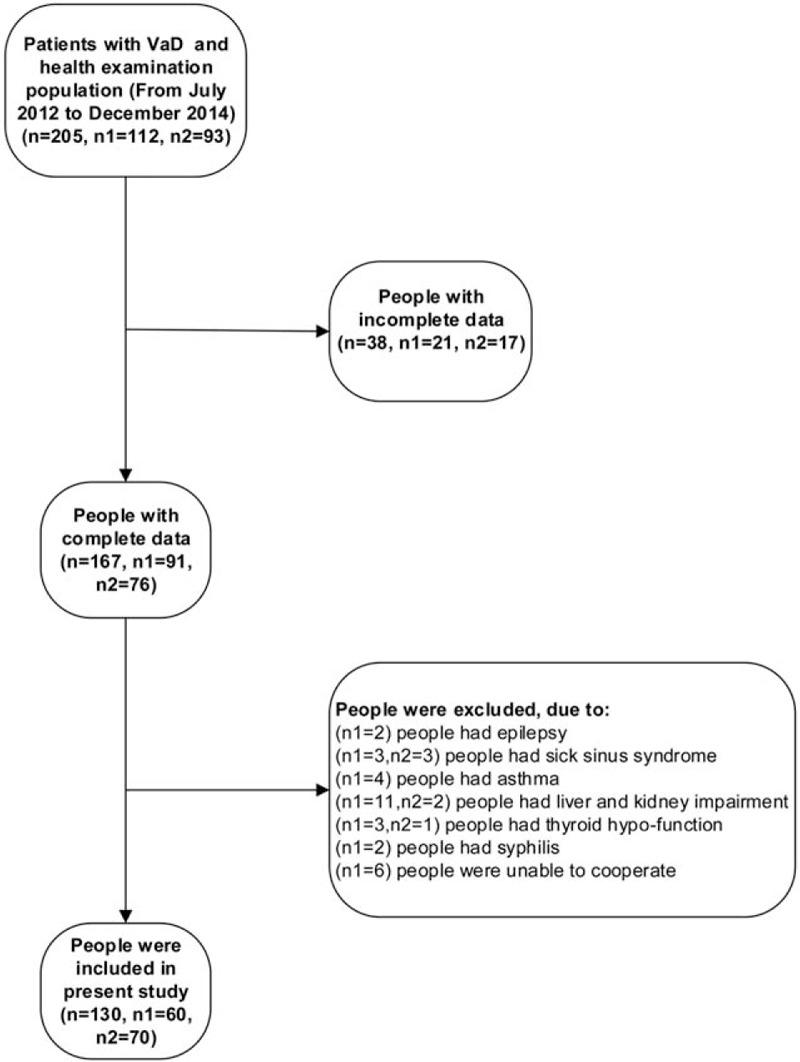
Flow diagram of the patient selection process (n: the total number of included people; n1: the number of VaD group; n2: the number of healthy control group).
2.2. Inclusion criteria
Both groups: (1) Hamilton depression scale (HAMD) score < 10. (2) Patients must be in good physical and mental condition before the treatment,[14] as well as be able to cooperate and complete the test.
VaD group: (3) Conformity with “vascular dementia” diagnostic criteria as outlined by the neurology branch of the Chinese Medical Association's (CMA) Vascular Dementia Diagnosis Standard Draft. (4) Patients were excluded if they had a past history of Alzheimer's disease, Parkinson's disease, and other types of dementia patients (such as vit B12 or folic acid deficiency). (5) Hachinski ischemic score ≥7.
Control group: (6) Mini-mental state examination (MMSE) score: Individuals who would not be selected in the study with scores, illiteracy ≤17, primary school ≤20, secondary school ≤ 22, university ≤23.
2.3. Exclusion criteria
(1) Other psychiatric disorders accompanied by cognitive impairment, such as epilepsy or schizophrenia. (2) Patients with a medical history of asthma, acute phase peptic ulcer, sick sinus syndrome (SSS), severe atrioventricular block, thyroid hypofunction, liver and kidney impairment, syphilis, HIV infections, and tumors. (3) Patients with disturbance of consciousness, severe aphasia, or otherwise unable to cooperate.
