Abstract
Rationale:
Medullary thyroid carcinoma (MTC) is a rare type thyroid carcinoma originating from the thyroid parafollicular cells (C cells). Chemotherapy has a limited efficacy for treating persistent or recurrent MTC.
Patient concerns:
A 46-year-old woman who underwent thyroidectomy for MTC in December 2007. She began experience recurring diarrhea in January 2015 and started to cough and feel shortness of breath in March 2016.
Diagnoses:
A chestcomputed tomography (CT) scan showed metastases in the bilateral lungs, pulmonary hilum, and mediastinal lymph nodes. Percutaneous biopsy of the pulmonary occupying lesions performed on March 21, 2016 indicated medullary carcinoma metastases at the right pulmonary hilum.
Interventions:
This patient was treated with oral apatinib (500 mg daily).
Outcomes:
The patient's symptoms of diarrhea, coughing, and shortness of breath disappeared. CT reexaminations for efficacy assessment at 1, 2, and 3 months after the treatment indicated partial remission. Systemic migrating bone and joint pains occurred during the treatment, which were considered to be adverse events of apatinib.
Lessons:
Treatment of MTC with apatinib has been shown to be effective in our case. Tyrosine kinase inhibitors (TKIs) that suppress rearranged during transfection (RET) and vascular endothelial growth factor receptor (VEGFR) should be considered as a effective therapeutic approaches.
Keywords: apatinib, medullary thyroid carcinoma, vascular endothelial growth factor receptor
1. Introduction
Medullary thyroid carcinoma (MTC) is a malignancy originating from the thyroid parafollicular cells (C cells). Although MTC only accounts for 3% to 8% of all thyroid cancers,[1,2] it represents up to 13.4% of thyroid cancer-related deaths.[3] The current treatments for patients with stage IV MTC are not satisfactory. According to the guidelines developed by the National Comprehensive Cancer Network (NCCN), the first-line treatments for MTC should be cabozantinib and vandetanib; however, it is difficult to purchase these drugs in China and their costs are beyond the economic ability of most patients. Therefore, a feasible and effective therapeutic approach with affordable drugs to treat advanced MTC patients is needed.
2. Case report
This study was approved by the Third Affiliated Hospital of Southern Medical University. Informed consent was obtained from all individual participants included in the study.
A 46-year-old woman underwent thyroidectomy for MTC on December 20, 2007 owing to a diagnosis of right thyroid cancer at the Third Affiliated Hospital of Sun Yat-Sen University on the same day. Postoperative pathological examinations indicated MTC, while the immunohistochemical findings were positive for calcitonin, cytokeratin (CK) 19, CgA, and carcinoembryonic antigen (CEA), weakly positive for neuron-specific enolase, and negative for thyroid peroxidase. Fifty micrograms levothyroxine was orally administered once daily postoperatively. Thyroid function and Doppler were monitored regularly and no abnormalities were observed. The patient began experiencing repeated diarrhea in January 2015. Colonoscopy findings were normal; therefore, a diagnosis of irritable bowel syndrome was made and antidiarrheal medications such as diosmectite were administered, although this did not fully alleviate the symptoms. In March 2016, she started to cough and feel short of breath. Detection of blood tumor markers showed that the levels of CEA and calcitonin were 407.6 ng/mL and 938 pg/mL, respectively. Enhanced chest computed tomography (CT) on March 4, 2016 showed the following: multiple occupying lesions in the bilateral lungs, considered as central lung cancer with intrapulmonary metastases, left lower pulmonary obstructive pneumonia, and atelectasis; hilar and mediastinal lymph node metastases; and hilar and mediastinal lymph node metastases (Fig. 1). No abnormalities were observed on abdominal CT scan.
Figure 1.
