Abstract
Background:
The laparoscopic mini-gastric bypass is a newly emerged surgical procedure in recent years. Owe to safe and simple process and effective outcomes, laparoscopic mini-gastric bypass has quickly become one of the most popular procedures in some countries. The safety and effectiveness of laparoscopic mini-gastric bypass versus laparoscopic sleeve gastrectomy remain unclear.
Methods:
A systematic literature search was performed in PubMed, Embase, Cochrane library from inception to May 20, 2017. The methodological quality of Randomized Controlled Trials and non-Randomized Controlled Trials were, respectively, assessed by Cochrane Collaboration's tool for assessing risk of bias and Newcastle–Ottawa scale. The meta-analysis was performed by RevMan 5.3 software.
Results:
Patients receiving mini-gastric bypass had a lot of advantageous indexes than patients receiving sleeve gastrectomy, such as higher 1-year EWL% (excess weight loss), higher 5-year EWL%, higher T2DM remission rate, higher hypertension remission rate, higher obstructive sleep apnea (OSA) remission rate, lower osteoarthritis remission rate, lower leakage rate, lower overall late complications rate, higher ulcer rate, lower gastroesophageal reflux disease (GERD) rate, shorter hospital stay and lower revision rate. No significant statistical difference was observed on overall early complications rate, bleed rate, vomiting rate, anemia rate, and operation time between mini-gastric bypass and sleeve gastrectomy.
Conclusion:
Mini-gastric bypass is a simpler, safer, and more effective bariatric procedure than laparoscopic sleeve gastrectomy. Due to the biased data, small sample size and short follow-up time, our results may be unreliable. Large sample and multicenter RCT is needed to compare the effectiveness and safety between mini-gastric bypass and sleeve gastrectomy. Future study should also focus on bile reflux, remnant gastric cancer, and long term effectiveness of mini-gastric bypass.
Keywords: bariatric, mini-gastric bypass, obesity, omega gastric bypass, single anastomosis gastric bypass, sleeve gastrectomy
1. Introduction
More and more people suffer from morbid obesity because of the increased living standard and decreased physical exercise in the past several decades. According to a recent report based on USA population, the incidence of obese among adults even reaches up to 34.9%.[1] Between 1980 and 2008, the mean global body mass index (BMI) was increasing by 0.4–0.5 kg/m2 per decade for both men and women.[2] Obesity and related comorbidities reduce life expectancy[3] and add economic burden,[4] which highlights the significance of bariatrics. The most effective therapy to treat obese and related comorbidities is bariatric surgery, in which Roux-en-Y gastric bypass (RYGBP) and sleeve gastrectomy (SG) are two most popular procedures[5,6]. Introduced by D.W. Hess et al in 1988 as part of the biliopancreatic diversion,[7,8] SG is one of the most popular procedures (37%) in the world.[9] SG is a technically less complex procedure with short learning curve and effective weight loss,[8] but it suffers from two outstanding disadvantages including high risk of weight regain and gastro-esophageal reflux disease (GERD).[10,11] Mini-gastric bypass (MGB), also known as single anastomosis gastric bypass or omega gastric bypass, is a newly emerged procedure originated from Rutledge.[12] Due to safe and simple process as well as effective outcomes, MGB has quickly become one of the most popular procedures in many countries.[13,14] Despite of popular status, the extension of MGB is still limited by some concerns such as gastric and oesophageal bile reflux, marginal ulcer, poor follow-up, and remnant gastric cancer.[15] During the past decade, many observational studies have proved the considerable short-term and long-term outcomes of MGB,[16,17] but comparative studies between MGB and SG are still scarce. For this reason, we conducted a meta-analysis to help the surgeon make a better selection between MGB and SG.
2. Material and methods
2.1. Search strategy
A systematic literature search was conducted in PubMed, Embase, and Cochrane library from inception to May 20, 2017. The search strategy for Medline is as follows which was applied to other databases:” (((((((((((mason's loop[Title/Abstract]) OR mini-gastric bypass[Title/Abstract]) OR mini-gastric bypass[Title/Abstract]) OR single anastomosis gastric bypass[Title/Abstract]) OR single-anastomosis gastric bypass[Title/Abstract]) OR single anastomosis (mini-) gastric bypass[Title/Abstract]) OR one anastomosis (mini-) gastric bypass[Title/Abstract]) OR one anastomosis gastric bypass[Title/Abstract]) OR one-anastomosis gastric bypass[Title/Abstract]) OR omega gastric bypass[Title/Abstract]) OR omega-loop bypass[Title/Abstract]) OR omega loop bypass[Title/Abstract]” Randomized control trials (RCTs), two-arm prospective studies, retrospective studies, and cohort studies were included. The reference list of potential studies was manually searched for eligibility by two independent reviewers, and if there was disagreement regarding inclusion, a third reviewer was consulted. Our study was approved by Ethics Committee of Beijing Tiantan Hospital.
2.2. Inclusion criteria
(1) Comparative studies between MGB and SG; (2) patients were adults, with age ranging from 20 to 70 years old; (3a) at least one of the following concerned endpoints was included: operation time, mortality, overall early complications, specific early complications, overall late complications, specific late complications, hospital stay, revision rate, remission rate of comorbidities,1-year %EWL or 5-year %EWL.
2.3. Exclusion criteria
(1) Observational studies or comparative studies between MGB and non-SG; (2) studies including adolescents or elderly patients; (3) no concerned endpoints were included; (4) duplicate studies; (5) low quality studies.
2.4. Data extraction
Data extraction was cross-checked synchronously between two authors to rule out any discrepancy. The third author made a final decision for the discrepancy. The following data were independently extracted for each included study: author, publication year, study design, sample size, proportion of female, patients’ mean age, preoperative BMI, operation time, blood loss, 1-year follow-up rate, mortality, overall early complications rate, specific early complications rate (leakage, bleed, abscess, dyspepsia, and wound infection), overall late complications rate, specific late complications rate (ulcer, stenosis, hypoalbuminemia, vomiting, anemia, reflux, internal hernia, GERD, malnutrition, and cholelithiasis), hospital stay, revision rate, remission rate of comorbidities (T2DM, hypertension, OSA, ostearthritis), 1-year %Excess Weight Loss (EWL) and 5-year %EWL. If data sets were overlapped or duplicated, only the most recent data were included. If necessary, the authors were contacted for additional information.