2.4. Data acquisition
Using a standard data-collection form, including age, sex, past history, measuring blood pressure, fasting blood glucose (FBG), glycosylated hemoglobin A1c (HbA1c), total cholesterol (TC), high-density lipoprotein (HDL), triglyceride (TG), low-density lipoprotein (LDL), homocysteine (HCY), and hypersensitive C-reactive protein (hs-CRP).
2.5. White matter lesion measuring
In terms of images, WML was defined as “hyperintensities in T2 and FLAIR sequences in magnetic resonance.”[15,16] According to the Cholinergic Pathway High Signal Rating Scale (CHIPS), severity of WMH was estimated by a 3-point scale for each region [0 = normal; 1 = mild (<50%); 2 = moderate-severe (≥50%)].[17] In our study, we took CHIPS combined with overlap analysis of magnetic resonance imaging (MRI) to estimate WML. People with WML was defined as follow the MRI standards and score of CHIPS ≥1. Brain MRI scanning was completed with a 1.5-tesla Magnetom (Simens Magnetom, Avanto, Germany). The scanning sequence and data extraction methods have been described in detail in a previous study.[18] All MR scans were examined by 2 raters. The reading of both raters was averaged.
2.6. Mini-mental state examination (MMSE)
MMSE was used to evaluate the degree of cognitive damage of VaD.
2.7. Framingham risk score (FRS)
Calculating the incidence of coronary events (angina, myocardial infarction) over a 10 years period according to the cholesterol level and noncholesterol level (such as age, plasma glucose, smoking, etc).
2.8. Sample size calculating
The sample size was calculated by taking the previously quoted study into account.[19,20] A significance level of 5% for a 2-sided t-test with a 80% power (1-β), 52 patients were required in each group. Assuming a 20% drop-out rate, we planned to randomize 60 patients to each group
2.9. Statistical processing
Data were presented as mean value ± standard deviation, frequencies, or interquartile ranges. Comparisons between 2 groups were made by independent samples t-test for normally distributed data, Mann–Whitney U test for non-normally distributed data, and Pearson Chi-square for categorical variables. Correlations were performed by linear regression analysis for normally distributed data, and Spearman rank test for non-normally distributed data. Binary logistic regression with enter method was applied to individuate variable independently associated with VaD. Receiver operating characteristics (ROC) curve was used to evaluate the predictive value of FRS for VaD. A logistic regression model was used to combine the results of the ROC curve analysis (the diagnosis value of WMLs combined FRS for VaD). According to the diagnosis of VaD, and based on the logistic regression model, predictors were combined and then applied to establish the ROC curves. A 2-tailed P value <.05 was considered statistically significant. Statistical analysis was performed using the SPSS19.0 (IBM Corp., Armonk, NY) software system.
3. Results
3.1. Baseline characteristics of the patients
There was a significant difference between systolic blood pressure (SBP) (P < .001), diastolic blood pressure (DBP) (P = .001), pulse pressure (P = .002), TC (P = .031), HCY (P = .019), HbA1c (P = .010), FRS (P < .001), and cerebral WMLs (P < .001) between the 2 groups, even after adjusting for age (all P < .05) (Table 1). Table 2 summarizes that there was no significant difference between antihypertensive (48.5% vs 50.0%, P = .872), antidiabetic (21.4% vs 21.7%, P = .974), antiplatelet (15.7% vs 28.3%, P = .088), and antidyslipidemic (5.7% vs 8.3%, P = .561) agents. In VaD group, 25% of the patients took cholinesterase inhibitors, 41.7% took other anti-dementia agents (such as piracetam, vitamin E), and the remaining took no anti-dementia agents.
Table 1.
Characteristics of subjects with vascular dementia group and controls group.
