Supplemental Digital Content is available in the text
Keywords: drainage, neck dissection, thyroid cancer, thyroidectomy
Abstract
Background:
To evaluate the effect of no drainage in patients who underwent thyroidectomy and neck lymph node dissection.
Methods:
We followed the methodological standard expected by Cochrane. We searched the following databases by March 23, 2017: PubMed, The Cochrane Library, EMBASE via Ovid SP, and Medline via Ovid SP. Two reviewers screened the studies and extracted the data. Randomized controlled trials (RCTs) or nonrandomized interventional studies assessing the effect of no drainage following thyroidectomy with lymph node dissection were included.
Results:
Three studies with 387 participants were included. There was no statistical difference between groups for the overall perioperative complications (2 RCTs, n = 234, RR 1.56, 95% CI 0.53–4.64), or specific complications such as seroma (2 RCTs, n = 234, RR 1.81, 95% CI 0.46–7.07), hematoma (2 RCTs, n = 234, RR 0.72, 95% CI 0.11–4.83) or hemorrhage (1 RCT, n = 69, RR 0.29, 95% CI 0.01–6.87). One case required reoperation due to hemorrhage in the drainage group was reported in 1 study (n = 32). No mortality was reported. Two studies (n = 234) stated a longer hospital stay in the drainage group than that in the group without drainage. There was moderate or serious bias for the risk of bias of included studies.
Conclusion:
The effect of no-drainage in patients with thyroid cancer who received thyroidectomy with neck dissections remains uncertain, since there are very few studies that addressed the question. Drainage may lead to longer hospital stay than nondrainage. More randomized or nonrandmized studies are required to address this issue.
1. Introduction
It has been a controversial issue whether drainage should be used after thyroid surgery. The major concern after thyroid surgery is the development of hematoma or seroma. Although the incidence of hematoma is as low as 0% to 2.6%,[1–4] postoperative hematoma may lead to life-threatening airway compression. Majority of surgeons took for granted that drainage following thyroid surgery would obliterate the dead space and evacuate the accumulation of blood and serum. However, there is no supportive evidence so far. Accumulating studies have shown that insertion of a drainage tube did not benefit patients after thyroid surgery.[5–9] Several studies demonstrated that drainage did not reduce hematomas. And in case of severe bleeding, wound drainage may be obstructed by clots.[7,10] Moreover, it also prolonged the length of hospitalization and increased the rate of infection.[5,6,8,11–14] One study even found that more fluid collection and higher incidence of postoperative complications occurred in patients with drainage than those without drainage.[15]
This subject is seldom discussed in the current guidelines. Several systematic reviews explored this issue in patients with benign thyroid diseases.[6,9,11] The Cochrane review, published in 2007, concluded that no clear evidence showed drainage can bring benefits to patients’ outcome, inversely it is associated with longer hospitalization.[6] One systematic review conducted in 2014 stated that routine drainage of thyroid bed may not be necessary as draining resulted in higher morbidity and prolonged the hospital stay.[11] A more recent systematic review did not confer benefit of drainage either.[9] However, evidence mentioned above did not involve patients with thyroid malignancy. Surgery for thyroid malignancy usually requires central or lateral lymph node dissection, leading to larger dead spaces. In this case, insertion of a drainage tube is considered necessary. In contrast, in other types of surgeries with much larger potential dead spaces such as cholecystectomy, hepatectomy, and colonic anastomosis, drainage had also been questioned and these procedures are now routinely performed without drainage.[16–19] Clinical studies focusing on patients with thyroid cancer also showed no benefit of drainage after thyroidectomy with lymph node dissection.[10,14,20] Considering the absence of synthetic evidence in this field, we aimed to collect relevant randomized controlled trials (RCTs) to evaluate the effectiveness and safety of no drainage following thyroidectomy with lymph node dissection.
2. Materials and methods
2.1. Ethical statement
This work was based on previously published studies. Therefore, no ethical approval or patient consent was necessary.
2.2. Criteria for including studies
We included studies that met the following criteria: RCTs or nonrandomized studies of interventions (such as nonrandomized clinical controlled trials or retrospective studies); participants with thyroid cancer and received thyroidectomy with central or lateral neck lymph nodes dissection; trials evaluated the safety of no drains. We excluded trials with mixed population which involved both benign thyroid disease (such as Grave disease, goiter) and thyroid carcinoma. Our outcomes of interest included adverse events (incidence of any adverse events or specific adverse events such as respiratory distress, infection, hematoma, seroma, hemorrhage, and so on), rates of reoperation, mortality, and length of hospital stay.