2.5. Assessment of methodological quality
The methodological quality of included cohort studies was performed by NOS (Newcastle–Ottawa scale). We modified NOS according to our previous study. The concrete content included selection of patients, comparability, and assessment of results. When scored ≤5, the cohort study was assessed as low quality and excluded from our meta-analysis; when scored>5, the study was assessed as high quality and included in our meta-analysis. The methodological quality of included RCTs was performed by the Cochrane Collaboration's tool for assessing risk of bias. The concrete content included random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; selective reporting; other sources of bias.
2.6. Endpoints
The primary endpoints included 1-year %EWL, 5-year %EWL, and remission rate of comorbidities (T2DM, hypertension, OSA, ostearthritis). The secondary endpoints included overall early complications rate, leakage rate, and postoperative bleed rate, overall late complications rate, ulcer rate, vomiting rate, anemia rate, GERD rate, hospital stay, and revision rate. The last endpoint was operation time.
2.7. Statistical analysis
Statistical analysis was carried out by RevMan 5.3 software. Risk ratio (RR) was calculated to express the effect size of dichotomous variables such as remission rate of comorbidities, overall early complications rate, leakage rate, postoperative bleed rate, overall late complications rate, ulcer rate, vomiting rate, anemia rate, GERD rate, and revision rate. Standard mean difference (SMD) was calculated to express the effect size of continuous variables such as 1-year %EWL, 5-year %EWL hospital stay, and operation time. I2 statistic was used to show the heterogeneity between studies. The random effects model was used when there was significant heterogeneity between studies (I2 ≥ 50%); on the contrary, the fixed effect model was used when there was no significant heterogeneity between studies (I2 < 50%).
3. Results
One study[18] was obtained by referring the reference list of included studies. One low quality paper[19] was excluded after assessment of methodological quality. At last, a total of 12 cohort studies[8,11,13,18,20–27] and 2 RCTs[28,29] were included in our meta-analysis (The flowchart of selecting procedure is shown in Fig. 1). Our meta-analysis included 3862 patients (1998 patients in MGB group, 1864 patients in SG group, respectively). There were 2 studies[25,27] on super obese patients (preoperative BMI > 50 kg/m2) and 3 studies[13,18,24] on the patients with preoperative T2DM. The basic characteristics of included studies are presented in Table 1. The bias of risk of cohort studies and RCTs are presented in Table 2 and Figure 2, respectively.
Figure 1.
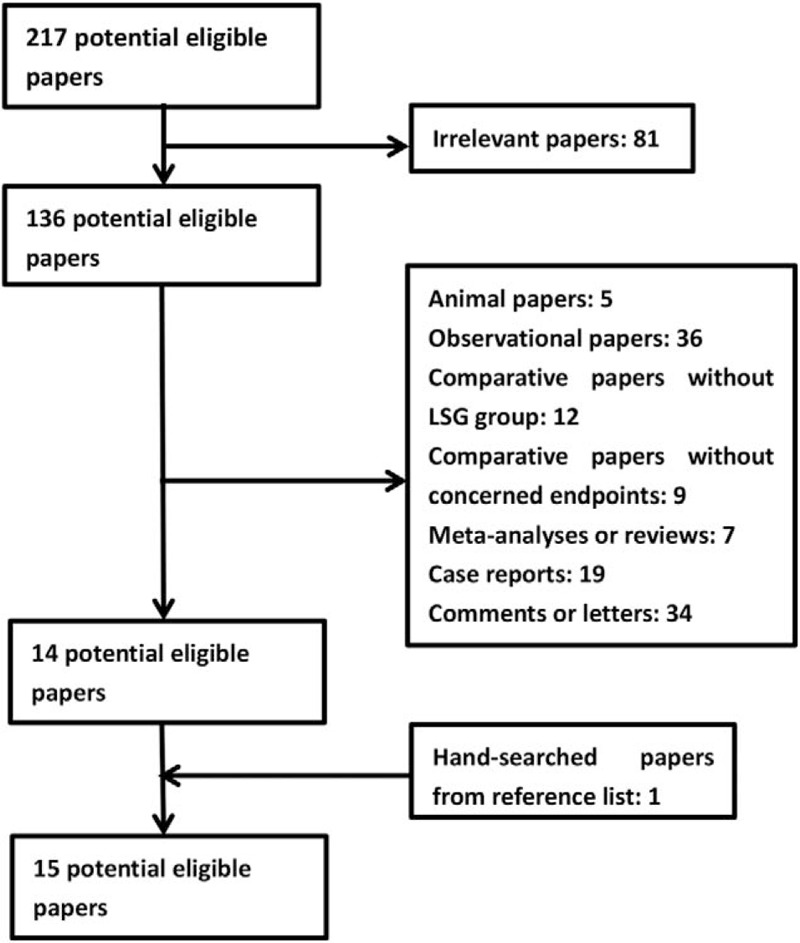
Flowchart of paper inclusion.
Table 1.
Basic characteristics of included studies (mini-gastric bypass/sleeve gastrectomy).
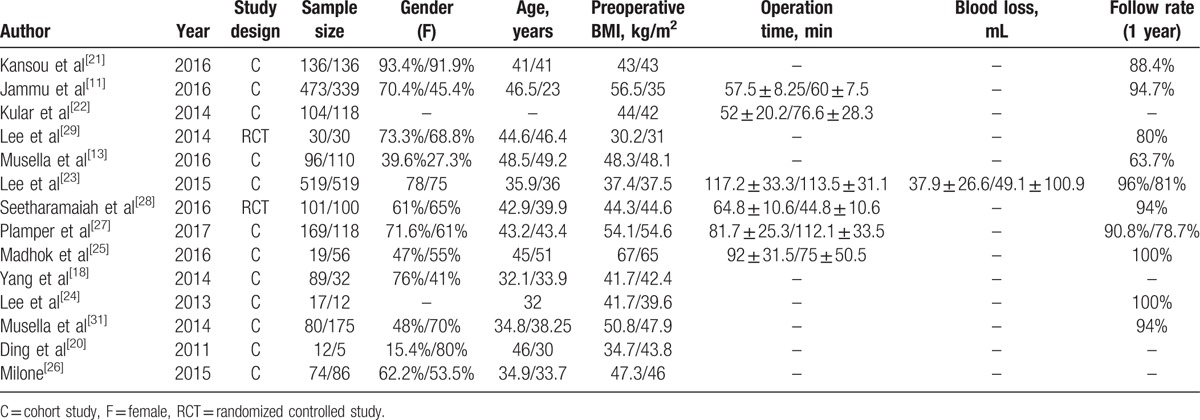
Table 2.
