Abstract
The combination of intermediate-dose cyclophosphamide (ID-CTX) and granulocyte colony-stimulating factor (G-CSF) fails to mobilize peripheral blood stem cells (PBSCs) in approximately 20% of treated patients with multiple myeloma (MM).
In this cohort study, patients with MM underwent PBSC mobilization with either an ID-CTX plus G-CSF plus recombinant human thrombopoietin (rhTPO) regimen (72 patients; TPO group), or an ID-CTX plus G-CSF regimen (70 patients; non-TPO group).
In the TPO group, the median CD34+ harvest was 5.36 × 106 per kg of body weight (0.50–22.39 × 106 per kg of body weight), with a harvest success rate of 91.7% (66/72), and an excellence rate of 55.6% (40/72). In the non-TPO group, the median CD34+ harvest was 3.30 × 106 per kg of body weight (0.20–21.14 × 106 per kg of body weight), with a harvest success rate of 75.7% (53/70), and an excellence rate of 25.7% (18/70). The median count of the CD34+ cells collected, success rate of collection, and excellence rate of collection were significantly higher in the TPO group than in the non-TPO group (P=.0001, P=.01, and P = .0001, respectively). Time to granulocyte and platelet engraftment was faster among patients in the TPO group than that in those from the non-TPO group. No platelet engraftment delay (>21 days) was observed among patients in the TPO group, while 3 patients in the non-TPO group displayed delayed platelet engraftment.
Adding rhTPO to the ID-CTX chemotherapy plus G-CSF regimen improved treatment efficacy in mobilizing PBSCs for autologous hematopoietic stem cell transplantation.
Keywords: autologous hematopoietic stem cell transplantation, mobilization, multiple myeloma, peripheral blood stem cells, thrombopoietin
1. Introduction
Multiple myeloma (MM) is a common hematological malignancy, which is routinely treated with autologous hematopoietic stem cell transplantation (ASCT). The application of new drugs in clinical practice, including bortezomib and lenalidomide, has significantly increased the overall survival in patients with MM,[1] elevating the treatment of MM into a “new-drug era.” However, ASCT remains an important component in the overall treatment strategy for MM in this new-drug era. Additional clinical studies are required to clarify how best to perform initial induction chemotherapy, as well as conditioning and maintenance therapies, following ASCT.[2,3]
One of the prerequisites for ASCT is the mobilization and collection of a sufficient number of autologous hematopoietic stem cells. Chemotherapy with cyclophosphamide (CTX) in combination with granulocyte colony-stimulating factor (G-CSF) is the most commonly used strategy for mobilization of peripheral blood stem cells (PBSCs) in patients with MM; however, suboptimal responses have been reported in some patients receiving this regimen.[4] The new stem cell-mobilization drugs that include plerixafor have the potential to further enhance mobilization of PBSCs by the combination of CTX and G-CSF, which can improve collection yield and purity[5,6]; unfortunately, many of these drugs are still unavailable in China. In addition, in countries where these novel drugs are available, their high price markedly limits their wide use. In order to identify a more effective strategy for mobilization of autologous PBSCs, we employed a combination of intermediate-dose CTX (ID-CTX) chemotherapy and G-CSF, with or without recombinant human thrombopoietin (rhTPO), to mobilize and collect PBSCs in 142 patients with MM. Herein, we report the findings from the use of these combinatorial regimens.
2. Methods
2.1. Patients
From January 2010 to June 2015, 142 patients with MM who underwent ID-CTX chemotherapy combined with G-CSF, with or without rhTPO, for the mobilization and collection of autologous PBSCs at our hospital were recruited. All patients met the diagnostic criteria of MM, as issued by the International Myeloma Working Group (IMWG).[7] The Durie-Salmon (DS) and International Staging System (ISS) criteria[8] were used for MM staging in these patients. Of the 142 patients, 81 and 61 were male and 61 female, respectively, with a median age of 51 years (range, 29–68 years). Seventy-two patients received ID-CTX and G-CSF along with rhTPO (referred to as the TPO group), and 70 patients received ID-CTX and G-CSF without rhTPO (the non-TPO group). Two patients in the non-TPO group received the rhTPO-containing strategy for secondary mobilization; in order to collect higher CD34+ cell counts, some patients received 2 mobilizations sequentially (the second mobilization was called “secondary mobilization”). All the patients provided informed consent. The mobilization and collection protocols were approved by the Beijing Chaoyang Hospital Ethics Committee.
