Abstract
Rationale:
Cases of bilateral acute angle closure have been reported after use of various drugs.
Patient concerns:
A 52-year-old woman visited the emergency room and complained of acute bilateral ocular pain and decreased vision accompanied by headache, nausea, and vomiting. One day before, she had started a herbal medicine containing Ma-huang for weight loss. On examinations, myopic shift, edematous cornea, increased intraocular pressure, shallow anterior chamber, and thickened choroid on both eyes were observed.
Diagnoses:
Angle closure glaucoma induced by drug (Ma-huang).
Interventions:
To promptly quit the offending drug and apply ocular hypotensives and cycloplegics.
Outcomes:
Her symptoms and signs were relieved after antiglaucoma medications and no significant recurrence has been occurred.
Lessons:
Physicians prescribing weight loss medications containing Ma-huang must be aware of the potentially sight-threatening adverse effect of bilateral acute angle closure.
Keywords: bilateral acute angle closure glaucoma, bilateral acute myopia, drug-induced angle closure glaucoma, Ma-huang
1. Introduction
Bilateral acute angle closure has been reported after use of topiramate,[1] hydrochlorothiazide,[2] selective serotonin reuptake inhibitors,[3,4] sildenafil citrate,[5] anticough mixtures,[6] antihistamine, decongestants,[7] and atropa belladonna,[8]a herbal ingredient containing the sympathomimetic agent pseudoephedrine. Here we report a case of acute angle closure and myopic shift in both eyes after use of Ma-huang for weight loss.
2. Case report
A 52-year-old female presented to the emergency department with complaints of acute bilateral ocular pain and decreased visual acuity accompanied by headache, nausea, and vomiting that started 1 day before presentation. Her past medical history and review of systems were unremarkable. One day before, she had started a herbal medicine containing Ma-huang for weight loss. The patient gave her written informed consent.
The initial examination revealed best corrected visual acuity of 0.7 in the right eye and 0.7 in the left eye. The spherical equivalent was +0.5 D in the right eye and +0.25 D in the left eye. Intraocular pressure (IOP) by Goldmann applanation tonometry was 64 mm Hg in the right eye and 68 mm Hg in the left eye. The pupils of both eyes were normal in size and reactive with no afferent defects.
On slit lamp examination, the cornea was mildly edematous. The central depth of the anterior chamber was shallow at 2.5 times the corneal thickness, and the peripheral depth of the anterior chamber was shallower than 1/4 of the corneal thickness. On gonioscopy, there was 360-degree-angle closure on both eyes. No peripheral anterior synechia was observed at indentation gonioscopy. On ocular ultrasonography, the choroid was thickened, but choroidal effusion was not observed (Fig. 1). Swept source OCT (optical coherence tomography) showed that subfoveal choroidal thickness was 350 μm in the right eye and 364 μm in the left eye, which were thicker than normal[9] (Fig. 2).
Figure 1.
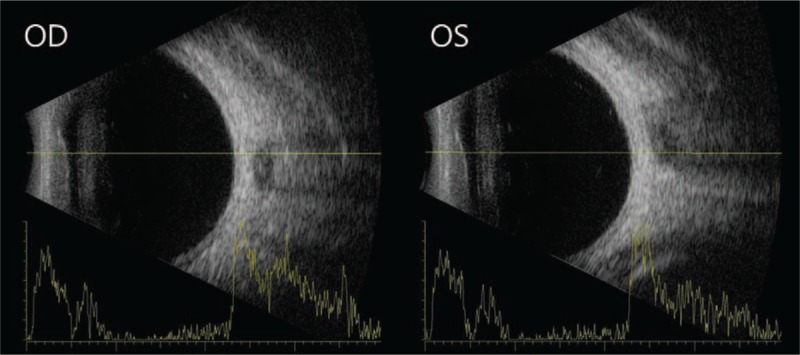
Ultrasonography showed thickened choroid in both eyes, but choroidal effusion was not observed.
Figure 2.
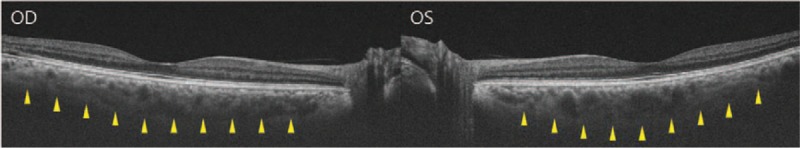
Markedly thickened choroid was observed on swept-source optical coherence tomography in both eyes.
