Abstract
Rationale:
Patients with the e6a2 BCR-ABL transcript, 1 of the atypical transcripts, have been reported to have a poor prognosis, and allogeneic stem cell transplantation (ASCT) can be considered as additional therapy. However, long-term survival after ASCT for this disease is rare.
Patient concerns:
This report concerns a 55-year-old female patient with e6a2 BCR-ABL-positive acute myeloid leukemia including the outcome of ASCT followed by donor lymphocyte infusion (DLI).
Diagnoses:
The breakpoint was confirmed by direct sequencing. Her minimal residual disease could be detected by nested reverse-transcription polymerase chain reaction using primers for the minor BCR-ABL (e1a2) transcript.
Interventions:
Treatment with tyrosine kinase inhibitors (TKIs) and ASCT followed by DLI.
Outcomes:
Despite multiple cytogenetic and molecular relapses after ASCT, she remains in molecular remission at 46 months after ASCT.
Lessons:
This case indicates the efficacy of the combination of the graft-versus-leukemia effect and TKIs for e6a2 BCR-ABL-positive acute leukemia. When the Philadelphia chromosome with an unusual chromosomal breakpoint is suggested, we should clarify the breakpoint because that information can aid molecular assessments and decisions to provide an additional or alternative therapy.
Keywords: BCR-ABL fusion gene, breakpoint, donor lymphocyte infusion, tyrosine kinase inhibitor
1. Introduction
The Philadelphia chromosome (Ph) results in the formation of the BCR-ABL fusion gene. The 3 types of widely recognized breakpoints are major BCR-ABL (e13[b2]a2/e14[b3]a2) in over 90% of chronic myeloid leukemia (CML) and one-third of acute lymphoblastic leukemia (ALL); minor BCR-ABL (e1a2), mainly in two-thirds of ALL; and micro BCR-ABL (e19a2) in CML and chronic neutrophilic leukemia.[1–3] In addition, it has been reported in some atypical transcripts, such as e8a2, e19a2, e13a3, e14a3, e1a3, and e6a2.[4,5]
The e6a2 BCR-ABL-positive leukemia is a rare disease reported in some cases of CML and AML, and the prognosis is known to be poor.[6–11] Here, we describe an e6a2 BCR-ABL-positive AML patient who obtained prolonged survival through tyrosine kinase inhibitors (TKIs) and donor lymphocyte infusion (DLI), despite multiple cytogenetic and molecular relapses after allogeneic stem cell transplantation (ASCT).
2. Case report
A 55-year-old, previously healthy female, visited our hospital, because of fever and anorexia. Complete blood counts at presentation showed a white blood cell count of 12.7 × 109/L with 40% blasts, despite an almost normal hemoglobin level and platelet count. Splenomegaly was absent. Bone marrow aspiration showed 72% of leukemic cells with positive myeloperoxidase and naphthol AS-D chloroacetate, and weakly positive α-naphthyl butyrate. Immunophenotyping of the leukemic blasts was positive for CD13, CD33, CD34, CD11b, CD117, and HLA-DR, and was negative for CD14, CD19, CD41, and CD56. Screening of peripheral blood by multiplex real-time polymerase chain reaction (PCR) detected no chimeric transcripts including major and minor BCR-ABL. We diagnosed her with AML (M4), and started induction chemotherapy with idarubicin (12 mg/m2, days 1–3) and cytarabine (100 mg/m2, continuous intravenous infusion for 7 days) according to the JALSG AML201 protocol.[12,13]
The result of the initial G-banding chromosomal assessment, which returned on the day 14 of the induction chemotherapy, was 46, XX, t(9;22)(q34;q11.2). Cytogenetic assessment by fluorescence in situ hybridization (FISH) using Carnoy-fixed born marrow cells at the initial diagnosis also confirmed t(9:22). Therefore, the final diagnosis was changed to Ph-positive AML (Ph+ AML). Although the initial screening of peripheral blood by multiplex real-time PCR detected no chimeric transcripts, single minor real-time quantitative (RQ)-PCR showed low copy numbers (200 copy/μg RNA) of transcript, and minor BCR-ABL nested reverse-transcription PCR (RT-PCR) showed a 472-bp band. Minor single-step RT-PCR of the same specimen showed an atypical band (approximately 900 bp) (Fig. 1 A), and the direct sequence of this product revealed a breakpoint of e6a2 (Fig. 1B).