2.3. Data source and study selection
We searched relevant trials by March 23, 2017 in the following databases: PubMed, The Cochrane Library, EMBASE via Ovid SP, and Medline via Ovid SP. We presented the search strategy in supplemental material (see search strategy, supplemental content, which presents the search strategy used in The Cochrane Library).
Two authors screened the study independently. Titles and abstracts were first inspected, then full texts of potentially relevant publications were obtained and screened. Any discrepancy was resolved by discussion between the 2 reviewers.
2.4. Data extraction and management
A data extraction form was predesigned for data collection. The form was piloted and revised. Any disagreement was resolved by discussion and consensus. The following items were collected:
-
1.
Study characteristics: first author, year of publication, locations, settings, patients characteristics (such as diagnosis, surgery type, key exclusion criteria, total number of participants, drop-outs).
-
2.
Study design (group allocation, assessment of selective reporting, blinding of participants, and study investigators or personal).
-
3.
Outcomes (reported outcomes of each study, definition of outcomes, and quantitative data).
2.5. Assessment of risk of bias in included studies
For randomized controlled trials, we made risk of bias judgment based on methods described in The Cochrane Handbook (Chapter 9).[21] We assessed 7 domains of risk of bias for each included study: randomization, allocation concealment, blinding of participants and personnel, blinding of outcome assessor, incomplete data, selective reporting, and other bias. For nonrandomized studies of intervention, we used Risk of Bias In Non-randomized Studies of Interventions (ROBINS-I) to assess the risk of bias.[22]
2.6. Data synthesis
Review Manager 5.3 was used to input and combine the data (http://community.cochrane.org/tools/review-production-tools/revman-5/revman-5-download). For dichotomous outcome data, we used risk ratios (RR) and its 95% confidence interval (CI) to measure the treatment effect. For continuous outcome data, we used the effect measure of mean differences and its 95% CI. We selected random-effect model to combine the data. Where the data was not applicable to combine, we simply described the findings of individual studies.
2.7. Assessment of heterogeneity
Heterogeneity in pooled results is usually resulted from clinical and methodological heterogeneity. An I2 estimate < = 50% accompanied by a statistically significant Chi[2] statistic (P<.1) was interpreted as evidence of substantial levels of heterogeneity.[21] Where heterogeneity was suspected, we explored the sources of heterogeneity from the above 2 aspects. If any source was identified, we planned to conduct post-hoc subgroup analysis. If the source was not identified, we combined the data in a random effect model and downgraded the quality of evidence for 1 level.
2.8. Assessment of reporting biases
We planned to use funnel plot to test the publication bias when the included number of studies in 1 meta-analysis was more than 10.[21] However, as only 3 studies were included in this review. The funnel plot was not conducted.
3. Results
3.1. Literature screening
The electronic search produced 1183 references. After removing the duplicates, 1009 references were screened. Of which, 983 references were excluded through viewing the titles and abstracts. Twenty-six full reports were checked for eligibility but only 3 studies were included (Fig. 1).
Figure 1.
