Supplemental Digital Content is available in the text
Keywords: body composition, body mass index, heart disease, hemodynamic, stroke
Abstract
The mechanisms linking short stature with an increase in cardiovascular and cerebrovascular disease risk remain elusive. This study tested the hypothesis that significant associations are present between height and blood pressure in a representative sample of the US adult population.
Participants were 12,988 men and women from a multiethnic sample (age ≥ 18 years) evaluated in the 1999 to 2006 National Health and Nutrition Examination Survey who were not taking antihypertensive medications and who had complete height, weight, % body fat, and systolic and diastolic arterial blood pressure (SBP and DBP) measurements; mean arterial blood pressure and pulse pressure (MBP and PP) were calculated. Multiple regression models for men and women were developed with each blood pressure as dependent variable and height, age, race/ethnicity, body mass index, % body fat, socioeconomic status, activity level, and smoking history as potential independent variables.
Greater height was associated with significantly lower SBP and PP, and higher DBP (all P < .001) in combined race/ethnic–sex group models beginning in the 4th decade. Predicted blood pressure differences between people who are short and tall increased thereafter with greater age except for MBP. Socioeconomic status, activity level, and smoking history did not consistently contribute to blood pressure prediction models.
Height-associated blood pressure effects were present in US adults who appeared in the 4th decade and increased in magnitude with greater age thereafter. These observations, in the largest and most diverse population sample evaluated to date, provide support for postulated mechanisms linking adult stature with cardiovascular and cerebrovascular disease risk.
1. Introduction
More than half a century has passed since Gertler et al[1] reported in 1951 that young men at risk for coronary artery disease were about 5 cm shorter than their healthy counterparts. Paffenbarger and Wing[2] extended these observations to the risk of developing a fatal stoke among longitudinally followed university students. Those succumbing to a stroke were 2 to 3 cm shorter than their classmates. Numerous studies have since reported inverse associations between height and the risk of developing ischemic heart disease and strokes in men and women.[3]
Multiple mechanisms for these adverse cardiovascular and cerebrovascular effects have been suggested,[3] although a single definitive causal factor remains elusive. An important potential group of proposed mechanisms encompass stature-related hemodynamic, circulatory, and blood pressure effects,[4–12] although there are several limitations of earlier studies. These include evaluation of small experimental samples (<200 healthy participants,[4,5,10–12] inclusion of limited adult age, sex, or race/ethnic groups,[6–8] or application of analysis strategies that are restricted in scope (see Supplemental Content 1 that summarizes these previous reports). The associations now recognized between height and blood pressure among adults thus leave important gaps that remain to be filled.
The aim of the present study was to fill this information void by establishing the relations between height and blood pressure across the full adult lifespan in a large nationally representative sample of non-Hispanic (NH) White, NH Black, and Mexican American participants of the US National Health and Nutrition Examination Survey (NHANES).[13]
2. Materials and methods
2.1. Study design and rationale
The study aim was to test the hypothesis that significant associations are present between height and blood pressure in the noninstitutionalized US adult (age ≥ 18 years) population after controlling for factors known to influence blood pressure including sex, age, race/ethnic group, body shape, and level of adiposity. Previous studies by our group established body mass index (BMI, weight/height2) in the NHANES sample as a height-independent measure of body shape.[14,15]
To test the hypothesis we first set out systolic blood pressure, diastolic blood pressure, pulse pressure, and mean arterial blood pressure (SBP, DBP, PP, and MBP) as dependent variables in multiple linear regression models that included age, BMI, % body fat, and height. We then additionally explored other potential model covariates including markers of socioeconomic status, activity levels, and smoking history.
2.2. Participants
The NHANES 1999 to 2006 dataset provided a nationally representative sample of US adult (age ≥ 18 years) participants among 3 race/ethnic groups including NH Whites, NH Blacks, and Mexican Americans. As in previous NHANES reports from our group,[14,15] we excluded the smaller “other Hispanic” and “other/multiracial” race/ethnic groups from the analysis sample.
The initial sample consisted of 29,026 participants. Of those in the initial sample, 3900 were removed because they were taking medications for high blood pressure. A subpopulation, or domain, analysis was conducted on the remaining 25,126 participants 18 years of age or older who were in the 3 identified race/ethnic groups (15,066 remaining participants). An additional 2078 participants had missing data (height, weight, blood pressure, or adiposity measurements[16]) resulting in a total sample size of 12,988 participants: 6799 men and 6189 women.
The NHANES protocol was approved by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention, and written informed consent was provided by all participants.
2.3. Demographic information
Subject self-reported socioeconomic status, activity level, and smoking history information was collected during the NHANES evaluations.[13] We estimated socioeconomic status as the subject's annual household income. Physical activity level was evaluated as whether or not the subject participated in vigorous physical activity over the past 30 days. Multiple smoking history variables were also examined.
2.4. Measurements
Height and weight were measured using standardized procedures and calibrated measurement devices as outlined in Supplemental Content 2. BMI was calculated as weight/height2.
2.4.1. Blood pressure
Rigorously controlled blood pressure measurements were made in NHANES[13] and the details of procedures involved are presented in Supplemental Content 2. SBP and DBP were each calculated as the average of 3 measurements. PP was calculated as the difference between the average SBP and DBP. MBP was calculated as: MBP = [2 × (DBP) + SBP]/3.
