Abstract
Rationale:
Idiopathic retroperitoneal abscesses are insidious, occult illnesses with high mortality if inadequately drained. Endoscopic ultrasound-guided drainage is an alternative to percutaneous or surgical drainage, it is not widely performed for retroperitoneal abscesses other than peripancreatic fluid collection.
Patient concerns:
We present a 76-year-old Japanese woman with abdominal pain, high fever, and a history of rheumatism on treatment with immunosuppressants.
Diagnoses:
The patient was diagnosed with idiopathic retroperitoneal abscess based on results obtained from her clinical course and findings on computed tomography.
Interventions:
We performed Endoscopic ultrasound—guided drainage. After we performed needle puncture via the descending portion of the duodenum, the fistula was expanded using a dilator, and a double-pigtail stent and endoscopic nasobiliary drainage tube were inserted.
Outcomes:
The patient was kept nil by mouth, together with intravenous antibiotic therapy, and repeated washing of the abscess cavity with saline was performed. After that, we confirmed disappearance of the cavity, and, after removing the tubes, commenced oral feeding. We were able to avoid surgery in this immunosuppressed patient.
Lessons:
Endoscopic ultrasound (EUS)-guided abscess drainage can be overall considered a safe and useful procedure. We also propose the double-stent method, with both internal and external stents, for the treatment of idiopathic retroperitoneal abscesses.
Keywords: abscess drainage, EUS-guided drainage, idiopathic retroperitoneal abscess, immunosuppressed condition, transduodenal puncture
1. Introduction
Idiopathic retroperitoneal abscesses are insidious and occult illnesses that have a high mortality rate if not adequately drained. Hence, they require early diagnosis and appropriate drainage.[1] Retroperitoneal abscesses develop from infections of retroperitoneal organs and other diseases, including malignancies, trauma, and perforation.[2] Sometimes, the cause of abscess formation is unknown, and could be because of immunosuppression for any reason. Conventionally, when the infection cannot be controlled, preoperative computed tomography (CT) or ultrasonography (US) -guided percutaneous drainage is performed. However, drainage using these imaging modalities is associated with the problems of inadequate visualization of some of the anatomic structures, such as blood vessels, which might be in the path of the puncture needle. Further, surgical drainage is an invasive procedure that should be avoided in immunocompromised individuals, if possible. Endoscopic ultrasound (EUS) provides greater spatial resolution and better anatomic detail than US and CT. EUS allows clear visualization of the needle and evaluation of blood flow along the path of the needle. EUS-guided drainage has been performed for various abscesses, including peri-pancreatic fluid collection, with a high success rate.[3–7] The method is safe and has good outcomes, and should be considered an alternative to percutaneous and surgical drainage. Placement of a double-pigtail plastic stent and endoscopic nasobiliary drainage (ENBD) tube into the abscess cavity via the duodenum allows histological and bacteriological evaluation of the abscess, along with repeated wash. We report here a case of retroperitoneal abscess in an immunocompromised patient that we drained using EUS, followed by ENBD tube insertion into the abscess cavity via the duodenum.
2. Case presentation
2.1. Preoperative evaluation
This case report was approved by the Medical Ethics Committee at Oita San-ai Medical Center and was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from the patient in writing.
A 76-year-old Japanese woman was admitted to the emergency room complaining of abrupt onset right abdominal pain with high fever. The patient had a history of rheumatism from the age of 60 years, for which she was on treatment with a drug combination that included immunosuppressants (bucillamine, 200 mg/d, and methotrexate, 4 mg/wk). Clinical examination revealed elevated body temperature (38.7°C) and right abdominal pain on palpation. Blood biochemistry revealed high blood leukocyte (13,330/mm3) and lactate levels (18 mg/dL). Other biological tests were normal. Unenhanced CT revealed a 9.1 cm × 4.2 cm × 11.7 cm large mass with increased peripheral fatty tissue in the retroperitoneal cavity below the right kidney and the horizontal portion of the duodenum. Contrast-enhanced CT revealed a hypo-enhanced mass (Fig. 1). Based on clinical and imaging findings, it appeared that the abscess was due to duodenal perforation or secondary to immunosuppression. However, since free air was not recognized and the patient's general condition was good, we conservatively observed the patient while keeping her nil by mouth and in conjunction with intravenous antibiotic therapy (meropenem: 1 g/d, clindamycin: 2.4 g/d) for 3 days. However, since her clinical symptoms and blood inflammatory parameters did not improve (blood leukocytes; 23,040/mm3), we decided to perform EUS-guided drainage.
