Abstract
Rationale:
Hepatic portal venous gas (HPVG) is a very rare radiological finding that occurs when gas enters the portal venous system. HGVG can be caused by various diseases, with the most common being intestinal ischemia or necrosis. While there are few reports of HPVG associated with colon cancer, we report a case of HPVG associated with advanced colon cancer.
Diagnosis:
The diagnosis of this patient was HPVG caused by colon cancer.
Interventions:
Left colon cancer resection, pancreatic tail resection, splenectomy, and transverse colostomy were performed.
Outcomes:
The patient recovered well, and postoperative paraffin pathology confirmed that the resected tumor was colon cancer.
Lessons:
Abdominal computed tomography is an effective method for diagnosing and monitoring HPVG. Klebsiella pneumonia is a potential gas-producing microorganism associated with HPVG, which may be confirmed by Blood culture or drainage culture. The prognosis of HPVG is closely related to the underlying pathology. Surgery should be performed early when there are signs of intestinal ischemia, necrosis, or perforation.
Keywords: hepatic portal venous gas, colon cancer, Klebsiella pneumonia, perforation
1. Introduction
Hepatic portal venous gas (HPVG) is a rare radiological finding, defined by the abnormal accumulation of gas in the portal system. HPVG is most often seen in patients with intestinal ischemia and necrosis, which is associated with a poor prognosis and a high mortality rate.[1] With the extensive use of computed tomography (CT), more and more cases with HPVG have been reported.[2] However, to the best of our knowledge, few cases have been reported in patients with malignant tumors. Here, we report a case of HPVG in a patient with advanced colon cancer who received emergency surgery due to bowel perforation.
2. Case report
A 65-year-old man was initially referred to our department at the China-Japan Union Hospital of Jilin University for an incomplete intestinal obstruction. He presented with left upper abdominal pain and reduced defecation for 5 days. He had no surgical history and no chronic diseases, such as hypertension or diabetes. His performance status was good, and his initial body temperature was 36.9°C, his pulse rate was 72 beats/min, his breath rate was 20 breaths/min, and his blood pressure was 93/58 mmHg. A physical examination revealed slight abdominal distention with mild tenderness of the left upper abdomen, but there was no muscle guarding or rebound tenderness. A palpable mass could be felt on the left upper abdomen, which was about 6 × 6 cm in size. The mass was hard and could be pushed. Bowel sounds were active.
Blood tests showed that the patient's white blood cell count was 13.25 × 109/L (4.00–10.00), the neutrophil ratio was 87.3% (50.0–70.0), the platelets were 47 × 109/L (100–300), alanine aminotransferase (ALT) was 45.90 IU/L (5.00–40.00), aspartate aminotransferase (AST) was 49.56 IU/L (8.00–40.00), CA 199 was 40.57 U/mL (0.10–37.00), and CA 724 was 11.95 U/mL (0.20–6.90). An abdominal CT scan noted classic findings for HPVG, which showed as a large amount of gas in the hepatic portal vein and branches, particularly at the right lobe (Fig. 1A and B). Gas was also present in the superior mesenteric vein and splenic vein (Fig. 1C and D). A space-occupying lesion was also found in the splenic flexure of the colon, which was considered to be colon cancer that might have invaded the tail of the pancreas and the spleen (Fig. 1E). As the patient was in good general health and he needed to have a colonoscopy, we chose to treat him using conservative management with total parenteral nutrition and empiric antibiotic therapy (sulbenicillin 8 g/day and metronidazole 2 g/day). The patient ate some solid food without authorization on the second day after admission. He then developed acute abdominal pain and had a fever of 39.0°C and chills. A physical examination revealed tenderness all over the abdomen, and signs of peritoneal irritation were found. Blood tests showed that the patient's white blood cell count was 13.97 × 109/L, the neutrophil ratio was 87.3%, and the platelets were 62 × 109/L. Blood culture showed the growth of Klebsiella pneumonia (results were returned 5 days later). An abdominal CT showed that the amount of gas in hepatic portal vein had decreased visibly (Fig. 2A); however, emerging intra-abdominal gas was found around the spleen, which suggested the possibility of intestinal perforation (Fig. 2B). Considering that there were indications for surgery, an emergency exploratory laparotomy was immediately performed. We found about 200 mL of pus in the abdominal cavity during the operation, and a tumor was seen in the splenic flexure of the colon that had invaded the pancreas and spleen. An abscess was formed between the tumor and the spleen. Multiple metastatic nodules were seen at the root of the mesentery. The proximal colon was dilated and the distal colon was empty. Left colon cancer resection, pancreatic tail resection, splenectomy, and transverse colostomy were performed. The specimens are shown in Figure 3. Gastrointestinal decompression, total parenteral nutrition, and intravenous antibiotic therapy were given after the operation. An abdomen CT showed that the portal venous gas had disappeared 1 week later (Fig. 4). Postoperative paraffin pathology confirmed that the resected tumor was colon cancer (Fig. 5), and the tumor, nodes, and metastasis (TNM) staging was T4bN2bM0. The patient recovered well and was discharged 2 weeks after the operation.