2.4.2. Body composition
Whole body dual-energy X-ray absorptiometry (DXA) scans (Hologic, Inc., Bedford, MA) were used to evaluate adiposity that was quantified as % body fat.[16] Participants wore examination gowns for the scan and were asked to remove all objects, such as jewelry, from their body. The manufacturer recommended calibration and acquisition procedure was followed for the evaluation of each subject's scan. Details of the imaging protocol are reported in the NCHS technical documentation.[17]
Due to the relatively large number of missing DXA values as well as the apparent nonrandom nature of whole or partially missing scans, the NCHS released 5 datasets where the missing DXA measurements were derived using a multiple imputation procedure.[18] Analyses were conducted on all 5 datasets and the between set variability was accounted for in estimating standard errors of the regression coefficients.
2.5. Statistical methods
We conducted analyses that describe height–blood pressure relations generalizable to the noninstitutionalized portions of the US adult (age ≥ 18 years) population across 3 race/ethnic groups. The analyses, conducted on participants not taking antihypertensive medications, were carried out using the survey package in R (R Core Team; 2016) to produce nationally representative estimates while accommodating for the complex, multistage sampling design of NHANES. These were weighted linear regression models using weights supplied by the NCHS as part of the NHANES datasets. Subpopulation analyses were conducted using the subset argument of the svyglm function in R. Standard error estimates were adjusted to account for both the complex sampling scheme and the multiple imputations using the stratum and masked variance units provided in the NHANES database and the mitools package in R, respectively. Taylor Series Linearization was used to account for the complex survey design, as recommended by the NCHS.[19]
Estimates for baseline characteristics were calculated using svymean and represented as mean ± standard error. These characteristics were weighted to account for the oversampling of minority groups.
Annual household income was chosen to represent socioeconomic status and whether or not the subject reported vigorous physical activity over the past 30 days was used as an overall indictor of activity level. When adjusting for the other variables of interest, neither variable was statistically significant and further models did not include these terms. None of the variables for smoking status were good univariate predictors of blood pressure and they were not considered for subsequent models.
Four weighted linear regression models were fit, 1 for each outcome (i.e., SBP, DBP, PP, and MBP). Initial explanatory variables included all of those previously listed plus all possible 2-way interactions with height. While all main effects were kept in the model regardless of P value, interaction terms were removed 1 at a time using backwards elimination until only statistically significant (P < .05) terms remained. No attempts were made to collapse categories of the categorical variables. Any time, 1 level of a categorical variable was significantly different than the reference category; all terms involving that variable were included. Statistically significant interactions with height remained in all models with the exception of MBP for women, making an interpretation of an overall effect of height in these models inappropriate. A combined model using sex as a covariate is provided in Supplemental Content 3.
3. Results
3.1. Participants
The participant groups had mean ages of approximately 35 to 43 years and BMI ranged from about 27 to 30 kg/m2 (Table 1). Average group levels of % body fat were lower in men (∼26%) compared with women (∼39%). Mexican American men and women had the smallest mean heights compared with the other 2 race/ethnic groups.
Table 1.
Subject characteristics.
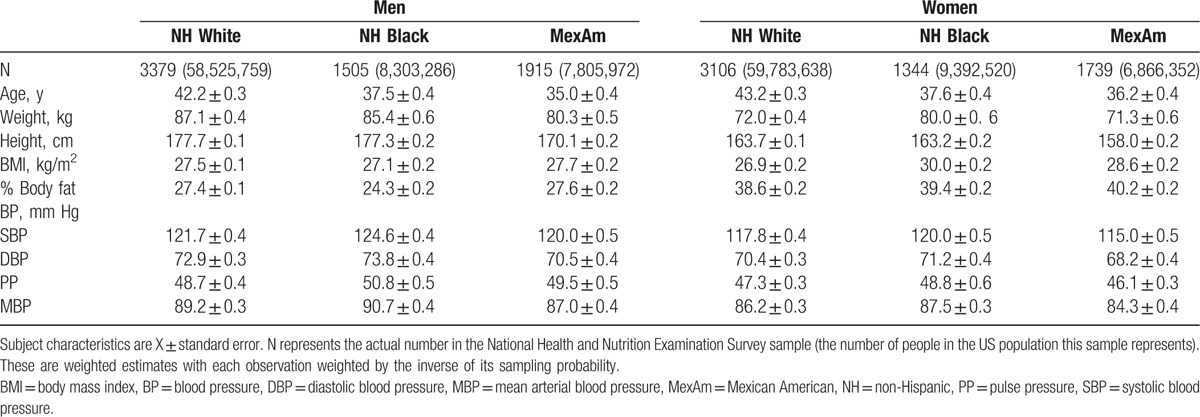
Women tended to have lower average SBP, DBP, PP, and MBPs compared with men (Table 1). Within sex groups, NH Black participants had consistently higher blood pressures compared with NH White participants while the lowest MBPs were present in the Mexican American men and women.
3.2. Blood pressure models
The 8 sex-specific regression models prepared are summarized in Table 2. Height, age, race/ethnicity, BMI, % body fat, and sex were significant model covariates along with their interaction terms in combined models. There were no significant height × sex interactions.
