Abstract
We performed this retrospective clinical study to examine the prognostic power of bone scintigraphy (BS) and 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) in terms of overall survival (OS) of breast cancer with bone-only metastasis.
We retrospectively evaluated 100 female invasive ductal breast cancer patients (mean age 48.1 years) with bone-only metastasis. Twenty-five patients had human epidermal growth factor receptor 2 (HER2)-positive tumors, 65 were estrogen receptor (ER) and/or progesterone receptor (PR)-positive, HER2-negative tumors, and 10 were triple negative tumors. The patients were treated properly with various treatments, including chemotherapy, radiotherapy, hormone, and bisphosphonate therapy, based on their clinical status. All patients underwent BS and FDG PET/CT at baseline and 1 year after treatment. The baseline and follow images were visually compared, and the patients were grouped as responders or nonresponders based on their images. OS was compared between the groups.
The mean OS after the diagnosis of bone-only metastasis was 57.6 months. Fifty-one patients (51%) died within 5 years after diagnosis of metastasis. No difference in survival was evident between responders and nonresponders based on BS imaging data (P = .090). The response status based on PET imaging data waste only significant independent prognostic factor on multivariate analysis (P = .001). Survival was lower in nonresponders than in responders based on PET imaging (32.7% vs 66.4%; P < .001).
Our findings suggest that the response status according to FDG PET imaging can be used to predict OS in breast cancer patients with bone-only metastasis.
Keywords: 18F-fluorodeoxyglucose positron emission tomography/computed tomography, bone scintigraphy, bone-only metastasis, breast cancer, overall survival
1. Introduction
The skeleton is the most frequently involved organ of distant metastasis in advanced breast cancer,[1–3] and bone metastasis develops in over 70% of metastatic breast cancer patients.[4,5] Despite skeletal metastasis-related morbidities, including pain, fractures, hypercalcemia, and spinal cord compression,[6] the survival of patients with bone metastases alone is relatively longer than that of patients with visceral disease.[6,7] Therefore, appropriate response monitoring of bone metastasis during therapy is vital in terms of cumulative morbidity and healthcare costs.[8]
In nuclear medicine, whole-body bone scintigraphy (BS) and 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) are widely used to evaluate bone metastasis in breast cancer patients. However, the uptake mechanisms in metastatic bone lesions of each imaging modality are very different, in that BS reflects osteoblastic responses in metastatic bones, and FDG PET/CT reveals high-level glucose metabolism bone sites.[9] Despite the superiority of FDG PET/CT in terms of evaluating osteolytic bone metastasis compared with BS, BS remains a valuable diagnostic method detecting osteosclerotic metastasis in patients exhibiting no or low FDG uptake.[1,10–13]
Assessing the response using several imaging methods is crucial in the management of breast cancer patients with bone metastasis. A previous study showed that osteolytic metastatic bone lesions frequently develop into sclerotic lesions during systemic treatment, and BS is of additional utility in such cases.[14] Other studies reported that FDG PET/CT was superior to BS in evaluating bone metastases regardless of whether the lesions were osteolytic or sclerotic.[13,15,16] Up to now, the evidence of efficacy and consensus regarding effective monitoring of a treatment response in imaging are lacking.[8,17] After treatment, it is not uncommon for the follow-up findings of metastatic breast cancer bone lesions to differ between BS and FDG PET/CT. Such differences in findings between imaging methods confuse the interpretation of patient status for clinicians in daily oncological practice.
We thus hypothesized that the prognostic values of BS and FDG PET/CT during follow-up are different, and we sought to establish which method is more valuable for predicting patient survival.