A diagnosis of bilateral angle-closure glaucoma was made, and the patient was treated with intravenous 20% mannitol, brimonidine (Alphagan, Allergan Pharmaceuticals, Irvine, CA), and dorzolamide-timolol (Cosopt, Merck & Co., Inc., Whitehouse Station, NJ). IOP decreased to 11 mm Hg in the right eye and 9 mm Hg in the left eye.
The following day, IOP was 7 mm Hg in the right eye and 7 mm Hg in the left eye. Depth of the central anterior chamber measured by IOL Master (Carl Zeiss Meditec, Oberkochen, Germany) was 2.34 mm in the right eye and 2.16 mm in the left eye. Axial length measured by IOL Master was 21.36 mm in the right eye and 21.62 mm in the left eye, which is shorter than average. Dilation of both eyes was performed; after mydriasis, the anterior chamber depth of both eyes was deeper than before (Fig. 3). The spherical equivalent was +1.875 D in the right eye and +1.125 D in the left eye after mydriasis.
Figure 3.
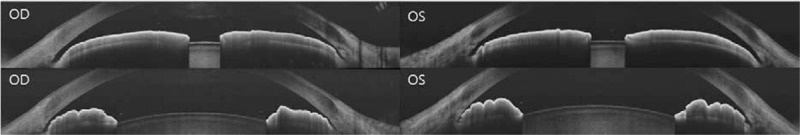
Anterior segment optical coherence tomography shows that anterior chamber depth after mydriasis (upper) was deeper than before mydriasis (lower).
After 4 weeks, her IOP was 13 mm Hg in the right eye and 12 mm Hg in the left eye. The central depth of the anterior chamber was 2.56 mm in the right eye and 2.57 mm in the left eye, which were deeper than before. No symptom recurrence or intraocular pressure elevation has been observed to this point.
3. Discussion
Ma-huang, or Ephedra, is a botanical source of ephedrine-type alkaloids which is derived from 3 kinds of plant, Ephedra sinica Staph, Ephedra intermedia Schrenk, and Ephedra equisetina Bge, and is used as a medicinal agent in Asia. The plant has 2 active ingredients, ephedrine and pseudoephedrine. Ephedrine is a sympathomimetic alkaloid with alpha-1, beta-1, and beta-2 receptor agonist properties, and pseudoephedrine has beta-2 agonist properties.[10]
Products containing the herb Ephedra were used in the United States in the early 1990s to increase weight loss and enhance athletic performance.[11,12] However, serious adverse events and even death[13,14] resulted in the FDA announcing that dietary supplements containing Ephedra represent “a significant or unreasonable risk of illness or injury.”[15] Although there have been no reports of ophthalmic complications, we experienced bilateral acute angle closure and myopic shift, caused by Ma-huang.
Acute angle closure is an urgent, dramatic symptomatic event accompanied by blurred vision, painful red eye, headache, nausea, and vomiting. It is diagnosed by high IOP, corneal edema, shallow anterior chamber depth, and a closed angle on gonioscopy.[16]
The mechanism of drug-induced acute angle closure involves relative pupillary block or choroidal exudation.[17–19] Relative pupillary block can be induced by agents such as adrenaline, anticholinergic drugs, tricyclic antidepressants, or tetracyclic antidepressants. It occurs when the posterior surface of the iris comes into contact with the anterior surface of the lens. This contact can block aqueous flow from the posterior chamber to the anterior chamber, creating pressure that pushes the iris root forward to close the angle. Because the area of the iris surface in contact with the lens is largest when the pupil is mid-dilated, acute angle closure can be triggered by drugs that induce pupil dilation,[17,18] especially in eyes with hyperopia, a narrow angle, or a thick lens. Choroidal exudation can be caused by drug-specific reactions of topiramate, hydrochlorothiazide, acetazolamide, or anticoagulants.[20,21] It might be caused by ciliary body effusion and secondary anterior movement of the lens. The zonules relax to thicken the lens, which simultaneously induces forward movement of the ciliary body. Therefore, the lens-iris diaphragm moves forward, and the anterior chamber becomes shallow. This series of reactions induces acute myopia and angle closure.[18,19] The exact pathophysiologic mechanism of ciliary body effusion remains unknown. With topiramate, elevated prostaglandin can induce effusion in the ciliary body, resulting in anterior movement of the lens-iris diaphragm and vitreous to cause acute myopia and angle closure.[22–25] The combination of these 2 mechanisms could contribute to angle closure attack, as in a case following sexual intercourse aided by sildenafil citrate.[5]
In this patient, acute angle closure was induced by ciliary body edema rather than relative pupillary block. Evidence for this mechanism includes the difference in refractive errors before and after mydriasis, as well as deepening of the anterior chamber after mydriasis. Ocular ultrasonography and swept source OCT also showed the thickening of choroid thickness.