Table 2.
Blood pressure regression model results.
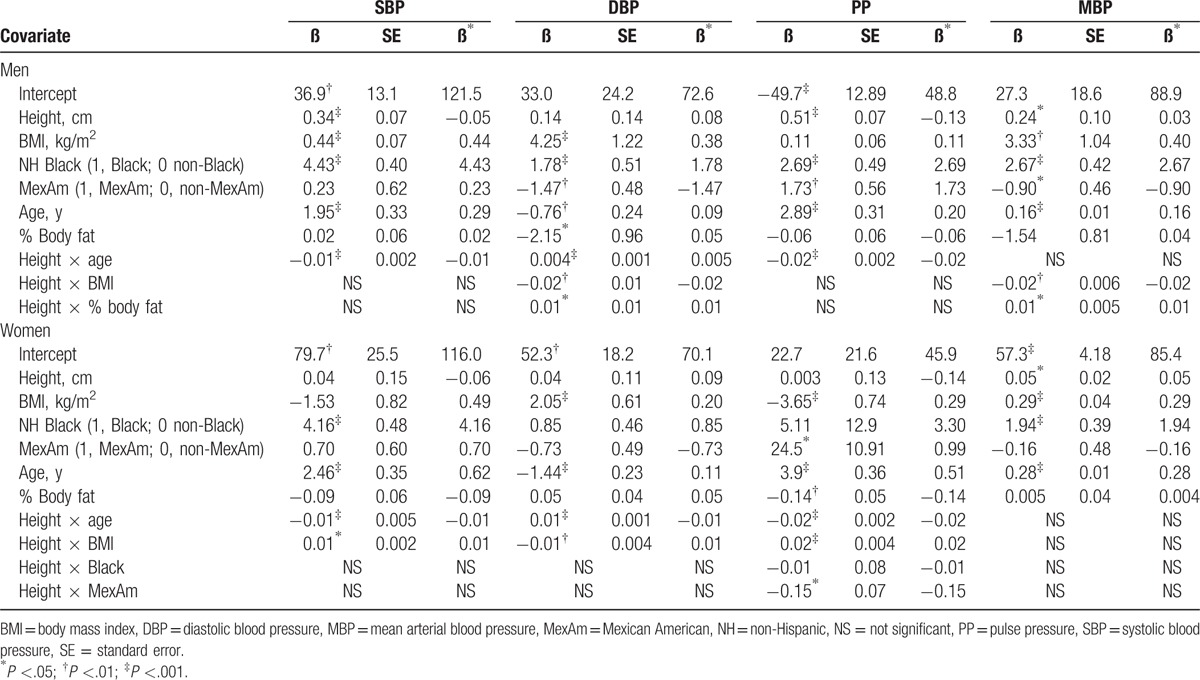
In the following sections, we provide example blood pressure model predictions for a representative man (BMI, 25 kg/m2; % body fat, 25) and woman (BMI, 25 kg/m2; % body fat, 40) who are at the 5th or 95th percentiles for height (man, 161.8 and 188.1 cm, respectively; woman, 150.0 and 173.0 cm, respectively).
3.2.1. Systolic blood pressure
In the model for SBP (Table 2), a significant interaction was found between height and age. The effect of height on SBP was thus not consistent across the age span. Prior to age 30 years, the height effect on SBP was positive and relatively small while beginning in the 4th decade the height effect was increasingly negative with greater age.
The difference in predicted SBP between the 5th (short) and 95th (tall) percentiles for height is shown in Fig. 1 at 3 ages (25, 50, and 75 years) for the representative male and female. SBP is predicted to be 2.4 mm Hg lower for the man who is short at age 25 years but is predicted to be 10.8 mm Hg higher than the tall man at age 75 years. The representative short woman has no difference in SBP at age 25 years and 11.5 mm Hg higher SBP than the tall woman at age 75 years.
Figure 1.
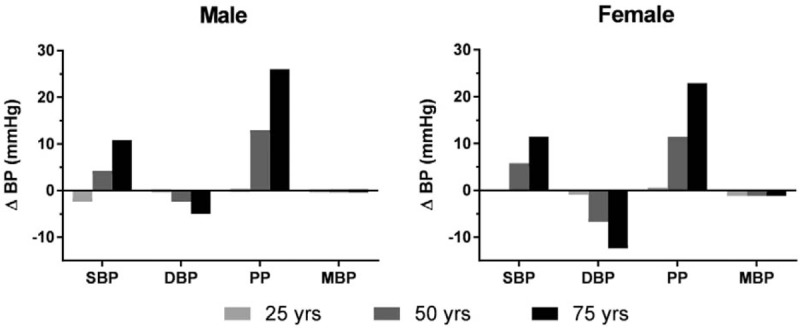
Predicted brachial artery blood pressure differences (ΔBP, short–tall BP) between the 5th and 95th height percentiles for a representative adult male (BMI, 25 kg/m2; % body fat, 25) and female (BMI, 25 kg/m2; % body fat, 40) at 3 ages. A race × height interaction was present for female PP and the predicted ΔBP was derived using values for non-Hispanic White. Variations in predicted ΔBP values for women of other race/ethnic groups are given in Section 3. BMI = body mass index, BP = blood pressure, DBP = diastolic blood pressure, MBP = mean arterial blood pressure, PP = pulse pressure, SBP = systolic blood pressure.