Assessment of methodological quality of cohort study.
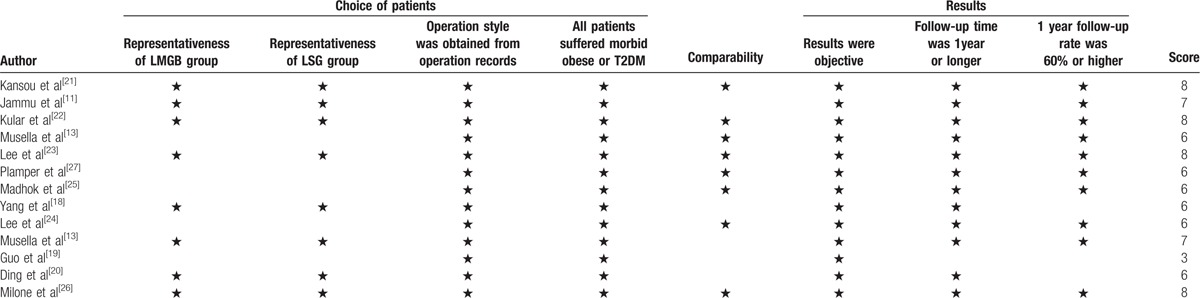
Figure 2.
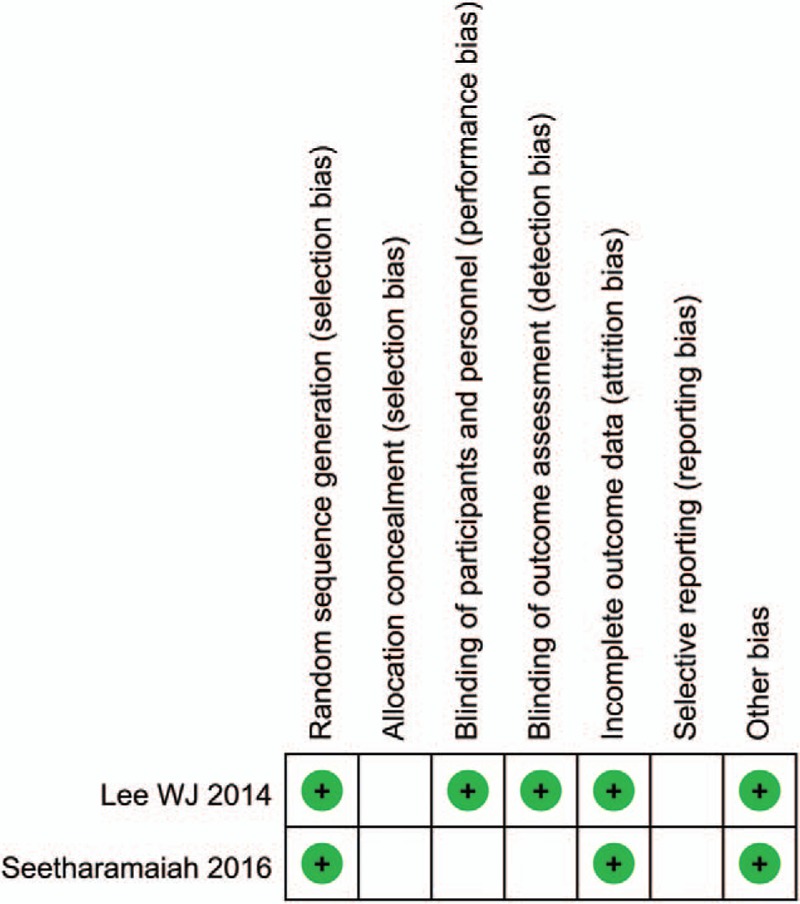
Risk of bias of included RCTs. RCT = randomized control trials.
3.1. Primary endpoints
3.1.1. One-year EWL%
A total of 7 studies[13,18,21,22,25,27,28] reported the 1-year EWL% in our meta-analysis (Table 3). I2 = 81%, so the random effects model was used to pool the 7 studies. The result indicated MGB group had a higher 1-year %EWL than SG group (P = .005) (Fig. 3A).
Table 3.
Resolution of comorbidities, %EWL and weight regain of included studies (mini-gastric bypass/sleeve gastrectomy).
Figure 3.
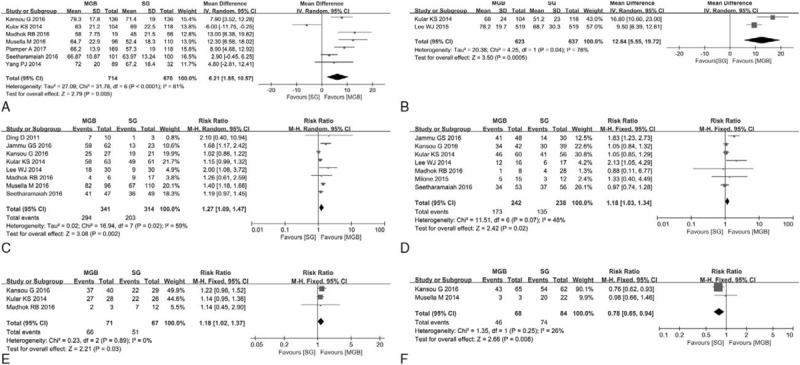
(A) 1 year EWL% of MGB versus SG. (B) 5 years EWL% of MGB versus SG. (C) T2DM remission rate of MGB versus SG. (D) Hypertension remission rate of MGB versus SG. (E) OSA remission rate of MGB versus SG. (F) Ostearthritis remission rate of MGB versus SG. EWL = excess weight loss, MGB = mini-gastric bypass, OSA = obstructive sleep apnea, SG = sleeve gastrectomy, T2DM = type 2 diabetes mellitus.