2.2. Mobilization strategies
ID-CTX chemotherapy combined with G-CSF, with/without rhTPO, was used to mobilize PBSCs in patients with MM. For the ID-CTX chemotherapy, a dose of 2.5 g/m2 was administered over 2 days. In addition, 10 μg/kg/d of G-CSF was administered when the white blood cell (WBC) count was lower than 1 × 109/L following chemotherapy, and never given later than 6 days after chemotherapy. G-CSF was subcutaneously administered once daily, until the stem cell collection was completed. For patients who received rhTPO, 15,000 U/d was administered subcutaneously once daily, 6 days after chemotherapy, and until the stem cell collection was completed. All the methods were carried out in accordance with relevant guidelines and regulations.
2.3. Collection of the PBSCs
Post administration of the mobilization regimen, when the WBC count recovered rapidly to >4 × 109/L and the CD34+ count was ≥20 cells/μL, femoral vein catheterization was performed and stem cells were collected using COM.TEC (Fresenius Kabi AG, Bad Homburg, German); PBMCs were isolated using a lymphocyte isolating program. The percentage of CD34+ cells in the collected PBMCs was routinely measured. The general target of the collection was to obtain CD34+ cells with a count ≥5 × 106 cells/kg of body weight. The PBSCs were collected for 1–3 continuous days according to the hemogram, and the CD34+ cell count was determined. The collection was considered excellent and successful if the CD34+ cell count was ≥5 × 106 cells/kg of body weight and ≥2 × 106 cells/kg body weight, respectively. A CD34+ cell count <2 × 106 cells/kg of body weight was considered a collection failure.
2.4. Statistical analysis
IBM SPSS Statistics software (version 20.0, IBM Corp, Armonk, NY) was used for statistical analysis. A P value <.05 indicated significant difference for the various tests. Normality testing was applied for continuous data. For data with a normal distribution frequency, values were expressed as mean ± standard deviation (SD), and the Student t test was used to compare data between 2 groups. The Pearson χ2 test was used to compare rates between groups. By contrast, for data that was non-normally distributed, the median (range: minimum–maximum) was used to describe the data, and the rank-sum test was used to compare data between groups. The Mann–Whitney U test or the Kruskal–Wallis H test was used to compare nonparametric data between groups.
3. Results
3.1. Baseline characteristics of the patients
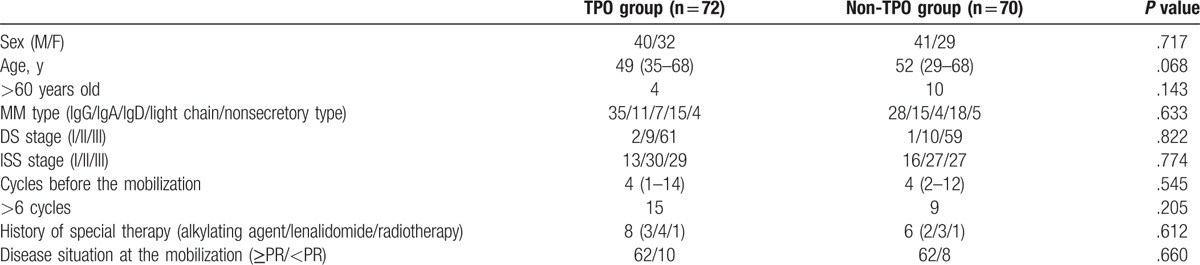
The baseline characteristics of the patients including age, sex, MM type, DS stage, ISS stage, treatment before mobilization, and disease status at mobilization (Table 1). The patients in the TPO and non-TPO groups had similar baseline characteristics. The general characteristics of the 2 patients in the non-TPO group who received a rhTPO-containing regimen in the secondary mobilization were as follows: patient 1 (male, aged 32 years, IgG-κ type, DS stage IIIA, and ISS stage I), received 4 cycles of chemotherapy with the TAD regimen (thalidomide, epirubicin, and dexamethasone) before mobilization, with the patient entering partial remission before mobilization; patient 2 (female, aged 58 years, λ light-chain type, DS stage IIIA, and ISS type III), received 4 cycles of chemotherapy with a PTD regimen (bortezomib, thalidomide, and dexamethasone) before mobilization, with the patient entering a phase of very good partial remission before mobilization.
