Abstract
The prognostic relevance of commonly used composite inflammation-based scores remains severely underdiscussed in pancreatic cancer (PC), especially for advanced PC. In this retrospective cohort study, we aimed to discuss the association between multiple inflammatory scores and the short-term overall survival (OS) of advanced pancreatic ductal adenocarcinoma (PDAC) patients. A total of 66 histologically confirmed PDAC patients were retrospectively analyzed. A multivariate Cox proportional hazards model was used to explore the association between 6 commonly used inflammatory scores measured right after diagnosis, Glasgow Prognostic Score (GPS), Modified Glasgow Prognostic Score (mGPS), neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), prognostic index (PI), prognostic nutritional index (PNI), and the short-term OS of advanced PDAC. Analytical results revealed that among GPS, mGPS, NLR, PLR, PI and PNI only PLR was significantly associated with short-term OS of PDAC. For both 1-year and 2-year OS, every 10 increase of PLR value resulted in 1.10 (95% CI: 1.04, 1.16) folds hazard ratio (HR). Further analysis identified a statistically significant dose–response relationship between PLR and HR. Our study results probably suggested that PLR is a promising prognostic factor of advanced PDAC; maintaining normally ranged platelet count may gain short-term survival benefit among such patients.
Keywords: advanced pancreatic ductal adenocarcinoma, inflammation-based scores, overall survival
1. Introduction
Pancreatic cancer (PC) is a common albeit lethal type of malignant tumor, with a reported 5-year survival rate as dismal as 0.4% to 4%.[1,2] Because the lack of specific symptoms in the early stage of the disease, many PC patients only can be diagnosed at an advanced stage, thus miss the opportunity for curative resection. It is estimated that only up to 20% of PC patients will be diagnosed with resectable disease.[1,3] For the majority of patients with advanced PC, gemcitabine-based palliative chemotherapy has long been used as a standard treatment. However, its usefulness in improving overall survival (OS) is still away from conclusive based on existing evidences.[4] Therefore, to identify other nontherapeutic characteristics which may influence the prognosis of advanced PC patients is a question of considerable clinical significance.
Among those characteristics, systemic inflammation status has been increasingly attracting the attention of researchers. In the last decade, a variety of systemic inflammation-based prognostic scores had been developed, among them, Glasgow Prognostic Score (GPS) and Modified Glasgow Prognostic Score (mGPS) based on serum C-reactive protein (CRP) and albumin, neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), prognostic index (PI) based on CRP and white blood cell count (WBC), and prognostic nutritional index (PNI) based on albumin and lymphocyte count are the most commonly used.[5] These scores and their associations with the survival of many types of cancer, such as lung,[6] esophageal,[7] colorectal,[8] and renal cancers,[9] as well as PC,[10–12] had been discussed.
Nevertheless, existing literature is far from sufficient to conclude a definitive association between inflammation scores and PC survival. At first, compare to other common types of cancer, the studies on PC are much scarce. Second, the majority of studies in this field discussed this association only in resectable PC patients, whereas for advanced PC patients, although many inflammatory indicators enumerated above have been investigated, like GPS, mGPS, NLR, PNI, PLR,[11,13–16] they remain severely underdiscussed; for most indicators, only 1 relevant study has been identified. Third, all studies available only analyzed the association between inflammatory scores and the general OS of advanced PC patients, no study has ever explored the possible difference in this association with regard to short-term OS, and this topic is certainly of clinical interest.
In this retrospective cohort study, we aimed to simultaneously analyze and compare the influence of 6 commonly used inflammatory-based scores (GPS, mGPS, NLR, PLR, PI, and PNI) which measured right after disease diagnosis in short-term OS of advanced PC patients, in hope of identifying some inflammatory indicators of prognostic relevance, and shed new light on possible clinical intervention measures.