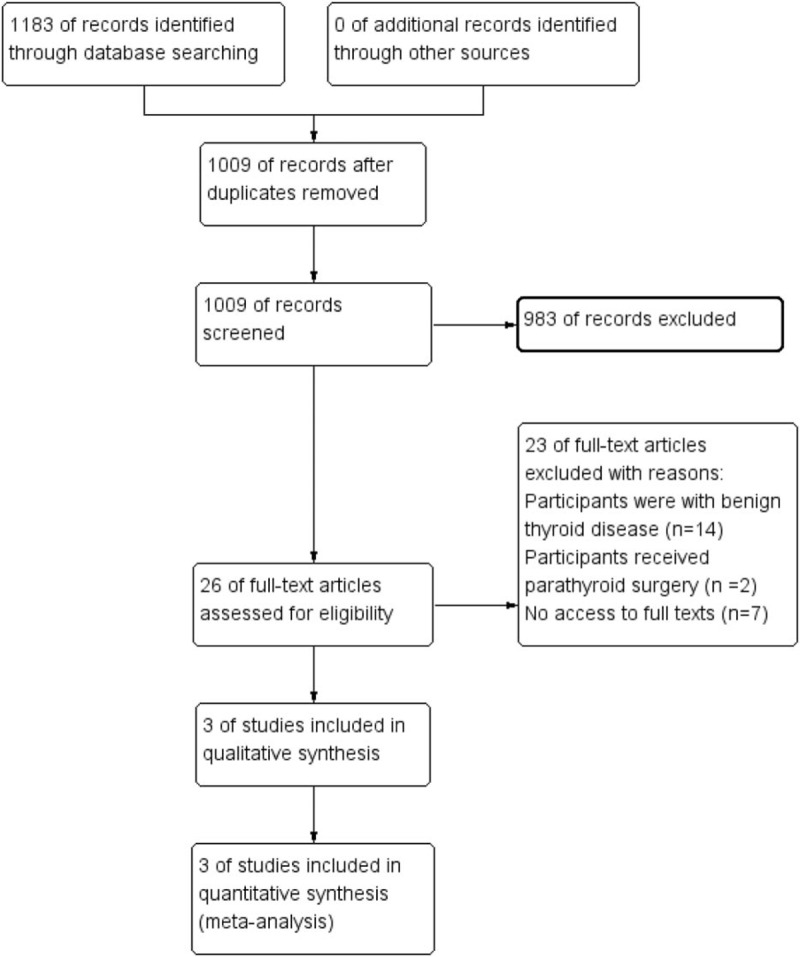
Study flow diagram.
3.2. Study characteristics
Three studies[10,20,23] with 387 participants who underwent thyroidectomy with lateral or central neck dissection (LND or CND) were included.
One RCT[20] was conducted in Korea enrolling participants between 2004 and 2005. The authors enrolled participants underwent hemi thyroidectomy, total thyroidectomy and total thyroidectomy with CND. One hundred one participants were randomly assigned to the drainage group while 97 were assigned to the control group. The incidences of local hemorrhage/seroma, intraoperative bleeding, operation time, volume of resected thyroid gland, length of hospitalization, duration of tube placement, and total amount of drainage were observed and compared between groups. Only results on local complications from the subgroup population who received CND were included in our review, with 37 participants from the drainage group and 32 from the control group. The central neck dissection was performed if the primary tumor size was larger than 1 cm or there was evidence of lymph node metastasis. The baseline characteristics of the subgroup population were not reported such as the mean age of subgroup population and the gender distribution. Another 2 observational studies[10,23] also explored this clinical question. Mekel et al[10] retrospectively analyzed data of 165 LNDs from 2001 to 2008. The patients were diagnosed with papillary carcinoma (85%), medullary carcinoma (10%), or other types of thyroid cancer. The age of the sample population ranges from 7 years old to 84 years old with a mean age of 44 years old. According to the medical records at the Massachusetts General hospital (USA), 102 LNDs were inserted with a draining tube after the operation, while 63 were not. The authors further subgrouped the population by the number of lymph nodes excised (participants with less than 14 excised lymph nodes vs participants with at least 14 excised lymph nodes). In each subgroup population, the authors stated the baseline characteristics were balanced between groups. The other observational study[23] reviewed the medical records of patients at Hotel Dieu de France Hospital in Lebanon from 1998 to 2011. The participants involved 205 patients with CND or LND and 121 patients underwent thyroidectomy without NDs. No participant received drainage after operation. The authors did not report the age and gender distribution or other baseline characteristics of the 205 CND or LND patients.
