Abstract
The condition known as cryptorchidism – undescended testis – is one of the most common congenital abnormalities found among males, and is one of the few known risk factors for testicular cancer (TC). Like testicular cancer, the key exposures in the occurrence of cryptorchidism remain elusive. Testicular descent is thought to occur during two hormonally-controlled phases – between 8–15 weeks and 25–35 weeks gestation – and while it is clear that a failure of testes to descend permanently is likely due to disruptions to one or both of these phases, the cause(s) and mechanism(s) of such disruption are still unclear. In this manuscript, we review the broad range of putative risk factors that have been evaluated in relation to the development of cryptorchidism to date, discuss their plausibility, and make suggestions regarding further approaches to understand aetiology. There are few exposures for which there is consistent evidence of an association with cryptorchidism; and in those cases where evidence appears unequivocal – for example, the relationship between cryptorchidism and gestational measures such as low birth weight – the measured exposure is likely to be a surrogate for the true causal exposure. The relative importance of each risk factor may vary considerably between mother/son pairs depending on an array of genetic, maternal, placental and foetal factors – all of which could vary between regions.
Introduction
Descent of the testes into the temperate environment of the scrotum represents a vital developmental step on the path toward successful human reproduction. Cryptorchidism – failure of one or both testes to permanently descend, otherwise referred to as undescended testis – is one of the most common congenital abnormalities among males:1 approximately 1–9% of all males are born with at least one cryptorchid testis.2–8 While about half of these cases will spontaneously descend during the first three months of life, approximately 1% of all males will remain cryptorchid at the end of their first year.3,8,9
Cryptorchidism is one of the few known risk factors for testicular cancer:10 men with a history of cryptorchidism have a three-11 to four-fold 12 increased risk of testicular cancer compared to those with no history of cryptorchidism. It is estimated that 5–9% of all men who develop testicular cancer have a history of persistent cryptorchidism.13,14 Cryptorchidism is also a risk factor for sub-fertility: it is estimated that men with a history of cryptorchidism are twice as likely to be sub-fertile compared to those without cryptorchidism.15 Cryptorchidism is also one component of theorised Testicular Dysgenesis Syndrome, a syndrome in which four individual conditions (cryptorchidism, TC, hypospadias and sub-fertility) possibly share the same prenatal risk factors.16 Hypospadias – a congenital anomaly where the urethra terminates on the underside of the penis, rather than at the tip of the glans 17 – is relatively more common among those with cryptorchidism; however, in absolute terms only a small proportion of boys born with cryptorchidism will also have hypospadias (approximately 2%).18 In rare cases, the testis will disappear due to torsion or some other unknown cause – a condition called Testicular Regression Syndrome, also known as ‘vanishing testis’.19
The primary treatment for cryptorchidism is the surgical repositioning of the cryptorchid testis into the scrotum, a procedure known as orchidopexy.20,21 In cryptorchid neonates it is recommended that this procedure be performed within one year of birth, while cases of ‘acquired’ cryptorchidism in early childhood should be treated immediately.22,23 Performing orchidopexy before puberty reduces the subsequent risk of testicular cancer relative to later correction:24 for example, cryptorchid boys who undergo orchidopexy after the age of 12 (or not at all) are 2–6 times more likely to have testicular cancer compared to those who receive the corrective treatment before the age of 12.25
The relationship between cryptorchidism and testicular cancer remains unclear. In unilateral cryptorchidism, the increased risk of testicular cancer is much weaker in the contralateral than in the ipsilateral testis (relative risk [RR] of testicular cancer in cryptorchid testis: 6.33, 95% CI 4.30–9.31; contralateral testis RR 1.74, 95% CI 1.01–2.98) 26 – suggesting that the link between cryptorchidism and testicular cancer is mostly related to variable manifestation of a shared risk factor, rather than a more generalised developmental abnormality that equally affects both testes.25,27 A testicular defect acquired in the intrauterine environment may be linked to both the development of cryptorchidism and to the risk of neoplasia within the cryptorchid testes, with a consequent increased risk of testicular cancer in later life.12,28
Temporal and geographic variation in disease occurrence
In terms of changes in the occurrence of cryptorchidism over time, we observe conflicting evidence – with some reports of increasing occurrence in recent decades,7,29,30 and others reporting stable or decreasing occurrence.2,31–33 This variation in temporal observations – as well as the wide variation in cryptorchidism occurrence as reported in the literature (1–9%) – is at least partially an indication of the wide geographical variation that has been observed between countries. For example, boys born in Denmark are more than four times as likely to have cryptorchidism as boys born in Finland, while boys born in Lithuania have an intermediate prevalence between Denmark and Finland.5,7 The reason for this geographic variation remains poorly understood – but at the very least suggests that the factors that predispose an individual to cryptorchidism are unevenly distributed between population groups.
Congenital vs. ‘acquired’ cryptorchidism
For the purposes of this manuscript, we define cryptorchidism as a congenital condition 34,35 – not because of the timing of condition presentation and/or discovery (which can vary), but rather the likely timing of disease aetiology. We acknowledge that there are several variations in terms of the former: a boy may present with/be discovered to have cryptorchidism at or soon after birth (‘congenital’ cryptorchidism, in the traditional sense); a boy may have normally-descended testes at birth, one or both of which subsequently ascend (‘acensus testis’), or a boy may have one or both initially-cryptorchid testes that spontaneously descend and then re-ascend (‘recurrent’ cryptorchidism).36 However for the purposes of this manuscript we consider the non-permanent descent of testes as being congenital in origin 37 – regardless of when this condition presents.
These variations in condition presentation and/or discovery may have important ramifications for measurement of disease occurrence. Firstly, measurements of disease prevalence at two years of age will exclude boys whose initially-cryptorchid testis have spontaneously descended; likewise, measurements taken at birth will exclude boys with normally-descended testis that subsequently ascend spontaneously. Secondly, cryptorchidism diagnosis can be performed via either clinical examination or be based on receipt of orchidopexy – with orchidopexy-confirmed cryptorchidism more likely to reflect ‘persistent’ cryptorchidism cases than clinically-diagnosed cryptorchidism.38 Thus, as well as reflecting geographic variance, the wide variation in disease occurrence (1–9%) may also reflect the difficulty in ascribing true disease prevalence when the condition occurs across a severity spectrum.