2. Methods
2.1. Patients
After Institutional Research Ethics Board of Fudan University approved, we performed a retrospective review in a mega population-based electronic inpatients database. This database has been thoroughly described in a previously published study.[17] We chose advanced PC patients, who were diagnosed between January 1, 2012 and December 31, 2013, from this database, the full-set of inclusion criteria include: histological confirmation of pancreatic ductal adenocarcinoma (PDAC); locally advanced or metastasis occurred, did not receive curative operation; not end-stage PC, defined as survival length after diagnosis exceeded 30 days; blood indicators for calculating inflammatory-based scores were tested within 20 days after histological confirmation; other vital information for analysis was complete, such as age, gender, and palliative chemotherapy agents. In the end, we successfully gleaned 66 advanced PDAC patients for further analysis.
The outcome of interest was OS, to be more specific, the short-term OS, which is defined as 1-year and 2-year OS. The date of death for all 66 advanced PDAC patients was determined through external matching with population-based death registration system.
2.2. Measurements of inflammation-based scores
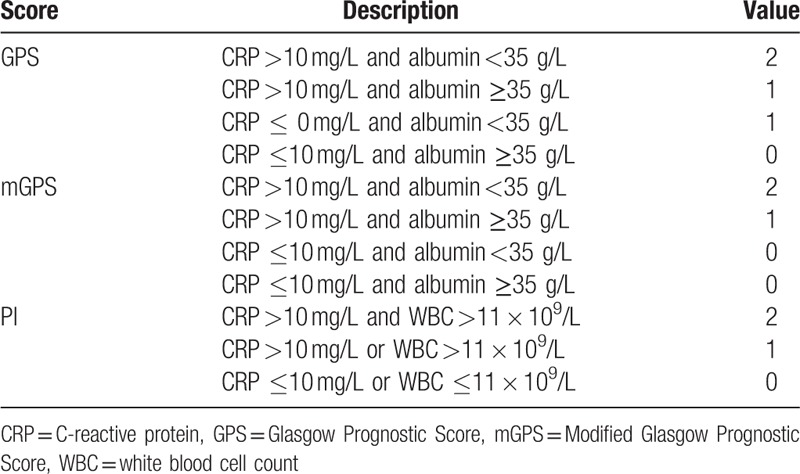
Measurements of inflammation-based scores we analyzed are summarized in Table 1. Briefly, patients with both an elevated CRP (>10 mg/L) and hypoalbuminemia (<35 g/L) were assigned a GPS and a mGPS of 2, patients with neither of the 2 indicators abnormal (CRP ≤10 mg/L and albumin ≥35 g/L) were assigned a GPS and a mGPS of 0, patients who only had an elevated CRP level were assigned a GPS and a mGPS of 1, and patients who only had hypoalbuminemia were assigned a GPS of 1 and a mGPS of 0. For PI, patients who had both elevated CRP (>10 mg/L) and WBC (>11 × 109/L) were assigned a score of 2, those with only 1 test abnormal were assigned a score of 1, both tests were normal were assigned a PI score of 0. PNI was defined as albumin (g/L) × lymphocyte count (×109/L). NLR, PLR, and PNI were treated as continuous variables to avoid arbitrarily assigned cut-offs in defining “normal” and “abnormal”.
Table 1.
Measurements of inflammation-based scores.
2.3. Statistical analysis
We used descriptive statistics to illustrate or compare distributional characteristics of advanced PDAC patients. Univariate and multivariate Cox proportional hazards model were used consecutively to estimate the crude and adjusted hazard ratios (HR) of inflammation-based scores in association with 1-year and 2-year OS of advanced PDAC patients, respectively. All statistical analyses were run by SAS (version 9.2, SAS Institute Inc, Cary, NC), with a predesignated 2-tailed significance level of P <.05.