3.3. Risk of bias
One randomized controlled trial[20] was included. However, we only used data from a subset population. The baseline characteristics of the drain and no drain group were not reported in this subgroup population which may indicate potential selection bias. The allocation concealment was also not stated by this trial. Another bias that may impact the result was the performance and detection bias, as the control group did not use draining tubes. It is not likely for the participants, investigators, or outcome assessors to be blinded. Another concern was that the subgroup sample size was too small to detect a clear difference between groups. For the nonrandomized controlled trial Mekel et al,[10] there were some biases in confounding, as the baseline characteristics may not be well balanced between groups. The other biases were not obvious. The authors viewed medical records of the participants, so there might be some selection biases in the selection of the analyzed cases. As the definition of drain and no drain is very clear, it is not likely that the definition of intervention may bias in the classification of participants. The missing data was not a concern either, as the intervention was conducted during hospitalization and the outcomes could be observed within several days post operation, so there was no missing data. The bias in the measurement of outcomes was also low. The selective reporting bias was unclear, as the protocol of this study was not available and the predefined outcome measurements and methods used to analyze data were not known. Overall, the risk of bias of this study[10] was judged as moderate according to the standards of overall risk of bias judgment described in ROBINS-I.[22] Another study[23] was judged as serious risk of bias. Instead of comparing drain with no drain in patients with NDs, this study compared the safety of no drain in patients who received NDs with those who did not receive NDs. The biases of participant selection and confounding were serious.
3.4. Effects of measurements
3.4.1. Adverse events
Three studies[10,20,23] reported the incidence of perioperative complications such as seroma, hematoma, or hemorrhage. Meta-analysis from 2 studies demonstrated that there was no statistical difference between the groups for the overall perioperative complications (2 RCTs, n = 234, RR 1.56, 95% CI 0.53–4.64, P = .42, Fig. 2), and specific complications such as seroma (2 RCTs, n = 234, RR 1.81, 95% CI 0.46–7.07, P = .40, Fig. 2), hematoma (2 RCTs, n = 234, RR 0.72, 95% CI 0.11–4.83, P = .73, Fig. 2), and hemorrhage (1 RCT, n = 69, RR 0.29, 95% CI 0.01–6.87, P = .44, Fig. 2). However, the result was imprecise as the confidence interval was wide, indicating that sample size was too small to detect a clear difference between groups. Lee et al[20] found that for patients who received CND, 6.25% (2/32) versus 8.10% (3/37) participants occurred with seroma in the drainage group and control group respectively. In the drainage group, 1 case occurred with severe hemorrhage 6 hours after operation which required reoperation. In control group, minor hematoma was observed in 1 patient, which was resolved by repeated needle aspiration and compressive dressing. In total, the authors stated that there was no statistical significant between 2 groups for local complications. Mekel et al[10] also stated that there was no statistical difference between groups for the incidence of seroma (1/102 vs 2/63) and hematoma (1/102 vs 1/63). Abboud et al[23] observed participants without drainage after operation. They grouped participants into 2 groups: 205 participants with CND or LND and 121 patients undergoing thyroidectomy without NDs. Twelve patients with CND or LND developed hematoma and/or seroma (12/205), while 5 participants without CND developed complications (5/121). Seven patients experienced seroma in the NDs group (7/205) while 4 experienced seroma in the non-NDs groups (4/121). Most seromas were identified within 2 weeks after discharge. Five patients experienced minor hematoma in the NDs group (5/205) while 1 experienced seroma in the non-NDs groups (1/121). There was no significant difference between the 2 groups. No major postoperative bleeding occurred in either group.
Figure 2.
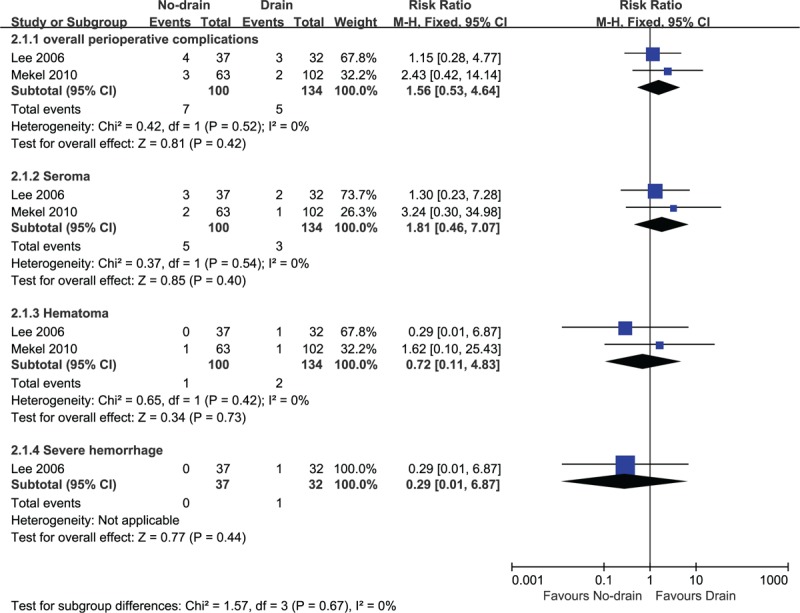
No-drain versus drain: adverse events.