Table 1.
General characteristics of the patients in the TPO and non-TPO groups.
3.2. Overall results of the mobilization and collection
A total of 165 mobilizations were performed for the included 142 patients. The median count of the CD34+ cells collected was 4.13 × 106 cells/kg of body weight (range, 0.20–22.39 × 106 cells/kg of body weight); the success rate of collection was 83.8% (119/142), while the excellence rate was 40.8% (58/142). Twenty-three of the 142 patients underwent secondary mobilization. Of the 23 secondary mobilizations, 20 were rescue mobilizations (performed due to collection-failure following the initial mobilization). Collection success was achieved in 15 of the 20 patients who underwent rescue mobilization. Collection failure was found in 23 of the 142 patients; among these patients, only 5 patients received secondary mobilization, while the remaining 18 did not receive secondary mobilization.
Of the 2 patients in the non-TPO group who received a TPO-containing regimen for secondary mobilization, the CD34+ cell count in the collected cells was 3.60 × 106 cells/kg of body weight (0.37 × 106 cells/kg of body weight after the initial mobilization and 3.23 × 106 cells/kg of body weight after the secondary mobilization) for the female patient, and 4.88 × 106 cells/kg of body weight (2.00 × 106 cells/kg of body weight after the initial mobilization and 2.88 × 106 cells/kg of body weight after the secondary mobilization) for the male patient. The collections in both patients were considered successful.
3.3. Results of the mobilization and collection in the TPO and non-TPO groups
Six patients in the TPO group failed collection; among these, 5 did not receive a secondary mobilization. Further, in the TPO group, a total of 5 patients received secondary mobilization; 4 of these were rescue mobilizations. Of the 4, collection success was achieved in 3 patients, including 1 that achieved an excellent collection. However, collection failure was still found in the other patient.
Collection failure was observed in 17 patients in the non-TPO group; of these 13 did not receive a secondary mobilization. Further, in the non-TPO group, a total of 18 patients underwent secondary mobilization, of which 16 were rescue mobilizations. Of the 16 patients, collection success was achieved in 12 (12/16), including 2 that achieved excellent collection (2/12). However, collection failure was still found in the remaining 4 patients (4/16).
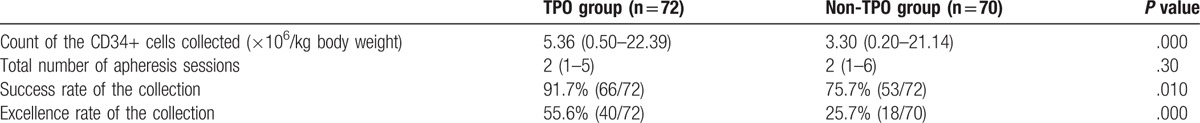
The number of CD34+ cells collected, as well as the success and excellence rates of collection, in patients from the TPO and non-TPO groups are shown in Table 2. When compared with the non-TPO group, the odds ratio (OR) of the success and excellence rates in the TPO group was 3.528 (95% CI: 1.300–9.576) and 3.611 (95% CI: 1.776–7.341), respectively.
Table 2.
Results of the mobilization and collection in the TPO and non-TPO groups.
