Abstract
Over the past 10 years, the resistances among microbes are increasing gradually in Europe and greater resistances are seen in southern countries. We studied the prevalence of community-onset ESBL-producing Escherichia coli urinary tract infections in children.
As secondary objectives, we analyzed associated risk factors and the resistance patterns in ESBL-producing E coli isolates.
Retrospective observational study in a tertiary care hospital about children ≤14 years old with community-onset E coli urinary tract infection. The variables studied were age, sex, ESBL-producing, antibiotic therapy 7 to 30 days before the infection, hospitalization 7 to 30 days before the infection, nefrourologic pathology, and vesicoureteral reflux.
Between January 1st, 2015 and December 31st, 2016, 229 isolates of E coli were obtained, of whom 21 (9.2%) where ESBL-producing E coli. Median age in non-ESBL-producing was 18 months versus 7 months in ESBL-producing group. Fourteen (66%) of the ESBL-producing group were men (P = .001), 5 (23.8%) were hospitalized 30 days before the infection (P = .001), 12 (57.1%) had nefrourological pathology (P = .003), 6 (28.5%) had vesicoureteral reflux (P = .032). Previous antibiotic therapy was not statistically significant. Multiple regression analyses between sex and 30 days previous hospitalization were r = 3.51 (P = .0001). Multidrug resistant isolates among ESBL-producing E coli was 12 (57%).
The retrospective study allowed assessing the problem of ESBL-producing isolates in the outpatient settings. Some risk factors from past studies were confirmed and a combined risk is suggested. The resistant spectrum should be taken into account when choosing antibiotic regimens.
Keywords: β-lactamases, community-acquired infections, Escherichia coli, spain/epidemiology, urinary tract infections
1. Introduction
Over the past 10 years, the resistances among microbes are increasing gradually in Europe.[1] This is especially important in Spain, where all the studies and reports suggest that our rates of antibiotic resistance are greater than other countries.[1–3]
Most of the studies were made in adult population, or at least not specifically in children.[4–12] This means that there is a gap of information that must be completed, in order to improve national health surveillance programs in our country.
On the other hand, the prevalence of urinary tract infections in children goes from 2% to 5%,[4] with a risk of developing permanent renal damage in children below 2 years old.[4]
Statistical data are quite different between countries. In the USA, reports show a prevalence of extended-spectrum β-lactamase (ESBL) producing Escherichia coli urinary tract infections (UTI) of 3%.[5] In Mexico, the studies show a prevalence of 16.3%.[6] In China, Iran, Saudi Arabia, and Israel, 10%,[7] 30.3%,[8] 41.9%,[9] and 5%,[10] respectively.
In Europe, greater resistances are seen in southern countries.[1] In Germany, the studies show a prevalence of 8%.[12] In France (very close to Spain) we can observe a prevalence of 3.3%[11] as seen in 2013.
The data of Spain are very limited, but in 2000 a report showed that the prevalence of ESBL-producing E coli was about 0.5%,[13] while in 2005 this number grew to 8.2%,[14] which is 16 times greater.
Bearing in mind the lack of information in this matter in children in Spain, we tried to study the prevalence of community-onset ESBL-producing E coli urinary tract infections.
As secondary objectives, we analyzed associated risk factors and the resistance patterns in ESBL-producing E coli isolates.
2. Material and methods
We performed a retrospective observational study in a tertiary care hospital between January 1st, 2015 and December 31st, 2016. We collected data of all children ≤14 years old with community-onset E coli urinary tract infection who visited our emergency department in Toledo (Spain). Data for demographic characteristics, medical conditions, medication, and urinary isolates are from the patient medical record.
We considered positive isolate when >50,000 colony-forming units in the urine sample obtained by urinary catheterization (those patients without urinary continence) or spontaneous urination. We excluded those cultures separated <7 days from the previous one (we consider them as a control of the evolution of the patient), and those who were receiving antibiotic prophylaxis.
E coli were identified by using biochemistry procedures. Resistance detection and in vitro susceptibility were made by broth microdilution (Siemens WalkAway, West Sacramento, CA).
The variables studied were age, sex, ESBL-producing, antibiotic therapy 7 days, 15 days, or 30 days before the infection, hospitalization 7 days, 15 days, or 30 days before the infection, nefrourologic pathology (defined as vesicoureteral reflux, intermittent catheterization, pyelectasis, multicystic renal dysplasia, and/or renal agenesis), and vesicoureteral reflux.
SPSS v23 (SPSS Inc., Chicago, IL) was used for analysis. P < .05 was consider statistically significant.
Previous hospitalization, antibiotic therapy, and pyelectasis were analyzed using Fisher exact test. Sex, nefrourologic pathology, and vesicoureteral reflux were analyzed with Chi-squared test. Student t test was used for age analysis.
Sex and 30 days previous hospitalization combined were analyzed with multiple regression.