Figure 1.
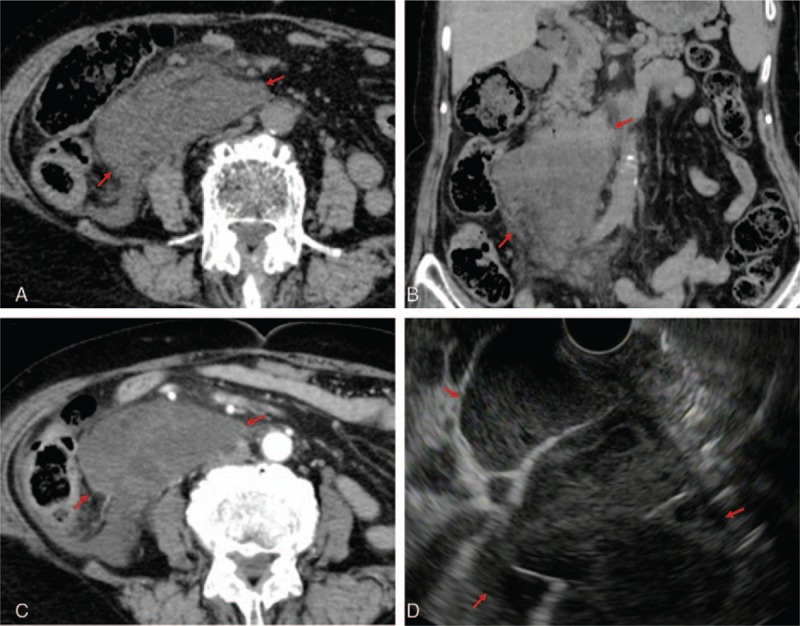
Idiopathic retroperitoneal abscess on CT and EUS. A and B, A 9.1 cm × 4.2 cm × 11.7 cm large mass in the retroperitoneal cavity below the right kidney and horizontal portion of the duodenum was revealed on unenhanced CT (surrounded by red arrows). C, A hypoenhanced mass was revealed on contrast-enhanced CT (in between the red arrows). D, EUS showed the peripheral rim of the abscess, solid necrotic structure, and partition wall inside the lesion (in between the red arrows). CT= computed tomography, EUS= endoscopic ultrasound.
2.2. The process of EUS-guided drainage
EUS via the descending and horizontal portions of the duodenum revealed the peripheral rim of the abscess, solid necrotic structure, and partition wall inside the abscess (Fig. 1). After confirming that there was almost no blood flow along the proposed needle track by Doppler, we performed needle puncture through the posterior wall of the descending part of the duodenum to the wall of the abscess using a 19-gauge needle (EZ shot 3 plus; Olympus Medical Systems, Tokyo, Japan), aspirated the whitish colored purulent fluid and injected iodixanol as the contrast agent. Then, we passed a 0.025-inch guidewire (VisiGlide 2; Olympus Medical Systems) into the abscess, inserted a double-lumen catheter (Uneven Double Lumen Cannula; Piolax Medical Devices, Tokyo, Japan), and placed another guidewire. Next, the fistula was expanded using an 8.5-Fr wire-guided diathermic dilator (Cysto-Gastro-Set; Endo-Flex GmbH, Voerde, Germany), following which we placed a 7-Fr 10 cm long double-pigtail catheter (Zimmon Biliary Stent; Cook Medical, Tokyo, Japan) from the abscess cavity to the duodenum and a 7-Fr 250 cm long ENBD tube, (Nasal Biliary Drainage Set; Cook Medical) (Fig. 2) to more efficiently wash the interior of the abscess.
