Abstract
Rationale:
Solitary fibrous tumors are mesenchymal tumors presenting as fibroblastic neoplasms with prominent branching vascular patterns, which are often generated from the pleura. Most solitary fibrous tumors are benign; however, some can turn malignant. High-grade sarcomas from solitary fibrous tumors include multidirectional histopathological components.
Patient concerns:
We describe our experience of a giant high-grade sarcoma with mixed components generated from a solitary fibrous tumor of the pleura in a 67-year-old female patient presenting with cough and left-sided chest pain. The patient had been diagnosed with a pleural mass in the left chest by X-ray about 30 years earlier. However, the tumor was allowed to grow, without surgical intervention, for a long time.
Interventions:
Thoracic surgeons performed the removal of the giant pleural tumor; the tumor measured 18.0 × 14.5 × 10 cm in size, and was considered a giant tumor generated from the pleura of the left chest cavity.
Diagnoses:
The surgically removed tumor was solid and light brownish, and included myxoid and arabesque pattern lesions. The tumor also showed hemorrhagic and necrotic lesions. Moreover, spindle cells with less atypia, resembling fibroblasts, were noted. These spindle tumor cells were CD34- and Stat6-positive, suggesting a solitary fibrous tumor. Some of the spindle tumor cells were surrounded by thick collagenous fibers. Considering that the tumor originated from the parietal pleura, the tumor was defined as a solitary fibrous tumor in origin. The tumor also comprised high-grade sarcomatous components; these included lipid-rich, rhabdomyosarcomatous, and pleomorphic components. The high-grade sarcoma component included bizarre tumor cells with severe atypia.
Outcomes:
Tumor recurrence occurred in the left chest about 4 months after the surgery, and the patient died 8 months postoperatively.
Lessons:
The present case clearly demonstrates that a solitary fibrous tumor can develop into high-grade sarcomatous overgrowth, including lipid-rich, rhabdomyosarcoma, and pleomorphic sarcoma components, if left untreated for a prolonged period. This case provides profound insights about the natural history, histogenesis, differentiation, and malignant transformation of solitary fibrous tumors.
Keywords: chemotherapy, giant tumor, lipid-rich component, pleura, rhabdomyosarcoma, solitary fibrous tumor
1. Introduction
Solitary fibrous tumors of the pleura are fibroblastic neoplasms that frequently show a prominent branching vascular pattern and exhibit varying degrees of biological behavior.[1–6] Most of them demonstrate benign biological behaviors[7]; however, some can turn malignant, resulting in a poor prognosis.[8,9] Malignant solitary fibrous tumors, also known as dedifferentiated solitary fibrous tumors, often contain histologically multidirectional components, including, for example, pleomorphic/epithelioid, undifferentiated small round cell (Ewing-like or synovial sarcoma-like), rhabdomyosarcomatous, or pleomorphic osteochondro-like components.[8,9]
Herein, we describe our experience of a rare case of a giant solitary fibrous tumor of the pleura with high-grade sarcomatous overgrowth, which contained a lipid-rich component in addition to rhabdomyosarcomatous and pleomorphic components. To the best of our knowledge, malignant solitary fibrous tumors of this type and size are very rare.[10] Therefore, we report this unique case along with the clinicopathological findings, and discuss its natural history of malignant transformation and histogenesis.
2. Case presentation
2.1. Clinical summary
A 67-year-old woman visited a nearby clinic because of cough and left-sided chest pain. Chest X-ray and computed tomography (CT) performed at that clinic revealed a mass lesion and pleural effusion in the left thoracic cavity. Thus, the patient was referred to our hospital for further examination. Her past medical history and family history were unremarkable except for a left thoracic abnormal shadow that had been pointed out by medical checks at approximately age 34 years; this lesion had been conservatively followed up as a benign tumor such as lipoma. She had an occupational history of exposure to dust (wheat). She was a nonsmoker and did not drink alcohol.
CT showed a pleural tumor, measuring about 150 mm in greatest diameter, located in the lower portion of the left thoracic cavity and compressing the left lung. The tumor edge was smooth and contrast-enhanced nodules were observed within the tumor. Despite its largeness, the pleural tumor showed less invasive tendencies. Moreover, hemorrhage was also noted on chest CT (Fig. 1A–D). These findings suggested that the tumor was a solitary fibrous tumor of the pleura.
Figure 1.