3.4.2. Rates of reoperation
Only 1 study[20] stated that 1 participant underwent reoperation due to hemorrhage in those who received suction drainage after thyroidectomy with CND (n = 32). Other studies did not report any case with reoperation.
3.4.3. Mortality
No study reported mortality.
3.4.4. Length of hospitalization
Two studies stated a longer hospital stay in the drainage group in patients with NDs.[10,20]
4. Discussion
We only found 3 studies with 387 participants investigating whether no drainage was applicable after thyroidectomy with NDs. Although all studies demonstrated no difference between groups with or without drainage for perioperative complications such as seroma, hematoma, or hemorrhage, the findings were doubtable, as the estimated effect was imprecise and unclear. The very low incidence of events and very small sample sizes from included studies made it difficult to detect clear difference between compared groups. Among 387 participants only 1 case suffered from severe hemorrhage which was resolved by reoperation. No case of mortality due to severe complications was reported. The length of hospital stay may be longer in drainage group in patients with NDs than that in control group. However, there was no difference on hospital stay between patients treated with or without NDs if drainage was not implicated.
There were several previous systematic reviews investigating the effect of drainage in patients with benign thyroid tumors.[6,11,24] These systematic reviews included RCTs that compared the usage of drainage versus no drainage in patients who underwent thyroid or parathyroid surgery without NDs. The results from all reviews indicated that drainage after routine thyroid surgery did not confer benefits to patients, such as lowering incidence of perioperative complications. Nonetheless drainage prolonged the hospital stay. Moreover, data from 1 observational study[14] involving 1066 patients who underwent thyroidectomy even found that the rate of reoperation due to life-threatening postoperative hemorrhage and wound infection was higher in the drainage group.
However, limited data investigated whether drainage should be used in patients who received thyroidectomy and NDs. The common dogma is that surgeries with enlarged dead space of the wound should be drained to facilitate the wound healing. Another important reason for drainage after NDs is to prevent severe complications such as severe hemorrhage which may cause airway compression. However, it seemed hemorrhage was neither prevented nor resolved by a drainage tube. Aspiration or reoperation was more effective solution to resolve severe hemorrhage.
Our systematic review collected current studies evaluating the effect of drainage for patients with thyroidectomy and neck dissection. We found that this clinical issue was seldom explored. Although only a few studies with unclear effect estimates were included, we still considered it as valuable findings. Like other systematic reviews, our review also had some limitations. First, our review included a small number of studies with small sample size due to limited availability. As the low incidence of complications, the data was insufficient to detect any difference between drainage and no-drainage groups. Second, there were selection or confounding biases with the included studies. This may also impact the reliability of the findings. Most importantly, the limited total sample size also made it difficult to detect firm findings.
5. Conclusions
5.1. Implication for clinicians
The incidence of perioperative complications after thyroid surgery, such as seroma, hematoma, and hemorrhage, is very low. However, the current evidence is insufficient to detect a clear difference on safety between patients with thyroid cancer treated with or without drainage after neck dissections.
5.2. Implication for research
More trials, either randomized controlled trials or observational studies with larger sample size, are required to evaluate the safety of drainage for thyroid cancer patients underwent neck dissection.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, CND = central neck dissection, LND = lateral neck dissection, MD = mean difference, RCT(s) = randomized controlled trial (s), RR = risk ratio.
LL and HC contributed equally to this work.