3.4. Data of ASCT in the TPO and non-TPO groups
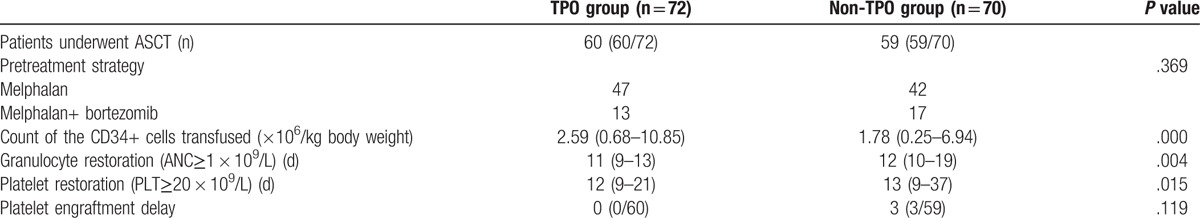
Of the 142 study patients, 119 underwent ASCT. The conditioning regimen for the patients before transplantation was melphalan (200 mg/m2, 2 days prior to ASCT), with some patients also receiving bortezomib (1.3 mg/m2, 6 and 2 days prior to ASCT, and 1 and 4 days post-ASCT). The median CD34+ cell count was 2.27 × 106 cells/kg of body weight (range, 0.25–10.85 × 106 cells/kg of body weight) in 119 patients. For all these ASCT patients, granulocyte and platelet engraftments were found at 11 days (range, 9–19 days) and 12 days (range, 9–37 days) after transplantation, respectively. Three patients had platelet engraftment delay (>21 days), and no transplantation-related mortality was found in any patients (Table 3). Among patients who underwent ASCT, the median-infused CD34+ cell count was significantly higher in patients from the TPO group than that in patients from the non-TPO group.
Table 3.
Characteristics of the ASCT in the TPO and non-TPO groups.
4. Discussion
Mobilization and collection of a sufficient number of CD34+ cells is a precondition for ASCT. Many researchers believe that ≥2 × 106 cells/kg of body weight is a safe cut-off value for the infusion of CD34+ cells for ASCT.[9] Recent studies show that increasing the dose of transplanted CD34+ cells could not only shorten the time to granulocyte and platelet engraftment after ASCT, but could also promote improved long-term platelet engraftment and help improve overall survival.[10,11] Therefore, the American Society for Blood and Marrow Transplantation recommends that for the collection of autologous PBSCs the target count of CD34+ cells should be ≥5 × 106/kg body weight.[12] Furthermore, as patients with MM have a high likelihood of requiring secondary ASCT, the IMWG recommends a target CD34+ cell count of 8–10 × 106/kg body weight for the collection of autologous PBSCs in patients with MM.[13] In light of these findings and recommendations, the Italian Group for Bone Marrow Transplantation (GITMO)[14] and the Mayo Clinic criteria[15] were used in the present study to evaluate the collection of autologous PBSCs in patients with MM. A harvest of ≥2 × 106 CD34+ cells/kg of body weight was considered a successful collection, and ≥5 × 106 Cd34+ cells/kg of body weight was considered an excellent collection; and <2 × 106 CD34+ cells/kg of body weight was considered a collection failure.
Previous studies show that collection failure may occur in about 25% of the patients who are otherwise eligible for ASCT.[4] The previous data collected at our center also show that among patients with MM who received a combination of chemotherapy and G-CSF for mobilization, the success and optimal rates of collection were 74.5% and 27.5%, respectively.[16] These findings suggest that new strategies are needed to improve the mobilization of CD34+ cells.
Plerixafor, a new mobilization-drug, when combined with G-CSF has demonstrated superior PBSC-mobilization, when compared with G-CSF alone[5,6]; however, plerixafor is still unavailable in China. In addition, the relatively high price of plerixafor has restrained its wide application, even in countries where it is accessible. Therefore, the commonly used mobilization strategy of CTX chemotherapy in combination with G-CSF was selected as the basic regimen going forward, and other stem cell-mobilizing drugs that are currently available in the Chinese market were used in combination to improve mobilization.