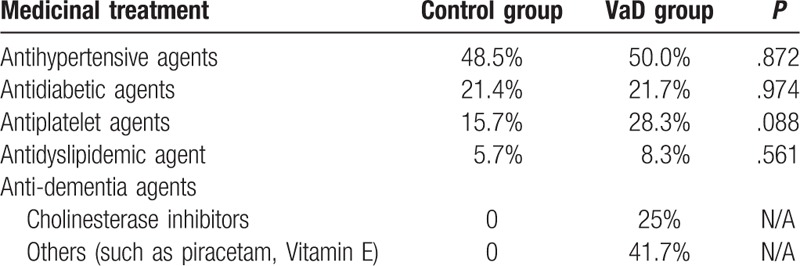
Table 2.
The medication of the selected patients.
3.2. Logistic regression analysis of the independent risk factors for VaD
Binary logistic regression analysis with forward (Likelihood Ratio Test) method was performed to evaluate the independent risk factors for VaD (Table 3). According to the results, age (OR = 1.20; P = .002), FRS (OR = 1.55; P = .006), and WMLs (OR = 10.17; P = .011) were proved to be the independent risk factors for VaD.
Table 3.
The logistic regression analysis with forward (Likelihood Ratio Test) method of independent risk factors of VaD.

3.3. Predictive value of the FRS for the diagnosis of VaD
The ROC curve was defined in terms of the sensitivity of the FRS for the diagnostic prediction of VaD as abscissa and 1-specificity as the vertical coordinate. The area under ROC curves (AUC) was 0.830 (95% CI: 0.730∼ 0.929) (Fig. 2). FRS, with a cutoff level of 9.5%, provided 60% sensitivity and 95% specificity.
Figure 2.
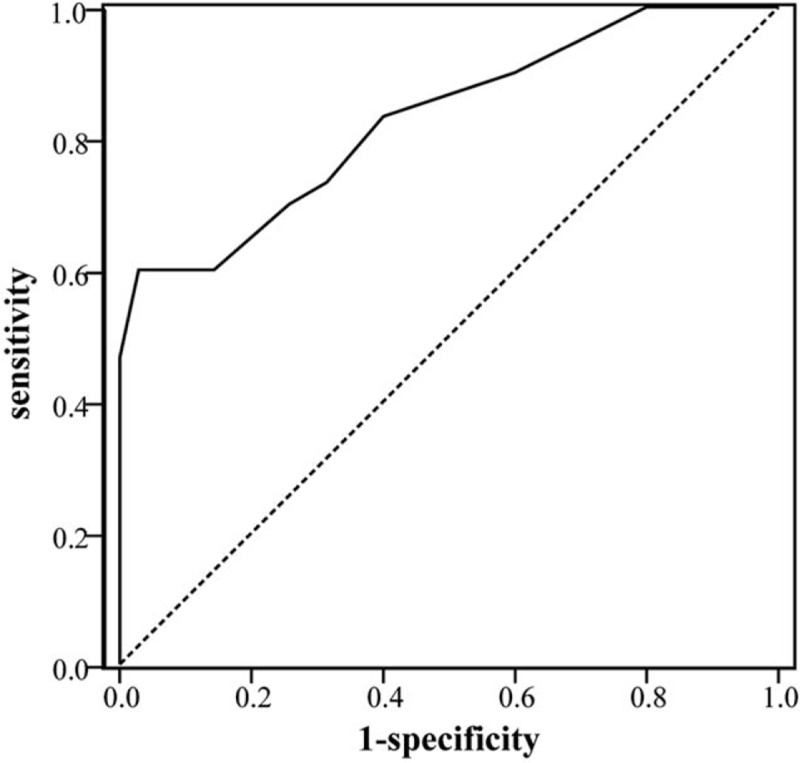
Predictive value of FRS for the diagnosis of VaD. The ROC curves analysis using FRS for identifying VaD. The estimated optimal cut-off value of FRS was 9.5% (AUC = 0.830, 95% CI: 0.730∼ 0.929).
The ROC curve was defined in terms of the sensitivity of WMLs and FRS combined WMLs for the diagnostic prediction of VaD as abscissa and 1-specificity as the vertical coordinate (Fig. 3), respectively. The AUC were 0.788 (95% CI: 0.667∼0.880) and 0.863 (95% CI: 0.754∼0.936) (P = .049), respectively.
Figure 3.