The anatomy of testicular descent
Comprehensive reviews of the process of testicular descent can be found elsewhere.18,39 Briefly, the primary factors that drive testicular descent during prenatal/perinatal life are thought to occur during two hormonally-controlled phases – between 8–15 weeks and 25–35 weeks gestation (Figure 1). A failure of one or both testes to descend permanently must be caused by some disruption to these phases. In this manuscript, we review the broad range of putative risk factors hypothesised to be associated with the development of cryptorchidism to date, discuss their plausibility with respect to influence on descent of the testis in utero, and make suggestions regarding those risk factors that require further investigation.
Figure 1. Descent of the testis.
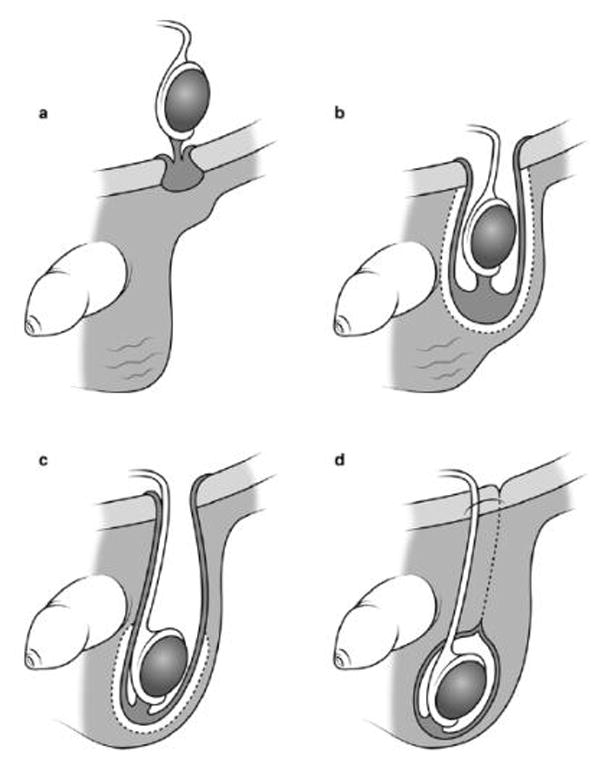
(Modified with permission from Hutson JM, Thorup JM, Beasley SW. Descent of the testis. 2nd ed: Springer, 201618) a) After gestational week 8, the developing testes in males are found lying in an intra-abdominal position. Sometime between the 8h and 15th week of gestation, the testes begin the first phase of their descent. b) The second phase of testicular descent (the inguinoscrotal phase) occurs during 25–35 weeks’ gestation. The gubernaculum bulges out from the inguinal region of the abdominal wall and begins to migrate toward the scrotum.28 c) Around week 35 of gestation, the gubernaculum (with its testis contained inside the processus vaginalis) finally comes to rest in the scrotal sack. d) Once the descent is complete, the processus vaginalis pouch closes at the top of the scrotum. Also at this time, the gubernaculum adheres to the scrotal wall – anchoring both itself and the contained testis into their terminal position 18 – while the remnants of the processus vaginalis above the scrotum regress.
Hormones
Since testicular descent is a hormonally-driven event, it is possible that the exposures discussed throughout this review are causes of (or proxies for) some abnormality in intrauterine hormonal function.40 Several authors have directly measured intrauterine hormone levels and compared these between those mothers of sons who developed cryptorchidism and those that did not. Based on their role in testicular descent, these investigations have primarily focussed on estrogens, androgens, human chorionic gonadotrophin (hCG) and the protein hormone Insulin-Like 3 (INSL3).
Estrogen
The estrogen hypothesis proposed by Sharpe and Skakkebaek in 1993 41 suggests foetal exposure to high levels of endogenous estrogen may be associated with maldescent of the testes (among other urogenital conditions). However, there is little 42 or no 43,44,45,46–48 evidence that this is the case; in fact, two previous studies have observed lower serum estrogen levels among mothers of cryptorchid cases compared to mothers of controls.43,44 Some authors have observed higher levels of serum alpha-fetoprotein (AFP) – a protein thought to mediate how the developing foetus responds to estrogen – among mothers of cryptorchid boys compared to mothers of non-cryptorchid boys;43,49 however, other authors have found no such association 50 – and given the lack of evidence regarding the role of maternal estrogen levels, it seems more likely that this difference in AFP levels may reflect some other dysfunction of the placenta.43,49 The role of the synthetic estrogen diethylstilbestrol (DES) is discussed later in this review (‘Pregnancy-related medications’).
Testosterone
Testosterone is critical to successful testicular descent: for instance, cryptorchidism can be achieved in animal models by exposing the animal to pure anti-androgens.51–53 It has therefore been hypothesised that low maternal levels of testosterone might be associated with development of cryptorchidism in the son. However, studies that have directly measured maternal testosterone levels in serum have reported little 45 or no 42,43 evidence of an association with cryptorchidism. Similarly, studies that have measured testosterone levels in cord blood found no evidence of an association between reduced testosterone levels and the development of cryptorchidism.55,56
Human Chorionic Gonadotrophin
Testosterone is produced (and regulated) by the developing testes primarily in response to human chorionic gonadotrophin (hCG), a hormone produced by the placenta.50,57 Some authors have hypothesised that disruption to hCG production may be associated with maldescent of the developing testes, with Chedane et al. 50 recently showing that the placentas of boys born with cryptorchidism had somewhat lower total hCG levels compared to controls (case mothers: 21.4 kilounits/litre, control mothers: 27.7 kilounits/litre). The authors suggest that the lower hCG values observed in the placentas of boys with cryptorchidism could reflect lower testosterone production, which in turn could lead to deficiencies in testicular descent;50 however, this relationship remains unclear.
Insulin-Like 3 (INSL3)
Like testosterone, the protein hormone INSL3 is crucial to testicular descent: cryptorchidism can be achieved in animal models by knocking out the gene that encodes INSL3.58 In recent years, it has been reported that cord blood levels of INSL3 are lower in children born with cryptorchidism 56,55,59 and might be reduced by exposure to exogenous endocrine-disrupting chemicals.56,60 However more direct observation of the pathway between exogenous stimuli (such as chemicals), altered intrauterine INSL3 levels and cryptorchidism development is required in order to substantiate causality. The role of endocrine-disrupting chemicals as a risk factor for cryptorchidism is discussed elsewhere in this review.
Assisted reproduction
Children conceived by in-vitro fertilisation (IVF) are 30% more likely to have a congenital malformation compared to those conceived naturally; however the absolute risk of malformation remains low.61 The mechanism of causality – if one exists – is suspected to be multifactorial, and perhaps involves endocrine disruption caused by stimulation of the ovaries in order to produce multiple oocytes, as well as factors directly related to the IVF procedure itself.62
However, there is limited 63,64,65,66,67,68,69 or no 70 evidence of an independent association between assisted reproduction procedures (ART) and development of cryptorchidism in the resulting child. In two studies a crude association between conception and cryptorchidism was substantially reduced after adjustment for birthweight. This suggests that the increased propensity for ART children to be born prematurely and with low birthweight 63 – discussed later in this manuscript as risk factors for cryptorchidism – may act as confounders or mediators in the association between ART and cryptorchidism.