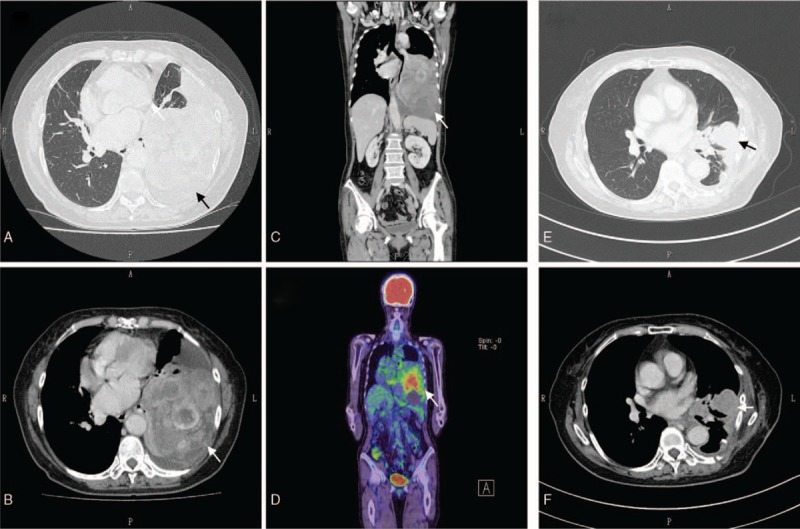
(A) Preoperative transverse-plane simple computed tomography (CT). The arrowhead indicates the pleural tumor in the left chest cavity. (B) Preoperative transverse-plane contrast-enhanced CT. The arrowhead indicates the pleural tumor in the left chest cavity. Hemorrhage inside the tumor can be observed. (C) Preoperative coronal-plane contrast-enhanced CT. The arrowhead indicates the pleural tumor in the left chest cavity. (D) Preoperative coronal-plane positron emission tomography. The arrowhead indicates the pleural tumor in the left chest cavity. (E) Postoperative transverse-plane CT. The arrowhead indicates the recurrent pleural tumor in the left chest cavity. (F) Postoperative transverse-plane contrast-enhanced CT. The arrowhead indicates the recurrent pleural tumor in the left chest cavity.
We performed removal of the giant pleural tumor and partial resection of the S6 region of the left lung by left anterior side incision. At the time of operation, the tumor was found to be protruding from the parietal pleura and compressing the lower lobe of the left lung. The intraoperative cytologic diagnosis of the left pleural effusion was a benign condition accompanied by reactive mesothelial cells and inflammatory cells. The postoperative course was uneventful and the patient was discharged from our hospital 13 days after the operation.
Unfortunately, tumor recurrence was found in the left thoracic cavity on CT about 4 months after the surgery (Fig. 1E, F). Although the patient underwent systemic chemotherapy for the recurrent tumor using doxorubicin, followed by treatment with pazopanib, a multityrosine kinase inhibitor,[11] the recurrent tumor was enlarged and the patient complained of chest pain and cough.
Subsequently, she underwent palliative care and remained alive with progressive disease for 4 months after the recurrence was noted. However, approximately 8 months postoperatively, the patient died because of the tumor. Informed consent was given for this study.
2.2. Histopathological findings
Grossly, the resected tumor measured 18 × 14.5 × 10 cm in size, harbored a stalk on the surgical margin of the parietal pleura, was well demarcated, and was surrounded by a thin fibrous capsule (Fig. 2). The tumor was attached to, but was not invasive into, the lung. Figure 2A shows the macroscopic tumor findings, including the stem part of the tumor, namely, the pleural side, which was the surgical margin to the pleura of the left chest cavity. The tumor was solid and light brownish, and the tumor slices revealed myxoid and arabesque pattern lesions (Fig. 2B). The tumor also included hemorrhagic or necrotic lesions, as well as small calcified lesions. We carefully examined the slice sections of the tumor using 129 blocks.
Figure 2.
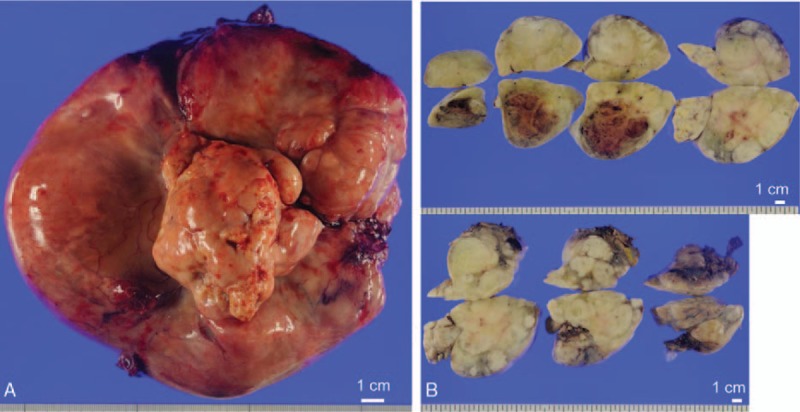
(A) Macroscopic findings of the giant tumor before the fixation. The central stem part was the surgical margin of the tumor, which was connected to the parietal pleura of the left chest cavity. The ruler shows 1.0 cm. (B) Macroscopic picture of the sections of the tumor. The ruler shows 1.0 cm.