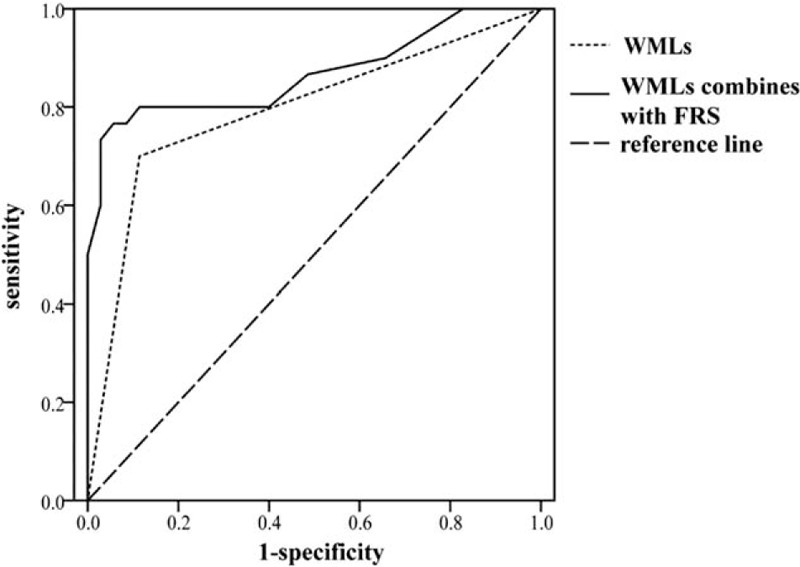
Predictive value of WMLs and FRS for the diagnosis of VaD. Comparison of predictive value between WMLs and FRS combined WMLs for the diagnosis of VaD (AUC = 0.788 vs 0.863, P < .05). FRS = Framingham risk score, WMLs = white matter lesions.
3.4. Correlation analysis between cardiovascular risk factors and the MMSE score
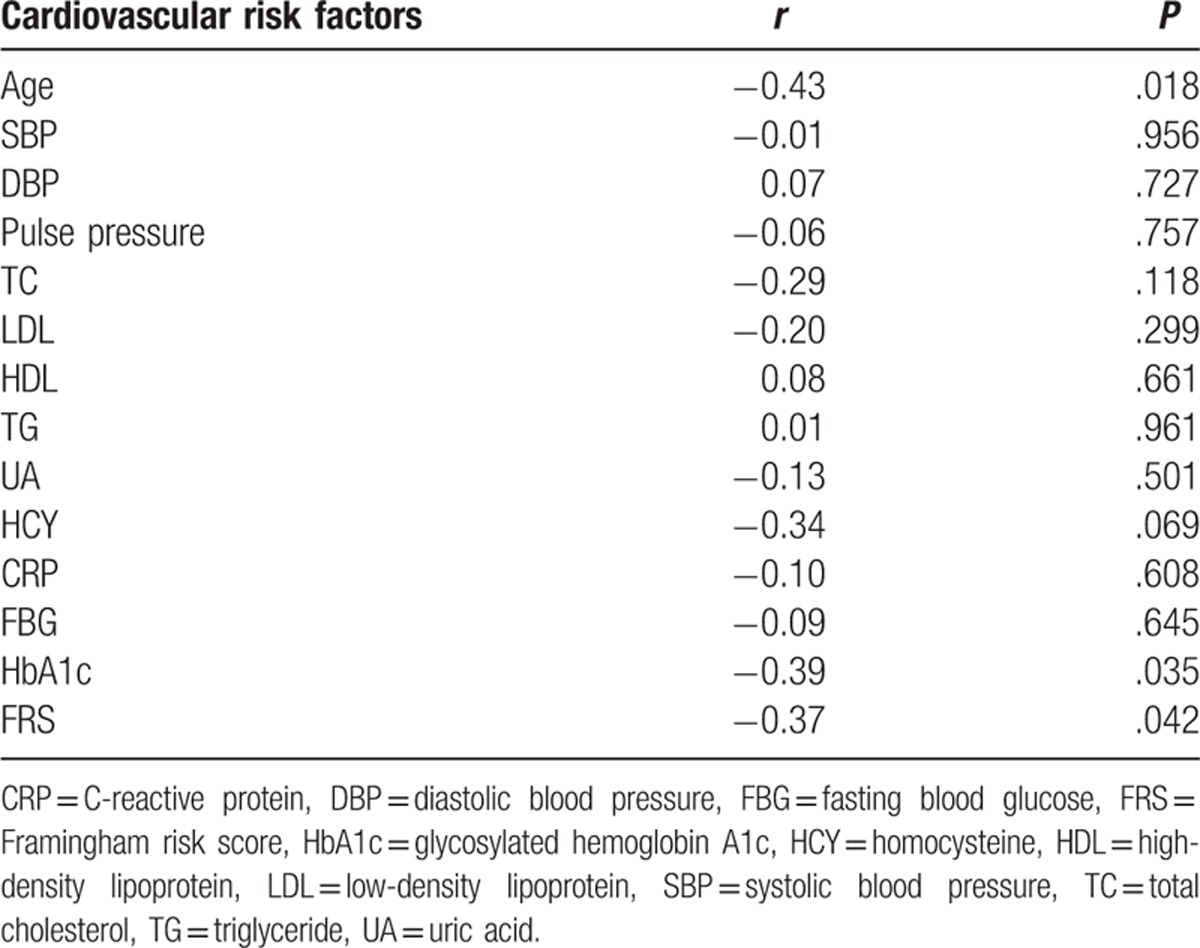
Age (r = −0.43, P = .018), HbA1c (r = −0.39, P = .035), and FRS (r = −0.37, P = .042) were negatively correlated with the MMSE score in the VaD group (Table 4).
Table 4.
Correlation between the MMSE score and cardiovascular risk factors.
3.5. Multiple stepwise regression analysis of MMSE scores and various influencing factors
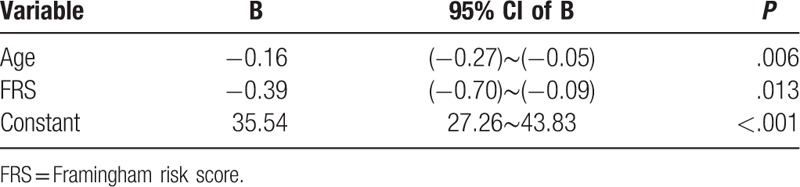
We used a multiple stepwise linear regression analysis with the MMSE score as a response variable and the factors correlated with MMSE (age, HbA1c, and FRS) as possible influencing factors (Table 5). The MMSE score regression equation was Y = 35.54+ (−0.16) ∗ age + (−0.39) ∗ FRS. Both the age and FRS influenced the MMSE score.
Table 5.
Multiple stepwise linear regression analysis with the MMSE score and various influencing factors.
4. Discussion
VaD is one of the most common diseases in China, which brings with it a heavy burden to the family and society. A series of CVDs such as decreased cerebral perfusion, cerebral micro-thrombus, and lacunar infarction influence cognitive function. Particularly, hypertension, lipid disorders, and diabetes are closely related to cognitive impairment and VaD.[21–23]
The Honolulu-Asia Aging Study (HAAS) found that hypertension without treatment was closely associated with VaD. A 10 mm Hg increase in systolic blood pressure contributed to a 7% increase in risk of cognitive impairment.[24] Sharp et al[25] conducted meta-analyses and confirmed that hypertension was significantly associated with an increased incidence of VaD (P < .00001), according to five longitudinal studies (OR 1.59, 95% CI: 1.29–1.95) and the 6 cross-sectional studies (OR 4.84, 95% CI: 3.52–6.67). Our study found a significant difference in SBP, DBP, and pulse pressure between the 2 groups. However, the indexes did not remain independent predictors of VaD after subsequent analysis using the logistic regression model. The negative result may be partly due to treatment for hypertension. A meta-analysis that include the SHEP, Syst-Eur, and SCOPE tests found no significant difference with antihypertensive treatment and risk of dementia (OR 0.89, 95% CI: 0.69–1.16).[26] Currently, the drugs that have been certified with positive effects include calcium antagonist (such as lercanidipine) and angiotensin II receptor blockers (such as telmisartan), among others.[27] However, both the dosage of these antihypertensive drugs and the mechanism of anti-dementia need to be investigated further.