Figure 1.
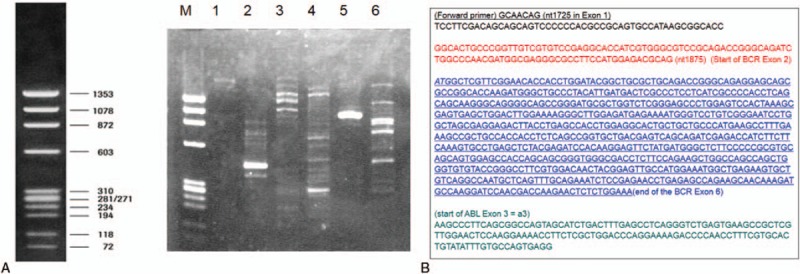
(A) Detection of the e6a2 transcript. M is a Marker X174 DNA Hae III digests. The cDNA major BCR-ABL-positive controls are shown in lanes 2, 4, and 6. The primers to detect b2(e13)a2/b3(e14)a2 and e19/a2, in lanes 1 and 3, respectively, revealed no atypical band. In lane 5, the atypical band was revealed by the primer to detect e1a2. (B) Sequence analysis. An amplified band was formed when analyzed with forward and reverse primers contained in the BCR e1 and ABL a2 regions. The BCR e6 (underlined) region was confirmed, followed by the ABL a2 region.
On day 41 of the induction chemotherapy, we confirmed complete hematological remission by bone marrow aspiration. However, FISH revealed 11% of t(9;22) signal. Minor nested RT-PCR was also positive. On day 49 of the induction chemotherapy, we performed the first cycle of consolidation therapy (mitoxantrone 7 mg/m2 for 3 days and cytarabine 100 mg/m2 for 5 days). Since a bone marrow examination at the recovery phase was positive for minor RT-PCR and FISH, imatinib 400 mg/d was used for 15 days (from day 35 to 49 of the first cycle of consolidation). The second cycle of consolidation chemotherapy (daunorubicin 50 mg/m2 for 3 days and cytarabine 200 mg/m2 for 5 days) was started on day 103 of the induction chemotherapy. A recovery phase examination was again positive for minor nested RT-PCR and FISH. From day 50 of the second consolidation, imatinib 400 mg/d was again administered; however, imatinib was soon changed to dasatinib (140 mg/d) because of severe nausea.
The patient underwent 1 allele mismatched (C-locus) unrelated allogeneic reduced intensity stem cell transplantation. Before ASCT, she was in hematological CR, but not in cytogenetic remission; FISH revealed 0.8% of t(9;22) signal in bone marrow cells. The conditioning regimen was fludarabine (25 mg/m2, day −6 to day −2) and melphalan (70 mg/m2, day −3 and −2), and the graft-versus-host disease (GVHD) prophylaxis was tacrolimus and short-term methotrexate. An engraftment was successfully achieved, and peripheral blood and bone marrow chimerism analyses confirmed 100% donor hematopoiesis at day 28. Minor nested RT-PCR at day 50 confirmed molecular remission. Skin acute GVHD of stage 3 (grade II) was observed, which was well controlled by topical corticosteroid.