Microscopically, a portion of the tumorous area was characterized by proliferation of fibroblast-like spindle cells accompanied by varying degrees of collagen fiber deposition (Fig. 3A, B). These spindle cells were immunohistochemically positive for CD34 and Stat6, the typical pattern of solitary fibrous tumors (Fig. 3C, D).[12–15]
Figure 3.
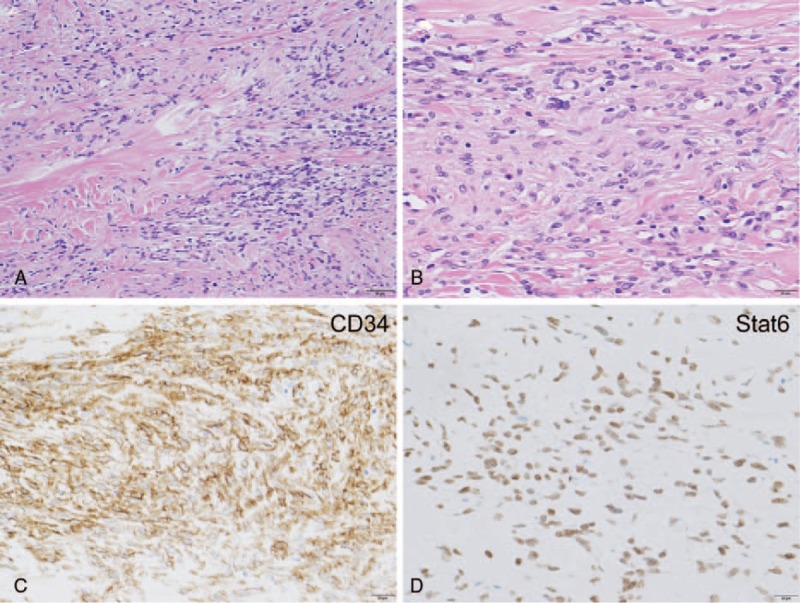
(A) The tumor spindle cells with less atypia in the giant tumor were found to be conventional solitary fibrous tumor components. The spindle cell tumor cells were surrounded by collagenous fibers. The scale bar shows 50 μm. (B) Magnified view of the conventional solitary fibrous tumor components in the giant tumor. The scale bar shows 20 μm. (C) The tumor spindle cells were CD34-positive, which is characteristic of solitary fibrous tumors. The scale bar shows 20 μm. (D) The tumor spindle cells were Stat6-positive, which is characteristic of solitary fibrous tumors. The scale bar shows 20 μm.