Previous study showed that the level of TC ≥ 240 mg/dL was associated with VaD HR of 1.26 (95% CI: 0.82–1.96).[28] Thus, high cholesterol level was considered as one of the risk factors of VaD. However, an increase in cholesterol level later in life may actually reduce the risk of VaD.[29] In Our study, we found that the TC level in the VaD group was significantly higher than the control group. Previous studies have identified that Asp/Asp gene-phenotype of Glutathione S-transferase omega-1 (GSTO1) could increase the risk of VaD (P = .02, OR = 2.2).[30] Currently, the correlation between lipid metabolism and VaD is still controversial, and further large-scale clinical trials are required to evaluate this factor.
A number of studies have indicated that diabetes can independently influence cognitive function, and an elevated HbA1c value was significantly associated with decreased velocity and reaction.[31] Similarly, according to a meta-analysis of 19 longitudinal studies, the relative risk (RR) of diabetic patients with VaD was 2.48.[32] In this study, we found a significant difference in HbA1c between the 2 groups. This suggests that blood glucose level and VaD are closely related, which is consistent with the view above. In addition, we found that HbA1c and MMSE were negatively correlated, indicating that cognitive impairment is aggravated with an increase in blood glucose level. The potential pathophysiological mechanisms of a brain affected by diabetes included oxidative stress, immune inflammation, mitochondrial damage, and a series of pathological injuries, which lead to cognitive impairment.[33–36]
Traditional cardiovascular risk factors were closely related to the occurrence and development of VaD.[37,38] The FRS is considered a classic predictor for the incidence of coronary events (angina, myocardial infarction) over a 10-year period. In this study, FRS increased significantly in the VaD group. As a predictor of VaD (OR = 1.55; P < .01), FRS had a moderate predictive value for VaD diagnosis (AUC = 0.830, 95% CI: 0.730∼ 0.929). Furthermore, FRS was negatively associated with the MMSE score, which was an independent factor of MMSE score. Namely, the higher the FRS, the more severe the cognitive impairment.
Cerebral WMLs are mainly determined through brain imaging [such as a head computed tomography (CT) or head MRI] and are useful for identification of abnormal white matter signals in periventricular and subcortical brain regions. In recent years, many studies have confirmed that WMLs and cognitive dysfunction are closely related.[39] WMLs could be used as an imaging biomarker, predicting VaD occurrence and development.[40] In this study, the ROC curve analysis showed that WMLs had a moderate predictive value for VaD, which is consistent with previous reports. On the basis of the results of the ROC curve analysis, we further explored the predictive value of combining 2 indicators (FRS and WMLs) for the diagnosis of VaD, which showed an increase in AUC (P < .05). Thus, the predictive value of FRS for VaD was confirmed again through another angle.
Overall, the results of this study reveal that FRS and VaD were significantly related. FRS, an independent predictor of VaD, has a moderate predictive value in diagnosing VaD, and the diagnostic value may improve by combining it with other indicators. Further study is required to estimate the extent to which prevention or correction of certain cardiovascular risk factors along with reasonable treatment of CVDs could reduce the occurrence and development of VaD. Furthermore, this study provides clues for therapeutic primary prevention as well as secondary prevention of VaD.
The limitation of this study is the small number of participants. Therefore, further large-scale clinical trials are required to confirm the results of our study.
Footnotes
Abbreviations: 95% CI = 95% confidence interval, AUC = area under the ROC curve, DBP = diastolic blood pressure, FRS = Framingham risk score, HbA1c = glycosylated hemoglobin A1c, HCY = homosysteine, MMSE = mini-mental state examination, OR= odds ratio, ROC = receiver-operating characteristic curve, SBP = systolic blood pressure, TC = total cholesterol, VaD = Vascular dementia, WMLs = cerebral white matter lesions.