3.2.2. Diastolic blood pressure
Significant interactions between height and age and height and BMI were found for the DBP model with lower BMI ranges associated with a greater height effect (Fig. 2).
Figure 2.
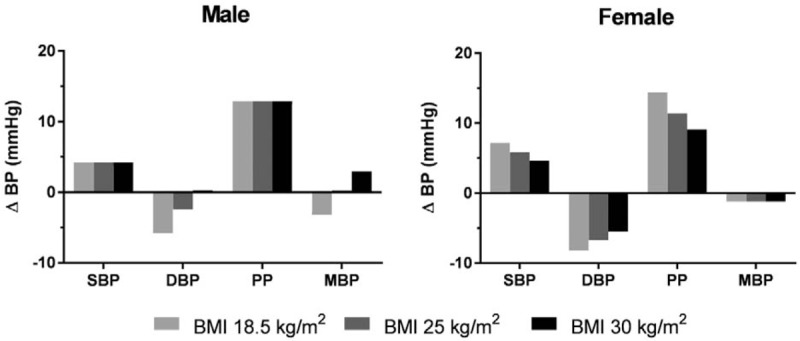
Predicted brachial artery blood pressure differences (ΔBP, short–tall BP) between the 5th and 95th percentiles for a representative male (age, 50 years; % body fat, 25) and female (age, 50 years; % body fat, 40) at 3 levels of BMI. The predicted values were derived using non-Hispanic White. Other race/ethnic groups for men and women displayed the same differences in blood pressure readings with the exception of female PP, although the same trend occurred. BMI = body mass index, BP = blood pressure, DBP = diastolic blood pressure, MBP = mean arterial blood pressure, PP = pulse pressure, SBP = systolic blood pressure.
The predicted DBP differences (short–tall) at ages 25, 50, and 75 years between the 5th and 95th percentiles for height are shown in Fig. 1 for the representative male and female. DBP is predicted to be 0.3 mm Hg lower for the short man at age 25 years and 5.0 mm Hg lower for the short man at age 75 years. At age 25 and 75 years, the short woman's DBP is predicted to be 0.9 and 12.4 mm Hg lower, respectively.
3.2.3. Pulse pressure
The effect of height on PP in developed regression models depends on BMI, age, and race/ethnicity for women. The PP differences between the 5th and 95th percentiles for height predicted for the representative male and female is shown in Fig. 1 for a person who is NH White. Predicted PP differences are 0.3 mm Hg higher at age 25 years and 26.0 mm Hg higher at age 75 years for the representative short man. The representative short woman who is NH White has a PP that is 0.1 mm Hg higher than the tall woman at age 25 years and 22.9 mm Hg higher at age 75 years. Corresponding women who are NH Black and Mexican American have larger PP differences: at age 75 years the predicted PP difference increases to 23.2 and 26.4 mm Hg, respectively.
Similar to DBP, the differences in predicted PP between the 5th and 95th height percentiles vary at different BMI's for women. At lower BMI's, the effect of PP on height is more pronounced (Fig. 2).
3.2.4. Mean arterial blood pressure
The height effect on MBP is not dependent on age.
Predicted MBP differences between the 5th and 95th percentiles for height is 0.3 mm Hg higher for the short man (Fig. 1) and 1.2 mm Hg lower for the short woman.
4. Discussion and conclusions
Prompted by epidemiological literature spanning more than half a century relating adult height to cardiac and cerebrovascular diseases,[3,20] we set out to firmly establish the associations between stature and blood pressure in a large race/ethnically diverse and carefully evaluated sample of the noninstitutionalized US adult population. Our findings in NHANES participants who were not taking antihypertensive medications confirm and extend earlier reports that a significant association is present between adult stature and blood pressure.[7,8,10–12]
Specifically, we observed consistent relations across men and women between height and 4 blood pressure measures after controlling for age, BMI, and level of adiposity. Using race/ethnicity and sex-combined and sex-specific regression models, we found for subjects in the evaluated sample that short stature beginning during the 4th decade is accompanied by a higher SBP and PP with a corresponding lower DBP and MBP than tall stature, effects that become larger with greater age. Moreover, we found significant blood pressure effects of several added model covariates in addition to height and age (i.e., race/ethnicity and BMI) that are consistent in effect size with earlier observations.[21–23] Adiposity, as assessed by measured % body fat, was an inconsistent covariate in blood pressure models, perhaps owing to the close association between % body fat and BMI in large population samples.[24] We did not find any consistent significant associations between blood pressure and selected measures of socioeconomic status, activity level, and smoking history.