2. Materials and methods
2.1. Patients
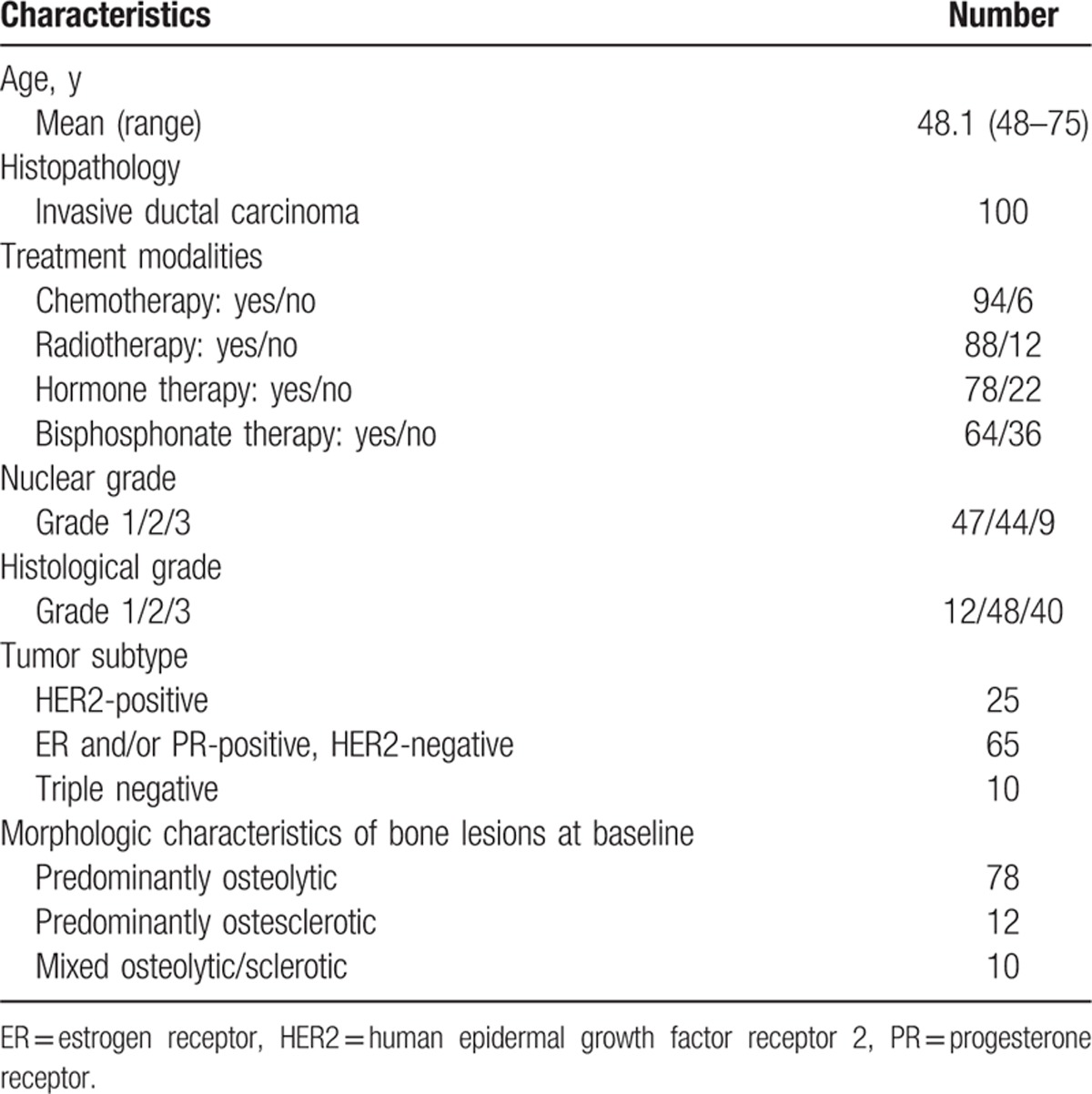
In all, 100 female patients (mean age ± standard deviation [SD] 48.1 ± 9.8 years; range 48–75 years) diagnosed with invasive ductal breast cancer with bone-only metastasis from March 2004 to March 2012 at a single institution (Ajou University Hospital, Suwon, Korea) were included in this study. All patients underwent both BS and FDG PET/CT at baseline (initial diagnosis of bone metastasis) and at follow-up 1 year after treatment. The patients’ clinical characteristics, including age, histology, and treatment modalities, were obtained by chart review blinded to the BS and FDG PET/CT results. Clinical follow-up was performed at least every 6 months, and the mean follow-up duration was 45.0 ± 23.7 months (range 15–131 months). Patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics.
The clinical design of this retrospective study was approved by the institutional review board of Ajou University (AJIRB-MED-MDB-17-162). The need for informed consent was waived.
2.2. Bone scintigraphy protocol
Whole-body bone scintigraphy was performed 4 hours after the injection 740 MBq of Tc-99m methylene diphosphonate. Anterior and posterior views were acquired using a dual-headed gamma camera equipped with low-energy, high-resolution collimator (Varicam, GE Healthcare, Milwaukee, WI).