Authorship: SSL and KZ conceived and designed the experiments; JZ and HYW performed the experiments; MZ, BM and SSL analyzed the data; SSL and KZ contributed to the writing of the manuscript; SSL and KZ revised the manuscript.
Funding/support: The work was supported by the National Natural Science Foundation of China (grant no.:81301053 and 81300387) and Provincial Nature Science Foundation of Hubei Province (2014CFB386).
The authors have read and approved the submission and declare no conflict of interest.
References
- [1].Versijpt J. Effectiveness and cost-effectiveness of the pharmacological treatment of Alzheimer's disease and vascular dementia. J Alzheimers Dis 2014;42(Suppl 3):S19–25. [DOI] [PubMed] [Google Scholar]
- [2].Sachdev P, Kalaria R, O’Brien J, et al. Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis Assoc Disord 2014;28:206–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Venkat P, Chopp M, Chen J. Models and mechanisms of vascular dementia. Exp Neurol 2015;272:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wortmann M. Dementia: a global health priority-highlights from an ADI and World Health Organization report. Alzheimers Res Ther 2012;4:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Killin LO, Starr JM, Shiue IJ, et al. Environmental risk factors for dementia: a systematic review. BMC Geriatr 2016;16:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gudmundsson P, Olesen PJ, Simoni M, et al. White matter lesions and temporal lobe atrophy related to incidence of both dementia and major depression in 70-year-olds followed over 10 years. Eur J Neurol 2015;22:781–8. e749–750. [DOI] [PubMed] [Google Scholar]
- [7].O’Brien JT, Erkinjuntti T, Reisberg B, et al. Vascular cognitive impairment. Lancet Neurol 2003;2:89–98. [DOI] [PubMed] [Google Scholar]
- [8].Hebert R, Brayne C. Epidemiology of vascular dementia. Neuroepidemiology 1995;14:240–57. [DOI] [PubMed] [Google Scholar]
- [9].Song J, Lee WT, Park KA, et al. Association between risk factors for vascular dementia and adiponectin. Biomed Res Int 2014;2014:261672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Seetlani NK, Kumar N, Imran K, et al. Alzheimer and vascular dementia in the elderly patients. Pak J Med Sci 2016;32:1286–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tadic M, Cuspidi C, Hering D. Hypertension and cognitive dysfunction in elderly: blood pressure management for this global burden. BMC Cardiovasc Disord 2016;16:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wilson PW, D’Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837–47. [DOI] [PubMed] [Google Scholar]
- [13].Clerici F, Caracciolo B, Cova I, et al. Does vascular burden contribute to the progression of mild cognitive impairment to dementia? Dement Geriatr Cogn Disord 2012;34:235–43. [DOI] [PubMed] [Google Scholar]
- [14].Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ 1992;305:160–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hachinski VC, Potter P, Merskey H. Leuko-araiosis: an ancient term for a new problem. Can J Neurol Sci 1986;13:533–4. [DOI] [PubMed] [Google Scholar]
- [16].Munoz DG. Leukoaraiosis and ischemia: beyond the myth. Stroke 2006;37:1348–9. [DOI] [PubMed] [Google Scholar]
- [17].Bocti C, Swartz RH, Gao FQ, et al. A new visual rating scale to assess strategic white matter hyperintensities within cholinergic pathways in dementia. Stroke 2005;36:2126–31. [DOI] [PubMed] [Google Scholar]
- [18].Godin O, Dufouil C, Maillard P, et al. White matter lesions as a predictor of depression in the elderly: the 3C-Dijon study. Biol Psychiatry 2008;63:663–9. [DOI] [PubMed] [Google Scholar]
- [19].Zhang Y, Chen J, Zhang K, et al. Combination of high ankle-brachial index and hard coronary heart disease Framingham Risk Score in predicting the risk of ischemic stroke in general population. PLoS One 2014;9:e106251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pencina MJ, D’Agostino RB, Sr, Larson MG, et al. Predicting the 30-year risk of cardiovascular disease: the Framingham heart study. Circulation 2009;119:3078–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gorelick PB. Risk factors for vascular dementia and Alzheimer disease. Stroke 2004;35:2620–2. [DOI] [PubMed] [Google Scholar]