As the post-transplantation therapy, we began 100 mg/d of imatinib at day 91 after transplantation. However, due to intolerance, we changed imatinib to dasatinib 50 mg/d at day 99 after transplantation. Since cytogenetic relapse was confirmed by G-banding of bone marrow at day 99 after transplantation, tacrolimus was rapidly tapered and discontinued at day 126. Although acute GVHD did not relapse, chronic GVHD of the skin and oral cavity became apparent along with the tapering of tacrolimus; however, no additional treatment was required for the chronic GVHD. At day 133, a donor lymphocyte infusion (DLI) was performed. A CD3-positive cell of 1.0 × 107/kg was administered. The result of a minor nested RT-PCR was negative (molecular remission) in the bone marrow just before the first DLI. No GVHD aggravation was observed after DLI. Molecular remission was also confirmed 28 days after the first DLI (day 161 after transplantation).
Fourteen months after the first DLI (18 months after transplantation), the second molecular relapse was confirmed by minor nested RT-PCR. Therefore, the second DLI (CD3-positive cells of 1.0 × 107/kg) was soon performed. At 54 days after the second DLI (21 months after ASCT), the patient experienced lower gastrointestinal bleeding. A colonoscopic examination revealed oozing blood from polyps in the transverse and descending colon. However, a histopathological analysis of the colon biopsy specimens revealed no intestinal GVHD. Since dasatinib was considered to be a possible cause,[14] we changed from dasatinib to nilotinib 600 mg/d.
Seventy-seven days after the second DLI, the patient developed a skin rash. A histopathological analysis of the skin biopsy specimens revealed acute GVHD. Since it was grade III acute GVHD after DLI (skin stage 3, gut stage 2), we started methylpredonisolone 1 mg/kg. Since the GVHD gradually improved, the steroid was slowly tapered.
Despite reducing the dose of nilotinib to 400 mg/d due to liver toxicity at 27 months after transplantation, the patient remains in molecular remission at the last follow-up (46 months after transplantation). The patient developed chronic GVHD (skin score 2, mouth score 2, eyes score 1)[15] and is still administered a low dose of prednisolone (2.5 mg/d). The course of treatment is summarized in Fig. 2. The patient provided informed consent for the protocol.
Figure 2.
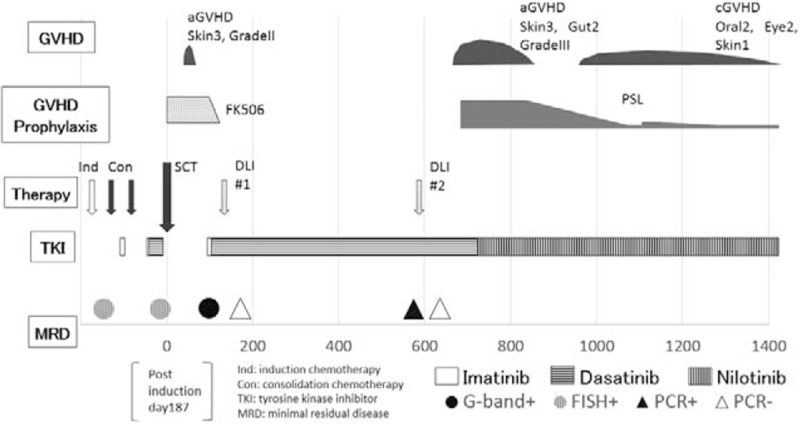
Summarized course of treatment from diagnosis to the last follow-up.
3. Discussion
Well-known breakpoints of BCR-ABL are (e13[b2]a2/e14[b3]a2), e1a2, and e19a2, which are transcribed into major, minor, and micro BCR-ABL messenger RNA, respectively.[1,2,16,17] E6a2, which was identified in this case, has been reported in 5 CML cases, but in only 1 AML case so far.