2.3. FDG PET/CT protocol
After fasting for at least 6 hours, patients were administered 5 MBq/kg FDG intravenously. The blood glucose level at the time of FDG injection was <150 mg/dL in all patients. Patients were instructed to rest comfortably for 60 minutes and to urinate before scanning. Whole-body PET/CT images were obtained using the Discovery ST scanner (GE Healthcare, Milwaukee, WI). Seven or 8 frames (3 min/frame) of emission PET data were acquired in 3-dimensional mode after noncontrast CT scanning from the base of the skull to the upper thigh (120 kV, 30–100 mA in the Auto mA mode, 3.75 mm section width). Emission PET images were reconstructed using an iterative method (ordered subset expectation maximization with 2 iterations and 20 subsets, 600 mm field of view, 3.27 mm slice thickness) and attenuation-corrected by noncontrast CT.
2.4. Image analysis
Images were assessed visually by consensus between 2 experienced nuclear medicine physicians (SP with 6 years of experience and YSA with 12 years of experience) who were blinded to all other clinical information. The morphologic characteristic of metastatic bone lesions (predominantly osteolytic, osteosclerotic, or mixed osteolytic/sclerotic) was determined on bone window setting CT images from baseline FDG PET/CT on an AW workstation (version 4.4). Baseline and follow-up images were compared for response evaluation. A patient was confirmed as a responder if disappearance of all lesions or a reduction in uptake activity in bone lesions was documented on follow-up scans. Follow-up images without significant interval changes or increased uptake activity of bone lesions compared with baseline with or without new lesions were indicative of nonresponders. The responders and nonresponders were evaluated using each imaging modality (BS and FDG PET/CT).
2.5. Histopathological evaluation
Surgical specimens from macroscopic tumors were sliced serially at 5-mm intervals, prepared as paraffin wax-embedded sections, and stained with hematoxylin and eosin. The specimens were evaluated according to the following histopathological features: histological type of carcinoma, black nuclear grade (nuclear grade 1, poorly differentiated; grade 2, moderately differentiated; and grade 3, well-differentiated), and modified Bloom–Richardson histological grade (histological grade 1, well-differentiated; grade 2, moderately differentiated; and grade 3, poorly differentiated). Expression levels of the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) were evaluated in surgically removed specimens using standard avidin–biotin complex immunohistochemical staining methods. All primary antibodies used were monoclonal, as follows: ER (1:50; Dako Corp., Carpinteria, CA), PR (1:50; Dako Corp.), and c-erbB2 (1:200; Novocastra Laboratories Ltd., Newcastle-Upon-Tyne, UK). ER or PR positivity was defined as the presence of at least 1% positively stained nuclei at ×10 magnification. The intensity of c-erbB-2 staining was scored as 0, 1+, 2+, or 3+. Tumors with a 3+ score for c-erbB-2 staining were classified as HER2-positive, and tumors with a 0 or 1+ score were classified as HER2-negative; in tumors with a 2+ score, gene amplification using fluorescence in situ hybridization was used to determine the HER2 status. All specimens were reviewed by a pathologist with 18 years of experience.
2.6. Statistical analysis
A sample size calculation was performed using MedCalc (ver. 14.8.1; MedCalc Software bvba, Ostend, Belgium). A significance (α) level of 5% and a statistical power (1-β) level of 80% were used and considered acceptable for the purpose of the study. A sample size of 95 was required to attain an appropriate confidence range; thus, the sample size of our study (n = 100) was sufficient to perform the statistical analysis.
The Kolmogorov–Smirnov test was used to assess whether parameter distributions differed significantly from a normal distribution. All data were normally distributed; thus, parametric analyses were used, and all data are presented as means with SDs.
Level of agreement between BS and PET results was quantified using the kappa statistics. The kappa value was interpreted according to the criteria presented by Altman (with 0.81–1 being very good agreement; 0.61–0.80 being good agreement; 0.41–0.60 being moderate agreement; 0.21–0.40 being fair agreement; ≤0.20 being poor agreement).[18] Overall survival (OS) was measured to assess the prognosis of patients and was defined as the interval from the initial diagnosis of bone metastasis to death from any cause. To assess the prognostic significance of clinicopathological and imaging parameters, univariate and multivariate analyses using a Cox proportional hazards regression model were performed. Covariates that achieved a significance level of <0.2 in the univariate model were included in the multivariate model. Survival functions of parameters were estimated using the Kaplan–Meier method and compared using the log-rank test. The MedCalc software package was used for all statistical analyses. P values <0.05 were considered to indicate statistical significance.