Another significant confounding factor – which cannot be easily managed in study design or analysis – is that those seeking such fertility treatment are, by definition, sub-fertile. Subfertility is itself a risk factor for congenital malformation: for example, the children of sub-fertile parents (that is, those who took longer than 12 months to naturally conceive) are 20–40% more likely to have some congenital malformation compared to those who took less than 12 months to conceive.71 Massaro et al.72,73 suggest that parental subfertility (regardless of which parent[s] are affected) is likely to make a more substantial contribution to risk of congenital malformation than the means of conception.72,73 Kallen et al. 74 observed that controlling for characteristics of the parent – including length of ‘involuntary childlessness’ – almost entirely explained the increased risk of congenital abnormality found among those conceived via IVF.74 In other words, it is possible that the association between ART and cryptorchidism is due to parental and neonatal characteristics that are common to those seeking and receiving this treatment.
Maternal age, parity and health
Maternal age
While little difference in mean maternal age has been observed between the mothers of cryptorchid compared to non-cryptorchid sons 75,76,77,78,79,80,81,68,6,5,46, some authors have observed an association between both ‘older’ (>30 years) 77,82,83 and ‘younger’ (<20 years) 76,77,84,85 maternal age and cryptorchidism. In contrast to the latter, both Jones et al. 48 and McGlynn et al. 82 observed that young age appeared to be protective against cryptorchidism development. In the absence of consistent observations, it is difficult to draw firm conclusions regarding the role of maternal age in the aetiology of cryptorchidism.
Parity
Any relationship between parity (and/or birth order) and risk of cryptorchidism is unclear: while several authors have found no association,76,78,86,6,5,75 others have found an increased risk of cryptorchidism among sons born to both primiparous mothers 87,88,89,48,83,84 – while still others have found increased risk among sons born to multiparous mothers.77 It is worth noting that those studies that observed an increased risk of cryptorchidism among sons born to multi- 77 or primiparous mothers 48,83,84,87–89 tended to have larger sample sizes than those that found no association,5,6,75,76,78,86 and thus had greater power to detect an association, and that among those larger studies there was more consistency towards an increased risk among firstborn men. It has been hypothesized that this association is related to hormonal differences in nulliparous women compared with multiparous women. 17 Women in their first pregnancy have higher levels of free estradiol than in subsequent pregnancies,28 and
Pregnancy-related health
A multitude of factors related to the health of the mother have been investigated as potential risk factors for cryptorchidism development, perhaps due to their relevance to pregnancy. However, there is little consistent evidence that these maternal factors are strong risk factors in the development of cryptorchidism – and as such, we have reserved a detailed description of these factors for supplementary material (see Supplementary Material 1).
Alcohol
When viewed collectively, the literature to date provides little 93 or no 79,94,99,96,6,46,78,95,76,92,89 evidence that maternal alcohol consumption during pregnancy is associated with cryptorchidism development. A recent meta-analysis reported a null association (adjusted pooled odds ratio: 0.97, 95% CI 0.87–1.07).100
However, there is limited evidence that ‘heavy’ drinking (relative to reported abstinence) might be associated with cryptorchidism development: whether via chronic exposure in terms of a relatively large number of drinks per week 98,97 or via binge drinking episodes. 97,99,96 For example, Damgaard et al. 97 observed that the sons of mothers who regularly consumed at least 5 drinks per week during pregnancy had three times greater odds of developing cryptorchidism compared to those not exposed (adjusted OR: 3.10, 95% CI 1.05–9.10). However, the authors found no conclusive evidence of an association between binge drinking episodes (i.e. instances where the mother was “noticeably inebriated”) and cryptorchidism development (adjusted OR: 1.18, 95% CI 0.77–1.83), findings which largely echo those observed by Jensen et al. 96 and Strandberg-Larsen et al..99 It is worth noting that observations regarding the impact of ‘heavy’ drinking have been made based on small proportion of exposed cases – for instance, of the n=2,477 mothers investigated in Damgaard et al.,97 only 34 consumed at least 5 drinks per week during pregnancy – of which 6 had a son who developed cryptorchidism (out of the total 128 cases) and 28 did not. Strandberg-Larsen et al.99 had the greatest power to detect an association between ‘heavy’ drinking and cryptorchidism development, with a cohort of more than n=41,000 and n=1,598 cryptorchidism cases; still, they found no such association.
Based on evidence to date, it would appear that alcohol is not a major risk factor in the development of cryptorchidism; however, there are a number of issues with exposure assessment that require further consideration. Firstly there is likely to be social desirability bias when using self-report to measure alcohol consumption during pregnancy.95 Secondly the aggregation of studies with different exposure assessment of alcohol consumption (e.g. binge vs ever drinking) may mask any true associations. Thirdly, the lack of consistent definitions of the same exposure (e.g. what constitutes binge drinking) also means it is possible that a true association may not be apparent due to poor, and thus misclassified, exposure assessment. Given a possible association between sustained heavy consumption or binge drinking and cryptorchidism development, standardisation of how these exposures are measured is necessary.