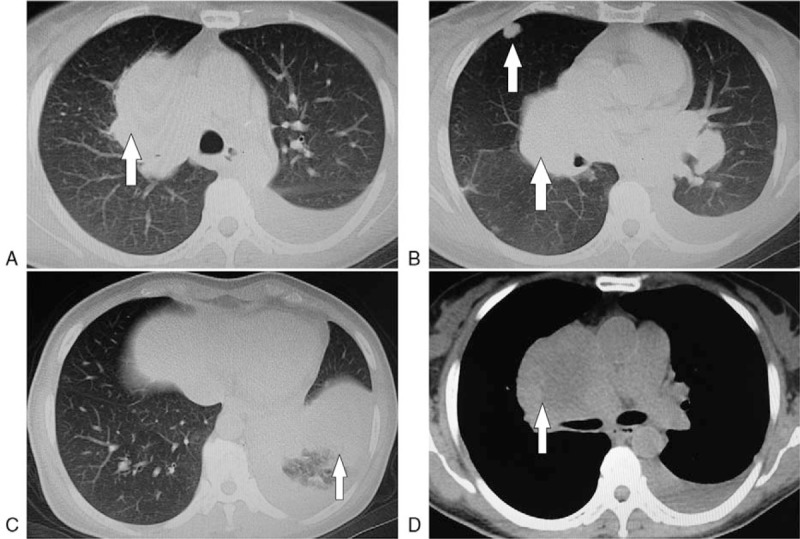
Metastatic nidi on the chest computed tomography images taken in March 2016. A: Right hilar metastatic nidi, B: Right hilar and intrapulmonary metastatic nidi, C: Left lower pulmonary obstructive pneumonia, and atelectasis, D: Right hilar and mediastinal lymph node metastases.
Percutaneous biopsy of the pulmonary occupying lesions was performed on March 21, 2016. The patient's clinical history and the pathological findings, which were consistent with the immunochemical findings (calcitonin [+], CK7 [+], TTF-1 (5,2′,4′-trihydroxy-6,7,5′-trimethoxyflavone) [+], CK5/6 [−], p40 [−], Napsin A [−], Syn [+], CgA (Chromogranin A) [+], CD56 [+], and CD117 [+]), indicated medullary carcinoma metastases at the right pulmonary hilum (Fig. 2).
Figure 2.
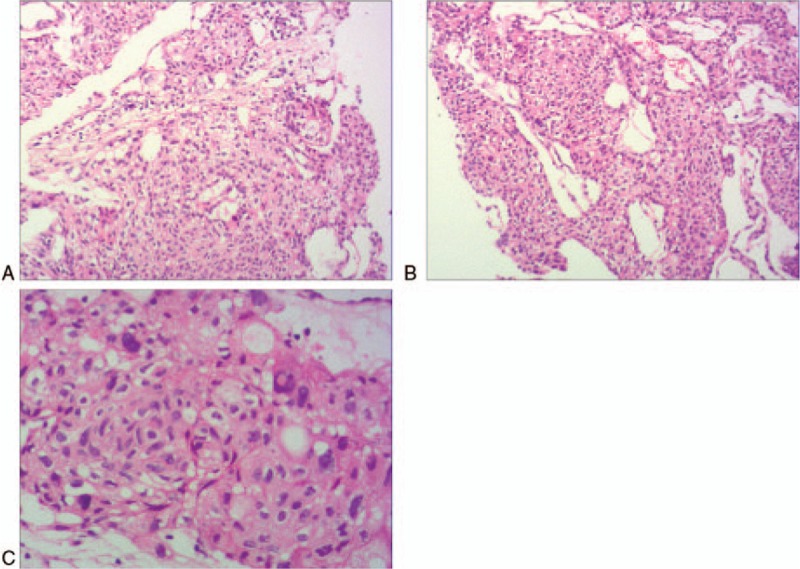
The images of postoperative pathological exams confirmed right lung metastatic carcinoma. (HE stain, A and B: X100; C: X200).
Collectively, presentations of recurrence of thyroid carcinoma after thyroidectomy were clearly confirmed for the patient, and her diagnoses included stage IV MTC recurrence, hilar and mediastinal lymph node metastases, and bilateral pulmonary metastases.