3. Results
3.1. Assessment of the agreement between BS and PET findings
According to BS images, 27% (27/100) of patients were classified as responders and 73% (73/100) as nonresponders. On the contrary, based on FDG PET/CT, 48% (48/100) of patients were identified as responders and 52% (52/100) as nonresponders. The kappa value showed a poor agreement between BS and PET findings (kappa 0.20, 95% confidence interval [CI] 0.03–0.38).
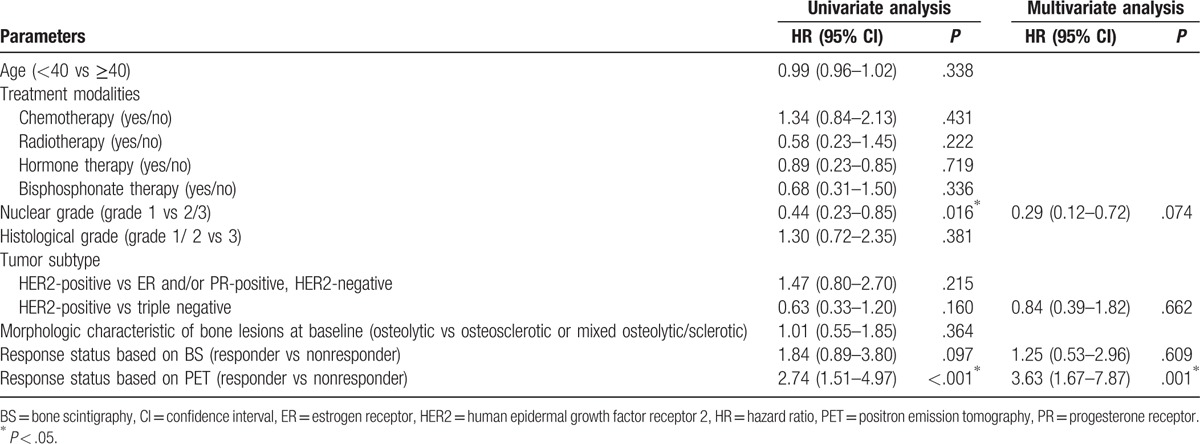
3.2. Assessment of prognostic parameters for OS
In all, 36 patients (36%) were alive during the follow-up period (45.0 ± 23.7 months). The mean OS after the diagnosis of bone-only metastasis was 57.6 (95% CI 49.7–65.5) months.
The OS rate at 5 years was 49%, and the remaining 51% of patients died within 5 years of initial diagnosis of bone-only metastasis. On univariate analysis, the response statuses based on FDG PET/CT imaging and nuclear grade were identified as significant prognostic factors for 5-year OS (P < .001 and P = .016, respectively). Other factors including age, histologic grade, tumor subtype, treatment modalities, morphologic characteristics of bone lesions, and response status based on BS imaging did not show statistical significance as prognostic factors (all P > .05; Table 2). Multivariate analyses showed that only response status based on PET imaging was independently prognostic of OS (P = .001; Table 2).
Table 2.
Univariate and multivariate analyses for factors influencing overall survival in breast cancer patients with bone-only metastasis.
The Kaplan–Meier survival estimates at 5 years based on response status as revealed by BS and FDG PET/CT imaging are shown in Fig. 1. The OS rate at 5 years according to BS imaging was higher for responders than nonresponders (66.7% vs 42.5%), but statistical significance was not attained (P = .090, Fig. 1A). The OS rate based on PET imaging was significantly poorer for nonresponders than responders (32.7% vs 66.4%; P < .001; Fig. 1B).
Figure 1.
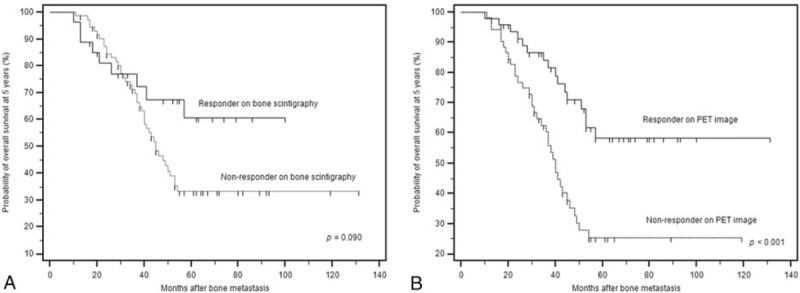
Kaplan–Meier estimates of overall survival at 5 years by response status based on image evaluation. (A) Responders as determined by BS imaging experienced a somewhat higher survival rate than that of nonresponders, but without significance (P = .090, hazard ratio [HR] 1.83, 95% confidence interval [CI] 0.99–3.38). (B) The prognosis of nonresponders as determined by PET imaging was significantly poorer than that of responders (P < .001, HR 2.69, 95% CI 1.55–4.67).