Tobacco
The disruptive impact of tobacco exposure on the intrauterine environment is unequivocal.101 In addition to the various means by which tobacco exposure might affect testicular maldescent (including cellular genetic mutation and vasoconstriction),102 it has also been shown that the endocrine system – upon which successful descent of the testes depends – is disrupted by cigarette smoke.103
Maternal smoking during pregnancy
There is some evidence of a weak positive association between maternal smoking during pregnancy and subsequent development of cryptorchidism. 94,46,5,89,75,80,92,82,40,76,104,105 Two separate meta-analyses have confirmed this association,100,106 with the most recent including information from 25 studies and observing a 17% increased risk of cryptorchidism among boys born to mothers who smoked tobacco during pregnancy (pooled OR: 1.17, 95% CI: 1.11–1.23).100 To put this into perspective, the association between maternal smoking and the development of cryptorchidism is comparable to that observed for smoking and cleft lip defect (OR range 1.2–1.6).101
Further evidence of an association was provided by Thorup et al.,107 who observed that among children born with cryptorchidism, chances of bilateral cryptorchidism (maldescent of both testes) is 50% among children born to mothers who smoked at least 10 cigarettes per day during pregnancy, but only 18% among those mothers who did not smoke during pregnancy, adjusted for birth weight.107
However, there are some caveats to the evidence around maternal smoking and risk of cryptorchidism. First, there is the issue of study quality: there has been a high degree of both inconsistency and imprecision in the measurement of maternal smoking during pregnancy. Most studies to date have employed an ‘any-use’ means of measuring tobacco exposure, and/or have collected smoking status data at relatively arbitrary times during the antenatal period. 46,5,48,89,108,80,94,6,75,78,40,82,98 This means that most studies ignore plausible dose-response relationships between the number of cigarettes smoked per day and cryptorchidism development, for which there is some evidence: Jensen et al. observed increasing risk of cryptorchidism with increasing numbers of cigarettes smoked per day (albeit with wide confidence intervals).105 It also means that most studies ignore the possibility that the timing of tobacco exposure is important (including timing of cessation among those who quit during pregnancy). One conceivable (though probably simplistic) explanation for why several studies have observed a null 6,68,108,79 or weak 94,46,5,48,80,78,92,40,82,98 association between maternal smoking and cryptorchidism might be due to poor data quality and/or incomplete analytical accounting for the intensity and timing of smoking. An exception to this is the study by Mongraw-Chaffin et al.,95 which collected comprehensive prospective data on tobacco exposure during pregnancy and found a null (if not weak negative) result. These data were collected between 1959–1967, well before the 1980 Surgeon General’s Report on the Health Consequences of Smoking for Women 109 – and as such, the prevalence of maternal smoking was high within both case (49%) and control groups (58%).95 However, it should be noted that the multivariable models run by Mongraw-Chaffin et al. 95 only included data on n=68 cases and n=212 controls – and thus the author’s ability to detect potentially-important associations due to the comprehensive nature of data collection may been diluted by the small size of their sample.
Second, the issue of social desirability bias and possible under-reporting of tobacco use applies here. While recall bias not an issue for those studies which collect tobacco exposure data prospectively,95 under-reporting is still highly likely regardless of study design and in prospective studies will likely result in the measure of association being biased towards the null.101 One approach to dealing with error in self-reported smoking status is to use a biologic measurement (e.g. via serum cotinine). This would seem a critical future step in validating the association between maternal smoking and cryptorchidism development.102
Paternal smoking
There appears to be consistent (albeit weak) evidence of an association between paternal smoking and cryptorchidism in the son. 75,80,79,105,104 For example, Jensen et al. 105 observed that paternal smoking during pregnancy increased the odds of cryptorchidism by 60% (unadjusted OR: 1.6, 95% CI 1.2–2.3).
It is difficult to discern whether the association between paternal smoking and cryptorchidism development is a causal or spurious one. Jensen et al. 105 observed that the relationship between paternal smoking and cryptorchidism development did not disappear when additionally adjusted for maternal smoking; and it is conceivable that paternal smoking is associated with cryptorchidism via passive maternal exposure 104,75,105 or genetic ‘damage’ to the sperm involved in conception.79,104 However, it is possible that the association may simply be the result of a strong correlation between maternal smoking (which may be underreported by mothers) and paternal smoking (for whom social desirability bias may have less impact on self-report).105 As suggested by Jensen et al.,105 measurement of serum cotinine (or a similar biomarker) would be a useful means of testing the independence of the relationship between paternal and maternal smoking and cryptorchidism development.
Drugs
Broadly speaking, drugs that have been studied for an association with cryptorchidism can be grouped as a) medications for conditions directly related to the pregnancy and/or fertility, b) medications for conditions unrelated to the pregnancy (excluding analgesics), c) analgesics, and d) recreational drugs (or non-therapeutic use of medicines).
Pregnancy-related medications
Multiple studies have investigated the association between medications that may be taken to facilitate (or prevent) pregnancy; prevent pregnancy loss or complication(s); or treat nausea and vomiting. Studies have reported either little or no consistent evidence of an association between cryptorchidism risk and maternal use of anti-nausea/vomiting medications,76,78 fertility medications 76 or contraceptive medications.76,78,110 In terms of medications used to prevent pregnancy loss or complications, some authors have found no association;76,78 however, Palmer et al. 111 observed an association between the synthetic estrogen diethylstilbestrol (DES) – widely given to women throughout the mid-20th century, ostensibly to prevent pregnancy complications – and development of cryptorchidism in sons (adjusted RR: 1.9, 95% CI 1.1–3.4). It is worth noting that most of these studies had relatively small samples sizes, and thus their power to detect associations was limited; for example, of the n=2,235 mothers investigated in Palmer et al.,111 only 54 had children who developed cryptorchidism – of which 38 were exposed to DES and 17 were not.76,78,110,111
Mavrogenesis et al. 110 also observed an association between dydrogesterone treatment (commonly for pre-pregnancy endometriosis) and development of cryptorchidism (adjusted OR: 2.75, 95% CI 1.04–7.28) – although the authors acknowledge the preliminary nature of this observation, since it was based on only 5 (out of 2,052) cases and 22 (out of 24,814) controls.
Medications for conditions unrelated to the pregnancy (excluding analgesics)
Several studies have investigated medications that may be taken by a mother during pregnancy to treat conditions unrelated to pregnancy – including anti-retrovirals, 112 antibiotics for fungal or bacterial infection,113,114 anti-depressives, 115 laxatives, 116 cough medications, 117 anti-anaemics 46, hypnotics, 46 and anti-epileptics. 118,119,120 In the majority of these studies, cryptorchidism was one of many congenital anomalies under investigation, and each study generally included a small number of cryptorchidism cases. These studies have found little or no association between use of these medications and subsequent cryptorchidism risk – with the possible exception of Bartfai et al.,117 who observed that boys born to mothers who reported using Prenoxdiazine (cough medicine) at some point during pregnancy appeared to be at increased risk of cryptorchidism (adjusted OR: 1.8, 95% CI 0.9–3.5). However, given the small number of cases upon which this association was made (n=21), further research is required to substantiate this observation.
Analgesics
Perhaps the most investigated group of drugs in the context of congenital cryptorchidism are analgesics, such as paracetamol and ibuprofen. Analgesics have been implicated as endocrine disruptors, and it has been shown that clinically-relevant concentrations can cause endocrine disturbances in the human fetal testis.121 Several authors have observed a strong positive association between maternal use of analgesics and cryptorchidism in their sons.94,122–124 For example, Snijder et al. 122 observed that women who used mild analgesics during their second trimester had more than twice the odds of giving birth to sons who had cryptorchidism (adjusted OR: 2.12, 95% CI 1.17–3.83), and after calculating population-attributable fractions (PAFs) concluded that up to 24% of all cryptorchidism cases in their cohort could be attributed to the maternal use of mild analgesics during pregnancy.