In addition to the typical solitary fibrous tumor component, a considerable portion of the tumor contained high-grade sarcomatous components, including a lipid-rich component, a rhabdomyosarcomatous component, and an anaplastic pleomorphic component (Figs. 4–6).
Figure 4.
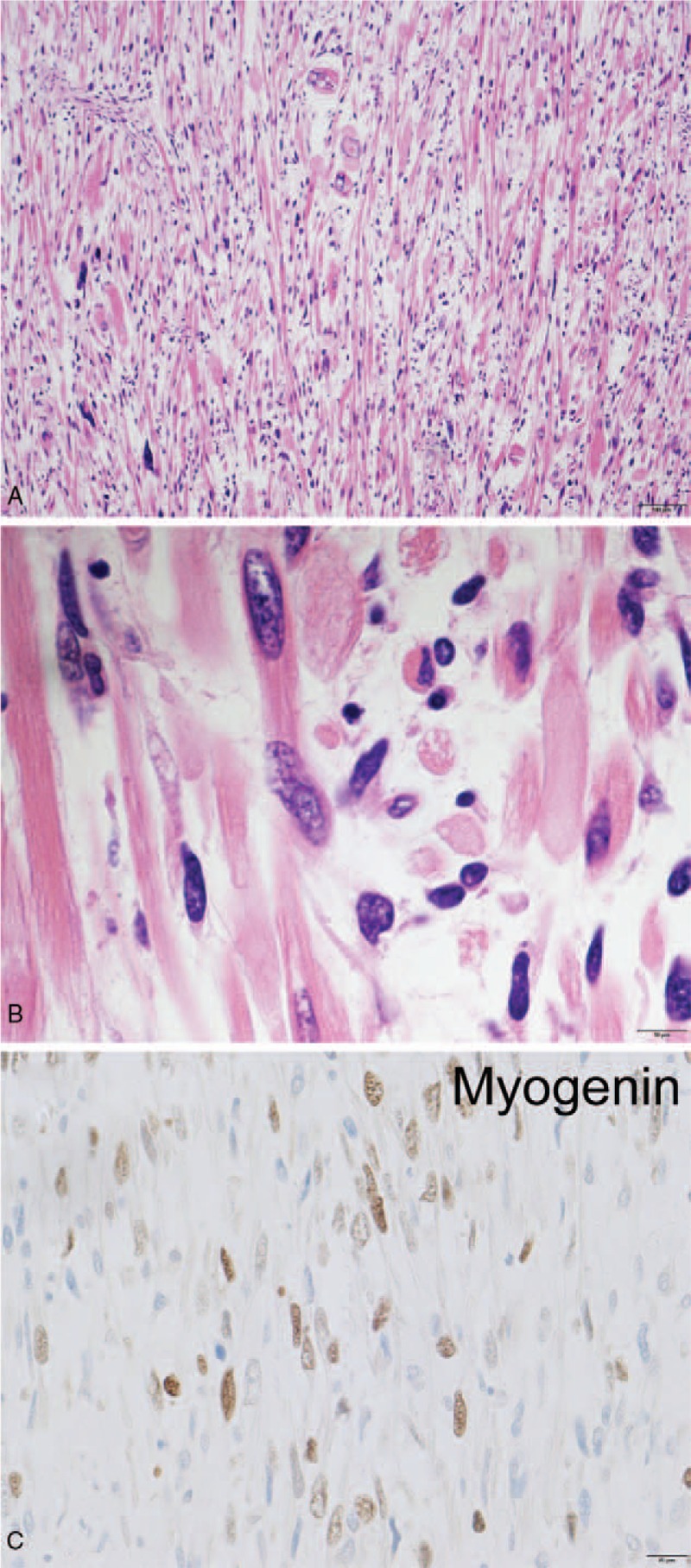
(A) The rhabdomyosarcoma component in the tumor. The tumor included bizarre cells. The scale bar shows 100 μm. (B) Some of the rhabdomyosarcoma cells showed striation, which clearly showed that the tumor was rhabdomyosarcoma. The scale bar shows 10 μm. (C) The rhabdomyosarcoma component was Myogenin-positive. The scale bar shows 20 μm.
Figure 6.
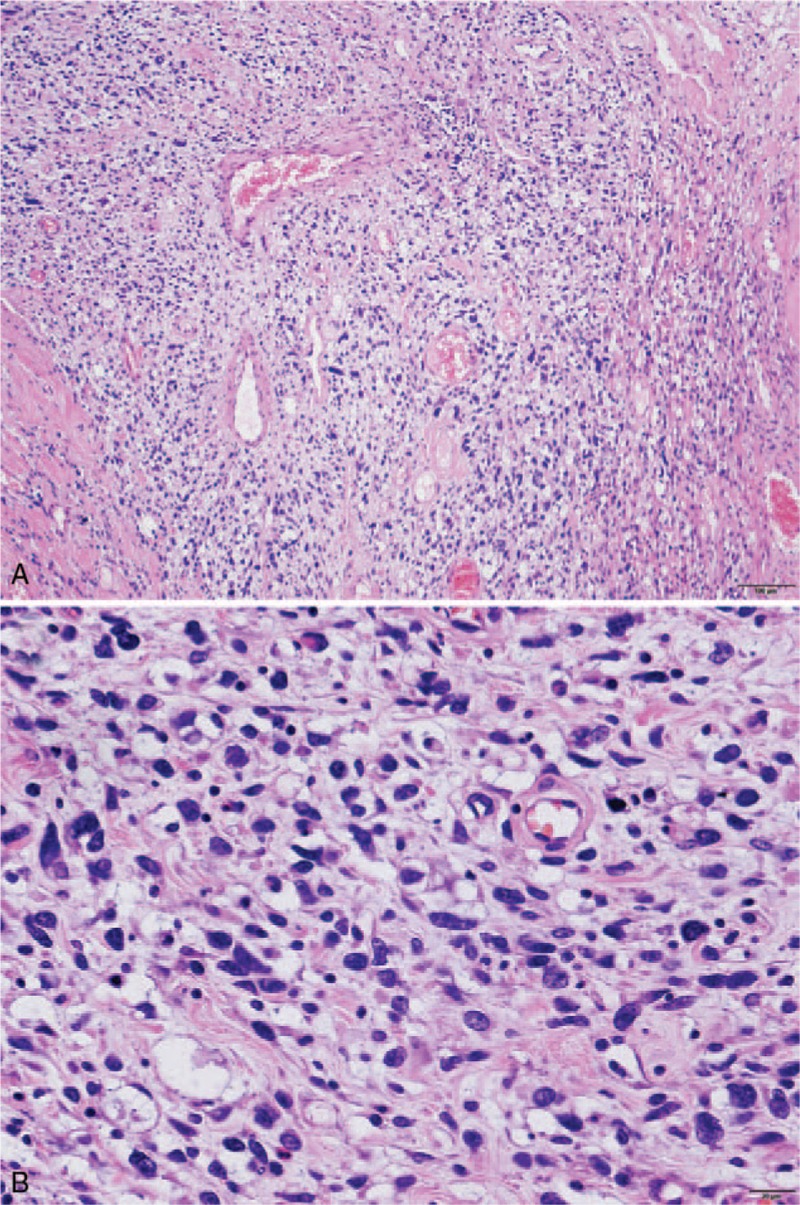
(A) The giant tumor included pleomorphic components. The scale bar shows 100 μm. (B) Magnified view of the pleomorphic sarcoma components. The scale bar shows 20 μm.