Therapeutic choice: Based on the NCCN guidelines, the first-line medications for stage IV MTC should be cabozantinib and vandetanib; however, these 2 drugs are unavailable in Guangzhou. It is reported that sorafenib can serve as a therapeutic option for MTC[4]; however, we ruled out this option owing to economic considerations. We recommended apatinib, a domestically developed targeted drug costing only one-tenth to one-fifth of the expense of sorafenib, to this patient.
Therapeutic efficacy: On April 2, 2016, the patient initiated the once-daily oral medication of 500 mg apatinib and was free from diarrhea, coughing, and shortness of breath 2 weeks later, with continuously declining CEA and calcitonin levels. CT reexaminations on April 8, May 4, and June 7, 2016 all indicated significantly reduced pulmonary metastases; therefore, the results of the efficacy assessments were all rated as partial response (PR) (Figs. 3 and 4).
Figure 3.
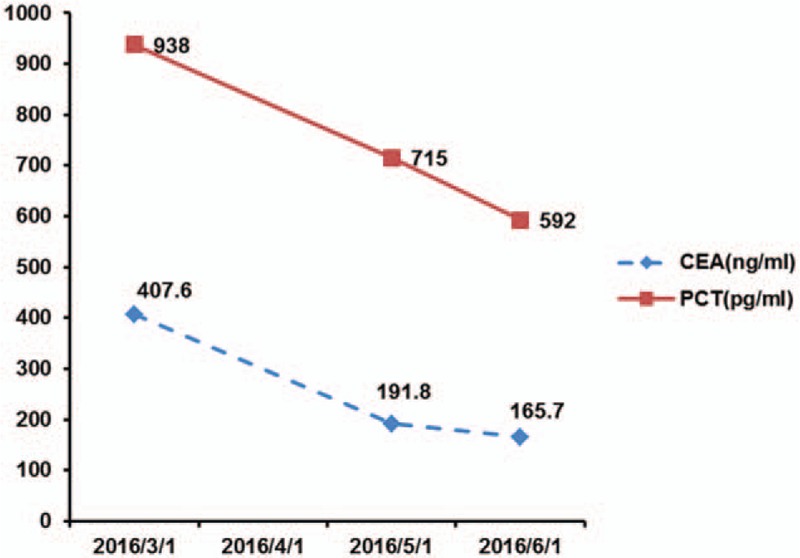
Levels of carcinoembryonic antigen and calcitonin before and after treatment. CEA = carcinoembryonic antigen, PCT = calcitonin.
Figure 4.
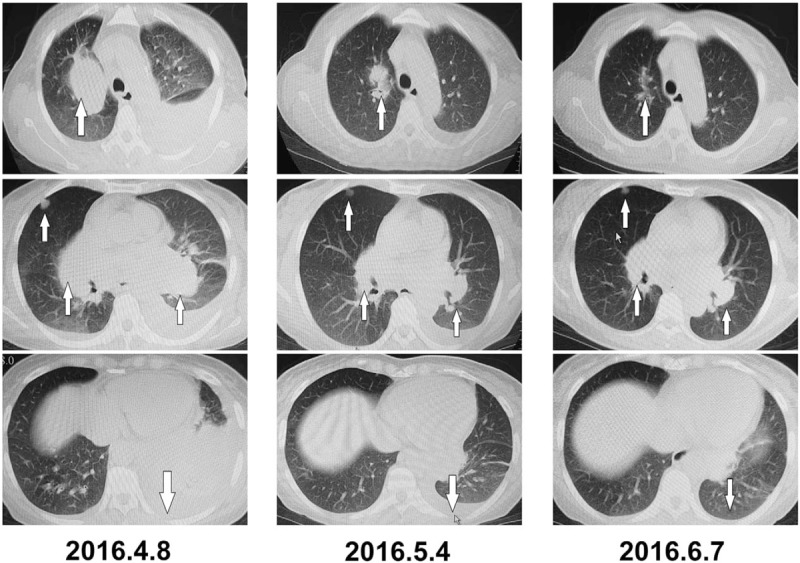
Metastatic nidi on the chest computed tomography images before and after treatment.