Figure 1.
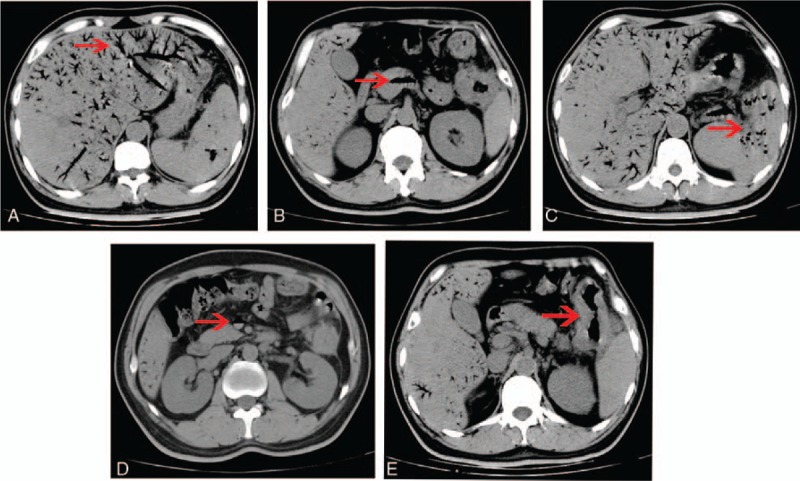
Abdominal CT showing (A) extensive gas within the branches of hepatic vein (arrow), (B) gas in the hepatic portal vein (arrow), (C) gas in the splenic vein (arrow), (D) gas in the superior mesenteric vein, and (E) tumor in the splenic flexure of colon which invaded the tail of the pancreas and spleen (arrow). CT = computed tomography.
Figure 2.
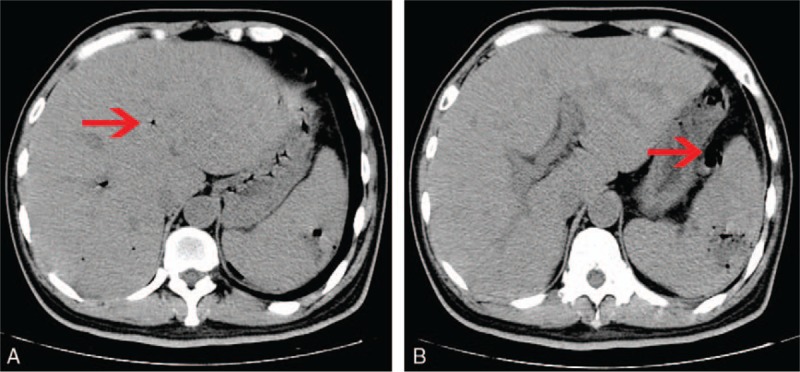
Abdominal CT performed before operation showing (A) the gas in hepatic portal vein decreased obviously (arrow), (B) emerging intra-abdominal gas around the spleen (arrow). CT = computed tomography.
Figure 3.
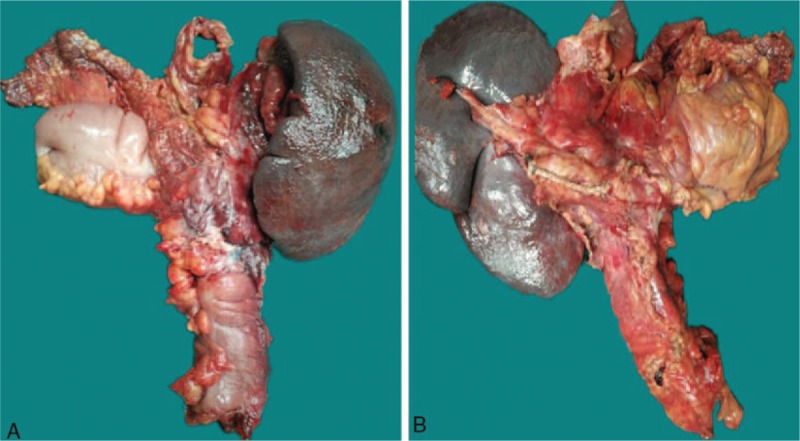
The specimens of left colon cancer resection, pancreatic tail resection, and splenectomy. (A) anterior view, (B) posterior view.