However, there is also conflicting evidence regarding an association between maternal use of analgesics and cryptorchidism, with several authors finding no 6,78 or weak/limited 46,125,126 evidence. We cannot easily attribute this conflict to differing exposure and outcome classification between studies: for example, Kristensen et al. 123 compared the association between maternal use of mild analgesics (including acetaminophen) and development of cryptorchidism in both Denmark and Finland, and observed a dose-dependent positive association in Denmark (e.g. adjusted OR for use of mild analgesics for more than two weeks during first and second trimester: 2.47, 95% CI 1.02–5.96) but not in Finland (same OR: 0.56, 95% CI 0.13–2.45). While some heterogeneity in exposures might be expected between countries and study power the extent of this divergence is difficult to explain – and adds some confusion regarding the possible role of analgesia in the development of cryptorchidism. The authors themselves state that rather than having a directly causal relationship with cryptorchidism development, it is entirely possible that maternal use of analgesics may in fact be a “sentinel” of other (unmeasured) factors.127 It is also worth noting that, with the possible exception of Jensen et al.,124 the studies that have investigated analgesia exposure have generally involved a relatively small number of cases – thus limiting the power of these studies to detect an association.
The ubiquity of analgesia use makes this exposure particularly important from a public health perspective – and given this fact, further well-powered, multivariate-adjusted investigations and meta-analyses are required to better assess this exposure.
Recreational drug use
Very few studies have investigated the association between maternal use of drugs which might be considered ‘recreational’ and the subsequent development of cryptorchidism in the son. Berkowitz and Lapinski 94 observed that maternal ‘drug abuse’ had no effect on cryptorchidism risk. While marijuana use by males has been reported to be associated with testicular cancer,128–131 there have been no studies to date examining maternal use of marijuana and cryptorchidism.
Endocrine-disrupting chemicals
A number of chemicals have been identified as potential disruptors of endocrine system function – and numerous animal studies have shown that exposure to these chemicals can interrupt normal testicular descent.132,133,134,135,136,137 There is an extensive body of literature examining the impact of these endocrine-disrupting chemicals on the prevalence of cryptorchidism in humans – and it is possible to classify this literature as referring to either occupational or environmental exposure.
Occupational exposure
Multiple authors have investigated the association between certain occupations and the development of cryptorchidism, with occupation acting as a proxy for exposure(s) that are thought to cause cryptorchidism. With a few rare exceptions, the underlying exposures of interest are synthetically-manufactured chemicals, particularly pesticides used in the agricultural industry. Several studies have observed an increased risk of cryptorchidism arising from maternal agri/horticultural occupation during pregnancy.98,138–140 For example, Jorgensen et al. 138 observed in a large Danish cohort that sons of mothers who farmed during pregnancy were nearly a third more likely to develop cryptorchidism compared to sons of mothers who did not farm (adjusted HR: 1.31, 95% CI 1.12–1.53). Several authors have also observed an increased risk of cryptorchidism among boys born to fathers who are agri/horticultural workers,75,98,141 although the mechanism by which paternal exposure affects the developing foetus remains unclear.
Morales-Surez-Varela et al. 142 observed that occupations with increased risk of paternal exposure to heavy metals were associated with an increased risk of cryptorchidism in sons (adjusted HR: 1.9, 95% CI 1.1–2.7); however, maternal exposure during pregnancy was not associated (adjusted HR: 1.0, 95% CI 0.3–1.7).142 Similarly, Vaktskjold et al. 143 observed no association between pregnant women working in a nickel factory and cryptorchidism (adjusted OR: 0.76, 95% CI 0.40–1.47).
Hairdressing has been investigated by one study as a proxy for solvent exposure, though there is little evidence of an association with cryptorchidism – with Jorgensen et al. 144 observing that boys born to women who worked as hairdressers during pregnancy were no more likely to develop cryptorchidism than sons whose mothers worked in other occupations (adjusted HR: 0.91, 95% CI 0.77–1.08). Finally, there is limited evidence that the sons of soldiers exposed to the endocrine-disrupting chemical dibutyl phthalate during the 1940’s-1960’s were more likely to be born cryptorchid; however this evidence is based on a very small cohort.145
Environmental exposure – indirect measurement
Other studies have investigated the association between environmental exposure to endocrine disrupting chemicals and cryptorchidism. Bornman et al. 146 observed that sons born to women who lived in areas sprayed with dichlorodiphenyltrichloroethane (DDT) were more than twice as likely to be born cryptorchid (adjusted OR: 2.1, 95% CI 1.14–3.92).
Several authors have estimated likely pesticide exposure according to the geographic region in which the child was born. This classification has been based on intensity of agricultural industry in the region,147,148 representative samples taken as part of a geological survey,149 or proximity to chemical plants.150,151 With respect to the latter, there is some evidence that proximity to a chemical plant is related to cryptorchidism risk. Czeizel et al. 150 observed in a large Hungarian cohort that the risk of cryptorchidism increased with proximity to the local acrylonitrile factory (a chemical used in plastic manufacturing) – while Kim et al. 151 observed that the risk of cryptorchidism was higher in those regions of South Korea that have petrochemical estates, compared to the national average.151 While offering little insight into the specific chemical exposures that may be associated with cryptorchidism development, these observations suggest – at least ecologically – that exposure to potentially-endocrine-disrupting chemicals may increase the risk of cryptorchidism development.
Environmental exposure – direct measurement
Rather than indirectly measure exposure to potential endocrine-disrupting chemicals via a proxy such as geographical location or occupation, a number of researchers have collected biological specimens and directly tested for the presence of these chemicals. The medium for this analysis varies: many studies have used maternal blood taken during pregnancy,152–158 although neonatal serum, 159,160 amniotic fluid, 60 breast milk,159,161–166 placental tissue/cord blood 159–162,166–172 and adipose tissue biopsy from the child 173,174 have also been employed. Those studies investigating early-life environmental exposure to endocrine-disrupting chemicals as a risk factor for cryptorchidism have generally drawn blood from the children themselves.175,176
Despite extensive evaluation, the role of direct exposure to endocrine-disrupting chemicals remains uncertain. Some authors have observed higher levels of the compounds bisphenol A,176 dibutylin 169 dioxin,164,173 heptachloroepoxide,174 hexachlorobenzene,174 polychlorinated biphenyls 164,173,177 and polybrominateddiphenyl ethers 159,164 among boys (and/or their mothers) who developed cryptorchidism compared to those who did not; however a substantial number of studies (at least some of which were adequately powered to detect an association) have directly evaluated these and other biologically-plausible compounds and found no such association.56,60,152–157,160,163,168,172,178 In some cases, studies have found substantial regional heterogeneity, including conflicting results between countries within the same study.161,162,164,169 For example, Rantakokko et al. 169 observed that maternal exposure to high levels (>0.15ng/g) of dibutylin was associated with increased risk of cryptorchidism in Danish sons (adjusted OR vs. <0.10ng/g: 4.01, 95% CI 1.42–11.33), but was inversely associated in Finnish sons (adjusted OR: 0.16, 95% CI 0.03–0.75).