3.1.2. 5-year EWL%
A total of 3 studies[22,23,29] reported the 5-year EWL% in our meta-analysis (Table 3). Since there were 2 studies involving overlap of data sets,[23,29] the most recent study was used.[23]I2 = 76%, so the random effects model was used to pool the 2 studies. The result indicated MGB group had a higher 5-year %EWL than SG group (P < .001) (Fig. 3B).
3.2. Remission rate of T2DM
A total of 10 studies[8,11,13,20–22,24,25,28,29] reported the remission rate of T2DM in our meta-analysis (Table 3). As there were 4 studies with overlap of data sets,[8,13,24,29] the 2 most recent studies were used.[13,29]I2 = 59%, so the random effects model was used to pool the 8 studies.[11,13,20–22,25,28,29] The result indicated MGB group had a higher remission rate of T2DM than SG group (P = .002) (Fig. 3C).
3.3. Remission rate of hypertension
A total of 7 studies[11,21,22,25,26,28,29] reported the remission rate of hypertension in our meta-analysis (Table 3). I2 = 48%, so the random effects model was used to pool the 6 studies. The result indicated MGB group had a higher remission rate of hypertension than SG group (P = .02) (Fig. 3D).
3.4. Remission rate of OSA
A total of 3 studies[21,22,25] reported the remission rate of OSA in our meta-analysis (Table 3). I2 = 0%, so the fixed effects model was used to pool the 3 studies. The result indicated MGB group had a higher remission rate of OSA than SG group (P = .03) (Fig. 3E).
3.5. Remission rate of ostearthritis
A total of 2 studies[8,21] reported the remission rate of osteoarthritis in our meta-analysis (Table 3). I2 = 26%, so the fixed effects model was used to pool the 2 studies. The result indicated MGB group had a lower remission rate of ostearthritis than SG group (P = .008) (Fig. 3F).
3.6. Secondary endpoints
3.6.1. Overall early complications rate
A total of 7 studies[8,13,21,22,23,25,27] reported overall early complications rate in our meta-analysis (Table 4). Since there were 2 studies with overlap of data sets[8,13] overlapped, the most recent study was used. I2 = 51%, so the random effects model was used to pool the 6 studies. No difference of overall early complications rate was found between MGB and SG (P = .28) (Fig. 4A).
Table 4.
Mortality, morbidity and hospital stay of included studies (mini-gastric bypass/sleeve gastrectomy).
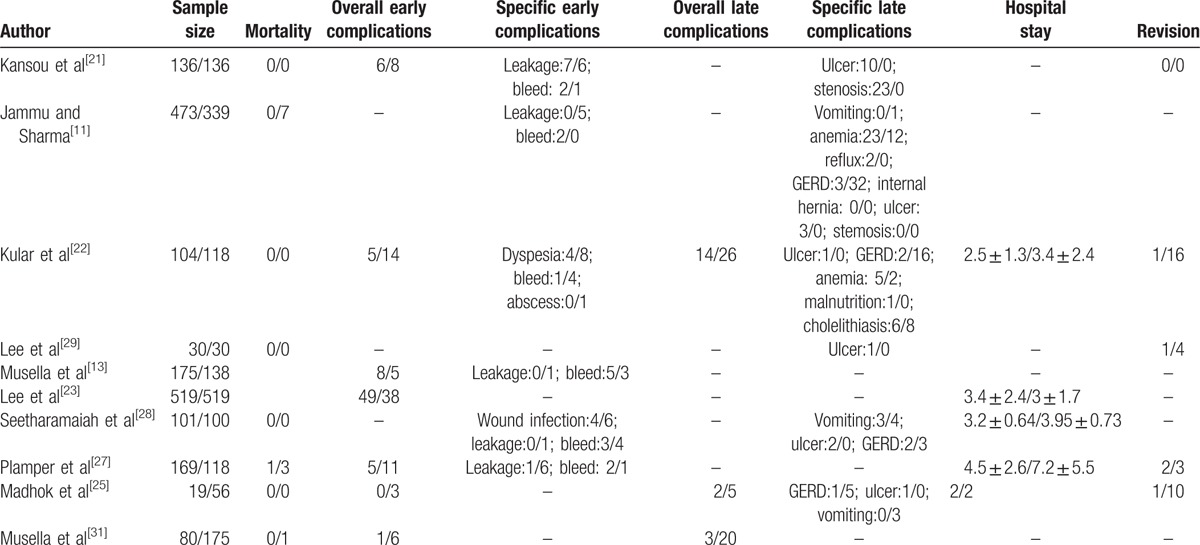
Figure 4.

(A) Overall early complications rate of MGB versus SG. (B) Leakage rate of MGB versus SG. (C) Bleed rate of MGB versus SG. (D) Overall late complications rate of MGB versus SG. (E) Ulcer rate of MGB versus SG. (F) Vomiting rate of MGB versus SG. (G) Anemia rate of MGB versus SG. (H) GERD rate of MGB versus SG. (i) Hospital stay of MGB versus SG. (J) Revision rate of MGB versus SG. (K) Operation time of MGB versus SG. GERD = gastroesophageal reflux disease, MGB = mini-gastric bypass, SG = sleeve gastrectomy.
3.6.2. Leakage rate
A total of 5 studies[11,13,21,27,28] reported leakage rate in our meta-analysis (Table 4). I2 = 41%, so the fixed effects model was used to pool the 5 studies. The result indicated MGB group had a lower leakage rate than SG group (P = .02) (Fig. 4B).
3.6.3. Bleed rate
A total of 6 studies[11,13,21,22,27,28] reported bleed rate in our meta-analysis (Table 4). I2 = 0%, so the fixed effects model was used to pool the 6 studies. No difference of leakage rate was found between MGB and SG (P = .95) (Fig. 4C).
3.6.4. Overall late complications rate
A total of 3 studies[8,22,25] reported overall late complications rate in our meta-analysis (Table 4). I2 = 0%, so the fixed effects model was used to pool the 3 studies. The result indicated MGB group had a lower overall late complications rate than SG group (P = .02) (Fig. 4D).