The rhabdomyosarcoma component was characterized by atypical striated myocytes with eosinophilic cytoplasms (Fig. 4B). This component was positive for Myogenin, Desmin, and Vimentin, but negative for MyoD1 and alpha-smooth muscle actin (Fig. 4C).
The liposarcoma-like lipid-rich component was characterized by atypical polygonal cells with clear and multivesicular cytoplasm, which was histochemically positive for Sudan III stain but negative for Alcian blue stain, thereby indicating intracytoplasmic lipid droplets (Fig. 5).[10,16] The lipid-rich component was immunohistochemically negative for S100 protein, MDM2, and CDK4.
Figure 5.
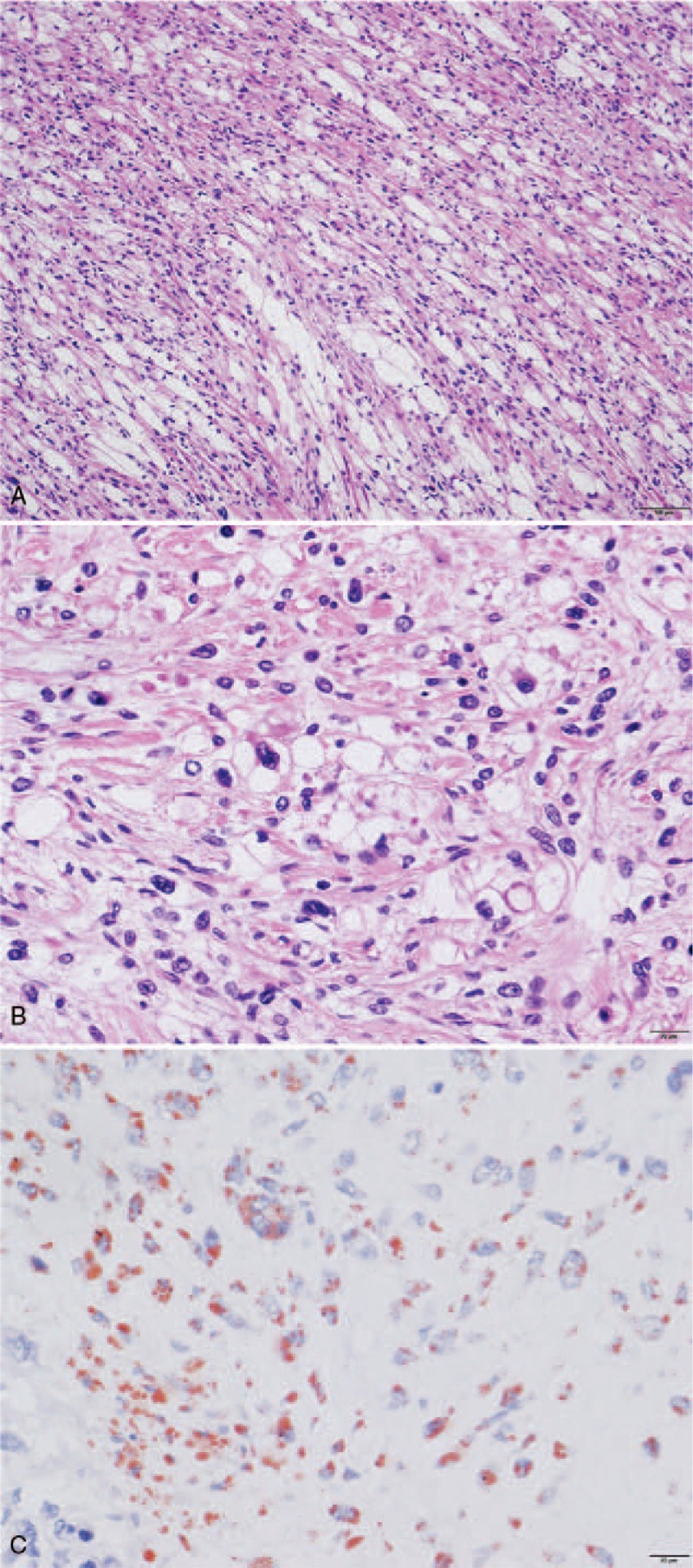
(A) The giant tumor included sarcoma with lipid-rich components. The scale bar shows 100 μm. (B) Magnified view of the sarcoma with lipid-rich components. The scale bar shows 20 μm. (C) Tumor area with lipid-rich components, stained by Sudan stain (lipid staining). The lipid parts are stained in red. The scale bar shows 20 μm.