4.1. Potential mechanisms
4.1.1. Hemodynamic and hydrostatic effects
Almost 4 decades ago, Voors et al[25] described a significant association between height and blood pressure in young adults. However, the authors adjusted body size in their sample for weight/height,[3] an index now known to be correlated with adult height[15] and thus making their results difficult to interpret. A decade after Voors’ report,[25] London and colleagues[11] showed for the first time that, after controlling for BMI, height in adults contributes to the observed magnitude of arterial wave reflection and peak SBP. As the systolic pressure pulse propagates across the central aorta and into the peripheral vascular system, a portion of the wave is reflected back and combines with the forward wave to amplify SBP and ventricular afterload.[26] Short participants in London's initial[11] and later follow-up study[10] had a larger reflected wave and SBP than participants who were tall. Smulyan et al,[12] working in the same group, reported several years later that individuals who are short appear to have a hemodynamic liability because the early appearance of reflected waves during systole contributes to aortic stiffening, an effect that also lowers aortic DBP. The present study observations confirm and extend the findings of London's group[10–12]: our cross-sectional blood pressure regression models predict that, beginning during the 4th decade, SBP increases and DBP decreases more in people who are short than those who are tall.
In a more recent series of reports, Langenberg et al[8] examined the relations between height and blood pressure in a cohort of over 3000 English men and women who were born in 1946 and who were age 53 years at the time of evaluation in 2003. After adjusting for potential covariates, PP was inversely correlated with both height and leg length in men and women; trends were similar and statistically significant for SBP but not DBP. The observed blood pressure effects became stronger with age in a longitudinal analysis of the cohort beginning at age 36 years.[7] The blood pressure-stature pattern reported by Langenberg et al[7,8] is similar to the 1 observed in the present study: a larger age-related increase in PP and SBP in people who are short that exceeds the corresponding blood pressure observations in people who are tall. Unlike Langenberg et al,[7,8] our findings of reduced DBP in people who are short also reached statistical significance, possibly owing to our larger sample size and wider age range. Korhonen et al[6] also recently reported significant inverse associations between ambulatory daytime SBP, PP, and MBP and height in 534 elderly Finnish adults. Nighttime blood pressures and 24-h heart rates did not differ significantly between the height groups.
With respect to the greater PP level in people who are short, PP increases with aorta and carotid artery stiffening and reductions in compliance, effects that can lead to pathological changes in microvascular structure/function and that may adversely influence high blood flow organs such as the brain beginning in the 4th decade.[27–29] Accordingly, greater arterial stiffness is a strong predictor of cardiovascular disease and stroke risk.[29,30] Cooper and colleagues demonstrated that the forward wave amplitude component of PP makes a larger impact on cardiovascular disease risk than the reflected wave amplitude, indicating that aortic stiffness and geometry contributes more to risk than wave reflection.[28] In the study of Ayyagari et al,[31] a 10 mm Hg increase in PP raised the risk of a stroke 3 years later by 3.8% after adjusting for age and related comorbidities. Okada et al[32] reported that PP is an independent predictor for stroke in people with normal SBP levels (<140 mm Hg); a 1-standard deviation elevation in PP led to a hazard ratio of 1.32. PP was the strongest predictor of coronary heart disease risk in the Framingham study at the age of 60 years and over.[30,33] In the present study, the predicted PP difference between an adult in the 5th and 95th percentiles for height over the age of 55 years, when coronary artery disease and stroke frequency increases, is between ∼10 and 25 mm Hg.
Reeve et al[34] recently examined central (aortic) and mid-arm brachial artery blood pressures in relation to height in a cohort of over 1000 Australian adults who on average were in their 7th decade. The associations between height and central SBP and augmentation index, a measure of wave reflection, were statistically significant whereas the associations between brachial blood pressures and aortic stiffness estimates were nonsignificant. The authors suggest that brachial blood pressure measurements underestimate the adverse hemodynamic exposures experienced by people who are short. Our large and diverse NHANES sample likely provided us with more power to detect small brachial artery blood pressure effects associated with height than in the study of Reeve et al.[34] Their observations, however, suggest the need for more advanced measures of cardiovascular dynamics that can further probe underlying mechanisms and their clinical implications.
In addition to SBP augmentation by reflected waves and arterial stiffening in people who are short, another potential stature-related effect is the hydrostatic force exerted by the blood column extending between the mid-brachial artery and brain.[4,5] Extensive cardiovascular studies in the giraffe reveal high heart-level pressures that are required to provide adequate brain perfusion while upright are also accompanied by increases in peripheral vascular resistance and cardiac hypertrophy.[27,35] A 1-cm vertical increase in blood column length will in theory raise blood pressure at the base by 0.76 mm Hg, an explanation often provided for why young children have lower blood pressure than their taller older counterparts.[36,37] Hydrostatic mechanisms should therefore lead to higher blood pressures in people who are tall and we are unable to specifically isolate these effects in the present study. Arvedsen et al[4,5] examined hydrostatic postural effects with tilt-table and centrifugation experiments and the investigators observed differing coordinated hemodynamic responses in short and tall adults. Taken collectively, these observations suggest that adult height plays a role in multiple components of the cardiovascular system that have yet to be fully evaluated and integrated.