4. Discussion
Bone scintigraphy and FDG PET/CT are convenient whole-body imaging tools used by physicians to evaluate bone metastasis in breast cancer patients. However, previous prognostic surveillance studies using BS and FDG PET to evaluate bone metastasis in breast cancer patients are very few in number. Cook et al[10] reported that FDG PET is superior to BS for the initial detection of osteolytic breast cancer metastasis, which is associated with a shorter OS than is osteosclerotic metastasis. To our knowledge, comparison of the prognostic utilities of BS and FDG PET in terms of response assessment in bone-only metastastic breast cancer patients has not been attempted.
We found that only the responses based on FDG PET imaging reliably predicted survival of breast cancer patients with bone-only metastases. Response assessment using BS was not of assistance. These results might be originated from that FDG PET acted as a tumor-specific tracer and reflected the glucose usage by tumor cells in viable metastatic lesions regardless of the characteristic of bone lesions (osteolytic, osteosclerotic or mixed osteolytic/sclerotic).[1,19] On the contrary, BS mainly reflects the altered bone microenvironment in metastatic bone lesions; persistent high uptake evident on BS imaging may be observed in osteosclerotic lesions that are already reduced in viability because of their response to treatment. In fact, in our present study, the responder group according to PET imaging findings was larger than that based on BS imaging findings (48 vs 27 patients), and a discrepancy between the PET and BS findings was evident (kappa 0.20).
The nuclear grade was a significant prognostic factor only on univariate analysis, but not on multivariate analysis, consistent with the finding of a previous study by Lee et al,[20] who showed poorer distant relapse-free survival of patients with an aggressive nuclear grade compared with breast cancer patients with bone-only metastasis. Furthermore, the nuclear grade was not significantly prognostic on multivariate analysis in the cited study. More studies may be required to validate the prognostic utility of the nuclear grade for bone-only metastatic patients.
Better survival of bone metastatic breast cancer patients without visceral disease was reported in previous studies.[6,7] In our study, the mean OS of patients with bone-only metastasis was 57.6 months, and 49% of these patients were alive 5 years from the time of the initial diagnosis of bone metastasis; the survival was quite different from the median survival of 40 to 55 months reported in previous studies.[3,6,7,21,22] The relatively long survival in these patients means that prediction of survival and proper management of bone metastastic disease are important to keep patients alive with a tolerable quality of life for as long as possible. In this context, our study provides useful information for clinicians in predicting the prognosis of their patients. Moreover, we enrolled a homogeneous group with only the invasive ductal type of breast cancer. In addition, the tumor subtype did not affect the response evident on PET imaging, being generally prognostic in our study. We thus expect that our results could be applied in clinical practice.
It is known that most breast cancer metastasis to bone results in osteolytic lesions.[23] In our study, 78% of the patients showed predominantly osteolytic features, which is consistent with previous known value (∼80%).[24] Although osteolytic metastases tend to be aggressive then sclerotic metastases,[10,25] the morphologic characteristics of bone at baseline did not appear to be a significant predictor of OS in our study. There are few previous studies that reported the relationship between morphologic characteristics of metastatic bone lesions and the survival, so it was hard to explain the reason for this result. One possible explanation is that the survival of patients might not be affected by morphologic characteristics of bone at initial diagnosis, if the patients were treated properly. Further studies will be necessary to clarify this issue.
In our study, to avoid the flare phenomenon, images obtained 1 year after treatment were selected as follow-up images. The flare phenomenon is well-known in BS, and it renders the differentiation between progression and a temporary healing osteoblastic response to successful therapy difficult.[26] Also, the flare phenomenon on FDG PET, known as metabolic flare, has been described after treatment of breast cancer.[17] To avoid the flare phenomenon on both BS and PET images, we assumed that the proper time lag for predicting prognosis via imaging response assessments in routine clinical practice is 1 year after therapy.