The giant tumor included pleomorphic components, some of which were characterized by bizarre cells (Fig. 6).
3. Discussion
Solitary fibrous tumors are defined as “intermediate, rarely metastasizing’ tumors in the World Health Organization classification.[2] The 5- and 10-year metastasis-free rates of solitary fibrous tumors have been reported as 74% and 55%, respectively.[17] The histopathological findings of solitary fibrous tumor transformations can provide profound insights about solitary fibrous tumors.
Metastasizing solitary fibrous tumors include high-grade sarcomatous overgrowth.[8] In the study by Collini et al, the final diagnoses of malignant solitary fibrous tumors were as follows: malignant solitary fibrous tumor (MSFT), MSFT + pleomorphic/epithelioid component, MSFT + pleomorphic/epithelioid and spindle cell, MSFT + undifferentiated small round cell sarcomas (USRCS) (Ewing sarcoma-like), MSFT + pleomorphic osteochondro-like component, USRCS (Ewing-like), USRCS (synovial-sarcoma like), MSFT + embryonal rhabdomyosarcoma, and epithelioid rhabdomyosarcoma.[8]
Mosquera et al described that malignant progression of solitary fibrous tumors is a rare event, with only 8 cases observed among 948 cases of solitary fibrous tumors.[9] In their study, the authors demonstrated bland fibroblastic morphology, a myxoid part, poorly differentiated epithelioid cell morphology, poorly differentiated round cell morphology, and pseudorosettes in the dedifferentiated solitary fibrous tumors.[9] In the present case, we observed lipid-rich, rhabdomyosarcoma, and pleomorphic sarcoma components in the giant solitary fibrous tumor (Fig. 4).
Although the tumor was MDM2-, Cdk4-, and S100-negative, the hematoxylin and eosin-stained samples showed sarcoma with lipid-rich components (Fig. 5). Hence, negative expressions of MDM2, Cdk4, and S100, which are all liposarcoma markers, might reflect poor differentiation of the high-grade sarcomatous components. We considered the possibility that the sarcoma with lipid-rich components might be a degenerated sarcoma originating from the solitary fibrous tumor because the lipid distribution of the sarcoma with lipid components did not clearly show the fully filled lipid structure seen in cases of lipoma or liposarcoma (Fig. 5). In fact, Lee et al reported 14 cases of malignant fat-forming solitary fibrous tumors.[10] Bai et al also reported a malignant solitary fibrous tumor of the pleura with liposarcomatous differentiation.[18] The lipid-rich components of our case might be explained as a malignant fat-forming solitary fibrous tumor. However, among the 14 cases of malignant fat-forming solitary fibrous tumors described in Lee et al's study, only 2 cases actually metastasized, and no obvious local recurrence was found.[10] In contrast, 7 of 10 patients with “high-grade sarcomatous overgrowth in solitary fibrous tumors” reported by Collini et al[8] and 3 of 8 patients with “dedifferentiated solitary fibrous tumors” reported by Mosquera et al[9] died.
The definition of biological grading of sarcomas is difficult based on the histopathological findings. For example, kaposiform hemangioendothelioma, which is defined as an “intermediate malignancy,” biologically behaves as a malignant tumor.[19] Our case of malignant solitary fibrous tumor included lipid-rich components, which are associated with better prognosis, and also simultaneously included pleomorphic components, which are associated with a worse prognosis. Ultimately, our case showed a poor prognosis. To match the biological behaviors and prognoses of malignant solitary fibrous tumors, further investigations may be necessary for defining the criteria thereof from a histopathological point of view. The present case was important for demonstrating that malignant and/or high-grade sarcomatous solitary fibrous tumors with lipid-rich components can generate local recurrence in a short time postoperatively.