4.1.2. Other proposed mechanisms
In addition to blood pressure effects, other proposed mechanisms for stature-related differences in vascular disease risk and mortality rates include smaller coronary artery diameters, and thus increased occlusion risk, in people who are short[38–40]; poorer lung function in people who are short[41]; and genetic effects that influence both height and coronary artery disease risk.[42,43]
Several studies have examined the hypothesis that early life exposures influence growth, adult height, metabolic profile, vascular integrity, and disease risk.[3,44–46] These collective observations raise the possibility that underlying hormonal mechanisms, possibly linked with early life nutrition and genetic effects, lead to differing large vessel protein composition and stiffness and related PP in adults who have large differences in stature. Intrauterine growth restriction in animal models leads to reorganization of the extracellular matrix, increased collagen engagement, and stiffness of large arteries such as the thoracic and abdominal aortas,[44–46] effects that can lead to adult high blood pressure. Growth factors such as insulin-like growth factor 1, levels of which are sensitive to nutrition and genetic contributions that impact adult height, stimulate elastin production in the aorta during the developmental period of life when levels are maximal and growth rates are rapid.[47,48] Growth factors, including IGF genes, are associated with body size and height attainment in both dogs and humans.[49–51]
Another previously evaluated early life exposure is socioeconomic status that can impact on intrauterine growth and adult height. In the present study, we used household income, level of vigorous physical activity, and smoking history as proxies for current and by inference early life socioeconomic status and these measures failed to enter blood pressure prediction models. Similarly, leg length in adults is a marker of early nutrition and socioeconomic status[52] that was significantly correlated with blood pressure in the study of Langenberg et al,[8] although childhood social class or birth weight did not enter into their blood pressure prediction models.[8]
4.2. Study limitations
While we have identified a clear stature-related brachial artery blood pressure pattern in a large, race/ethnically diverse, and carefully evaluated population sample, we were unable to probe further into underlying hemodynamic and hydrostatic mechanisms that require technology and testing facilities unavailable as part of NHANES. Cardiovascular system and anatomic variables of potential importance, such as resting heart rate, leg length, and sitting height were unavailable to us. Our analysis was focused on the relations between height and blood pressure and we did not explore other disease risk factors of potential importance in the current context such as insulin sensitivity, blood lipid levels, kidney function, lung function, and circulating hormones and growth factors. Our sample size was reduced by limited data for some measures such as current socioeconomic status and the NHANES database does not include detailed demographic information on adult participants at the time of their birth. Reliable data on alcohol ingestion, oral contraceptive use, and hormone replacement therapy were unavailable to us. We used the cross-sectional 1999 to 2006 NHANES database with DXA body composition estimates to develop our blood pressure models and these predictions ideally should be validated in comparably large longitudinal samples.
5. Conclusions
A distinct cross-sectional height-brachial arterial blood pressure pattern was observed beginning in the 4th decade in men and women not taking antihypertensive medications across 3 US race/ethnic groups. The basis for these effects is likely a combination of hemodynamic and hydrostatic mechanisms that are not yet fully quantified and integrated. The observed blood pressure pattern is consistent with an increased cardiovascular disease and stroke risk in people who are short. These observations, as with all adults, emphasize the particular importance of managing hypertension in individuals who are short. Other mechanisms, including hormonal vascular effects, are likely involved and require additional studies for their full elucidation.
Supplementary Material
Acknowledgment
The authors extend their appreciation to Emily F. Mire, MS, for her assistance with NHANES data acquisition.
Footnotes
Abbreviations: BMI = body mass index, DBP = diastolic blood pressure, DXA = dual-energy X-ray absorptiometry, MBP = mean blood pressure, NCHS = National Center for Health Statistics, NH = non-Hispanic, NHANES = National Health and Nutrition Examination Survey, PP = pulse pressure, SBP = systolic blood pressure.
BB, KW, DMT, OC, FBH, MH, and SBH designed research, analyzed data, and had primary responsibility for final content; and BB, KW, and SBH conducted research and wrote the paper.
This work was partially supported by the National Institutes of Health NORC Center Grants P30DK072476, Pennington/Louisiana; and pennington biomedical research foundation P30DK040561, Harvard; and R01DK109008, Shape UP! Adults.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Gertler MM, Garn SM, White PD. Young candidates for coronary heart disease. J Am Med Assoc 1951;147:621–5. [DOI] [PubMed] [Google Scholar]