Medication-related adverse events: In May 2016, the patient had systemic migrating bone and joint pains (grade 2 based on the NCI CTC 4.0 criteria). Results from the autoimmune antibody examination were normal. Results from the total-body bone scan performed on June 8, 2016 indicated no abnormalities except a mild accumulated shadow at the left posterior 5th rib. Temporary apatinib discontinuation or oral medication with 200 mg celecoxib twice daily was administered for analgesia and the pain was relieved. In June 2016, the patient suffered from grade II hypertension (140/90 mm Hg), and oral medication with 150 mg irbesartan once daily was administered, achieving good blood pressure control. Thus far, the patient has been followed up through November 2016 and both her general condition and the statuses of the lesions were stable.
3. Discussion
MTC is a malignancy originating from the thyroid parafollicular cells (C cells). Although MTC only accounts for 3% to 8% of all thyroid cancers,[1,2] it represents up to 13.4% of thyroid cancer-related deaths.[3] C cell is the endocrinal cell that originates in the spinal cord, namely amine precursor uptake decarboxylation cell, which secretes 5-serotonin, histamine, prostaglandins, and adrenocorticotropic hormone like substances. Thus, results in painless intractable diarrhea, bone pain, facial flushing, and other endocrinal symptoms in a portion of patients. Some reports[2,5] exhibited diarrhea as a relatively specific clinical manifestation of MTC, and could reflect the differentiation level of MTC, with better prognosis for patient's having diarrhea. MTC can be classified as sporadic or familial. Domestic data show that MTC cases are mainly sporadic and no familial types have been found in China, even though familial MTCs account for 20% of total MTC cases, as reported by foreign studies.[6] MTC is a multiple endocrinal carcinoma that mainly manifests as multiple bilateral lesions. Serum calcitonin has been universally regarded as a sensitive and specific marker for MTC, which is of great significance for the early diagnosis, assessment of efficacy and residual reoccurrence and metastases, and postoperative observations in MTC cases. Some studies[7–9] reported that an increased level of calcitonin was correlated with poor prognosis of MTC.
In our case, the patient had no familial history and only suffered from unilateral lesions; therefore, it is likely that this was sporadic MTC. After the surgery, regular calcitonin monitoring was not achieved for this patient. Within 1 year of starting treatment, she started to suffer from painless diarrhea and a diagnosis of irritable bowel syndrome was made. Antidiarrheal medications such as diosmectite were administered by an outside hospital, and this interfered with the calcitonin monitoring. Then, we prescribed apatinib for antitumor treatment, and symptoms of diarrhea disappeared 2 weeks later. According to previous studies,[2,5] MTC patients may experience painless intractable diarrhea, bone pain, facial flushing, and other endocrine symptoms. However, diarrhea can reflect the differentiation of MTC; therefore, we considered the symptoms of diarrhea as endocrine symptoms caused by the reoccurrence of MTC.