No ethical approval was needed because it is an observational study, all the data were obtained from the patient medical record, without any contact with them. It is also impossible to identify through the database any of the patients included.
3. Results
During the study period, 229 E coli isolates were identified from sterile samples from children in the emergency department. Twenty one (9.2%) of them were ESBL-producing E coli.
The median age was 17 months in all isolates, 18 months in non-ESBL-producing E coli, and 7 months in ESBL-producing E coli.
The risk factors statistical analyses and results are detailed in Table 1.
Table 1.
Analysis of risk factors in E coli isolates from January 2015 to December 2016 in children between 0 and 14 years old.

Multiple regression analyses between sex and 30 days previous hospitalization were r = 3.51 (P = .0001)
The resistances observed in ESBL-producing E coli sample are detailed in Table 2.
Table 2.
Resistances among ESBL-producing E coli isolates from January 2015 to Decembre 2016 in children between 0 and 14 years old.
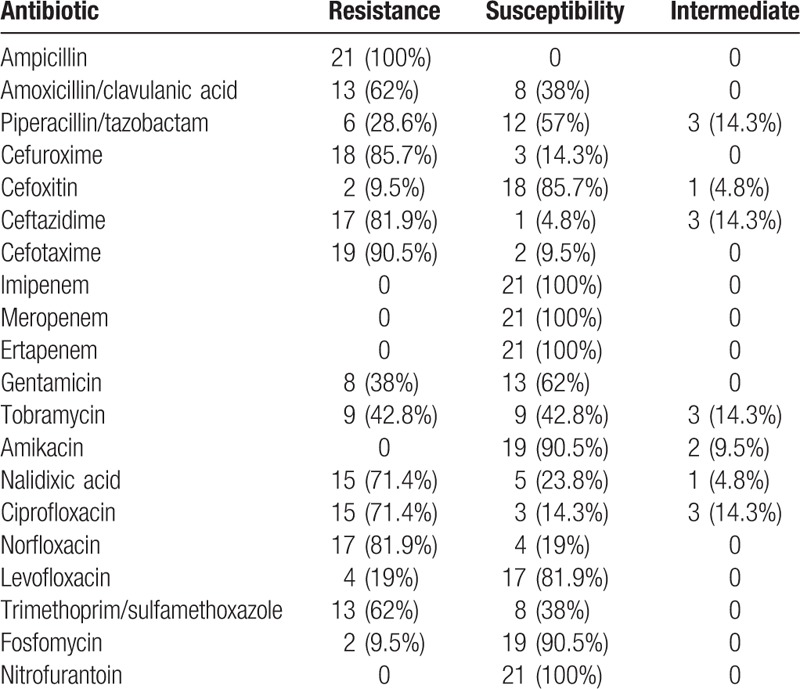
Moreover, 7 (33%) of them were resistant to cephalosporins, aminoglycosides, and quinolones, and 5 (23.8%) to cephalosporins and quinolones, but not aminoglycosides. The total amount of multidrug resistant isolates among ESBL producing E coli was 12 (57%).
4. Discussion
We find that among children, the isolation of ESBL-producing E coli is becoming common across pediatric age groups in Spain, consistent with previous reports in adults,[13,14] and children over the world.[8,15]
The spread of ESBL is plasmid-mediated, and can be transferred to other Gram-negative bacteria.[16] This fact is important to understand the easily spread of resistances in a country, and the need of national healthcare programs.
In 2015, the prevalence of ESBL-producing E coli among UTI caused by E coli was 7.1% versus 14.6% in 2016. As we can see, this rate is increasing very fast among pediatric population.
The upward trend in ESBL-producing isolates with age,[10] is not seen in our study (median age of 7 months in ESBL-producing group vs 18 months in E coli isolates). This may be because the E coli behavior in children is different from adults.
As seen in previous studies, being male, hospitalization 30 days previous to infection,[6,15] nefrourologic pathology and vesicoureteral reflux are proposed as risk factors.
Moreover, male sex and hospitalization 30 days before infection have a combined risk of 3.51 (P = .0001), which should be taken into account to choose the best antibiotic regime for these children.
In our sample, 57% of the ESBL-producing E coli were multidrug resistant, which means that at least 5.24% of the children included have a multidrug resistant infection. They remain highly susceptible to fosfomycin, nitrofurantoin, and carbapenems.
In conclusion, the retrospective study allowed assessing the problem of ESBL-producing isolates in the outpatient settings. Some risk factors from past studies were confirmed and a combined risk is suggested. The resistant spectrum should be taken into account when choosing antibiotic regimens.
Our study has the proper limitations of an observational study.
Further studies are needed to reveal the real impact of these microorganisms in national healthcare.
Footnotes
Abbreviations: ESBL = extended-spectrum beta-lactamase, UTI = urinary tract infections.
Funding: None.