- [2].Paffenbarger RS, Jr, Wing AL. Characteristics in youth predisposing to fatal stroke in later years. Lancet 1967;1:753–4. [DOI] [PubMed] [Google Scholar]
- [3].Stefan N, Haring HU, Hu FB, et al. Divergent associations of height with cardiometabolic disease and cancer: epidemiology, pathophysiology, and global implications. Lancet Diabetes Endocrinol 2016;4:457–67. [DOI] [PubMed] [Google Scholar]
- [4].Arvedsen SK, Damgaard M, Norsk P. Body height and blood pressure regulation in humans during anti-orthostatic tilting. Am J Physiol Regul Integr Comp Physiol 2012;302:R984–9. [DOI] [PubMed] [Google Scholar]
- [5].Arvedsen SK, Eiken O, Kolegard R, et al. Body height and arterial pressure in seated and supine young males during +2 G centrifugation. Am J Physiol Regul Integr Comp Physiol 2015;309:R1172–7. [DOI] [PubMed] [Google Scholar]
- [6].Korhonen PE, Kautiainen H, Eriksson JG. The shorter the person, the higher the blood pressure: a birth cohort study. J Hypertens 2017;35:1170–7. [DOI] [PubMed] [Google Scholar]
- [7].Langenberg C, Hardy R, Breeze E, et al. Influence of short stature on the change in pulse pressure, systolic and diastolic blood pressure from age 36 to 53 years: an analysis using multilevel models. Int J Epidemiol 2005;34:905–13. [DOI] [PubMed] [Google Scholar]
- [8].Langenberg C, Hardy R, Kuh D, et al. Influence of height, leg and trunk length on pulse pressure, systolic and diastolic blood pressure. J Hypertens 2003;21:537–43. [DOI] [PubMed] [Google Scholar]
- [9].Latham RD, Westerhof N, Sipkema P, et al. Regional wave travel and reflections along the human aorta: a study with six simultaneous micromanometric pressures. Circulation 1985;72:1257–69. [DOI] [PubMed] [Google Scholar]
- [10].London GM, Guerin AP, Pannier B, et al. Influence of sex on arterial hemodynamics and blood pressure. Role of body height. Hypertension 1995;26:514–9. [DOI] [PubMed] [Google Scholar]
- [11].London GM, Guerin AP, Pannier BM, et al. Body height as a determinant of carotid pulse contour in humans. J Hypertens Suppl 1992;10:S93–5. [PubMed] [Google Scholar]
- [12].Smulyan H, Marchais SJ, Pannier B, et al. Influence of body height on pulsatile arterial hemodynamic data. J Am Coll Cardiol 1998;31:1103–9. [DOI] [PubMed] [Google Scholar]
- [13].Center for Disease Control and Prevention/National Center for Health Statistics. National Health and Nutrition Examination Survey: Questionnaires, Datasets, and Related Documentation 2017; 2017. Available at: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx. Accessed March 7, 2017. [Google Scholar]
- [14].Schuna JM, Jr, Peterson CM, Thomas DM, et al. Scaling of adult regional body mass and body composition as a whole to height: relevance to body shape and body mass index. Am J Hum Biol 2015;27:372–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Heymsfield SB, Peterson CM, Thomas DM, et al. Scaling of adult body weight to height across sex and race/ethnic groups: relevance to BMI. Am J Clin Nutr 2014;100:1455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Schoeller DA, Tylavsky FA, Baer DJ, et al. QDR 4500A dual-energy X-ray absorptiometer underestimates fat mass in comparison with criterion methods in adults. Am J Clin Nutr 2005;81:1018–25. [DOI] [PubMed] [Google Scholar]
- [17].Center for Disease Control and Prevention/National Center for Health Statistics. National Health and Nutrition Examination Survey (NHANES): Dual Energy X-ray Absorptiometry (DXA) Procedures Manual. In: Services USDoHaH, ed. Hyattsville, MD: Center for Disease Control and Prevention; January 2007. [Google Scholar]
- [18].Schenker N, Borrud LG, Burt VL, et al. Multiple imputation of missing dual-energy X-ray absorptiometry data in the National Health and Nutrition Examination Survey. Stat Med 2011;30:260–76. [DOI] [PubMed] [Google Scholar]
- [19].Center for Disease Control and Prevention/National Center for Health Statistics. National Health and Nutrition Examination Survey 2012; 2017. Available at: https://www.cdc.gov/nchs/tutorials/. Accessed March 7, 2017. [Google Scholar]
- [20].Karhunen PJ, Meinander T. Coronary artery disease: complex association between height and CHD-size matters. Nat Rev Cardiol 2015;12:385–6. [DOI] [PubMed] [Google Scholar]
- [21].Dyer AR, Elliott P, Shipley M. Body mass index versus height and weight in relation to blood pressure. Findings for the 10,079 persons in the INTERSALT study. Am J Epidemiol 1990;131:589–96. [DOI] [PubMed] [Google Scholar]
- [22].Wright JD, Hughes JP, Ostchega Y, et al. Mean systolic and diastolic blood pressure in adults aged 18 and over in the United States. Natl Health Stat Report 2001–2008;2011:1–22. [PubMed] [Google Scholar]
- [23].Harlan WR, Hull AL, Schmouder RL, et al. Blood pressure and nutrition in adults. The National Health and Nutrition Examination Survey. Am J Epidemiol 1984;120:17–28. [DOI] [PubMed] [Google Scholar]
- [24].Sun Q, van Dam RM, Spiegelman D, et al. Comparison of dual-energy X-ray absorptiometric and anthropometric measures of adiposity in relation to adiposity-related biologic factors. Am J Epidemiol 2010;172:1442–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Voors AW, Harsha DW, Webber LS, et al. Relation of blood pressure to stature in healthy young adults. Am J Epidemiol 1982;115:833–40. [DOI] [PubMed] [Google Scholar]