Figure 4.
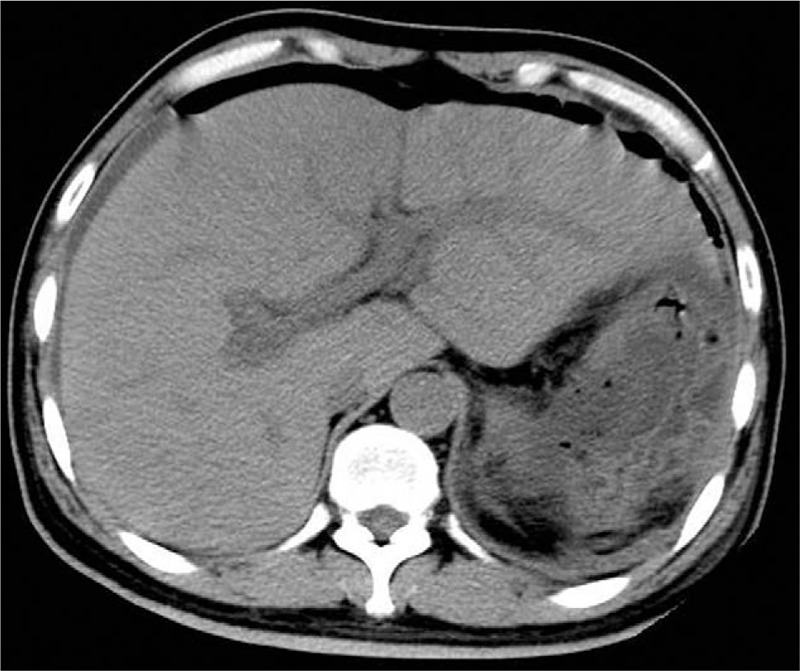
Abdominal CT performed 1 week after operation showing the gas in the hepatic portal vein disappeared completely. CT = computed tomography.
Figure 5.
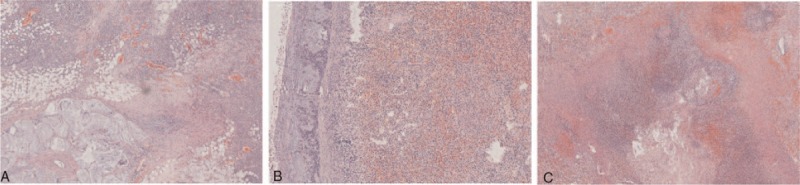
Postoperative paraffin pathology showing (A) colon cancer, (B) tumor invaded the spleen, and (C) the abscess between colon and spleen.
3. Discussion
HPVG is not an independent disease. It is usually accompanied by digestive tract diseases, and it often has a relatively short duration. HPVG was first described by Wolfe and Evans in 1955 in infants with fatal necrotizing enterocolitis.[3] The first case of HPVG in an adult was reported in 1960 by Susman and Senturia in a patient with superior mesenteric artery thrombosis.[4] Since then, HPVG has been reported to occur with a variety of abdominal diseases, such as inflammatory bowel disease, bowel ischemia, bowel obstruction, gastric ulcer, closed abdominal trauma, intra-abdominal abscess, and diverticulitis.[1] HPVG is a critical condition as the mortality was as high as 75% in a 1978 review of 64 cases.[5] A 2001 review of 182 cases revealed an overall mortality of 39%, in which only 3% of the HPVG cases were associated with an intraperitoneal tumor.[6] Thus, cases of HPVG complicated by colon cancer are very rare. Recently, Ginesu et al reported 1 case of HPVG in a patient who underwent a left colectomy for descending colon cancer; the patient was treated conservatively, and his symptoms resolved.[7] In addition, 2 cases of HPVG occurred during the course of chemotherapy in advanced colorectal cancer patients.[8,9] In our case, HPVG was found before the operation, and the patient was surgically cured.