Figure 2.
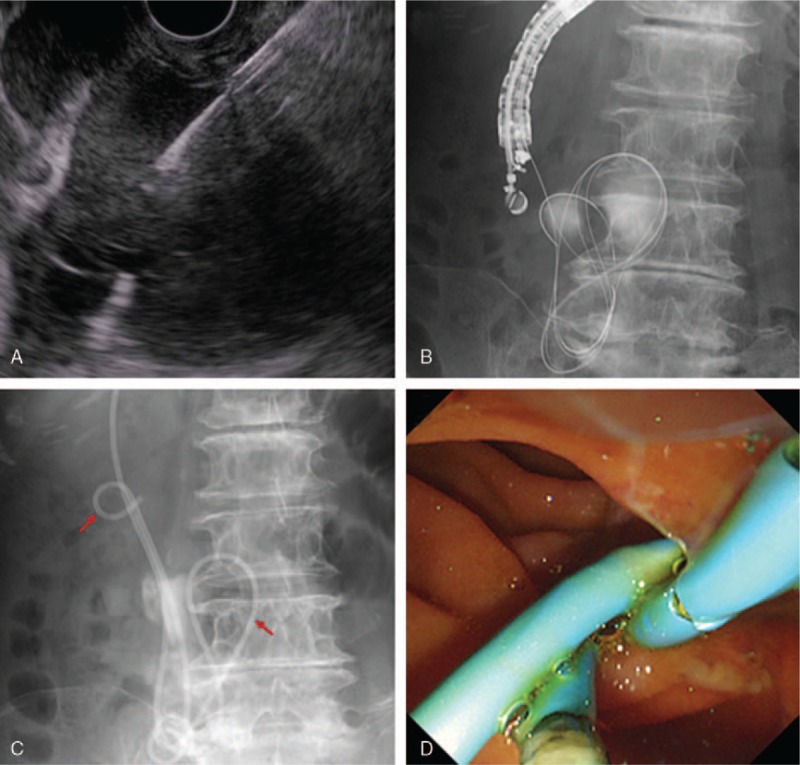
EUS-guided idiopathic retroperitoneal abscess drainage with internal and external stent placement. After confirming that there was almost no blood flow along the path of the needle by Doppler, we performed needle puncture via the descending portion of the duodenum using a 19-gauge needle. Then, we passed a 0.025-inch guidewire into the abscess, inserted, a double-lumen catheter, and placed another guidewire. After expanding the fistula with an 8.5-Fr wire-guided diathermic dilator, we placed a 7-Fr, 10 cm double-pigtail stent (left red arrow) and 7-Fr, 250 cm endoscopic nasobiliary drainage (ENBD) tube (right red arrow). D, Under endoscopy, the internal stent and ENBD tube were placed via a duodenal fistula into the abscess. Infected fluid was seen draining out of the stent and fistula into the duodenum by endoscopy. EUS= endoscopic ultrasound.
2.3. Postoperative observation
Microbiological evaluation of the abscess fluid revealed the presence of E coli and the results of cytology were not malignant. We treated the patient conservatively by with-holding oral intake and giving intravenous antibiotic therapy (melopenem and clindamycin), along with washing the abscess cavity with 40 mL saline 6 times a day via the ENBD tube. On day 14 after the abscess drainage, improvement in her clinical symptoms and inflammatory parameters on blood tests (blood leukocytes: 5790/mm3, C reactive protein: 0.03 mg/dL, maximum: 18.1 mg/dL) were observed. We changed the antibiotic to sulbactam/cefoperazone 2 g/d and continued maintaining the patient on parenteral nutrition. On day 31, after confirming the disappearance of the abscess cavity on contrast X-ray (Fig. 3A) and CT, we pulled out the ENBD tube and stent under gastrointestinal endoscopic visualization and commenced oral feeding. On day 41, the patient was discharged from the hospital. CT follow-up after 3 months revealed that the abscess had not recurred (Fig. 3B). With our management strategy, we were able to avoid surgery in this immunocompromised patient.