A previous study by Ahn et al[22] reported that bisphosphonate treatment was a significant prognostic factor for predicting patient's survival. However, in our study, the response status based on PET image was only significant independent prognostic factor for OS, irrespective of treatment modality. To date, the optimal treatment for bone-only metastastic patients remains unclear[21]; more studies are needed.
There are several limitations to our study. First, we did not include standardized uptake values (SUVs) obtained from FDG PET in our imaging analysis. Most of our patients (97/100) had multiple metastatic bone lesions, and it was difficult to compare SUVs lesion by lesion on before and after-treatment images. Moreover, SUVs are useful when evaluating PET images of soft-tissue metastases only; bone lesions remain “nonmeasurable.”[27,28] Thus, we did not use SUV data in our study. Second, we did not include data on the morphological changes of bone lesions as evident on CT images. A previous study by Tateishi et al[29] showed that only PET changes predicted progression of bone metastasis, CT changes did not. We thus focused on metabolic changes on PET images. Third, the guideline for treatment of bone metastases was changed within the study period,[30] so the enrolled patients were not treated with the same guideline. Given that this type of study is retrospective, we could not control for this factor that may influence outcome. The final limitation of our study was that, although solitary bone metastases are significant prognostic factors in patients with skeletal metastasis,[20,31] we could not explore this topic in our study, because only 3 patients had single bone metastases. Further studies including more patients with solitary bone metastases may be needed.
5. Conclusion
In conclusion, the response evident on FDG PET images after treatment predicts OS in breast cancer patients with bone-only metastases.
Footnotes
Abbreviations: BS = bone scintigraphy, CI = confidence interval, ER = estrogen receptor , FDG PET/CT = 18F-fluorodeoxyglucose positron emission tomography/computed tomography, HER2 = human epidermal growth factor receptor 2, OS = overall survival, PR = progesterone receptor, SUV = standardized uptake value.
Funding: This work was supported by a National Research Foundation of Korea (NRF) grant, funded by the Korean government (MEST) (no. NRF-2015R1C1A2A01052005).
The authors report no conflicts of interest.
References
- [1].Koolen BB, Vegt E, Rutgers EJ, et al. FDG-avid sclerotic bone metastases in breast cancer patients: a PET/CT case series. Ann Nucl Med 2012;26:86–91. [DOI] [PubMed] [Google Scholar]
- [2].Hortobagyi GN. Bone metastases in breast cancer patients. Semin Oncol 1991;18(suppl 5):11–5. [PubMed] [Google Scholar]
- [3].Coleman RE, Smith P, Rubens RD. Clinical course and prognostic factors following bone recurrence from breast cancer. Br J Cancer 1998;77:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jung SY, Rosenzweig M, Sereika SM, et al. Factors associated with mortality after breast cancer metastasis. Cancer Causes Control 2012;23:103–12. [DOI] [PubMed] [Google Scholar]
- [5].Manders K, van de Poll-Franse LV, Creemers G-J, et al. Clinical management of women with metastatic breast cancer: a descriptive study according to age group. BMC Cancer 2006;6:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Domchek SM, Younger J, Finkelstein DM, et al. Predictors of skeletal complications in patients with metastatic breast carcinoma. Cancer 2000;89:363–8. [DOI] [PubMed] [Google Scholar]
- [7].Plunkett TA, Smith P, Rubens RD. Risk of complications from bone metastases in breast cancer: implications for management. Eur J Cancer 2000;36:476–82. [DOI] [PubMed] [Google Scholar]
- [8].Cook GJ, Azad GK, Goh V. Imaging bone metastases in breast cancer: staging and response assessment. J Nucl Med 2016;57(suppl 1):27S–33S. [DOI] [PubMed] [Google Scholar]
- [9].Sahin E, Zincirkeser S, Baris Akcan A. Is (99m) Tc-MDP whole body bone scintigraphy adjuvant to (18) F-FDG-PET for the detection of skeletal metastases. J BUON 2014;19:291–6. [PubMed] [Google Scholar]
- [10].Cook GJ, Houston S, Rubens R, et al. Detection of bone metastases in breast cancer by 18FDG PET: differing metabolic activity in osteoblastic and osteolytic lesions. J Clin Oncol 1998;16:3375–9. [DOI] [PubMed] [Google Scholar]