Our previous findings, using a relatively small sample of patients, showed that chemotherapy with ≥4.0 g/m2 of CTX could not improve the harvesting of CD34+ cells[16]; on the contrary, this chemotherapeutic strategy might increase the risk of infection after chemotherapy, possibly requiring a blood transfusion.[17] In light of our previous findings and those reported by other researchers,[16–18] the ID-CTX strategy, comprising of a total dose of 2.5 g/m2 CTX over 2 days, was selected in the present study as the chemotherapeutic regimen. Further, combining G-CSF with other cytokines has been demonstrated to improve the harvest of CD34+ cells.[4] However, in our previous study, we obtained less than ideal results when we tested G-CSF in combination with granulocyte macrophage colony-stimulating factor (GM-CSF) or IL-11 as the mobilization regimen.[16] Furthermore, TPO improves the proliferation of stem/progenitor cells in vitro, and maintains and even enhances the activities of hematopoietic stem cells in mice.[19] Studies in primates show that mobilization with G-CSF combined with PEGylated megakaryocyte growth and development factor (pegMGDF) improves the CD34+ cell count in the peripheral blood, suggesting that combinatorial regimens with TPO-receptor agonists could improve mobilization and collection of stem cells.[4] In addition, studies in patients with breast cancer or other solid tumors also show that using a combination of chemotherapy and G-CSF with rhTPO achieves a higher stem-cell yield.[20–22] Therefore, in the present study, a combination of ID-CTX chemotherapy and G-CSF with rhTPO was used for the mobilization of stem cells.
Previous studies have shown that several factors including age, number of chemotherapy cycles before collection, and treatment history (especially, chemotherapy with alkylating agents, and radiotherapy) affect stem-cell collection. In our study, these factors were similar between the TPO and non-TPO groups, and therefore the mobilization and collection results in these 2 groups were comparable. The success and excellence rates of collection were 91.7% and 55.6% in the TPO group, respectively, which were significantly higher than those observed in the non-TPO group (see in Table 2). The OR for both the success and excellence rates in the TPO group, when compared to the non-TPO group, was >3. Collectively, these findings show that using TPO along with the combination of ID-CTX chemotherapy and G-CSF mobilization could achieve a higher stem-cell yield.
The success rate in the TPO group in the present study was similar to the success rate reported by the Mayo Clinic (86%); however, the excellence rate was comparatively lower in the current study (55.6% vs 70%).[15] The reasons for this difference might include the following: In the Mayo Clinic study, the frequency of patients who underwent secondary mobilization was 15.7% (157/997). Of these, 63.7% underwent secondary mobilizations due to suboptimal initial mobilization (CD34+ count: ≥2 × 106 cells/kg of body weight, but <5 × 106 cells/kg of body weight), rather than mobilization failure. Furthermore, the number of cycles of chemotherapy in these patients, after the first partial remission and within 4 months after induction therapy, was relatively fewer. By contrast, most patients in the present study underwent secondary mobilization, and this was due to collection failure but not suboptimal collection after the initial mobilization. In addition, a relatively large proportion of the patients (20.8%, 15/72) underwent more than 6 cycles of chemotherapy before mobilization in the present study, and over half (6/10) of the patients refused secondary mobilization, both of which affected the overall success and excellence rates.
Our data showed that after ASCT, granulocyte and platelet engraftment in the TPO group was faster than that observed in the non-TPO group. No platelet-engraftment delay was found in the TPO group, while 3 patients in the non-TPO group exhibited a delay in platelet engraftment. However, the difference in the delay between the 2 groups was not statistically significant (P = .119) (see in Table 3). This could be associated with the fact that for patients administered ID-CTX chemotherapy plus G-CSF with rhTPO for mobilization, more autologous PBSCs could be harvested, and thus sufficient CD34+ cells could be infused subsequently in ASCT. Therefore, the granulocyte and platelet engraftment was quicker.[10,11] The dose of CD34+ cells infused into the patients in the non-TPO group with delayed platelet engraftment was 0.25–0.64 × 106 cells/kg body weight; this relatively lower dose might have contributed to the engraftment delay.[10,11] The lowest dose of CD34+ cells infused into patients from the TPO group was 0.68 × 106 cells/kg body weight, suggesting that the relatively higher dose of CD34+ cells that were infused might have prevented a delay in platelet engraftment for these patients.