There is some evidence that rather than an individual chemical type being associated with cryptorchidism development, it is the mixture 167,171 or total burden165,170,173,177 of chemical exposure that is important. For example, Damgaard et al. 165 observed no (or very limited) association between individual compounds extracted from breast milk and cryptorchidism in the son – however, when the eight most commonly-occurring compounds (dichlordiphenyldichloroethylene [DDE], DDT, β-hexachlorocyclohexane], hexachlorobenzene], α-endosulfan, cis-heptachloroepoxide, oxychlordane and dieldrin) were combined, the authors observed higher levels of these pesticides among boys with cryptorchidism compared to boys without.
Overall, the evidence regarding the association between cryptorchidism and exposure to potential endocrine-disrupting chemicals suggests a weak and inconsistent association – perhaps stronger in some contexts than others 161,162,164,169 for reasons that are not presently clear. It also remains unclear if the higher levels of compounds observed among cryptorchid boys (or their mothers) in some studies is indicative of heightened exposure to these chemicals, or indicates inability to metabolise those chemicals.166 We should also note that exposure to some of these chemicals has been in decline over recent decades,173 as production of chemicals such as PCB’s have been prohibited in many parts of the world.174
Seasonality
Some authors have proposed that cryptorchidism occurs in cycles according to calendar period of birth – with seasonal peaks that occur between September to November and again sometime between January and May (in the Northern Hemisphere).1,81,87,88,179–181 While it has been suggested that these seasonal peaks may coincide with hypothesised seasonal peaks in testosterone levels,81 this has not been substantiated.
Diet
Giordano et al. 93 found an association between maternal consumption of smoked food products during pregnancy (adjusted OR: 2.46, 95% CI 1.15–5.29), and suggested that this may be evidence of a link between potentially-toxic components of food and disruption of endocrine processes. Brantsæter et al. recently observed no association between maternal consumption of organic foods during pregnancy and subsequent development of cryptorchidism in the son.182
There is conflicting evidence in terms of the existence of an association between maternal use of caffeine and development of cryptorchidism: Berkowitz and Lapinski 94 observed no association between either maternal coffee (crude OR: 0.97, 95% CI 0.58–1.63 for >=1 cup per day) or tea (OR: 1.04, 95% CI 0.59–1.81 for >=1 cup per day) drinking during pregnancy and cryptorchidism development, while Mongraw-Chaffin et al. 95 observed that coffee drinkers appeared more likely to have sons with cryptorchidism (adjusted OR: 1.43, 95% CI 1.06–1.93 for 3 cups of coffee/day). However, Mongraw-Chaffin et al. 95 suggested that, if Berkowitz and Lapinski 94 had set their threshold for coffee use higher than >=1 cup/day, they too may have observed an association between caffeine exposure and cryptorchidism development. Further work is required to substantiate this association – particularly given the abrupt rise in the consumption of caffeinated ‘energy’ drinks over the past decade.183
Birth presentation
In 1983, Swerdlow and colleagues 84 observed that boys born with cryptorchidism were considerably more likely to present in the breech position at the time of delivery than non-cryptorchid boys. The authors postulated that this might suggest causality between the obstetric trauma of a breech pregnancy and cryptorchidism, citing evidence from previous studies that had found testicular bruising and lesions among children who had presented in the breech position.184–186 The authors also suggested this observation was further grounds for protective Caesarean section to deliver these children.84
However, while multiple authors have also observed an association between cryptorchidism and breech presentation 89,81,48,68,187,188 and/or mode of delivery, 6,89,81,88,78,87,76 it is possible that this association is primarily driven by shared aetiology rather than direct causality.89 In other words, the intrauterine factors that cause a child to settle into breech could be the same (or similar) to those that lead to maldescent of the testes; a theory supported by the observations of Damgaard et al.,68 who found that the association between breech presentation and cryptorchidism remained strong even when adjusted for multiple covariates, including mode of delivery (adjusted OR: 2.59, 95% CI 1.12–5.97). The authors suggest that the association between breech presentation and cryptorchidism may actually be an indirect marker of placental impairment.68
Gestational factors
Cryptorchidism has been consistently shown to be strongly associated with low birth weight, gestational age, and size for gestational age. 5,34,48,81,83,89,189,190,88,68,87,75,86,40,187,82,84,80,77,8,92 For example, recent observations from a New Zealand birth cohort showed that the prevalence of all three of these markers within boys with orchidopexy-confirmed cryptorchidism was approximately twice that observed among non-cryptorchid boys.189 In addition, relative foetal growth restriction is also associated with cryptorchidism: Jensen et al. 34 observed that a twin born with cryptorchidism was, on average, 136g lighter (95% CI 70–202) than a non-cryptorchid male twin.
In combination, these observations suggest that these could be risk factors for cryptorchidism; however, rather than being risk factors per se, birth weight and fetal growth restriction may either have a shared aetiology with cryptorchidism, or be on the causal pathway between causative factors and cryptorchidism. In this case, the true aetiological factors would be exposures in the intrauterine environment that affect foetal development – likely reflecting a combination of the genetic and/or environmental exposures discussed elsewhere in this review. Low birth weight, for example, may be the result of a multitude of maternal factors – such as smoking during pregnancy, nutrition, pre-pregnancy weight and age;191,192 thus, it is difficult to determine the true nature of the association between these markers of gestation and testicular maldescent.
Twinning
Weidner et al. 86 observed a protective effect among twin boys (compared to singletons) even after adjusting for birthweight (adjusted OR: 0.76, 95% CI 0.63–0.92) – while Jensen et al.193 noted that the rate of concordance (i.e. cryptorchidism occurrence in a pair of brothers) is substantially higher among twin boy pairs compared to full brother pairs (from separate pregnancies) – but not different between mono- and dizygotic twins (concordance rate: full brother pairs 8.8%; dizygotic twin pairs 24.1%; monozygotic twin pairs 27.3%). This suggests that this concordance may more strongly related to the shared intrauterine environment, rather than a strong genetic component. Consistent with this, Schnack et al.,194 estimated that the risk of cryptorchidism concordance in male-male twin pairs was 2.6 times higher than what would be expected from genetic contributions alone.