In terms of treatment, for patients with persistent or recurrent MTC, chemotherapy should not be the first-line option owing to the low response rate. For MTC patients with significant tumor nidi, obvious symptoms, or progressive metastatic nidi, tyrosine kinase inhibitors (TKIs) that suppress rearranged during transfection (RET) and vascular endothelial growth factor receptor (VEGFR) are considered to be the first-choice systemic therapeutic approaches.[10,11] From the perspective of clinical features, it is concluded that MTC was robustly invasive and susceptible to the metastasis occurring in the early stages via lymphatic and vascular transportation. The mechanism of angiogenesis plays an important role in metastasis. Immunohistochemistry results from a study[12] displayed that VEGFA, VEGFR1, and VEGFR2 were overexpressed in >90% of MTC patients, indicating the essential role of VEGFR transactivation in rapid tumor growth. VEGFR activates downstream signaling pathways of the network, including phosphatidylinositol-3-kinase/protein kinase B pathway, and hence promotes tumor angiogenesis and blood supply. Its biological activities include promotion of endothelial cell proliferation and survival, enhancement of endothelial cell migration and invasion, which subsequently increases the vascular permeability, promotes chemotaxis and homing of bone marrow-derived vascular progenitor cells.[13] Therefore, VEGFR-associated angiogenesis remains to be a critical target for molecular-targeted therapy of MTC.[14]
RET oncogene is another critical target for molecular-targeted therapy of MTC. The mechanism under RET-targeted drug therapy attributes to the signal transduction pathway of RET/PTC-RAS-RAF-mitogen-activated protein kinase/extracellular-regulated protein kinase-mitogen-activated protein kinase. Blockade of RET mismatched repair and fusion, together with inhibition of transactivation of the rearranged gene in the thyroid follicular cell occurs in this signaling pathway, which leads to the impairment of uncontrolled cell proliferation; or, blockade of epigenetic mutation at specific sites inhibit RET protein aberrations. This in turn inhibits tyrosine kinase self-phosphorylation and pathogenesis of MTC from the thyroid parafollicular cell. Many new drugs targeting these 2 genes have been developed (Table 1).
Table 1.
Multitarget kinase inhibitors used in the treatment of thyroid cancer and their main cellular targets[13].
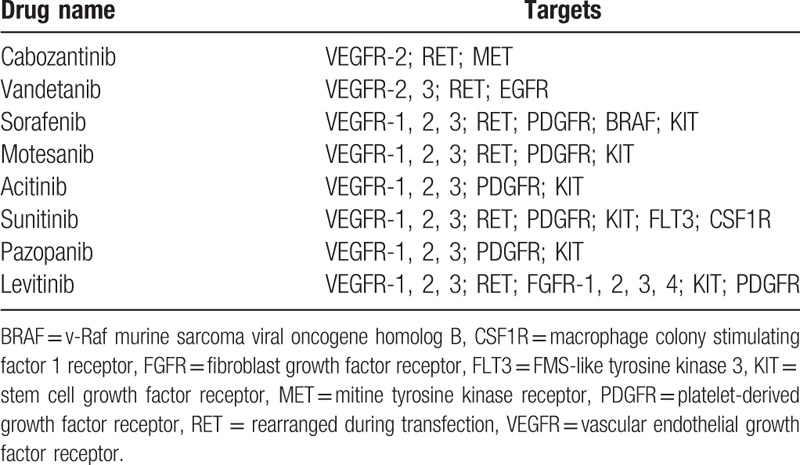
According to the 2013 NCCN Clinical Practice Guidelines for thyroid carcinoma,[15] targeted therapy with cabozantinib or vandetanib is the type I recommendation for recurrent, persistent, or metastatic MTC. Vandetanib is a nonspecific TKI that specifically targets VEGFR-2, EGFR, and RET, and is approved by Food and Drug Administration for the treatment of advanced MTC. Cabozantinib is also a TKI that targets VEGFR-2 and MET, but had higher affinity to RET than Vandetanib. However, phase III trials of vandetanib[16] and cabozantinib[17] for MTC have only shown improved progression-free survival, not long-term survival, in MTC patients. Other trials for drugs targeting MTC (such as sorafenib, axitinib, and motesanib) are still at phase II and lack evidence from phase III trials.
Since the patient did not have access to vandetanib or cabozantinib and her economic condition did not allow her to receive some of the more expensive therapies, she and her relatives opted for the domestically developed targeted drug apatinib. As an orally administered low-molecular weight VEGFR-targeting TKI recently developed in China, apatinib can effectively inhibit the VEGF signaling pathway, demonstrating a particularly high selectivity for VEGFR-2.[18,19] Apatinib blocks the signal transduction pathway emanated from VEGF binding with the cognate receptor, which thus potently inhibits the tumor angiogenesis, playing an antitumor role. At higher concentrations, apatinib can also inhibit platelet-derived growth factor receptor, C-KIT, SRC, and other kinases. Clinical studies show that apatinib is effective and well-tolerated during treatment of multiple cancers, such as advanced gastric cancer, liver cancer, breast cancer, and advanced liposarcoma.[19,20] Lin et al[21] have investigated the antitumor activities and mechanisms of apatinib in RET-rearranged lung adenocarcinoma, finding that the KIF5B-RET fusion protein promoted cell invasion and migration, possibly via the Src signaling pathway. The antitumor effects of apatinib are realized via cytotoxicity and inhibition of the RET/Src signaling pathway, which further inhibit metastasis and invasion. These results support the potential activity of apatinib for the treatment of KIF5B-RET-driven tumors. Also, RET gene mutations are believed to be associated with MTC pathogenesis.