These findings suggest that for patients with MM deemed eligible for transplantation or potentially requiring ASCT, the target-count of CD34+ cells in the collected PBSCs should be increased (ideal count of 8–10 × 106 and minimum count of ≥ 5 × 106 cells/kg body weight), with the aim of achieving a successful ASCT. For the patients who achieved successful collection after the initial mobilization, but did not reach the target count, a secondary mobilization and collection should be considered.
In summary, our findings show that the combination of ID-CTX chemotherapy and G-CSF, with rhTPO is an effective mobilization strategy; however, collection failure was still detected in about 5% of the patients. These findings suggest that for newly diagnosed patients that were eligible for transplantation, adverse factors that may affect treatment (i.e., numbers of treatment cycles, type of drugs, and treatment regimens) and the subsequent stem-cell collection should be avoided. In addition, more clinical studies are needed to help accurately identify the patients who are at a high risk for poor mobilization to develop more effective regimens for mobilization in these patients.
Footnotes
Abbreviations: ASCT = autologous hematopoietic stem cell transplantation, CTX = cyclophosphamide, DS = Durie–Salmon, G-CSF = granulocyte colony-stimulating factor, ID-CTX = intermediate-dose cyclophosphamide, IMWG = International Myeloma Working Group, ISS = International Staging System, MM = multiple myeloma, PBSCs = peripheral blood stem cells, rhTPO = recombinant human thrombopoietin.
Author contributions: Dr GW and Prof WC wrote the main manuscript text, Dr GW prepared Tables 1–3. Dr GW, Prof WC, Ms YW, Mr YL, Ms YL, and Ms AL conducted the mobilization and collection protocols. All authors reviewed the manuscript.
This study has did not receive funding support. All procedures performed involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
The authors have no conflicts of interest to disclose.
References
- [1].Rajkumar SV. Treatment of multiple myeloma. Nat Rev Clin Oncol 2011;8:479–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Moreau P, Attal M. All transplantation-eligible patients with myeloma should receive ASCT in first response. Hematology Am Soc Hematol Educ Program 2014;2014:250–4. [DOI] [PubMed] [Google Scholar]
- [3].Mohty M, Richardson PG, McCarthy PL, et al. Consolidation and maintenance therapy for multiple myeloma after autologous transplantation: where do we stand? Bone Marrow Transplant 2015;50:1024–9. [DOI] [PubMed] [Google Scholar]
- [4].Larsen SR, Chng K, Battah F, et al. Improved granulocyte colony-stimulating factor mobilization of hemopoietic progenitors using cytokine combinations in primates. Stem Cells 2008;26:2974–80. [DOI] [PubMed] [Google Scholar]
- [5].DiPersio JF, Stadtmauer EA, Nademanee A, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood 2009;113:5720–6. [DOI] [PubMed] [Google Scholar]
- [6].Milone G, Martino M, Spadaro A, et al. Plerixafor on-demand combined with chemotherapy and granulocyte colony-stimulating factor: significant improvement in peripheral blood stem cells mobilization and harvest with no increase in costs. Br J Haematol 2014;164:113–23. [DOI] [PubMed] [Google Scholar]
- [7].Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia 2009;23:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol 2005;23:3412–20. [DOI] [PubMed] [Google Scholar]
- [9].Wuchter P, Ran D, Bruckner T, et al. Poor mobilization of hematopoietic stem cells—definitions, incidence, risk factors, and impact on outcome of autologous transplantation. Biol Blood Marrow Transplant 2010;16:490–9. [DOI] [PubMed] [Google Scholar]