Genetics
Genes that encode the molecules that facilitate testicular descent could be related to the risk of cyptorchdism;195 for example, experimental studies have shown that ‘knocking-out’ the gene that encodes INSL3 will result in bilateral cryptorchidism.195 It follows, then, that if abnormalities in these genes are handed from mother or father to son – and/or if epigenetic aberrations cause such abnormalities post-conception – then testicular descent will be directly affected by a genetic (or epigenetic) pathway.
In humans, there is evidence that brothers and sons of men both with cryptorchidism are at increased risk of cryptorchidism: some authors have shown an increased familial risk that declines with decreasing degree of relativity.6,196,187,86,188 Jensen et al.193 and Schnack et al.194 both observed substantially higher concordance in cryptorchidism rates among maternal half-brothers compared to paternal half-brothers (concordance rate: maternal half-brothers 6%, paternal half-brothers 3.4%) – with the authors suggesting that given this observation (and the aforementioned observation regarding twinning), future aetiological work should focus on maternal genes (particularly the X chromosome) and the intrauterine environment.193
Variants in more than 15 genes have thus far been implicated in the development of cryptorchidism in humans via candidate gene studies (see Supplementary Material 2); however, only one genome-wide association study (GWAS) has been performed in ‘non-syndromic’ cryptorchidism (i.e. cryptorchidism in the absence of other congenital anomalies).197 In a GWAS of 844 boys with cryptorchidism and 2,718 controls, no individual SNPs reached a level of genome-wide significance. Pathway analysis, however, suggested that loci important in cyptoskeleton-dependent function may be of importance. The authors noted that their findings might reflect the fact that susceptibility to this disease is highly heterogeneous and possibly driven by environmental causes and/or rare genetic variants.197
With respect to the latter, rare mutations in INSL3 and its receptor RXFP2 have been reported at low frequencies (1–4%) in boys born with cryptorchidism,198,199 while rare mutations have also been found in NR5A1, which is involved in several reproductive processes.200 However, given the rarity of these mutations, they can only explain a very small proportion of cryptorchidism cases.
It is possible that inherited genetic variants may make an individual more or less susceptible to endocrine disruption via pathways such as exposure to chemicals, by disrupting the metabolism of these elements. For example an (albeit un-replicated) association reported by Qin et al.,201 was with a variant of the Aryl hydrocarbon receptor nuclear translocator 2 (ARNT2) gene (OR for minor homozygous genotype rs5000770 [AA vs. GG]: 3.5, 95% CI 1.7–7.3). This gene is part of a family of transcription factors that, among other roles, regulates several physiological pathways including responses to environmental contaminants. In this case, the likely causal mechanism by which genetic traits of the individual are influencing the likelihood of cryptorchidism is via interaction with environmental exposures – with this interaction leading to a ‘supra-multiplicative’ increase in cryptorchidism risk, beyond that which might be expected based on the individual associations. Such phenomena are referred to as non-additive (multiplicative) gene-environment interactions. There is, however, no evidence yet that such interactions exist in cryptorchidism.
It is important to note that a number of discoveries in the cryptorchidism context – for example, regarding the role of INSL3 in testicular descent – were made using animal models, and that the way in which testicular descent occurs in non-human models varies in several important respects to the same process in humans. For example, in humans the processus vaginalis (Fig. 1) disappears following testicular descent; while in rodents, it remains intact – enabling the testis to ascend back into the peritoneum later in life.18 Because of key differences in testicular descent between species, findings in animal models are not necessarily transferable to humans – unless, as in the case of INSL3, evidence exists from both experimental (non-human) models 195 and human studies.198,199
Ethnicity
There is some evidence of varying risk of cryptorchidism by ethnic grouping. In the United States, McGlynn et al. 82 observed that cryptorchidism was somewhat less likely in Black males compared to White males; while in New Zealand, Māori males are 20% more likely to be born with cryptorchidism than the non-Māori/Pacific/Asian population (adjusted RR: 1.20, 95% CI 1.11–1.30), while Pacific (0.89, 95% CI 0.80–0.99) and Asian (0.68, 95% CI 0.59–0.79) males have lower risk.189 It has also been noted in New Zealand that ethnic trends in cryptorchidism incidence mirror those observed for testicular cancer.202,203
It is possible that these ethnic trends are driven by predisposing genetic variants that are present in some ethnic groups but not others – however, it is important to note that ethnicity is a social construct, to which individuals self-identify (or are identified by their parents, in the case of newborns). However it is possible that individuals within a specific ethnic group are more likely to share some common ancestry, which may be associated with a high likelihood of specific predisposing genetic variants.
Recommendations for future work
Further systematic reviews and meta-analyses
For those risk factors in which there is a body of epidemiological evidence, additional high-quality systematic reviews, which include quality assessment of the studies, and meta-analyses are required in order to provide best-estimates regarding the current state of evidence with respect to a given exposure. The heterogeneity of exposure measurement (and outcome measurement, for that matter) will present a challenge for these studies.
Strengthening causal inference
Future work should aim at using approaches that can strengthen casual inference by separating spurious associations from causal effects. For example, future work aiming to provide further evidence for a causal role for smoking and alcohol in the development of cryptorchidism should consider using Mendelian randomisation. Using this approach, researchers could use genetic variants known to influence the risk of smoking and alcohol consumption, and assess their association with cryptorchidism. This approach is not subject to experimental biases (including social desirability bias), and as such represents a viable alternative means of testing the observational associations for causality. While the rarity of cryptorchidism may affect the usefulness of this method in this context, it is certainly worthy of further consideration.
Development of a cryptorchidism consortium
As noted throughout this review, many individual studies investigating specific risk factors for cryptorchidism are underpowered to identify potential associations. There is therefore a great need for further high-quality studies that are well-powered and designed with sufficient detail to allow exploration of dose-response relationships and adjustment for multiple confounders, ideally with inclusion of genetic sampling to contribute to a large GWAS in cryptorchidism with thousands of cases. A key area of future emphasis in this regard will be the combination of study datasets across international contexts, such that these comprehensive multivariate analyses can be performed. This consortium dataset would help in the identification causal factors, but also allow us to estimate prevalence of cryptorchidism in a standardised fashion across different countries, which would be highly informative.