- [26].Mitchell GF. Arterial stiffness and wave reflection in hypertension: pathophysiologic and therapeutic implications. Curr Hypertens Rep 2004;6:436–41. [DOI] [PubMed] [Google Scholar]
- [27].Mitchell G, Skinner JD. An allometric analysis of the giraffe cardiovascular system. Comp Biochem Physiol A Mol Integr Physiol 2009;154:523–9. [DOI] [PubMed] [Google Scholar]
- [28].Cooper LL, Rong J, Benjamin EJ, et al. Components of hemodynamic load and cardiovascular events: the Framingham Heart Study. Circulation 2015;131:354–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Selvaraj S, Steg PG, Elbez Y, et al. Pulse pressure and risk for cardiovascular events in patients with atherothrombosis: from the REACH registry. J Am Coll Cardiol 2016;67:392–403. [DOI] [PubMed] [Google Scholar]
- [30].Franklin SS, Wong ND. Pulse pressure: how valuable as a diagnostic and therapeutic tool? J Am Coll Cardiol 2016;67:404–6. [DOI] [PubMed] [Google Scholar]
- [31].Ayyagari R, Vekeman F, Lefebvre P, et al. Pulse pressure and stroke risk: development and validation of a new stroke risk model. Curr Med Res Opin 2014;30:2453–60. [DOI] [PubMed] [Google Scholar]
- [32].Okada K, Iso H, Cui R, et al. Pulse pressure is an independent risk factor for stroke among middle-aged Japanese with normal systolic blood pressure: the JPHC study. J Hypertens 2011;29:319–24. [DOI] [PubMed] [Google Scholar]
- [33].Franklin SS, Larson MG, Khan SA, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation 2001;103:1245–9. [DOI] [PubMed] [Google Scholar]
- [34].Reeve JC, Abhayaratna WP, Davies JE, et al. Central hemodynamics could explain the inverse association between height and cardiovascular mortality. Am J Hypertens 2014;27:392–400. [DOI] [PubMed] [Google Scholar]
- [35].Mitchell G, Maloney SK, Mitchell D, et al. The origin of mean arterial and jugular venous blood pressures in giraffes. J Exp Biol 2006;209:2515–24. [DOI] [PubMed] [Google Scholar]
- [36].Kahn HS, Bain RP, Pullen-Smith B. Interpretation of children's blood pressure using a physiologic height correction. J Chronic Dis 1986;39:521–31. [DOI] [PubMed] [Google Scholar]
- [37].Martin-Du Pan RC, Benoit R, Girardier L. The role of body position and gravity in the symptoms and treatment of various medical diseases. Swiss Med Wkly 2004;134:543–51. [DOI] [PubMed] [Google Scholar]
- [38].Bjornsson E, Thorgeirsson G, Gudnason T. Adult height associates with angiographic extent of coronary artery disease. Atherosclerosis 2016;254:237–41. [DOI] [PubMed] [Google Scholar]
- [39].Nwasokwa ON, Weiss M, Gladstone C, et al. Effect of coronary artery size on the prevalence of atherosclerosis. Am J Cardiol 1996;78:741–6. [DOI] [PubMed] [Google Scholar]
- [40].Nwasokwa ON, Weiss M, Gladstone C, et al. Higher prevalence and greater severity of coronary disease in short versus tall men referred for coronary arteriography. Am Heart J 1997;133:147–52. [DOI] [PubMed] [Google Scholar]
- [41].Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest 2005;127:1952–9. [DOI] [PubMed] [Google Scholar]
- [42].Nelson CP, Hamby SE, Saleheen D, et al. Genetically determined height and coronary artery disease. N Engl J Med 2015;372:1608–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Nuesch E, Dale C, Palmer TM, et al. Adult height, coronary heart disease and stroke: a multi-locus Mendelian randomization meta-analysis. Int J Epidemiol 2016;45:1927–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Dodson RB, Miller TA, Powers K, et al. Intrauterine growth restriction influences vascular remodeling and stiffening in the weanling rat more than sex or diet. Am J Physiol Heart Circ Physiol 2017;312:H250–64. [DOI] [PubMed] [Google Scholar]
- [45].Dodson RB, Rozance PJ, Fleenor BS, et al. Increased arterial stiffness and extracellular matrix reorganization in intrauterine growth-restricted fetal sheep. Pediatr Res 2013;73:147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Dodson RB, Rozance PJ, Petrash CC, et al. Thoracic and abdominal aortas stiffen through unique extracellular matrix changes in intrauterine growth restricted fetal sheep. Am J Physiol Heart Circ Physiol 2014;306:H429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bartke A, Sun LY, Longo V. Somatotropic signaling: trade-offs between growth, reproductive development, and longevity. Physiol Rev 2013;93:571–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wolfe BL, Rich CB, Goud HD, et al. Insulin-like growth factor-I regulates transcription of the elastin gene. J Biol Chem 1993;268:12418–26. [PubMed] [Google Scholar]
- [49].Albertsson-Wikland K, Rosberg S. Analyses of 24-hour growth hormone profiles in children: relation to growth. J Clin Endocrinol Metab 1988;67:493–500. [DOI] [PubMed] [Google Scholar]
- [50].Benonisdottir S, Oddsson A, Helgason A, et al. Epigenetic and genetic components of height regulation. Nat Commun 2016;7:13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hoopes BC, Rimbault M, Liebers D, et al. The insulin-like growth factor 1 receptor (IGF1R) contributes to reduced size in dogs. Mamm Genome 2012;23:780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hermanussen M, Ipsen J, Mumm R, et al. Stunted growth. Proceedings of the 23rd Aschauer Soiree, Held at Aschauhof, Germany, November 7th 2015. Pediatr Endocrinol Rev 2016;13:756–67. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