3.6.5. Ulcer rate
A total of 6 studies[11,21,22,25,28,29] reported ulcer rate in our meta-analysis (Table 4). I2 = 0%, so the fixed effects model was used to pool the 6 studies. The result indicated MGB group had a higher ulcer rate than SG group (P = .001) (Fig. 4E).
3.6.6. Vomiting rate
A total of 3 studies[11,25,28] reported vomiting rate in our meta-analysis (Table 4). I2 = 0%, so the fixed effects model was used to pool the 3 studies. No difference of vomiting rate was found between MGB and SG (P = .36 (Fig. 4F).
3.6.7. Anemia rate
A total of 2 studies[11,22] reported anemia rate in our meta-analysis (Table 4). I2 = 0%, so the fixed effects model was used to pool the 3 studies. No difference of anemia rate was found between MGB and SG (P = .17 (Fig. 4G).
3.6.8. GERD rate
A total of 4 studies[11,22,25,28] reported GERD rate in our meta-analysis (Table 4). I2 = 52%, so the random effects model was used to pool the 4 studies. The result indicated MGB group had a lower GERD rate than SG group (P = .006) (Fig. 4H).
3.7. Hospital stay
A total of 4 studies[22,23,27,28] reported hospital stay in our meta-analysis (Table 4). I2 = 96%, so the random effects model was used to pool the 4 studies. The result indicated MGB group had a shorter hospital stay than SG group (P = .05) (Fig. 4I).
3.7.1. Revision rate
A total of 5 studies[21,22,25,27,29] reported revision rate in our meta-analysis (Table 4). I2 = 0%, so the fixed effects model was used to pool the 5 studies. The result indicated MGB group had a lower revision rate than SG group (P < .001) (Fig. 4J).
3.8. Operation time
A total of 6 studies[11,22,23,25,27,28] reported operation time in our meta-analysis (Table 1). I2 = 98%, so the random effects model was used to pool the 4 studies. No difference of operation time was found between MGB and SG (P = .58) (Fig. 4K).
4. Discussion
Proposed by Rutledge[12] in 2001, MGB has become one of the most popular surgical procedures for morbid obesity in many countries because of its high safety and effectiveness. Today, thousands of MGB cases have been reported and most of these cases showed MGB had similar or superior safety and effectiveness than SG or RYGB.[12,30–35] To our knowledge, a total of 8 large sample (≥1000), retrospective, and observational studies[12,31,33–35,36–38] have proven the safety and effectiveness advantage of MGB. However, comparative studies between MGB and SG are scarce or the sample size is too small. After RYGB, SG is now the second frequently used surgical procedure in the world. Many researches[39–41] have compared the safety and effectiveness of SG versus RYGB, and the results were variable. A meta-analysis including 62 comparative studies performed by Li et al[42] has shown patients receiving RYGB had a significantly higher percentage of excess weight loss and better resolution of hypertension, dyslipidemia, gastroesophageal reflux disease, and arthritis compared with those receiving SG. But Osland et al[43–45] have pooled the RCTs comparing RYGB with SG and concluded that SG and RYGB were comparable in weight loss outcomes, postoperative comorbid disease resolution (T2DM, hypertension, dyslipidemia, OSA, joint and musculoskeletal conditions, GERD) and early minor complications. Due to the variable results, we cannot make a conclusion about the effectiveness and safety between SG and RYGB. Large sample and multi-center RCT is needed to prove the better procedure between SG and RYGB in the future. Here, we performed a meta-analysis of MGB versus SG, in the hope of helping the bariatric surgeon make a better selection between MGB and SG.
4.1. EWL%
In the 13 included studies, the 1-year EWL% for MGB and SG were, respectively, 58% to 79.3% and 45% to 71.4%, while the 5-year EWL% were are 68% to 78.2% and 51.2% to 68.7%, respectively. We reviewed the observational studies and found a similar EWL% for MGB.[12,31,33–38] Our result indicated MGB had a superior 1-year EWL% and 5-year EWL% than SG. The maximum EWL% always occurs in 2 years after surgery according to previous studies,[33,46] in view of which, future studies should compare MGB and SG in terms of 2-year or longer EWL%. Although we pooled the 5-year EWL%, minor sample size may influence the stability of the result. The higher EWL% of MGB may be due to different mechanisms between MGB and SG. As we all know, SG is a restrictive procedure, but MGB is a restrictive and malabsorptive procedure.
4.2. Remission rate of comorbidities
The most common comorbidities of morbid obesity are T2DM, hypertension, OSA and ostearthritis, among which, T2DM is the most harmful one. Remission of T2DM was defined as HbA1C level < 6.5% in 3 studies,[13,20,28] <6% in 1 study,[22] untold in 4 studies.[11,21,25,29] Remission of hypertension, OSA, and ostearthritis was defined as normalization of baseline characteristics without using drugs or continuous positive-pressure airway machine.[8,21,22,25] The overall remission rate of T2DM, hypertension, OSA, and ostearthritis was 86%, 75%, 93%, 68% for MGB and 65%, 60%, 76%, 88% for SG, respectively. Previous large sample size and observational studies on MGB showed a remission rate of 84.1% to 94%,[16,33,34,37] 52.1% to 94%,[16,33,34] 50% to 90%,[33,34] and 18% to 36.5%[33,34] for T2DM, hypertension, OSA and ostearthritis, respectively. Our results indicated MGB had a higher remission rate of T2DM, hypertension, OSA and a lower remission rate of ostearthritis than SG. The higher remission rate of comorbidities of MGB may be explained by foregut and hindgut hypothesis.[47,48] Due to the small sample size, the results on remission rate of OSA and ostearthritis may be unreliable. Future studies should include the endpoints of OSA and ostearthritis.