HPVG is mainly diagnosed by x-ray, abdominal CT, and ultrasonography. The criterion of x-ray for HPVG is a branching radiolucency extending to within 2 cm of the liver capsule. However, the sensitivity is low, detection is difficult, and it is easily overlooked.[10] Ultrasonography and CT scan are superior to abdominal radiographs in identifying HPVG. Ultrasonography is very sensitive for HPVG detection. Moving echogenic particles in the portal venous system and highly echogenic patches within the hepatic parenchyma are the typical ultrasonographic features of HPVG.[11] However, the use of ultrasound is limited because it is more dependent on the operator's experience and it is difficult to detect the etiology. The CT scan has a higher sensitivity to diagnosis HPVG, and it can help to detect underlying diseases.[12,13] Case reports of HPVG associated with benign etiologies have increased with the wide use of CT, which might be one reason for the decrease in mortality of HPVG.[7] In addition, CT can predict the prognosis of HPVG. A recent study showed that outside of shock situations, HPVG involving 2 or fewer hepatic segments without pneumatosis intestinalis had a good predicted outcome.[2] The typical radiographic pattern of CT was represented by tubular lucency branching from the portal vein to the liver capsule, and the gas lucency can be noted even 2 cm beneath the liver capsule.[14] In this case, HPVG was detected by abdominal CT and a malignant tumor in the splenic flexure of the colon was found, which provided the basis for follow-up treatment. Therefore, an abdominal CT is an effective method for diagnosing and monitoring HPVG, and it should be used as the primary diagnostic tool.
At present, the mechanism for HPVG is not well understood. There are 2 main theories proposed for the pathophysiologic etiology of HPVG: mechanical versus bacterial. Intestinal obstruction, intestinal ischemia, inflammatory bowel disease, gastrointestinal neoplasms, and colonoscopy can damage the intestinal mucosa. Such an injury may provide a portal for intraluminal gas to enter the intestinal wall and eventually enter the portal venous system. However, HPVG has been associated with an intra-abdominal abscess without mucosal damage in some cases. In these circumstances, the gas in the portal venous was produced by a gas-forming organism.[15,16] We think there are 2 possible reasons why HPVG was so pronounced in this case. First, the blood culture and the intraoperative pus culture showed the growth of K pneumonia, which is an opportunistic pathogen. K pneumonia can produce gas and acid by decomposing glucose. K pneumonia is a common pathogen of pyogenic liver abscesses. The incidence of pyogenic liver abscesses caused by K pneumonia has been increasing in Asian countries and the United States.[17,18] A case of emphysematous gastritis caused by K pneumonia has been reported by Al-Jundi and Shebl, and gas was found in both the stomach wall and the liver portal venous by CT scan.[19] In our case, an abscess caused by K pneumonia was formed between the colon cancer and the spleen. K pneumonia propagated in the intestinal wall and portal vein system, which produced gas leading to HPVG. Second, this patient suffered from an incomplete intestinal obstruction. His intestinal mucosa was damaged due to the increased pressure in the intestine, the expansion of the intestinal canal, and ischemic necrosis of the tumor. As a result, the gas in the intestine could enter the portal system. Based on the above analysis, we speculate that the significant reduction of gas before the operation was also associated with 2 factors. First, the use of antibiotics inhibited the reproduction of K pneumonia. Second, perforation of the intestine reduced the internal pressure of the intestine and caused some of the gas to enter the abdominal cavity.
HPVG requires conservative treatment and surgical treatment, which should be based on the underlying cause of the disease. Nelson et al designed an “ABC” algorithm, which stressed that operative treatment, close monitoring, and medical treatment should be carried out according to the patient's condition.[16] Surgical intervention is generally recommended for cases of intestinal ischemia and necrosis, while close monitoring is advised in patients with a disease of benign etiology and nonischemic conditions.[14] Wayne et al established a scoring system for HPVG, which could help to determine whether mesenteric ischemia occurred.[15] In our case, the underlying cause of HPVG was colon cancer. At first, the patient was in good general condition, so he was treated conservatively while waiting for further examination. An intestinal perforation occurred during the treatment, so emergency surgery was immediately performed. HPVG disappeared completely 1 week after the operation and the patient recovered well, so the treatment for this patient was timely and appropriate. At the same time, we are also thinking that it may be better to operate before intestinal perforation, which will avoid the risk of severe abdominal infection, pancreatic leakage, and other complications associated with intestinal perforation.
Our case has the following characteristics. It is a case of adult HPVG, and the combined disease was colon cancer. The imaging performance was very typical, and large volumes of gas were found in the hepatic portal vein, splenic vein, and superior mesenteric vein. The gas may be produced by K pneumonia. The gas was significantly reduced after intestinal perforation. The prognosis of this patient was good after surgery.