- [22].Kherada N, Heimowitz T, Rosendorff C. Antihypertensive therapies and cognitive function: a review. Curr Hypertens Rep 2015;17:79. [DOI] [PubMed] [Google Scholar]
- [23].Yuan X, Mei B, Zhang L, et al. Enhanced penetration of exogenous EPCs into brains of APP/PS1 transgenic mice. Am J Transl Res 2016;8:1460–70. [PMC free article] [PubMed] [Google Scholar]
- [24].Launer LJ, Masaki K, Petrovitch H, et al. The association between midlife blood pressure levels and late-life cognitive function. The Honolulu-Asia Aging Study. JAMA 1995;274:1846–51. [PubMed] [Google Scholar]
- [25].Sharp SI, Aarsland D, Day S, et al. Hypertension is a potential risk factor for vascular dementia: systematic review. Int J Geriatr Psychiatry 2011;26:661–9. [DOI] [PubMed] [Google Scholar]
- [26].McGuinness B, Todd S, Passmore P, et al. Blood pressure lowering in patients without prior cerebrovascular disease for prevention of cognitive impairment and dementia. Cochrane Database Syst Rev 2009;Cd004034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Coca A. Hypertension and vascular dementia in the elderly: the potential role of anti-hypertensive agents. Curr Med Res Opin 2013;29:1045–54. [DOI] [PubMed] [Google Scholar]
- [28].Solomon A, Kivipelto M, Wolozin B, et al. Midlife serum cholesterol and increased risk of Alzheimer's and vascular dementia three decades later. Dement Geriatr Cogn Disord 2009;28:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Perez L, Heim L, Sherzai A, et al. Nutrition and vascular dementia. J Nutr Health Aging 2012;16:319–24. [DOI] [PubMed] [Google Scholar]
- [30].Kolsch H, Linnebank M, Lutjohann D, et al. Polymorphisms in glutathione S-transferase omega-1 and AD, vascular dementia, and stroke. Neurology 2004;63:2255–60. [DOI] [PubMed] [Google Scholar]
- [31].Jacobson AM, Musen G, Ryan CM, et al. Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med 2007;356:1842–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cheng G, Huang C, Deng H, et al. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J 2012;42:484–91. [DOI] [PubMed] [Google Scholar]
- [33].Acee AM. Type 2 diabetes and vascular dementia: assessment and clinical strategies of care. Medsurg Nurs 2012;21:349–53. 384. [PubMed] [Google Scholar]
- [34].Chatterjee S, Peters SA, Woodward M, et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care 2016;39:300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zheng K, Mei B. Effects of exogenous sonic hedgehog peptide on proliferation, adhesion, migration of endothelial progenitor cells from peripheral blood. J Huazhong Univ Sci Technolog Med Sci 2013;33:335–8. [DOI] [PubMed] [Google Scholar]
- [36].Srinivasan V, Braidy N, Chan EK, et al. Genetic and environmental factors in vascular dementia: an update of blood brain barrier dysfunction. Clin Exp Pharmacol Physiol 2016;43:515–21. [DOI] [PubMed] [Google Scholar]
- [37].Meguro K, Akanuma K, Meguro M, et al. Prognosis of vascular mild cognitive impairment includes vascular dementia onset and death by cardiovascular disease: reanalysis from the Osaki-Tajiri project. J Stroke Cerebrovasc Dis 2012;21:607–11. [DOI] [PubMed] [Google Scholar]
- [38].Kuller LH, Lopez OL, Jagust WJ, et al. Determinants of vascular dementia in the Cardiovascular Health Cognition Study. Neurology 2005;64:1548–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kuller LH, Lopez OL, Newman A, et al. Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology 2003;22:13–22. [DOI] [PubMed] [Google Scholar]
- [40].Stephan BC, Tzourio C, Auriacombe S, et al. Usefulness of data from magnetic resonance imaging to improve prediction of dementia: population based cohort study. BMJ 2015;350:h2863. [DOI] [PMC free article] [PubMed] [Google Scholar]