4.3. Early complications
The most common early complications include leakage, intraperitoneal bleed, wound infection, intraperitoneal abscess, and bowel obstruction. According to the results of our meta-analysis, the overall rate of early complications, leakage, and bleed of MGB were 6.5%, 0.76%, 1.3% versus 7.3%, 2.3%, 1.4% of SG. Rutledge et al[35] performed a retrospective and observational study on 2410 patients having MGB, results showed that the rate of early complications was 5.9% and rate of leakage was 1.08%. Noun et al[38] performed a similar study in 1000 consecutive patients, and results showed that the rate of early complications was 2.7% the rate of leakage was 0.43%, and the rate of bleeding was1.6%. Most recently, Taha et al[16] reported 1520 cases receiving MGB for consecutive 6 years, and results showed that the rate of early complications was 3.2% leakage rate was 0.1% and bleed rate was 1.7%. All the 3 large sample size observational studies presented the favorable rate early complications, which seemed superior than our results. Our results indicated MGB group had a similar overall rate early complications, similar bleed rate and lower leakage rate compared with SG group. The lower leakage rate in MGB group may be explained by the decreased intragastric pressures caused by pylorus exclusion.[42]
4.4. Late complications
The most common late complications include ulcer, stenosis, vomiting, anemia, bile reflux, GERD, and malnutrition. The overall rate of complications, ulcer, vomiting, anemia, and GERD were 9.4%, 2.1%, 0.51%, 4.9%, 1.1% for MGB versus 14.6%, 0%, 1.6%, 3.1%, 9.1% for SG. Previous large sample size and observational studies have reported the overall rate complications of 2% to 7.9%,[16,33,46] ulcer rate of 0.2% to 4%,[16,33–35,38,46] stenosis rate of 0.1% to 0.8%,[33,34,46] vomiting rate of,[34] anemia rate of 1.5% to 4.9%,[16,34,35] bile reflux rate of 0% to 1.6%,[16,33,38,46] GERD rate of 2%,[34] and malnutrition rate of 2%[34] for patients receiving MGB. Ulcer and stenosis generally happened at anastomosis area,[11,21,22,25,28,29] which may account for the higher ulcer rate in MGB patients, whereas no anastomosis existed in SG patients. The lower GERD rate may be due to decreased intragastric pressure in MGB patients, which has been proven by Tolone et al.[49] The authors hypothesized that the long narrow sleeve gastric tube could have caused an increase in intragastric pressure, triggering a rise in GERD. Bile reflux is the most concerned factor that limits the extension of MGB. Although previous observational studies reported a considerable rate of bile reflux (0%–1.6%), comparative studies on bile reflux between MGB and SG are rare. Tolone et al[49] performed a small sample size comparative study between MGB and SG, and concluded patients receiving MGB had significantly diminished total number of reflux episodes, including acidic, weakly acidic, and weakly alkaline reflux. The reason why bile reflux symptom was rarely described in previous reports on MGB was that the bile can be neutralized by gastric acid secreted by remnant gastric before flowing to the gastrointestinal anastomosis, and the neutralized bile had less stimulation on gastric mucosa. Our results showed MGB group had a lower vomiting rate and higher anemia rate than SG group, and there was no significant statistical difference between two groups. Only one study reported stenosis rate, bile reflux rate, and malnutrition rate in MGB and SG patients, so we did not pool these endpoints. Future comparative studies between MGB and SG should include the endpoints of bile reflux and malnutrition.
4.5. Hospital stay, operation time, and revision rate
Our results shown MGB patients had a shorter hospital stay, lower revision rate, and similar operation time than SG patients. The shorter hospital stay may be explained by less trauma in MGB. The major causes of revision were malnutrition,[22] bile reflux[27,29] for MGB patients, whereas weight regain,[22] severe GERD[22,25,27] for SG patients.
4.6. Previous meta-analysis
To our knowledge, there were 2 meta-analyses comparing MGB with SG published online. Quan et al[50] performed a meta-analysis of MGB versus SG, and concluded MGB group had the same %EWL (P = .51) and 1-year postoperative BMI (P = .38), lower revision rate (P = .004) and higher remission rate of T2DM (P = .004) than SG group. Only 6 studies were included in Quan, Y's meta-analysis, so the results were unreliable. Most recently, Magouliotis et al[51] performed a simple meta-analysis of MGB versus SG, wherein 10 English studies were included and most results (one-year EWL%, remission rate of T2DM, remission rate of hypertension, bleed rate, anemia rate, GERD rate, hospital stay, operation time, and revision rate) were similar to our meta-analysis. In our meta-analysis, MGB group had a higher remission rate of OSA and lower leakage rate than SG group (P = .03), which was different from the Magouliotis meta-analysis results. There were 3 main differences between our meta-analysis and the Magouliotis meta-analysis. First, there were more eligible studies and more patients included in our meta-analysis, which made our meta-analysis more reliable. Second, we pooled the additional endpoints of 5-year %EWL, remission rate of ostearthritis, overall rate of early complications, overall rate of late complications, ulcer rate, vomiting rate. Third, unlike the Magouliotis meta-analysis, the overlapped data were excluded from our meta-analysis. For example, data from the Milone[26] and Musella[13] study shared overlapped research time and their data were from the same hospital, and the data of the two studies were used by Magouliotis[26] to pool T2DM remission rate, while we excluded the earlier one.
4.7. Limitations
Our meta-analysis still had some limitations. First, only 2 of eligible studies were RCTs, the others were cohort studies with inherent selection bias. Second, small sample size and short follow-up time may influence the stability of result. Third, due to few eligible studies were included in our meta-analysis, so we did not perform an analysis of publication bias. Fourth, heterogeneity between studies was high in our meta-analysis, which may be explained by different basic patients’ characteristics of included studies and different surgical level of different hospital.
5. Conclusions
MGB is a simple, safe, and effective bariatric procedure. Due to the biased data, small sample size and short follow-up time, our results may be unreliable. Large sample and multicenter RCT is needed to compare the effectiveness and safety between mini-gastric bypass and sleeve gastrectomy. Future study should also focus on the endpoints of bile reflux, remnant gastric cancer and long term effectiveness in MGB patients.
Acknowledgments
Thanks to Zhao M and Cheng S for their advices on our meta-analysis.
Footnotes
Abbreviations: CS = cohort study, EWL = excess weight loss, GERD = gastroesophageal reflux disease, MGB = mini-gastric bypass, NOS = Newcastle–Ottawa Scale, OSA = obstructive sleep apnea, RCT = randomized control trial, RR = risk ratio, SG = sleeve gastrectomy, T2DM = type 2 diabetes mellitus.
Publication consent: Our paper does not contain any individual persons data, thus publication consent is not applicable.
Data availability statements: All our data supporting the results can be found.
The authors have no funding and no conflicts of interest to disclose.