If drug-induced angle closure is suspected in a patient with bilateral acute angle closure glaucoma, the drug should be investigated to determine which mechanism of acute angle closure glaucoma has occurred to ensure proper treatment. It is common to promptly quit the offending drug. If the mechanism is suspected to be relative pupillary block with drugs such as adrenergics, anticholinergics, tricyclic antidepressants, or tetracyclic antidepressants with mydriatic effect, ocular hypotensives and miotic drugs are necessary for treatment. In contrast, if a moderate degree of myopic shift and acute angle closure are observed on refraction test after use of medications such as oral sulfase, hydrochlorothiazide, or anticoagulant, the likely mechanism is ciliary body edema. In these cases, miotic drugs can aggravate angle closure by causing anterior movement of the lens and ciliary body spasm.[26,27] In this case, ocular hypotensives and cycloplegics strain the zonules and cause posterior movement of the lens, deepening the anterior chamber and opening the trabecular meshwork that is clogged by the iris.[28] In some cases, it is necessary to reduce suprachoroidal inflammation by adding local or systemic corticosteroids to resolve the anterior movement of the ciliary body caused by supraciliary fluid.[29]
In our case, we also stopped Ma-huang immediately to treat angle closure and acute myopia. Then, intravenous mannitol and ocular hypotensives were injected in the emergency room. The patient's myopic shift and angle closure improved after initiation of cycloplegics.
4. Conclusions
This is the first case of acute angle closure and myopic shift of Ma-huang contained herbal medicine for weight loss. Physicians prescribing weight loss medications containing Ma-huang must be aware of the potentially sight-threatening adverse effect of bilateral acute angle closure. Also, patients should be advised to seek immediate ophthalmic examination for blurred vision, eye pain, or headache after using medication.
Footnotes
Abbreviations: IOP = intraocular pressure, OCT = optical coherence tomography.
SJR and YUS equally contributed to this article as cofirst authors.
This research was supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (NRF-2017R1D1A1B03031934).
The authors have no conflicts of interest to disclose.
References
- [1].Chalam KV, Tillis T, Syed F, et al. Acute bilateral simultaneous angle closure glaucoma after topiramate administration: a case report. J Med Case Rep 2008;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chen SH, Karanjia R, Chevrier RL, et al. Bilateral acute angle closure glaucoma associated with hydrochlorothiazide-induced hyponatraemia. BMJ Case Rep 2014;2014: bcr2014206690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ezra D, Storoni M, Whitefield L. Simultaneous bilateral acute angle closure glaucoma following venlafaxine treatment. Eye (Lond) 2006;20:128–9. [DOI] [PubMed] [Google Scholar]
- [4].Kirwan JF, Subak-sharpe I, Teimory M. Bilateral acute angle closure glaucoma after administration of paroxetine. Br J Ophthalmol 1997;81:252–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lee WJ, Seong M. Bilateral simultaneous acute angle closure glaucoma following sexual intercourse aided by sildenafil citrate. J Korean Ophthalmol Soc 2011;52:1123–7. [Google Scholar]
- [6].Lai J, Liu D, Tham C, et al. Epidemiology of acute primary angle-closure glaucoma in the Hong Kong Chinese population: prospective study. Hong Kong Med J 2001;7:118–23. [PubMed] [Google Scholar]
- [7].Barrett V, Jordan T. Angle closure risk from proprietary medicines. Eye 2001;15:248–9. [DOI] [PubMed] [Google Scholar]
- [8].Rudkin AK, Gray TL, Awadalla M, et al. Bilateral simultaneous acute angle closure glaucoma precipitated by non-prescription cold and flu medication. Emerg Med Australas 2010;22:477–9. [DOI] [PubMed] [Google Scholar]