Interestingly, most of the high-grade sarcomatous components of our case did not immunohistochemically express CD34 and/or Stat6. This finding suggests that these components might be degenerated sarcoma surrounded by vacuolar degeneration. The tumor seemed to arise from, and retain the characteristics of, the original solitary fibrous tumor.[20] However, we could not exclude the possibility that the clear and/or hypocellular components in the solitary fibrous tumor included high-grade liposarcoma.
We found pleomorphic bizarre tumor cells in the malignant solitary fibrous tumor (Figs. 4 and 6). A case of malignant solitary fibrous tumor with pleomorphic malignant fibrous histiocytoma-like appearance has been previously reported.[21] Moreover, bizarre tumor cells with multiple nuclei in malignant solitary fibrous tumors have also been reported.[22,23] These pleomorphic and/or bizarre cells in malignant solitary fibrous tumors show that malignant solitary fibrous tumors can acquire high-grade sarcomatous characteristics with poor prognoses once they transform, despite originally having benign or well-differentiated characteristics.[2–4,8,10,17,22] In fact, the malignant solitary fibrous tumor in the present patient recurred only 4 months after the surgery, and the patient finally died 8 months postoperatively, even though the solitary fibrous tumor had grown into the giant size for about 30 years without metastasis.
The multidirectional sarcomatous components of this tumor suggest that the transformation of the tumor occurred at the immature tumor stem cell level. Ultrastructural observations have revealed that solitary fibrous tumors may originate from peculiar perivascular multipotent mesenchymal elements, which have features akin to those of pericytes and fibroblasts. Pericytes may differentiate into fibroblast-like cells, adipocytes, chondrocytes, and osteoblasts.[6,24–27] If we hypothesize that the solitary fibrous tumor of this case was generated from pericytes, which have the potential to differentiate into adipocytes and multiple other kinds of cells, we can reasonably explain why this tumor transformation generated sarcoma with lipid-rich components.[10,18]
As for the genetic characteristics of solitary fibrous tumors, NAB2-STAT6 gene fusions and subsequent enhanced expression of Stat6 have been reported.[13–15] Our case also showed enhanced Stat6 expression in the benign solitary fibrous part (Fig. 3D); however, this was not seen in the high-grade sarcomatous components. Although Stat6 is important for tumor formation of benign solitary fibrous tumors, sustained Stat6 expression may not be important for the growth and survival of transformed malignant high-grade sarcomatous components originating from solitary fibrous tumors. This question remains to be answered in the future.
In conclusion, the case presented herein provided profound insights about the natural history, origin, differentiation, and malignant transformation of solitary fibrous tumors.
Footnotes
Abbreviations: CT = computed tomography, MSFT = malignant solitary fibrous tumor, USRCS = undifferentiated small round cell sarcomas.
TN and HO equally contributed to this work.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Briselli M, Mark EJ, Dickersin GR. Solitary fibrous tumors of the pleura: eight new cases and review of 360 cases in the literature. Cancer 1981;47:2678–89. [DOI] [PubMed] [Google Scholar]
- [2].Fletcher CDM, World Health Organization, International Agency for Research on Cancer. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. Lyon: IARC Press; 2013. [Google Scholar]
- [3].England DM, Hochholzer L, McCarthy MJ. Localized benign and malignant fibrous tumors of the pleura. A clinicopathologic review of 223 cases. Am J Surg Pathol 1989;13:640–58. [DOI] [PubMed] [Google Scholar]
- [4].Magdeleinat P, Alifano M, Petino A, et al. Solitary fibrous tumors of the pleura: clinical characteristics, surgical treatment and outcome. Eur J Cardiothorac Surg 2002;21:1087–93. [DOI] [PubMed] [Google Scholar]
- [5].de Perrot M, Fischer S, Brundler MA, et al. Solitary fibrous tumors of the pleura. Ann Thorac Surg 2002;74:285–93. [DOI] [PubMed] [Google Scholar]
- [6].Lee SC, Tzao C, Ou SM, et al. Solitary fibrous tumors of the pleura: clinical, radiological, surgical and pathological evaluation. Eur J Surg Oncol 2005;31:84–7. [DOI] [PubMed] [Google Scholar]