References
- [1].Ogden CL, Carroll MD, Kit BK, et al. Prevalence of obesity among adults: United States, 2011–2012. NCHS Data Brief 2013;1–8. [PubMed] [Google Scholar]
- [2].Finucane MM, Stevens GA, Cowan MJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet 2011;377:557–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fontaine KR, Redden DT, Wang C, et al. Years of life lost due to obesity. JAMA 2003;289:187–93. [DOI] [PubMed] [Google Scholar]
- [4].Abegunde DO, Mathers CD, Adam T, et al. The burden and costs of chronic diseases in low-income and middle-income countries. Lancet 2007;370:1929–38. [DOI] [PubMed] [Google Scholar]
- [5].Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004;292:1724–37. [DOI] [PubMed] [Google Scholar]
- [6].Maggard MA, Shugarman LR, Suttorp M, et al. Meta-analysis: surgical treatment of obesity. Ann Int Med 2005;142:547–59. [DOI] [PubMed] [Google Scholar]
- [7].Prevot F, Verhaeghe P, Pequignot A, et al. Two lessons from a 5-year follow-up study of laparoscopic sleeve gastrectomy: persistent, relevant weight loss and a short surgical learning curve. Surgery 2014;155:292–9. [DOI] [PubMed] [Google Scholar]
- [8].Musella M, Milone M, Gaudioso D, et al. A decade of bariatric surgery. What have we learned? Outcome in 520 patients from a single institution. Int J Surg 2014;12(suppl 1):S183–8. [DOI] [PubMed] [Google Scholar]
- [9].Angrisani L, Santonicola A, Iovino P, et al. Bariatric surgery worldwide 2013. Obes Surg 2015;25:1822–32. [DOI] [PubMed] [Google Scholar]
- [10].Howard DD, Caban AM, Cendan JC, et al. Gastroesophageal reflux after sleeve gastrectomy in morbidly obese patients. Surg Obes Rel Dis 2011;7:709–13. [DOI] [PubMed] [Google Scholar]
- [11].Jammu GS, Sharma R. A 7-year clinical audit of 1107 cases comparing sleeve gastrectomy, Roux-En-Y gastric bypass, and mini-gastric bypass, to determine an effective and safe bariatric and metabolic procedure. Obes Surg 2016;26:926–32. [DOI] [PubMed] [Google Scholar]
- [12].Rutledge R. The mini-gastric bypass: experience with the first 1,274 cases. Obes Surg 2001;11:276–80. [DOI] [PubMed] [Google Scholar]
- [13].Musella M, Apers J, Rheinwalt K, et al. Efficacy of bariatric surgery in type 2 diabetes mellitus remission: the role of mini gastric bypass/one anastomosis gastric bypass and sleeve gastrectomy at 1 year of follow-up. A European survey. Obes Surg 2016;26:933–40. [DOI] [PubMed] [Google Scholar]
- [14].Lee WJ, Lin YH. Single-anastomosis gastric bypass (SAGB): appraisal of clinical evidence. Obes Surg 2014;24:1749–56. [DOI] [PubMed] [Google Scholar]
- [15].Mahawar KK, Carr WR, Balupuri S, et al. Controversy surrounding ’mini’ gastric bypass. Obes Surg 2014;24:324–33. [DOI] [PubMed] [Google Scholar]
- [16].Taha O, Abdelaal M. Outcomes of omega loop gastric bypass, 6-years experience of 1520 cases. Obes Surg 2017;27:1952–60. [DOI] [PubMed] [Google Scholar]
- [17].Lessing Y, Pencovich N, Khatib M, Meron-Eldar S, Koriansky J, Abu-Abeid S. One-anastomosis gastric bypass: first 407 patients in 1 year. Obes Surg. 2017. [DOI] [PubMed] [Google Scholar]
- [18].Yang PJ, Lee WJ, Tseng PH, et al. Bariatric surgery decreased the serum level of an endotoxin-associated marker: lipopolysaccharide-binding protein. Surg Obes Rel Dis 2014;10:1182–7. [DOI] [PubMed] [Google Scholar]
- [19].Guo X, Yin K, Chen D, et al. Impacts of laparoscopic bariatric surgery on GLP-1 and Ghrelin level in patients with type 2 diabetes mellitus. Zhonghua Wai Ke Za Zhi 2013;51:323–7. [PubMed] [Google Scholar]
- [20].Ding D, Chen D, Hu X, et al. Outcomes after laparoscopic surgery for 219 patients with obesity. Zhonghua Wei Chang Wai Ke Za Zhi 2011;14:128–31. [PubMed] [Google Scholar]
- [21].Kansou G, Lechaux D, Delarue J, et al. Laparoscopic sleeve gastrectomy versus laparoscopic mini-gastric bypass: one year outcomes. Int J Surg 2016;33(pt A):18–22. [DOI] [PubMed] [Google Scholar]
- [22].Kular KS, Manchanda N, Rutledge R. Analysis of the five-year outcomes of sleeve gastrectomy and mini-gastric bypass: a report from the Indian sub-continent. Obes Surg 2014;24:1724–8. [DOI] [PubMed] [Google Scholar]
- [23].Lee WJ, Pok EH, Almulaifi A, et al. Medium-term results of laparoscopic sleeve gastrectomy: a matched comparison with gastric bypass. Obes Surg 2015;25:1431–8. [DOI] [PubMed] [Google Scholar]
- [24].Lee YC, Lee WJ, Liew PL. Predictors of remission of type 2 diabetes mellitus in obese patients after gastrointestinal surgery. Obes Res Clin Pract 2013;7:e494–500. [DOI] [PubMed] [Google Scholar]
- [25].Madhok B, Mahawar KK, Boyle M, et al. Management of super-super obese patients: comparison between mini (one anastomosis) gastric bypass and sleeve gastrectomy. Obes Surg 2016;26:1646–9. [DOI] [PubMed] [Google Scholar]
- [26].Milone M, Lupoli R, Maietta P, et al. Lipid profile changes in patients undergoing bariatric surgery: a comparative study between sleeve gastrectomy and mini-gastric bypass. Int J Surg 2015;14:28–32. [DOI] [PubMed] [Google Scholar]
- [27].Plamper A, Lingohr P, Nadal J, et al. Comparison of mini-gastric bypass with sleeve gastrectomy in a mainly super-obese patient group: first results. Surg Endosc 2017;31:1156–62. [DOI] [PubMed] [Google Scholar]