Figure 3.
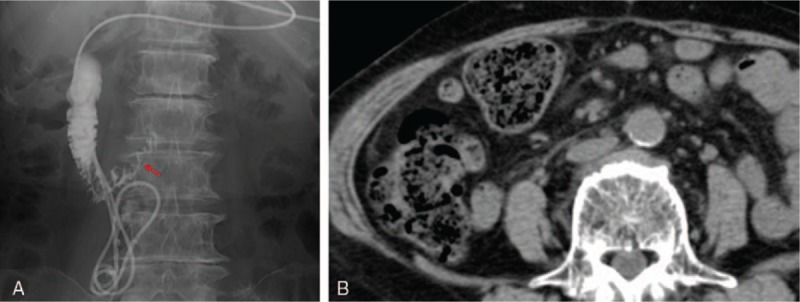
Resolution of the abscess. On day 31, we confirmed disappearance of the abscess cavity on contrast X-ray. The red arrow shows traces of the abscess cavity. The abscess did not recur, as seen on CT performed 3 months after discharge. CT= computed tomography.
3. Discussion
Early diagnosis and appropriate drainage of idiopathic retroperitoneal abscesses are essential for preventing prolonged sepsis and the associated high mortality.[1]
The retroperitoneum is a potential space between the peritoneum and transversalis fascia with defined boundaries. The source of retroperitoneal infections is usually from organs such as the kidneys, ureters, duodenum, pancreas, and portions of the ascending and descending colon. Intestinal perforation secondary to malignancies or diverticulitis, appendicitis, pancreatitis, biliary tract disease, peptic ulcer disease, trauma and inflammatory bowel disease, and osteomyelitis of vertebral bodies can all cause retroperitoneal abscesses.[2] A majority of patients with retroperitoneal abscesses are immunosuppressed. For uncontrolled infection, treatment usually consists of surgical drainage in conjunction with intravenous antibiotic therapy.[1] CT- and US-guided percutaneous drainage, which are the usual procedures for preoperative evaluation and treatment, have many drawbacks. US-guidance is sometimes preferred over CT because the needle is visualized in real time. However, it is difficult to visualize the needle due to overlying bowel gas and inability to clearly delineate intervening tissues.[8]
EUS provides greater spatial resolution and allows better visualization of anatomic details than US and CT. It offers clear and consistent visualization of the needle in real time, allowing avoidance of intervening vasculature, and with less interference by bowel gas.[3] Originally, EUS-guided fine needle aspiration (FNA) was performed for preoperative evaluation of idiopathic abdominal masses, including abscesses, with a sensitivity and specificity of diagnosis of 83% and 100%, respectively.[8] EUS-guided drainage is currently performed as an alternative to surgery for peri-pancreatic fluid collections, including pancreatitis, abscesses and pseudocysts, and is also performed for hepatic, splenic, sigmoid diverticular, perirectal, and pelvic abscesses, with a high success rate.[3,4] The utility of EUS-guided drainage of peri-pancreatic fluid collections and pelvic abscesses, in particular, has been previously adequately reported.[5,6] In a systematic review, the clinical success and adverse events rates of EUS-guided pancreatic pseudocyst drainage appeared to be comparable with that of surgical or percutaneous drainage, although the EUS approach reduced hospital stay and cost, and improved postprocedure quality of life.[5] In another study, the technical and clinical success rates of EUS-guided pelvic abscess drainage were 100% and 91.9% respectively, and the long-term success rate was 86.5% at a median follow-up period of 64 months.[6] In a study on liver abscess drainage, EUS-guided liver abscess drainage using a fully covered metallic stent resulted in a short hospital stay, high clinical success rate (100%), and low adverse event rate (0%) compared with percutaneous drainage.[7] Intra-abdominal abscesses arise from intraperitoneal (liver, spleen, stomach) and retroperitoneal (kidney, pancreas, spine, muscular elements) viscera.[8] If an abscess can be visualized by EUS, EUS-guided drainage can be performed. EUS-guided retroperitoneal abscess drainage is safer and less invasive, especially for patients in an immunocompromised state, compared with other drainages. We usually place a double-pigtail plastic stent or metallic stent as the internal fistula, and/or ENBD tube as the external fistula. In this case, by placing a pigtail plastic stent and ENBD tube, we could evaluate the abscess histologically, to determine whether or not it was malignant, and bacteriologically. We could choose the appropriate intravenous antibiotic therapy depending on the drug sensitivity of the species. We could also repeatedly wash the abscess cavity using the ENBD tube, and could evaluate the cavity using contrast injected via the ENBD tube, which significantly contributed to the reduction in the size of the abscess cavity.