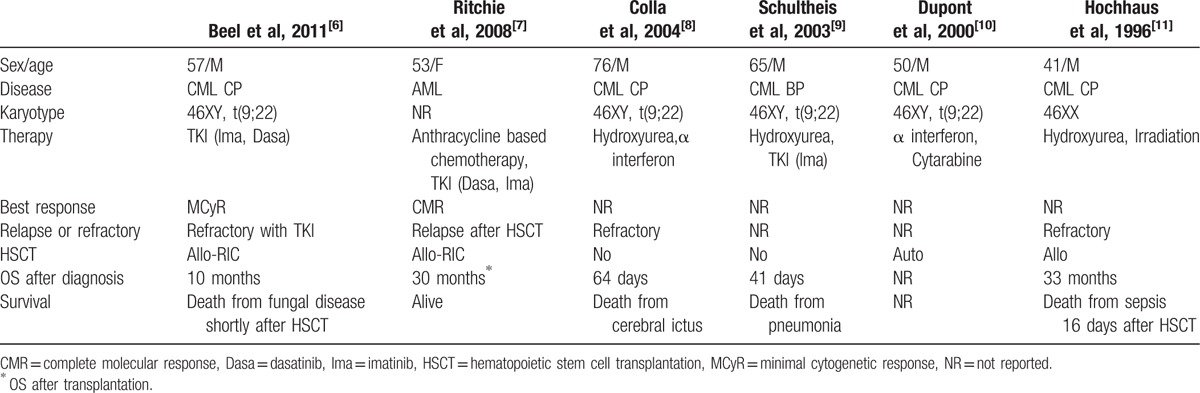
Table 1 is a summary of the clinical features of e6a2 BCR-ABL-positive leukemia patients in previous reports. This subtype was clinically aggressive, and the prognosis was poor because of treatment resistance.[6–11] In our case, although cytogenetic relapse was confirmed shortly after transplantation, long-term survival was achieved with TKIs and DLIs. To our knowledge, a 52-month survival after diagnosis was the longest in an e6a2 BCR-ABL-positive leukemia patient. It was suggested that the disease with e6a2 could be controlled by the combination of TKIs and allogeneic stem cell transplantation (including DLI).
Table 1.
The clinical features of e6a2 bcr-abl-positive leukemia patients in previous reports.
Primers to detect the e6a2 transcripts are not routinely available in most clinical laboratories. However, the therapeutic response can be assessed using minor nested RT-PCR. Amplification failure at the initial screening by multiplex real-time PCR may be caused by low extension/elongation efficiency because of the approximately doubled PCR product. Another cause might be that the amount of primer used to detect the e1a2 transcripts contained in the mixed primers for a multiplex real-time PCR was smaller than that included in a single RT-PCR.
This case confirms the importance of assessing the molecular response, where we could perform the second DLI immediately after the reappearance of the e6a2 BCR-ABL transcripts before hematological relapse. Indeed, it may not be necessary to clarify the breakpoint for a response evaluation because a molecular assessment was possible by commercially based examination. However, clarifying the breakpoint helped us to understand the clinical significance of the minor nested RT-PCR, which has an extremely high sensitivity for detecting the e6a2 BCR-ABL fusion gene.
4. Conclusions
In conclusion, we report the longest-surviving patient with e6a2 BCR-ABL-positive AML who was treated with TKIs and ASCT followed by DLIs. When Ph with an unusual chromosomal breakpoint is suspected, it is important to clarify the breakpoint because that information can help in molecular assessments of the disease. Consequently, these assessments can lead to a decision to administer an additional or alternative therapy with appropriate timing.
Footnotes
Abbreviations: ASCT = allogeneic stem cell transplantation, DLI = donor lymphocyte infusion, TKIs = tyrosine kinase inhibitors.
Funding: This study was supported in part by JSPS KAKENHI Grant number JP17K16186.
The authors declare no conflict of interest.