Apart from MTC, a team led by Professor Yansong Lin from the Department of Nuclear Medicine, Peking Union Medical College Hospital initiated a phase II trial for apatinib in patients with radioactive iodine-refractory differentiated thyroid cancer in early 2016.[22] Ten patients were enrolled in the study. Rapid and conspicuous efficacy was observed after treatment with apatinib in the findings of serology, CT imaging, and tumor metabolic characteristics. After 2-week treatment with apatinib, Tg reduction reached up to 70% in majority (80%) of the patients. Their results showed that 90% of the patients showed PR after 8 weeks of treatment, achieving a superior short-term remission rate compared with the results of previously reported studies on sorafenib or levitinib. This result has further indicated the application prospects of apatinib in radioactive iodine-refractory differentiated thyroid cancer. A national multicenter phase III clinical register study by Professor Yansong Lin's group is currently ongoing (ClinicalTrials.gov ID: NCT03048877). Also a newly paper shows that apatinib also has a good effect on osteosarcoma with pulmonary metastases and may be related to the rich vascular supply of osteosarcoma.[23]
In terms of adverse events, milder adverse events have been observed with apatinib compared with other targeted drugs, which may be because of its high selectivity for VEGFR-2. These adverse events mainly include hypertension, proteinuria, hand–foot syndrome, and increased bleeding. In our case, adverse events related to the use of apatinib were systemic migrating bone and joint pains and grade II hypertension. For systemic migrating bone and joint pains, we conducted a total-body bone scan but observed no metastases to the bone. In addition, all of the autoimmune indicators were normal once the pain was relieved after discontinuing apatinib. We later administered oral celecoxib in combination with apatinib to the patient, and her pain was then tolerable. Thus, we considered these symptoms to be adverse effects of apatinib, even though bone pain has not been previously reported as an adverse event.
Our follow-up duration for this patient was rather short, and more cases and continuous follow-up are needed to further identify the efficacy and side effects of apatinib in the treatment of MTC. Unfortunately, neither the patient nor her family underwent genetic testing for RET because of economic considerations. Nevertheless, we still infer that the roles of apatinib in MTC involve antiangiogenesis and genetic inhibition of dysfunctional RET signaling. We believe that more patients will benefit from the use of apatinib if future basic studies and clinical trials can further determine the therapeutic mechanisms and indications of apatinib for MTC.