3. Results
3.1. Patients characteristics
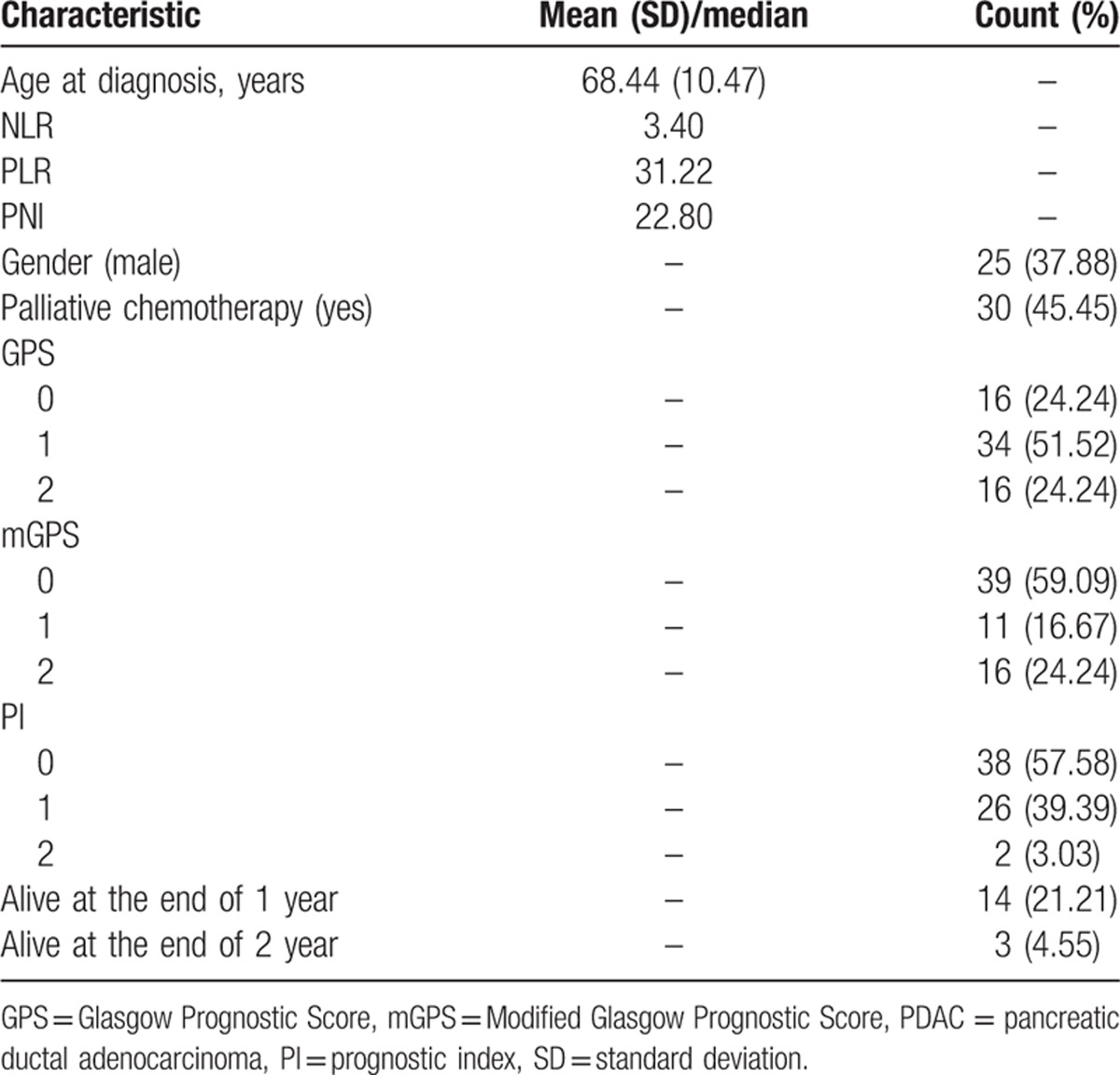
Demographical and clinical characteristics of patients are listed in Table 2. The mean of age at diagnosis for all patients was 68.44 years with a standard deviation of 10.47 years. Males were accounted for a comparatively small part, <40%. Nearly a half of patients received palliative chemotherapy subsequently. The medians for baseline NLR, PLR, and PNI were 3.40, 31.22, and 22.80, respectively. Over a half of advanced PDAC patients had a GPS of 1, mGPS of 0, and PI score of 0. By the end of 1-year survival length, 14 patients were still alive, whereas by the end of 2-year survival length, only 3 patients were still alive.