One possible disadvantage of our method, which requires mention, is the possibility of needle tract seeding from malignancies. However, the rate of tumor seeding after EUS-FNA is probably very low.[9,10]
EUS-guided abscess drainage can be overall considered a safe and useful procedure. We also propose the double-stent method, with both internal and external stents, for the treatment of idiopathic retroperitoneal abscesses. We reported the valuable case of idiopathic retroperitoneal abscess treated by EUS-guided drainage without surgery.
Acknowledgments
The author thank endoscopy engineers of Oita San-ai Medical Center, for their helpful technical assistance with EUS-guided drainage.
Footnotes
Abbreviations: CT= computed tomography, ENBD= endoscopic nasobiliary drainage, EUS= endoscopic ultrasound, FNA= fine needle aspiration, US= ultrasonography.
The authors have no conflicts of interest to disclose.
References
- [1].Crepps JT, Welch JP, Orlando R., 3rd Management and outcome of retroperitoneal abscesses. Ann Surg 1987;205:276–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Oluwole SF, Adekunle A, Akintan B. Retroperitoneal abscess. J Natl Med Assoc 1983;75:693–700. [PMC free article] [PubMed] [Google Scholar]
- [3].Prasad GA, Varadarajulu S. Endoscopic ultrasound-guided abscess drainage. Gastrointest Endosc Clin N Am 2012;22:281–90. [DOI] [PubMed] [Google Scholar]
- [4].Seewald S, Ang TL, Teng KY, et al. Endoscopic ultrasound-guided drainage of abdominal abscesses and infected necrosis. Endoscopy 2009;41:166–74. [DOI] [PubMed] [Google Scholar]
- [5].Teoh AY, Dhir V, Jin ZD, et al. Systematic review comparing endoscopic, percutaneous and surgical pancreatic pseudocyst drainage. World J Gastrointest Endosc 2016;8:310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Poincloux L, Caillol F, Allimant C, et al. Long-term outcome of endoscopic ultrasound-guided pelvic abscess drainage: a two-center series. Endoscopy 2017;49:484–90. [DOI] [PubMed] [Google Scholar]
- [7].Ogura T, Masuda D, Saori O, et al. Clinical outcome of endoscopic ultrasound-guided liver abscess drainage using self-expandable covered metallic stent (with Video). Dig Dis Sci 2016;61:303–8. [DOI] [PubMed] [Google Scholar]
- [8].Catalano MF, Sial S, Chak A, et al. EUS-guided fine needle aspiration of idiopathic abdominal masses. Gastrointest Endosc 2002;55:854–8. [DOI] [PubMed] [Google Scholar]
- [9].Ngamruengphong S, Xu C, Woodward TA, et al. Risk of gastric or peritoneal recurrence, and long-term outcomes, following pancreatic cancer resection with preoperative endosonographically guided fine needle aspiration. Endoscopy 2013;45:619–26. [DOI] [PubMed] [Google Scholar]
- [10].Zhu H, Jiang F, Zhu J, et al. Assessment of morbidity and mortality associated with endoscopic ultrasound-guided fine-needle aspiration for pancreatic cystic lesions: a systematic review and meta-analysis. Dig Endosc 2017;29:667–75. [DOI] [PubMed] [Google Scholar]