- [7].Gold JS, Antonescu CR, Hajdu C, et al. Clinicopathologic correlates of solitary fibrous tumors. Cancer 2002;94:1057–68. [PubMed] [Google Scholar]
- [8].Collini P, Negri T, Barisella M, et al. High-grade sarcomatous overgrowth in solitary fibrous tumors: a clinicopathologic study of 10 cases. Am J Surg Pathol 2012;36:1202–15. [DOI] [PubMed] [Google Scholar]
- [9].Mosquera JM, Fletcher CD. Expanding the spectrum of malignant progression in solitary fibrous tumors: a study of 8 cases with a discrete anaplastic component—is this dedifferentiated SFT? Am J Surg Pathol 2009;33:1314–21. [DOI] [PubMed] [Google Scholar]
- [10].Lee JC, Fletcher CD. Malignant fat-forming solitary fibrous tumor (so-called “lipomatous hemangiopericytoma”): clinicopathologic analysis of 14 cases. Am J Surg Pathol 2011;35:1177–85. [DOI] [PubMed] [Google Scholar]
- [11].van der Graaf WTA, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012;379:1879–86. [DOI] [PubMed] [Google Scholar]
- [12].Yokoi T, Tsuzuki T, Yatabe Y, et al. Solitary fibrous tumour: significance of p53 and CD34 immunoreactivity in its malignant transformation. Histopathology 1998;32:423–32. [DOI] [PubMed] [Google Scholar]
- [13].Robinson DR, Wu YM, Kalyana-Sundaram S, et al. Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat Genet 2013;45:180–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Doyle LA, Vivero M, Fletcher CDM, et al. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod Pathol 2014;27:390–5. [DOI] [PubMed] [Google Scholar]
- [15].Yoshida A, Tsuta K, Ohno M, et al. STAT6 immunohistochemistry is helpful in the diagnosis of solitary fibrous tumors. Am J Surg Pathol 2014;38:552–9. [DOI] [PubMed] [Google Scholar]
- [16].Carneiro SS, Scheithauer BW, Nascimento AG, et al. Solitary fibrous tumor of the meninges: a lesion distinct from fibrous meningioma—a clinicopathologic and immunohistochemical study. Am J Clin Pathol 1996;106:217–24. [DOI] [PubMed] [Google Scholar]
- [17].Demicco EG, Park MS, Araujo DM, et al. Solitary fibrous tumor: a clinicopathological study of 110 cases and proposed risk assessment model. Mod Pathol 2012;25:1298–306. [DOI] [PubMed] [Google Scholar]
- [18].Bai H, Aswad BI, Gaissert H, et al. Malignant solitary fibrous tumor of the pleura with liposarcomatous differentiation. Arch Pathol Lab Med 2001;125:406–9. [DOI] [PubMed] [Google Scholar]
- [19].Nakaya T, Morita K, Kurata A, et al. Multifocal kaposiform hemangioendothelioma in multiple visceral organs: an autopsy of 9-day-old female baby. Hum Pathol 2014;45:1773–7. [DOI] [PubMed] [Google Scholar]
- [20].Rubin BP, Fletcher JA, Renshaw AA. Clear cell sarcoma of soft parts - Report of a case primary in the kidney with cytogenetic confirmation. Am J Surg Pathol 1999;23:589–94. [DOI] [PubMed] [Google Scholar]
- [21].Hanau CA, Miettinen M. Solitary fibrous tumor: histological and immunohistochemical spectrum of benign and malignant variants presenting at different sites. Hum Pathol 1995;26:440–9. [DOI] [PubMed] [Google Scholar]
- [22].Schirosi L, Lantuejoul S, Cavazza A, et al. Pleuro-pulmonary solitary fibrous tumors: a clinicopathologic, immunohistochemical, and molecular study of 88 cases confirming the prognostic value of de Perrot staging system and p53 expression, and evaluating the role of c-kit, BRAF, PDGFRs (alpha/beta), c-met, and EGFR. Am J Surg Pathol 2008;32:1627–42. [DOI] [PubMed] [Google Scholar]
- [23].Magro G, Emmanuele C, Lopes M, et al. Solitary fibrous tumour of the kidney with sarcomatous overgrowth. Case report and review of the literature. APMIS 2008;116:1020–5. [DOI] [PubMed] [Google Scholar]
- [24].Ide F, Obara K, Mishima K, et al. Ultrastructural spectrum of solitary fibrous tumor: a unique perivascular tumor with alternative lines of differentiation. Virchows Arch 2005;446:646–52. [DOI] [PubMed] [Google Scholar]
- [25].Gengler C, Guillou L. Solitary fibrous tumour and haemangiopericytoma: evolution of a concept. Histopathology 2006;48:63–74. [DOI] [PubMed] [Google Scholar]
- [26].Song S, Ewald AJ, Stallcup W, et al. PDGFR beta(+) perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol 2005;7:870–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Allt G, Lawrenson JG. Pericytes: Cell biology and pathology. Cells Tissues Organs 2001;169:1–1. [DOI] [PubMed] [Google Scholar]