Table 2.
Characteristics of advanced PDAC patients (N = 66).
3.2. Univariate and multivariate analysis of 1-year and 2-year OS
Based on the univariate Cox model, we found that NLR and PLR were significantly associated with both 1-year and 2-year OS of advanced PDAC, whereas mGPS and PI were only significantly associated with 1-year OS. After multivariate adjustment, only PLR stayed prominent: for both endpoints, every 10 increase in PLR score was associated with a constant 10% increase in death hazard (Table 3). We further dichotomized PC patients into 2 subgroups by using the median of baseline PLR. Kaplan–Meier curves and log-rank test results revealed that both 1-year and 2-year survival were distinctively different between the 2 subgroups. PC patients with higher baseline PLR had deteriorated 1-year or 2-year OS (Fig. 1).
Table 3.
Univariate and multivariate Cox model fitting results for 1-year and 2-year OS of advanced PC patients.
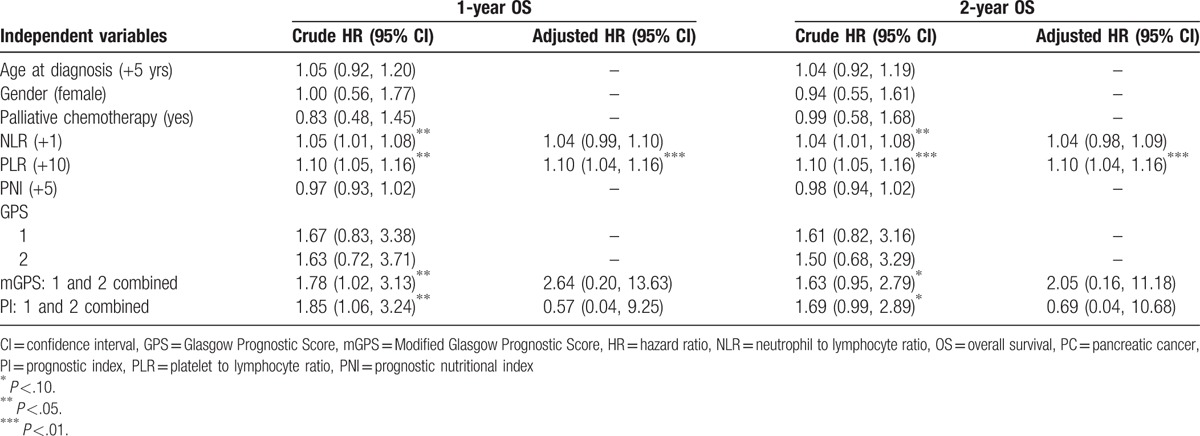
Figure 1.
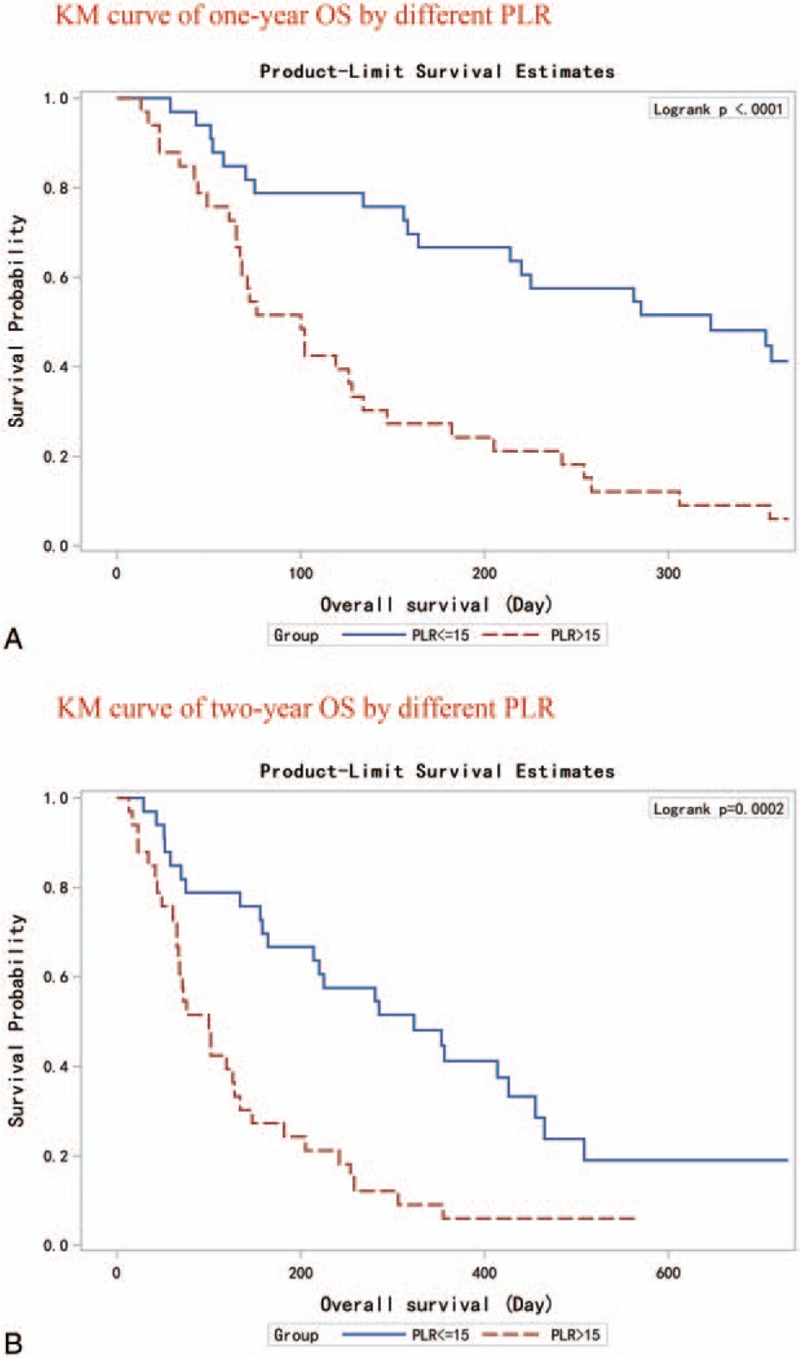
Kaplan–Meier curves for PC patients with different baseline PLR. (Panel A) 1-year OS. (Panel B) 2-year OS. OS = overall survival, PC = pancreatic cancer, PLR = platelet to lymphocyte ratio.