This work is supported by National Natural Science Foundation of China (NSFC) grants (81402357, 81773048, 81702397, 81402444) and Chinese Ministry of Science and Technology (MOST) grant (14C26213601925).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Kennedy SA, Irvine RA, Westerberg BD, et al. Meta-analysis: prophylactic drainage and bleeding complications in thyroid surgery. J Otolaryngol Head Neck Surg 2008;37:768–73. [PubMed] [Google Scholar]
- [2].Herranz J, Latorre J. [Drainage in thyroid and parathyroid surgery]. Acta Otorrinolaringol Esp 2007;58:7–9. [PubMed] [Google Scholar]
- [3].Harding J, Sebag F, Sierra M, et al. Thyroid surgery: postoperative hematoma—prevention and treatment. Langenbecks Arch Surg 2006;391:169–73. [DOI] [PubMed] [Google Scholar]
- [4].Colak T, Akca T, Turkmenoglu O, et al. Drainage after total thyroidectomy or lobectomy for benign thyroidal disorders. J Zhejiang Univ Sci B 2008;9:319–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tubergen D, Moning E, Richter A, et al. [Assessment of drain insertion in thyroid surgery?]. Zentralbl Chir 2001;126:960–3. [DOI] [PubMed] [Google Scholar]
- [6].Samraj K, Gurusamy KS. Wound drains following thyroid surgery. Cochrane Database Syst Rev 2007;Cd006099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Memon ZA, Ahmed G, Khan SR, et al. Postoperative use of drain in thyroid lobectomy—a randomized clinical trial conducted at Civil Hospital, Karachi, Pakistan. Thyroid Res 2012;5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Khanna J, Mohil RS, Chintamani, et al. Is the routine drainage after surgery for thyroid necessary? A prospective randomized clinical study [ISRCTN63623153]. BMC Surg 2005;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Alexiou K, Konstantinidou E, Papagoras D. The use of drains in thyroid surgery. Hellenic J Surg 2015;87:97–100. [Google Scholar]
- [10].Mekel M, Stephen AE, Gaz RD, et al. Surgical drains can be safely avoided in lateral neck dissections for papillary thyroid cancer. Am J Surg 2010;199:485–90. [DOI] [PubMed] [Google Scholar]
- [11].Woods RS, Woods JF, Duignan ES, et al. Systematic review and meta-analysis of wound drains after thyroid surgery. Br J Surg 2014;101:446–56. [DOI] [PubMed] [Google Scholar]
- [12].Tabaqchali MA, Hanson JM, Proud G. Drains for thyroidectomy/parathyroidectomy: fact or fiction? Ann R Coll Surg Engl 1999;81:302–5. [PMC free article] [PubMed] [Google Scholar]
- [13].Schoretsanitis G, Melissas J, Sanidas E, et al. Does draining the neck affect morbidity following thyroid surgery? Am Surg 1998;64:778–80. [PubMed] [Google Scholar]
- [14].Ozlem N, Ozdogan M, Gurer A, et al. Should the thyroid bed be drained after thyroidectomy? Langenbecks Arch Surg 2006;391:228–30. [DOI] [PubMed] [Google Scholar]
- [15].Irfan Is, Javed I, Rana A, et al. The role of drainage after thyroid surgery. Is it mandatory? APMC 2008;2:46–9. [Google Scholar]
- [16].Gurusamy KS, Koti R, Davidson BR. Routine abdominal drainage versus no abdominal drainage for uncomplicated laparoscopic cholecystectomy. Cochrane Database Syst Rev 2013;CD006004. [DOI] [PubMed] [Google Scholar]
- [17].Gurusamy KS, Samraj K, Davidson BR. Routine abdominal drainage for uncomplicated liver resection. Cochrane Database Syst Rev 2007;CD006232. [DOI] [PubMed] [Google Scholar]
- [18].Johnson CD, Lamont PM, Orr N, et al. Is a drain necessary after colonic anastomosis? J R Soc Med 1989;82:661–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lewis RT, Goodall RG, Marien B, et al. Simple elective cholecystectomy: to drain or not. Am J Surg 1990;159:241–5. [DOI] [PubMed] [Google Scholar]
- [20].Lee SW, Choi EC, Lee YM, et al. Is lack of placement of drains after thyroidectomy with central neck dissection safe? A prospective, randomized study. Laryngoscope 2006;116:1632–5. [DOI] [PubMed] [Google Scholar]
- [21].Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from http://handbook.cochrane.org. [Google Scholar]
- [22].Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ V 355 2016;i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Abboud B, Tannoury J, Sleilaty G, et al. Cervical neck dissection without drainage in papillary thyroid carcinoma. J Laryngol Otol 2013;127:299–302. [DOI] [PubMed] [Google Scholar]
- [24].Tian J, Li L, Liu P, et al. Comparison of drain versus no-drain thyroidectomy: a meta-analysis. Eur Arch Otorhinolaryngol 2017;274:567–77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