Disclosure of Potential Conflicts of Interest: Authors have nothing to disclose.
The authors have no conflicts of interest to disclose.
References
- [1].European Centre for Disease Prevention and Control. Antimicrobial Resistance Surveillance in Europe 2015. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net); 2017. Stockholm: ECDC. [Google Scholar]
- [2].Kahlmeter G, Poulsen HO. Antimicrobial susceptibility of Escherichia coli from community-acquired urinary tract infections in Europe: the ECO SENS study revisited. Int J Antimicrob Agents 2012;39:45–51. [DOI] [PubMed] [Google Scholar]
- [3].Kahlmeter G, Menday P. Cross-resistance and associated resistance in 2478 Escherichia coli isolates from the Pan-European ECO.SENS Project surveying the antimicrobial susceptibility of pathogens from uncomplicated urinary tract infections. J Antimicrob Chemother 2003;52:128–31. [DOI] [PubMed] [Google Scholar]
- [4].Rodrigo Gonzalo de Liria C, Méndez Hernández M, Azuara Robles M. Diagnostic-therapeutic protocols of the AEP: Pediatric Infectology. Chapter 14: urinary infection. Ed Ergon. 3 edición. 2011. Available in http://www.aeped.es/sites/default/files/documentos/itu.pdf. [Google Scholar]
- [5].Thaden JT, Fowler VG, Sexton DJ, et al. Increasing incidence of extended-spectrum β-lactamase-producing Escherichia coli in community hospitals throughout the Southeastern United States. Infect Control Hosp Epidemiol 2016;37:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Morfín-Otero R, Mendoza-Olazarán S, Silva-Sánchez J, et al. Characterization of Enterobacteriaceae isolates obtained from a tertiary care hospital in Mexico, which produces extended-spectrum β-lactamase. Microb Drug Resist 2013;19:378–83. [DOI] [PubMed] [Google Scholar]
- [7].Ho PL, Wong RC, Yip KS, et al. COMBAT study group. Antimicrobial resistance in Escherichia coli outpatient urinary isolates from women: emerging multidrug resistance phenotypes. Diagn Microbiol Infect Dis 2007;59:439–45. [DOI] [PubMed] [Google Scholar]
- [8].Rezai MS, Salehifar E, Rafiei A, et al. Characterization of multidrug resistant extended-spectrum Beta-lactamase-producing Escherichia coli among uropathogens of pediatrics in North of Iran. Biomed Res Int 2015;2015:309478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Al Yousef SA, Younis S, Farrag E, et al. Clinical and laboratory profile of urinary tract infections associated with extended spectrum β-lactamase producing Escherichia coli and Klebsiella pneumoniae. Ann Clin Lab Sci 2016;46:393–400. [PubMed] [Google Scholar]
- [10].Toubiana J, Timsit S, Ferroni A, et al. Community-onset extended-spectrum β-lactamase-producing Enterobacteriaceae invasive infections in children in a University Hospital in France. Medicine (Baltimore) 2016;95:e3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Martin D, Fougnot S, Grobost F, et al. ONERBA-ville network. Prevalence of extended-spectrum beta-lactamase producing Escherichia coli in community-onset urinary tract infections in France in 2013. J Infect 2016;72:201–6. [DOI] [PubMed] [Google Scholar]
- [12].Kresken M, Pfeifer Y, Hafner D, et al. Working Party ‘Antimicrobial Resistance’ of the Paul-Ehrlich-Society for chemotherapy. Occurrence of multidrug resistance to oral antibiotics among Escherichia coli urine isolates from outpatient departments in Germany: extended-spectrum β-lactamases and the role of fosfomycin. Int J Antimicrob Agents 2014;44:295–300. [DOI] [PubMed] [Google Scholar]
- [13].Hernández JR, Pascual A, Cantón R, et al. Grupo de Estudio de Infección Hospitalaria. GEIH. [Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in spanish hospitals (GEIH-BLEE Project 2002)]. Enferm Infecc Microbiol Clin 2003;21:77–82. [PubMed] [Google Scholar]
- [14].García MV, Gallardo MM, Rodríguez-Ortega R, et al. [Distribution of patterns of sensitivity and associated phenotypes of resistance in nosocomial and community acquired Escherichia coli during 2005]. Rev Esp Quimioter 2008;21:157–65. [PubMed] [Google Scholar]
- [15].Dayan N, Dabbah H, Weissman I, et al. Urinary tract infections caused by community-acquired extended-spectrum β-lactamase-producing and nonproducing bacteria: a comparative study. J Pediatr 2013;163:1417–21. [DOI] [PubMed] [Google Scholar]
- [16].Wragg R, Harris A, Patel M, et al. Extended spectrum beta lactamase (ESBL) producing bacteria urinary tract infections and complex pediatric urology. J Pediatr Surg 2017;52:286–8. [DOI] [PubMed] [Google Scholar]