3.3. Dose–response association of PLR in 1-year and 2-year OS
We further analyzed the dose–response association between PLR and short-term OS of advanced PDAC. The 33rd and 66th percentiles were used to regroup the patients. Results are shown in Figure 2: the hazard of death was picking up the trend along with the increase of PLR in both 1-year and 2-year OS. Compared with PDAC patients who reported the lowest one-third PLR value, HRs for PDAC patients with the central one-third and the highest one-third PLR values were 2.45 (95% CI: 1.15, 5.24) and 3.00 (95% CI: 1.31, 6.83) for 1-year OS, 2.07 (95% CI: 1.04, 4.12) and 2.30 (95% CI: 1.06, 4.96) for 2-year OS. The dose–response association was statistically prominent in both groups.
Figure 2.
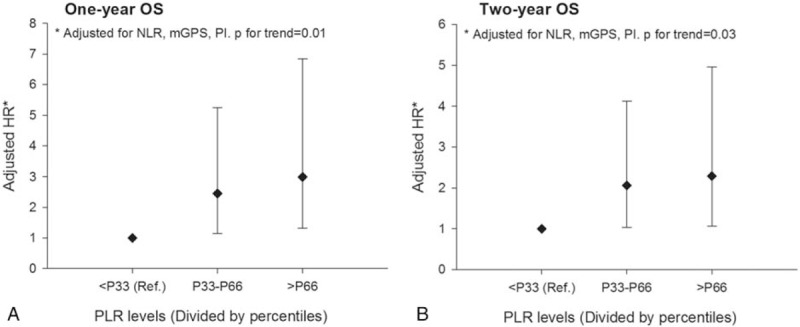
Dose–response association between postdiagnosis PLR and the short-term OS of advanced PDAC. HR = hazard ratio, mGPS = Modified Glasgow Prognostic Score, NLR = neutrophil to lymphocyte ratio, OS = overall survival, PDAC = pancreatic ductal adenocarcinoma, PI = prognostic index, PLR = platelet to lymphocyte ratio.