- [11].Nakai T, Okuyama C, Kubota T, et al. Pitfalls of FDG-PET for the diagnosis of osteoblastic bone metastases in patients with breast cancer. Eur J Nucl Med Mol Imaging 2005;32:1253–8. [DOI] [PubMed] [Google Scholar]
- [12].Shie P, Cardarelli R, Brandon D, et al. Meta-analysis: comparison of F-18 fluorodeoxyglucose-positron emission tomography and bone scintigraphy in the detection of bone metastases in patients with breast cancer. Clin Nucl Med 2008;33:97–101. [DOI] [PubMed] [Google Scholar]
- [13].Morris PG, Lynch C, Feeney JN, et al. Integrated positron emission tomography/computed tomography may render bone scintigraphy unnecessary to investigate suspected metastatic breast cancer. J Clin Oncol 2010;28:3154–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Du Y, Cullum I, Illidge TM, et al. Fusion of metabolic function and morphology: sequential [18F] fluorodeoxyglucose positron-emission tomography/computed tomography studies yield new insights into the natural history of bone metastases in breast cancer. J Clin Oncol 2007;25:3440–7. [DOI] [PubMed] [Google Scholar]
- [15].Yang S, Liang J, Lin F, et al. Comparing whole body 18 F-2-deoxyglucose positron emission tomography and technetium-99m methylene diphosphonate bone scan to detect bone metastases in patients with breast cancer. J Cancer Res Clin Oncol 2002;128:325–8. [DOI] [PubMed] [Google Scholar]
- [16].Ohta M, Tokuda Y, Suzuki Y, et al. Whole body PET for the evaluation of bony metastases in patients with breast cancer: comparison with 99Tcm-MDP bone scintigraphy. Nucl Med Commun 2001;22:875–9. [DOI] [PubMed] [Google Scholar]
- [17].Woolf DK, Padhani AR, Makris A. Assessing response to treatment of bone metastases from breast cancer: what should be the standard of care? Ann Oncol 2015;26:1048–57. [DOI] [PubMed] [Google Scholar]
- [18].Altman DG. Practical Statistics for Medical Research Chapman & Hall London Google Scholar. 1991. [Google Scholar]
- [19].Fogelman I. Osteoblastic bone metastases in breast cancer: is not seeing believing? Eur J Nucl Med Mol Imaging 2005;32:1250–2. [DOI] [PubMed] [Google Scholar]
- [20].Lee SJ, Park S, Ahn HK, et al. Implications of bone-only metastases in breast cancer: favorable preference with excellent outcomes of hormone receptor positive breast cancer. Cancer Res Treat 2011;43:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Niikura N, Liu J, Hayashi N, et al. Treatment outcome and prognostic factors for patients with bone-only metastases of breast cancer: a single-institution retrospective analysis. Oncologist 2011;16:155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ahn SG, Lee HM, Cho S-H, et al. Prognostic factors for patients with bone-only metastasis in breast cancer. Yonsei Med J 2013;54:1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chen Y-C, Sosnoski DM, Mastro AM. Breast cancer metastasis to the bone: mechanisms of bone loss. Breast Cancer Res 2010;12:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 2006;12:6243s–9s. [DOI] [PubMed] [Google Scholar]
- [25].O'Sullivan GJ, Carty FL, Cronin CG. Imaging of bone metastasis: an update. World J Radiol 2015;7:202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ford HT, Gazet JC. Assessment of response of bone metastases to systemic treatment in patients with breast cancer. Cancer 1983;52:610–4. [DOI] [PubMed] [Google Scholar]
- [27].Eisenhauer EA, Therasse P, Bogaerts J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- [28].Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. Breast Cancer 2005;12:S16–27. [Google Scholar]
- [29].Tateishi U, Gamez C, Dawood S, et al. Bone metastases in patients with metastatic breast cancer: morphologic and metabolic monitoring of response to systemic therapy with integrated PET/CT 1. Radiology 2008;247:189–96. [DOI] [PubMed] [Google Scholar]
- [30].Wong M, Pavlakis N. Optimal management of bone metastases in breast cancer patients. Breast Cancer (Dove Med Press) 2011;3:35–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Koizumi M, Yoshimoto M, Kasumi F, et al. Comparison between solitary and multiple skeletal metastatic lesions of breast cancer patients. Ann Oncol 2003;14:1234–40. [DOI] [PubMed] [Google Scholar]