- [28].Seetharamaiah S, Tantia O, Goyal G, et al. LSG vs OAGB—1 year follow-up data—a randomized control trial. Obes Surg 2016;27:948–54. [DOI] [PubMed] [Google Scholar]
- [29].Lee WJ, Chong K, Lin YH, et al. Laparoscopic sleeve gastrectomy versus single anastomosis (mini-) gastric bypass for the treatment of type 2 diabetes mellitus: 5-year results of a randomized trial and study of incretin effect. Obes Surg 2014;24:1552–62. [DOI] [PubMed] [Google Scholar]
- [30].Parmar CD, Mahawar KK, Boyle M, et al. Mini gastric bypass: first report of 125 consecutive cases from United Kingdom. Clin Obes 2016;6:61–7. [DOI] [PubMed] [Google Scholar]
- [31].Musella M, Susa A, Greco F, et al. The laparoscopic mini-gastric bypass: the Italian experience: outcomes from 974 consecutive cases in a multicenter review. Surg Endosc 2014;28:156–63. [DOI] [PubMed] [Google Scholar]
- [32].Kular KS, Manchanda N, Rutledge R. A 6-year experience with 1,054 mini-gastric bypasses-first study from Indian subcontinent. Obes Surg 2014;24:1430–5. [DOI] [PubMed] [Google Scholar]
- [33].Chevallier JM, Arman GA, Guenzi M, et al. One thousand single anastomosis (omega loop) gastric bypasses to treat morbid obesity in a 7-year period: outcomes show few complications and good efficacy. Obes Surg 2015;25:951–8. [DOI] [PubMed] [Google Scholar]
- [34].Carbajo MA, Luque-de-Leon E, Jimenez JM, et al. Laparoscopic one-anastomosis gastric bypass: technique, results, and long-term follow-up in 1200 patients. Obes Surg 2017;27:1153–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rutledge R, Walsh TR. Continued excellent results with the mini-gastric bypass: six-year study in 2,410 patients. Obes Surg 2005;15:1304–8. [DOI] [PubMed] [Google Scholar]
- [36].Kular KS, Manchanda N, Cheema GK. Seven years of mini-gastric bypass in type II diabetes patients with a body mass index <35 kg/m2. Obes Surg 2016;26:1457–62. [DOI] [PubMed] [Google Scholar]
- [37].Guenzi M, Arman G, Rau C, et al. Remission of type 2 diabetes after omega loop gastric bypass for morbid obesity. Surg Endosc 2015;29:2669–74. [DOI] [PubMed] [Google Scholar]
- [38].Noun R, Skaff J, Riachi E, et al. One thousand consecutive mini-gastric bypass: short- and long-term outcome. Obes Surg 2012;22:697–703. [DOI] [PubMed] [Google Scholar]
- [39].Lee WJ, Chong K, Ser KH, et al. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg (Chicago, IL: 1960) 2011;146:143–8. [DOI] [PubMed] [Google Scholar]
- [40].Peterli R, Wolnerhanssen BK, Vetter D, et al. Laparoscopic sleeve gastrectomy versus Roux-Y-gastric bypass for morbid obesity-3-year outcomes of the prospective randomized Swiss multicenter bypass or sleeve study (SM-BOSS). Ann Surg 2017;265:466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Keidar A, Hershkop KJ, Marko L, et al. Roux-en-Y gastric bypass vs sleeve gastrectomy for obese patients with type 2 diabetes: a randomised trial. Diabetologia 2013;56:1914–8. [DOI] [PubMed] [Google Scholar]
- [42].Li J, Lai D, Wu D. Laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy to treat morbid obesity-related comorbidities: a systematic review and meta-analysis. Obes Surg 2016;26:429–42. [DOI] [PubMed] [Google Scholar]
- [43].Osland E, Yunus RM, Khan S, et al. Changes in non-diabetic comorbid disease status following laparoscopic vertical sleeve gastrectomy (lvsg) versus laparoscopic Roux-En-Y gastric bypass (LRYGB) procedures: a systematic review of randomized controlled trials. Obes Surg 2017;27:1208–21. [DOI] [PubMed] [Google Scholar]
- [44].Osland E, Yunus RM, Khan S, et al. Postoperative early major and minor complications in laparoscopic vertical sleeve gastrectomy (LVSG) versus laparoscopic Roux-en-Y gastric bypass (LRYGB) procedures: a meta-analysis and systematic review. Obes Surg 2016;26:2273–84. [DOI] [PubMed] [Google Scholar]
- [45].Osland E, Yunus RM, Khan S, et al. Weight loss outcomes in laparoscopic vertical sleeve gastrectomy (LVSG) versus laparoscopic Roux-en-Y gastric bypass (LRYGB) procedures: a meta-analysis and systematic review of randomized controlled trials. Surg Laparosc Endosc Percutan Tech 2017;27:8–18. [DOI] [PubMed] [Google Scholar]
- [46].Bruzzi M, Rau C, Voron T, et al. Single anastomosis or mini-gastric bypass: long-term results and quality of life after a 5-year follow-up. Surg Obes Rel Dis 2015;11:321–6. [DOI] [PubMed] [Google Scholar]
- [47].Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg 2006;244:741–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Thaler JP, Cummings DE. Minireview: hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology 2009;150:2518–25. [DOI] [PubMed] [Google Scholar]
- [49].Tolone S, Cristiano S, Savarino E, et al. Effects of omega-loop bypass on esophagogastric junction function. Surg Obes Rel Dis 2016;12:62–9. [DOI] [PubMed] [Google Scholar]
- [50].Quan Y, Huang A, Ye M, et al. Efficacy of laparoscopic mini gastric bypass for obesity and type 2 diabetes mellitus: a systematic review and meta-analysis. Gastroenterol Res Pract 2015;2015:152852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Magouliotis DE, Tasiopoulou VS, Svokos AA, et al. One-anastomosis gastric bypass versus sleeve gastrectomy for morbid obesity: a systematic review and meta-analysis. Obes Surg 2017;27:2479–87. [DOI] [PubMed] [Google Scholar]