4. Discussion
In this retrospective cohort study, we analyzed and compared the prognostic value of multiple systemic inflammation-based scores in 1-year and 2-year OS of advanced PDAC patients. We found that, among GPS, mGPS, NLR, PLR, PI, and PNI, PLR was the only index that significantly associated with short-term OS of advanced PDAC patients. Generally, every 10 increase in the PLR value was associated with 10% additional death hazard in both 1-year and 2-year OS, and this association was further confirmed by subsequent analysis which identified a prominent dose–response relationship.
As a commonly used systemic inflammation-based biomarker, the prognostic significance of pretreatment PLR in various types of cancer, such as nonsmall cell lung cancer,[18] gastric cancer,[19] colorectal cancer,[20] and ovarian cancer,[21] has been discussed. Currently, for many types of cancer, the association between pretreatment PLR and survival is still away from confirmative with regard to contradicting results. For PC, this association has also been discussed albeit only by several published studies, and the majority of them were focused on resectable PC patients. For example, Smith et al,[12] Asari et al,[22] and Shirai et al[23] all reported that preoperative PLR was an independent predictor of OS in resectable PC patients, however, Bhatti et al[24] found that this association was insignificant. We only identified 2 newly published studies which discussed this association in advanced PC patients: one observed prominent association while the other did not.[15,17] However, these 2 studies all focused on general OS rather than short-term OS. From this point of view, our study is valuable in accumulating the initial evidence. Considering that we did not identify the same prominent association between NLR and short-term OS, we assumed that the elevated platelet count rather than decreased lymphocyte count plays a central role behind this association. Some possible mechanisms may be involved to explain the detrimental effect of elevated platelet count on PC survival. It has been revealed that platelets can secrete a group of proteins which directly promote tumor cell proliferation and angiogenesis.[25] Moreover, experimental studies suggested that platelets can facilitate cancer metastasis by cloaking stray cancer cells in blood stream, so as to prevent them to be recognized and cleared by the immune system.[26] Our finding perhaps suggested that, for advanced PDAC patients, correcting elevated platelet count may bring about short-term survival benefit. However, as we recently found that thrombocytopenia can also cause deteriorated OS of PC,[17] the correction of thrombocytosis should be dealt with caution in clinical practice.
Despite of insignificant finding, the possibility of true association between mGPS and OS of advanced PDAC cannot be precluded with regard to comparatively strong effect estimate (HR = 2.05). Small sample size could be the reason which prevented this association been successfully detected. This assumption can be partly supported by the reality that 2 newly published studies with bigger sample sizes have all found that higher mGPS score was associated with less optimistic OS in metastatic PC patients.[11,16]
Although 1 study compared the prognostic value of PI with other common inflammatory factors in PC patients,[27] the influence of PI in survival of advanced PC patients has not been exclusively discussed. We found that PI did not present noticeable effects on short-term OS of advanced PDAC, and this result was comparable to the conclusion reached by the aforementioned study. As to the rest 3 inflammatory scores we analyzed, NLR, GPS, and PNI, after adjustment, they all showed insignificant association with short-term OS of advanced PDAC. On the contrary, several published studies suggested that they could be independent prognostic markers of unresectable PC.[13–15] A possible explanation to this discrepancy could be different endpoints selected, as we chose short-term OS and these studies chose the general OS.