- [10].Bensinger W, Appelbaum F, Rowley S, et al. Factors that influence collection and engraftment of autologous peripheral blood stem cells. J Clin Oncol 1995;13:2547–55. [DOI] [PubMed] [Google Scholar]
- [11].Stiff PJ, Micallef I, Nademanee AP, et al. Transplanted CD34(+) cell dose is associated with long-term platelet count recovery following autologous peripheral blood stem cell transplant in patients with non-Hodgkin lymphoma or multiple myeloma. Biol Blood Marrow Transplant 2011;17:1146–53. [DOI] [PubMed] [Google Scholar]
- [12].Duong HK, Savani BN, Copelan E, et al. Peripheral blood progenitor cell mobilization for autologous and allogeneic hematopoietic cell transplantation: guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2014;20:1262–73. [DOI] [PubMed] [Google Scholar]
- [13].Cavo M, Rajkumar SV, Palumbo A, et al. International Myeloma Working Group consensus approach to the treatment of multiple myeloma patients who are candidates for autologous stem cell transplantation. Blood 2011;117:6063–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Olivieri A, Marchetti M, Lemoli R, et al. Proposed definition of ‘poor mobilizer’ in lymphoma and multiple myeloma: an analytic hierarchy process by ad hoc working group Gruppo Italiano Trapianto di Midollo Osseo. Bone Marrow Transplant 2012;47:342–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gertz MA, Wolf RC, Micallef IN, et al. Clinical impact and resource utilization after stem cell mobilization failure in patients with multiple myeloma and lymphoma. Bone Marrow Transplant 2010;45:1396–403. [DOI] [PubMed] [Google Scholar]
- [16].Wang GR, Chen WM, Li YC, et al. A retrospective analysis of autologous peripheral blood hematopoietic stem cell mobilizations and collections in 149 multiple myeloma patients. Zhonghua Xue Ye Xue Za Zhi 2015;36:367–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fitoussi O, Perreau V, Boiron JM, et al. A comparison of toxicity following two different doses of cyclophosphamide for mobilization of peripheral blood progenitor cells in 116 multiple myeloma patients. Bone Marrow Transplant 2001;27:837–42. [DOI] [PubMed] [Google Scholar]
- [18].Hamadani M, Kochuparambil ST, Osman S, et al. Intermediate-dose versus low-dose cyclophosphamide and granulocyte colony-stimulating factor for peripheral blood stem cell mobilization in patients with multiple myeloma treated with novel induction therapies. Biol Blood Marrow Transplant 2012;18:1128–35. [DOI] [PubMed] [Google Scholar]
- [19].Duchez P, Chevaleyre J, Vlaski M, et al. Thrombopoietin to replace megakaryocyte-derived growth factor: impact on stem and progenitor cells during ex vivo expansion of CD34+ cells mobilized in peripheral blood. Transfusion 2011;51:313–8. [DOI] [PubMed] [Google Scholar]
- [20].Gajewski JL, Rondon G, Donato ML, et al. Use of thrombopoietin in combination with chemotherapy and granulocyte colony-stimulating factor for peripheral blood progenitor cell mobilization. Biol Blood Marrow Transplant 2002;8:550–6. [DOI] [PubMed] [Google Scholar]
- [21].Somlo G, Sniecinski I, Ter Veer A, et al. Recombinant human thrombopoietin in combination with granulocyte colony-stimulating factor enhances mobilization of peripheral blood progenitor cells, increases peripheral blood platelet concentration, and accelerates hematopoietic recovery following high-dose chemotherapy. Blood 1999;93:2798–806. [PubMed] [Google Scholar]
- [22].Linker C, Anderlini P, Herzig R, et al. Recombinant human thrombopoietin augments mobilization of peripheral blood progenitor cells for autologous transplantation. Biol Blood Marrow Transplant 2003;9:405–13. [DOI] [PubMed] [Google Scholar]