4. Summary
Various diseases can cause HPVG, including colon cancer, although it is rare. The prognosis of HPVG is closely related to the underlying pathology. Abdominal CT is an effective method for diagnosing and monitoring HPVG. Blood culture or drainage culture is also an important method that can help to identify the underlying cause. K pneumonia is a potential gas-producing microorganism that may be associated with HPVG. We must attach great importance to HPVG in clinical practice, and surgery should be performed early when there are signs of intestinal ischemia, necrosis, or perforation.
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Footnotes
Abbreviations: CT = computed tomography, HPVG = Hepatic portal venous gas, TNM = tumor, nodes, and metastasis.
Informed consent has been obtained in writing from the patient and is available for review by the editor.
The authors report no conflicts of interest.
References
- [1].Alqahtani S, Coffin CS, Burak K, et al. Hepatic portal venous gas: a report of two cases and a review of the epidemiology, pathogenesis, diagnosis and approach to management. Can J Gastroenterol 2007;21:309–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Moussa M, Marzouk I, Abdelmoula K, et al. Role of Computed tomography in predicting prognosis of Hepatic portal venous gas. Int J Surg Case Rep 2017;30:177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wolfe JN, Evans WA. Gas in the portal veins of the liver in infants; a roentgenographic demonstration with postmortem anatomical correlation. Am J Roentgenol Radium Ther Nucl Med 1955;74:486–8. [PubMed] [Google Scholar]
- [4].Susman N, Senturia HR. Gas embolization of the portal venous system. Am J Roentgenol Radium Ther Nucl Med 1960;83:847–50. [PubMed] [Google Scholar]
- [5].Liebman PR, Patten MT, Manny J, et al. Hepatic–portal venous gas in adults: etiology, pathophysiology and clinical significance. Ann Surg 1978;187:281–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kinoshita H, Shinozaki M, Tanimura H, et al. Clinical features and management of hepatic portal venous gas: four case reports and cumulative review of the literature. Arch Surg 2001;136:1410–4. [DOI] [PubMed] [Google Scholar]
- [7].Ginesu GC, Barmina M, Cossu ML, et al. Conservative approach to hepatic portal venous gas: a case report. Int J Surg Case Rep 2017;30:183–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Platania M, Valeri B, Marchiano A, et al. Fatal case of hepatic portal venous gas following palliative stenting and chemotherapy for occlusive advanced colorectal cancer. Int J Colorectal Dis 2015;30:429–30. [DOI] [PubMed] [Google Scholar]
- [9].Zalinski S, Scatton O, Jacqmin S, et al. Portal venous gas following chemotherapy for colorectal cancer liver metastasis. Eur J Surg Oncol 2009;35:557–60. [DOI] [PubMed] [Google Scholar]
- [10].Gosink BB. Intrahepatic gas: differential diagnosis. AJR Am J Roentgenol 1981;137:763–7. [DOI] [PubMed] [Google Scholar]
- [11].Pan HB, Huang JS, Yang TL, et al. Hepatic portal venous gas in ultrasonogram–benign or noxious. Ultrasound Med Biol 2007;33:1179–83. [DOI] [PubMed] [Google Scholar]
- [12].Schindera ST, Triller J, Vock P, et al. Detection of hepatic portal venous gas: its clinical impact and outcome. Emerg Radiol 2006;12:164–70. [DOI] [PubMed] [Google Scholar]
- [13].Chan SC, Wan YL, Cheung YC, et al. Computed tomography findings in fatal cases of enormous hepatic portal venous gas. World J Gastroenterol 2005;11:2953–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Abboud B, El Hachem J, Yazbeck T, et al. Hepatic portal venous gas: physiopathology, etiology, prognosis and treatment. World J Gastroenterol 2009;15:3585–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wayne E, Ough M, Wu A, et al. Management algorithm for pneumatosis intestinalis and portal venous gas: treatment and outcome of 88 consecutive cases. J Gastrointest Surg 2010;14:437–48. [DOI] [PubMed] [Google Scholar]
- [16].Nelson AL, Millington TM, Sahani D, et al. Hepatic portal venous gas: the ABCs of management. Arch Surg 2009;144:575–81. discussion 81. [DOI] [PubMed] [Google Scholar]
- [17].Basu S. Klebsiella pneumoniae: an emerging pathogen of pyogenic liver abscess. Oman Med J 2009;24:131–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Qu K, Liu C, Wang ZX, et al. Pyogenic liver abscesses associated with nonmetastatic colorectal cancers: an increasing problem in Eastern Asia. World J Gastroenterol 2012;18:2948–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Al-Jundi W, Shebl A. Emphysematous gastritis: case report and literature review. Int J Surg 2008;6:e63–6. [DOI] [PubMed] [Google Scholar]