Several limitations of our study should be noticed. At first, because the detailed TNM classification information for each patient was unavailable, we could not fully adjust for its confounding. As tumor stage will directly influence systemic inflammation status of cancer patients, there lies a possibility that the positive association between PLR and short-term OS of advanced PDAC patients we identified was in essence of the association between more advanced tumor stage and deteriorated survival. But if it is true, the null association between other inflammation-based scores and short-term OS will be hard to interpret. Second, the sample size of the present study was small, and that definitely hampered statistical efficiency. For example, we identified a strong association between mGPS and OS based on point estimate of HR; however, after taking a big standard error into consideration, this association turned into insignificant. Nevertheless, under this scenario, all conclusions we reached will be comparatively conservative. Third, for analytical purpose, we only included advanced PDAC patients with complete vital information, and if the patients we analyzed were not comparable to patients we excluded on major characteristics, selection bias can be introduced into our study results.
To conclude, in this retrospective cohort study, we have identified that PLR measured after disease diagnosis could be a promising marker of the short-term OS of advanced PDAC patients. This conclusion probably hints the clinical significance of treating elevated platelet count in advanced PDAC patients, in order to gain possible survival benefits. More clinical studies with prospective design and large sample size are warranted to validate this finding.
Footnotes
Abbreviations: GPS = Glasgow Prognostic Score, mGPS = Modified Glasgow Prognostic Score, NLR = neutrophil to lymphocyte ratio, OS = overall survival, PC = pancreatic cancer, PDAC = pancreatic ductal adenocarcinoma, PI = prognostic index, PLR = platelet to lymphocyte ratio, PNI = prognostic nutritional index.
YX and ZX contributed equally as joint first authors.
Funding: This study was supported by National Natural Science Foundation of China (no. 81703324, no. 81273187) and National Science and Technology Major Project of the People's Republic of China (2012ZX09303-013-014).
The authors have no conflicts of interest to disclose.
References
- [1].Coopermann AM, Chamberlain RS. Saunders, The Surgical Clinics of North America. Philadelphia:2001. [Google Scholar]
- [2].Jemal A, Murray T, Samuels A, et al. Cancer statistics, 2003. CA Cancer J Clin 2003;53:5–26. [DOI] [PubMed] [Google Scholar]
- [3].Jemal A, Thomas A, Murray T, et al. Cancer statistics, 2002. CA Cancer J Clin 2002;52:23–4. [DOI] [PubMed] [Google Scholar]
- [4].Ciliberto D, Botta C, Correale P, et al. Role of gemcitabine-based combination therapy in the management of advanced pancreatic cancer: a meta-analysis of randomised trials. Eur J Cancer 2013;49:593–603. [DOI] [PubMed] [Google Scholar]
- [5].Yamamura K, Sugimoto H, Kanda M, et al. Comparison of inflammation-based prognostic scores as predictors of tumor recurrence in patients with hepatocellular carcinoma after curative resection. J Hepatobiliary Pancreat Sci 2014;21:682–8. [DOI] [PubMed] [Google Scholar]
- [6].Forrest LM, McMillan DC, McArdle CS, et al. A prospective longitudinal study of performance status, an inflammation-based score (GPS) and survival in patients with inoperable non-small-cell lung cancer. Br J Cancer 2005;92:1834–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Crumley AB, McMillan DC, McKernan M, et al. Evaluation of an inflammation-based prognostic score in patients with inoperable gastro-oesophageal cancer. Br J Cancer 2006;94:637–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].McMillan DC, Canna K, McArdle CS. Systemic inflammatory response predicts survival following curative resection of colorectal cancer. Br J Surg 2003;90:215–9. [DOI] [PubMed] [Google Scholar]