References
- [1].Melo JV. The diversity of BCR-ABL fusion proteins and their relationship to leukemia phenotype. Blood 1996;88:2375–84. [PubMed] [Google Scholar]
- [2].Saglio G, Guerrasio A, Rosso C, et al. New type of Bcr/Abl junction in Philadelphia chromosome-positive chronic myelogenous leukemia. Blood 1990;76:1819–24. [PubMed] [Google Scholar]
- [3].Pane F, Frigeri F, Sindona M, et al. Neutrophilic-chronic myeloid leukemia: a distinct disease with a specific molecular marker (BCR/ABL with C3/A2 junction). Blood 1996;88:2410–4. [PubMed] [Google Scholar]
- [4].Schnittger S, Bacher U, Kern W, et al. A new case with rare e6a2 BCR-ABL fusion transcript developing two new resistance mutations during imatinib mesylate, which were replaced by T315I after subsequent dasatinib treatment. Leukemia 2008;22:856–8. [DOI] [PubMed] [Google Scholar]
- [5].Torres F, Ivanova-Dragoeva A, Pereira M, et al. An e6a2 BCR-ABL fusion transcript in a CML patient having an iliac chloroma at initial presentation. Leuk Lymphoma 2007;48:1034–7. [DOI] [PubMed] [Google Scholar]
- [6].Beel KA, Lemmens J, Vranckx H, et al. CML with e6a2 BCR-ABL1 transcript: an aggressive entity? Ann Hematol 2011;90:1241–3. [DOI] [PubMed] [Google Scholar]
- [7].Ritchie DS, McBean M, Westerman DA, et al. Complete molecular response of e6a2 BCR-ABL-positive acute myeloid leukemia to imatinib then dasatinib. Blood 2008;111:2896–8. [DOI] [PubMed] [Google Scholar]
- [8].Colla S, Sammarelli G, Voltolini S, et al. e6a2 BCR-ABL transcript in chronic myeloid leukemia: is it associated with aggressive disease? Haematologica 2004;89:611–3. [PubMed] [Google Scholar]
- [9].Schultheis B, Wang L, Clark R E, et al. BCR-ABL with an e6a2 fusion in a CML patient diagnosed in blast crisis. Leukemia 2003;17:2054–5. [DOI] [PubMed] [Google Scholar]
- [10].Dupont M, Jourdan E, Chiesa J. Identification of E6A2 BCR-ABL fusion in a Philadelphia-positive CML. Leukemia 2000;14:2011–2. [DOI] [PubMed] [Google Scholar]
- [11].Hochhaus A, Reiter A, Skladny H, et al. A novel BCR-ABL fusion gene (e6a2) in a patient with Philadelphia chromosome-negative chronic myelogenous leukemia. Blood 1996;88:2236–40. [PubMed] [Google Scholar]
- [12].Ohtake S, Miyawaki S, Fujita H, et al. Randomized study of induction therapy comparing standard-dose idarubicin with high-dose daunorubicin in adult patients with previously untreated acute myeloid leukemia: the JALSG AML201 Study. Blood 2011;117:2358–65. [DOI] [PubMed] [Google Scholar]
- [13].Miyawaki S, Ohtake S, Fujisawa S, et al. A randomized comparison of 4 courses of standard-dose multiagent chemotherapy versus 3 courses of high-dose cytarabine alone in postremission therapy for acute myeloid leukemia in adults: the JALSG AML201 Study. Blood 2011;117:2366–72. [DOI] [PubMed] [Google Scholar]
- [14].Ono Y, Mori T, Kato J, et al. Hemorrhagic colonic ulcers caused by dasatinib for chronic myelogenous leukemia. Int J Hematol 2010;92:556–8. [DOI] [PubMed] [Google Scholar]
- [15].Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005;11:945–56. [DOI] [PubMed] [Google Scholar]
- [16].Melo JV, GordonF DE, CrossF NC, et al. The ABL-BCR fusion gene is expressed in chronic myeloid leukemia. Blood 1993;81:158–65. [PubMed] [Google Scholar]
- [17].Chissoe S L, Bodenteich A, Wang YF, et al. Sequence and analysis of the human ABL gene, the BCR gene, and regions involved in the Philadelphia chromosomal translocation. Genomics 1995;27:67–82. [DOI] [PubMed] [Google Scholar]