- [9].Hirata M, Tsujikawa A, Matsumoto A, et al. Macular choroidal thickness and volume in normal subjects measured by swept-source optical coherence tomography. Invest Ophthalmol Vis Sci 2011;52:4971–8. [DOI] [PubMed] [Google Scholar]
- [10].Tyler VE, Brady LR, Rpbbers JE. Pharmacognosy, vol. 9. 9th ed. Philadelphia: Lea & Fabiger; 1988. [Google Scholar]
- [11].Gurley BJ, Wang P, Gardner SF. Ephedrine-type alkaloid content of nutritional supplements containing Ephedra sinica (Ma-huang) as determined by high performance liquid chromatography. J Pharm Sci 1998;87:1547–53. [DOI] [PubMed] [Google Scholar]
- [12].Shekelle PG, Hardy ML, Morton SC, et al. Efficacy and safety of ephedra and ephedrine for weight loss and athletic performance: a meta-analysis. JAMA 2003;289:1537–45. [DOI] [PubMed] [Google Scholar]
- [13].Charatan F. Ephedra supplement may have contributed to sportsman's death. BMJ 2003;326:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Haller CA, Benowitz NL. Adverse cardiovascular and central nervous system events associated with dietary supplements containing ephedra alkaloids. N Engl J Med 2000;343:1833–8. [DOI] [PubMed] [Google Scholar]
- [15].US Food and Drug Administration. HHS acts reduce potential risks of dietary supplements containing ephedra. FDA News, February 28, 2003. [Google Scholar]
- [16].Cioffi G, Durcan F, Girkin C. Basic and Clinical Science Course, Section 10: Glaucoma. 1st ed.2011;New York: American Academy of Ophthalmology, 57–63. [Google Scholar]
- [17].Subak-Sharpe I, Low S, Nolan W, et al. Pharmacological and environmental factors in primary angle-closure glaucoma. Br Med Bull 2010;93:125–43. [DOI] [PubMed] [Google Scholar]
- [18].Lai JS, Gangwani RA. Medication-induced acute angle closure attack. Hong Kong Med J 2012;18:139–45. [PubMed] [Google Scholar]
- [19].Lachkar Y, Bouassida W. Drug-induced acute angle closure glaucoma. Curr Opin Ophthalmol 2007;18:129–33. [DOI] [PubMed] [Google Scholar]
- [20].Caronia RM, Sturm RT, Fastenberg DM, et al. Bilateral secondary angle-closure glaucoma as a complication of anticoagulation in a nanophthalmic patient. Am J Ophthalmol 1998;126:307–9. [DOI] [PubMed] [Google Scholar]
- [21].Wood WJ, Smith TR. Senile disciform macular degeneration complicated by massive hemorrhagic retinal detachment and angle closure glaucoma. Retina 1983;3:296–303. [DOI] [PubMed] [Google Scholar]
- [22].Ryan EH, Jr, Jampol LM. Drug-induced acute transient myopia with retinal folds. Retina 1985;6:220–3. [DOI] [PubMed] [Google Scholar]
- [23].Craig JE, Ong TJ, Louis DL, et al. Mechanism of topiramate-induced acute-onset myopia and angle closure glaucoma. Am J Ophthalmol 2004;137:193–5. [DOI] [PubMed] [Google Scholar]
- [24].Banta JT, Hoffman K, Budenz DL, et al. Presumed topiramate-induced bilateral acute angle-closure glaucoma. Am J Ophthalmol 2001;132:112–4. [DOI] [PubMed] [Google Scholar]
- [25].Medeiros FA, Zhang XY, Bernd AS, et al. Angle-closure glaucoma associated with ciliary body detachment in patients using topiramate. Arch Ophthalmol 2003;121:282–5. [DOI] [PubMed] [Google Scholar]
- [26].Abramson DH, Coleman DJ, Forbes M, et al. Pilocarpine: effect on the anterior chamber and lens thickness. Arch Ophthalmol 1972;87:615–20. [DOI] [PubMed] [Google Scholar]
- [27].Mapstone R. Acute shallowing of the anterior chamber. Br J Ophthalmol 1981;65:446–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fraunfelder F, Fraunfelder F, Keates EU. Topiramate-associated acute, bilateral, secondary angle-closure glaucoma. Ophthalmology 2004;111:109–11. [DOI] [PubMed] [Google Scholar]
- [29].Razeghinejad MR, Myers JS, Katz LJ. Iatrogenic glaucoma secondary to medications. Am J Med 2011;124:20–5. [DOI] [PubMed] [Google Scholar]