- [9].Ramsey S, Lamb GW, Aitchison M, et al. Evaluation of an inflammation-based prognostic score in patients with metastatic renal cancer. Cancer 2007;109:205–12. [DOI] [PubMed] [Google Scholar]
- [10].Morinaga S, Murakawa M, Katayama Y, et al. Glasgow prognostic score predicts clinical outcomes in patients with pancreatic cancer undergoing adjuvant Gemcitabine monotherapy after curative surgery. Anticancer Res 2015;35:4865–70. [PubMed] [Google Scholar]
- [11].Imaoka H, Mizuno N, Hara K, et al. Evaluation of modified Glasgow prognostic score for pancreatic cancer: a retrospective cohort study. Pancreas 2016;45:211–7. [DOI] [PubMed] [Google Scholar]
- [12].Smith RA, Bosonnet L, Raraty M, et al. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg 2009;197:466–72. [DOI] [PubMed] [Google Scholar]
- [13].Shimoda M, Katoh M, Kita J, et al. The Glasgow prognostic score is a good predictor of treatment outcome in patients with unresectable pancreatic cancer. Chemotherapy 2010;56:501–6. [DOI] [PubMed] [Google Scholar]
- [14].Xue P, Kanai M, Mori Y, et al. Neutrophil-to-lymphocyte ratio for predicting palliative chemotherapy outcomes in advanced pancreatic cancer patients. Cancer Med 2014;3:406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Geng Y, Qi Q, Sun M. Prognostic nutritional index predicts survival and correlates with systemic inflammatory response in advanced pancreatic cancer. Eur J Surg Oncol 2015;41:1508–14. [DOI] [PubMed] [Google Scholar]
- [16].Martin HL, Ohara K, Kiberu A, et al. Prognostic value of systemic inflammation-based markers in advanced pancreatic cancer. Intern Med J 2014;44:676–82. [DOI] [PubMed] [Google Scholar]
- [17].Xiao Y, Xie H, Xie Z, et al. Kinetics of postdiagnosis platelet count with overall survival of pancreatic cancer: a counting process approach. Cancer Med 2016;5:881–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhao QT, Yuan Z, Zhang H, et al. Prognostic role of platelet to lymphocyte ratio in non-small cell lung cancers: A meta-analysis including 3,720 patients. Int J Cancer 2016;139:164–70. [DOI] [PubMed] [Google Scholar]
- [19].Wang DS, Ren C, Qiu MZ, et al. Comparison of the prognostic value of various preoperative inflammation-based factors in patients with stage III gastric cancer. Tumour Biol 2012;33:749–56. [DOI] [PubMed] [Google Scholar]
- [20].Gu X, Gao XS, Qin S, et al. Elevated platelet to lymphocyte ratio is associated with poor survival outcomes in patients with colorectal cancer. PLoS One 2016;11:e0163523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Asher V, Lee J, Innamaa A, et al. Preoperative platelet lymphocyte ratio as an independent prognostic marker in ovarian cancer. Clin Transl Oncol 2011;13:499–503. [DOI] [PubMed] [Google Scholar]
- [22].Asari S, Matsumoto I, Toyama H, et al. Preoperative independent prognostic factors in patients with borderline resectable pancreatic ductal adenocarcinoma following curative resection: the neutrophil-lymphocyte and platelet-lymphocyte ratios. Surg Today 2016;46:583–92. [DOI] [PubMed] [Google Scholar]
- [23].Shirai Y, Shiba H, Sakamoto T, et al. Preoperative platelet to lymphocyte ratio predicts outcome of patients with pancreatic ductal adenocarcinoma after pancreatic resection. Surgery 2015;158:360–5. [DOI] [PubMed] [Google Scholar]
- [24].Bhatti I, Peacock O, Lloyd G, et al. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg 2010;200:197–203. [DOI] [PubMed] [Google Scholar]
- [25].Alvarez RH, Kantarjian HM, Cortes JE. Biology of platelet-derived growth factor and its involvement in disease. Mayo Clin Proc 2006;81:1241–57. [DOI] [PubMed] [Google Scholar]
- [26].Palumbo JS, Talmage KE, Massari JV, et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood 2005;105:178–85. [DOI] [PubMed] [Google Scholar]
- [27].Wang DS, Luo HY, Qiu MZ, et al. Comparison of the prognostic values of various inflammation based factors in patients with pancreatic cancer. Med Oncol 2012;29:3092–100. [DOI] [PubMed] [Google Scholar]