Footnotes
Abbreviations: CEA = carcinoembryonic antigen, CK = cytokeratin, CT = computed tomography, MTC = medullary thyroid carcinoma, NCCN = National Comprehensive Cancer Network, PR = partial response, RET = rearranged during transfection, TKIs = tyrosine kinase inhibitors, VEGFR = vascular endothelial growth factor receptor.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Tu G. Modern Head and Neck Cancer Surgery. Beijing: Science Press; 2004. [Google Scholar]
- [2].Kebebew E, Ituarte PH, Siperstein AE, et al. Medullary thyroid carcinoma: clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer 2000;88:1139–48. [DOI] [PubMed] [Google Scholar]
- [3].Kazaure HS, Roman SA, Sosa JA. Medullary thyroid microcarcinoma: a population-level analysis of 310 patients. Cancer 2012;118:620–7. [DOI] [PubMed] [Google Scholar]
- [4].Ahmed M, Barbachano Y, Riddell A, et al. Analysis of the efficacy and toxicity of sorafenib in thyroid cancer: a phase II study in a UK based population. Eur J Endocrinol 2011;165:315–22. [DOI] [PubMed] [Google Scholar]
- [5].Shang J, Wang K, Liu A. The prognostic factors of medullary thyroid carcinoma. Chin Arch Otolaryngol Head Neck Surg 2003;10:291–3. [Google Scholar]
- [6].Corsello SM, Lovicu RM, Migneco MG, et al. Diagnostic approach, genetic screening and prognostic factors of medullary thyroid carcinoma. Rays 2000;25:257–66. [PubMed] [Google Scholar]
- [7].Cohen R, Campos JM, Salaun C, et al. Preoperative calcitonin levels are predictive of tumor size and postoperative calcitonin normalization in medullary thyroid carcinoma. Groupe d’Etudes des Tumeurs a Calcitonine (GETC). J Clin Endocrinol Metab 2000;85:919–22. [DOI] [PubMed] [Google Scholar]
- [8].Scheuba C, Kaserer K, Kotzmann H, et al. Prevalence of C-cell hyperplasia in patients with normal basal and pentagastrin-stimulated calcitonin. Thyroid 2000;10:413–6. [DOI] [PubMed] [Google Scholar]
- [9].Brandao LG, Cavalheiro BG, Junqueira CR. Prognostic influence of clinical and pathological factors in medullary thyroid carcinoma: a study of 53 cases. Clinics (Sao Paulo) 2009;64:849–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Feng S, Liu C. An interpretation of the revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Chin J Endocrinol Metab 2016;32:356–60. [Google Scholar]
- [11].Wells SA, Jr, Asa SL, Dralle H, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015;25:567–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sherman SI. Advances in chemotherapy of differentiated epithelial and medullary thyroid cancers. J Clin Endocrinol Metab 2009;94:1493–9. [DOI] [PubMed] [Google Scholar]
- [13].Guleng B, Tateishi K, Ohta M, et al. Blockade of the stromal cell-derived factor-1/CXCR4 axis attenuates in vivo tumor growth by inhibiting angiogenesis in a vascular endothelial growth factor-independent manner. Cancer Res 2005;65:5864–71. [DOI] [PubMed] [Google Scholar]
- [14].Xu D, Dai W. Targeted therapies for medullary thyroid carcinoma. Chin J Bases Clin General Surg 2014;21:1579–82. [Google Scholar]
- [15].Robert I.Haddad, et al. National Comprehensive Cancer Network. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: thyroid carcinoma. Version 2, 2013. [DOI] [PubMed] [Google Scholar]
- [16].Wells SA, Jr, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol 2012;30:134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Elisei R, Schlumberger MJ, Muller SP, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol 2013;31:3639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Li J, Zhao X, Chen L, et al. Safety and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor YN968D1 in patients with advanced malignancies. BMC Cancer 2010;10:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Li J, Qin S, Xu J, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol 2013;31:3219–25. [DOI] [PubMed] [Google Scholar]
- [20].Dong M, Bi J, Liu X, et al. Significant partial response of metastatic intra-abdominal and pelvic round cell liposarcoma to a small-molecule VEGFR-2 tyrosine kinase inhibitor apatinib: a case report. Medicine (Baltimore) 2016;95:e4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lin C, Wang S, Xie W, et al. Apatinib inhibits cellular invasion and migration by fusion kinase KIF5B-RET via suppressing RET/Src signaling pathway. Oncotarget 2016;7:59236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lin Y, Wang C, Gao W, et al. Overwhelming rapid metabolic and structural response to apatinib in radioiodine refractory differentiated thyroid cancer. Oncotarget 2017;8:42252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhou Y, Zhang W, Tang F, et al. A case report of apatinib in treating osteosarcoma with pulmonary metastases. Medicine (Baltimore) 2017;96:e6578. [DOI] [PMC free article] [PubMed] [Google Scholar]