Conclusions
Painting a picture of the factors that lead to maldescent of the testes is a difficult task, as evidenced by the uncertainties noted in this review. We have presented a list of putative risk factors in Box 1, which lists risk factors according to the likelihood that they are associated with cryptorchidism development. However, there are few instances in which there is consistent evidence with respect to a given exposure; and in those cases where evidence appears unequivocal – for example, the relationship between cryptorchidism and gestational measures such as low birth weight – the measured exposure actually represents exposure(s) that we don’t yet fully understand in the cryptorchidism context. Perhaps these caveats provide a clue as to why a concrete understanding of the aetiology of this disease remains elusive: in a situation where myriad (and often ubiquitous) exposures have been associated with cryptorchidism, it is likely that the causal roots of this condition are multifactorial and highly variable between individuals. Rather than a handful of candidate exposures being responsible for the vast majority of cases, perhaps the relative importance of each risk factor varies considerably between mother/son pairs depending on an array of genetic, maternal, placental and foetal factors – all of which could vary between regional contexts. In short, the complexity of (and mystery surrounding) the aetiology of this disease is perhaps an appropriate reflection of the complexity of the biological mechanisms that drive testicular descent in the first place.
Box 1. Putative risk factors associated with cryptorchidism.
Likely to be associated
Unlikely to be associated
Inconsistent or limited evidence of an association
Intrauterine exposure to high levels of endogenous hormones (e.g. estrogen 42–50)
Maternal health (see Supplementary Material 1)
Maternal age 75,76,77,78,79,80,81,68,6,5,46,77,82,83,76,77,84,85,48,82
Maternal alcohol consumption during pregnancy 93,79,94,99,96,6,46,78,95,76,92,89,100
Maternal use of medications not related to pregnancy 46,113–120
Maternal recreational drug use 94
Maternal occupational exposure to endocrine-disrupting chemicals 98,138–140,143
Paternal occupational exposure to endocrine-disrupting chemicals 75,98,141,142
Geographic proximity to areas of intensive use of endocrine-disrupting chemicals 147–151
Maternal or childhood direct exposure to endocrine-disrupting chemicals 56,60,152–157,159,160,163,164,168,169,172–174,176–178
Supplementary Material
Review Criteria.
We conducted searches of the Scopus database using the following search terms: ((cryptorchidism) OR (cryptorchism) OR (undescended test*)) AND ((exposure*) OR (exposed) OR (risk) OR (risk factor*)) AND ((epidemiology) OR (case-control) OR (cohort) OR (association) OR (associated) OR (population)). In addition, we scanned the reference lists of relevant articles to identify additional papers. The final search was conducted on 8th June 2016.
Key Points.
Cryptorchidism is one of the most common congenital abnormalities found among males, and is one of the few known risk factors for testicular cancer (TC).
Like testicular cancer, the key exposures in the occurrence of cryptorchidism (undescended testes) remain elusive.
Despite a considerable body of aetiological research, there are few exposures for which there is consistent evidence of an association with cryptorchidism.
In those cases where evidence appears unequivocal, the measured exposure is likely to be a surrogate for the true causal exposure.
The relative importance of each risk factor may vary considerably between mother/son pairs depending on an array of genetic, maternal, placental and foetal factors – all of which could vary between regions.
References
- 1.Ansell PE, et al. Cryptorchidism: A prospective study of 7500 consecutive male births, 1984–8. Archives of Disease in Childhood. 1992;67:892–899. doi: 10.1136/adc.67.7.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdullah NA, et al. Birth prevalence of cryptorchidism and hypospadias in northern England, 1993–2000. Archives of Disease in Childhood. 2007;92:576–579. doi: 10.1136/adc.2006.102913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berkowitz GS, et al. Prevalence and natural history of cryptorchidism. Pediatrics. 1993;92:44–49. [PubMed] [Google Scholar]
- 4.Pierik FH, et al. The cryptorchidism prevalence among infants in the general population of Rotterdam, the Netherlands. International Journal of Andrology. 2005;28:248–252. doi: 10.1111/j.1365-2605.2005.00538.x. [DOI] [PubMed] [Google Scholar]
- 5.Preikša RT, et al. Higher than expected prevalence of congenital cryptorchidism in Lithuania: A study of 1204 boys at birth and 1 year follow-up. Human Reproduction. 2005;20:1928–1932. doi: 10.1093/humrep/deh887. [DOI] [PubMed] [Google Scholar]
- 6.Wagner-Mahler K, et al. Prospective study on the prevalence and associated risk factors of cryptorchidism in 6246 newborn boys from Nice area, France. International Journal of Andrology. 2011;34:e499–e510. doi: 10.1111/j.1365-2605.2011.01211.x. [DOI] [PubMed] [Google Scholar]
- 7.Boisen KA, et al. Difference in prevalence of congenital cryptorchidism in infants between two Nordic countries. Lancet. 2004;363:1264–1269. doi: 10.1016/S0140-6736(04)15998-9. [DOI] [PubMed] [Google Scholar]
- 8.Ghirri P, et al. Incidence at birth and natural history of cryptorchidism: A study of 10,730 consecutive male infants. Journal of Endocrinological Investigation. 2002;25:709–715. doi: 10.1007/BF03345105. [DOI] [PubMed] [Google Scholar]
- 9.Barthold JS, Gonzalez R. The epidemiology of congenital cryptorchidism, testicular ascent and orchiopexy. Journal of Urology. 2003;170:2396–2401. doi: 10.1097/01.ju.0000095793.04232.d8. [DOI] [PubMed] [Google Scholar]
- 10.Dieckmann KP, Pichlmeier U. Clinical epidemiology of testicular germ cell tumors. World Journal of Urology. 2004;22:2–14. doi: 10.1007/s00345-004-0398-8. [DOI] [PubMed] [Google Scholar]
- 11.Lip SZL, Murchison LED, Cullis PS, Govan L, Carachi R. A meta-analysis of the risk of boys with isolated cryptorchidism developing testicular cancer in later life. Archives of Disease in Childhood. 2013;98:20–26. doi: 10.1136/archdischild-2012-302051. [DOI] [PubMed] [Google Scholar]
- 12.Cook MB, et al. A systematic review and meta-analysis of perinatal variables in relation to the risk of testicular cancer - experiences of the son. International Journal of Epidemiology. 2010;39:1605–1618. doi: 10.1093/ije/dyq120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanto S, et al. Risk factors in past histories and familial episodes related to development of testicular germ cell tumor. International journal of urology : official journal of the Japanese Urological Association. 2004;11:640–646. doi: 10.1111/j.1442-2042.2004.00853.x. [DOI] [PubMed] [Google Scholar]
- 14.Prener A, Engholm G, Jensen OM. Genital anomalies and risk for testicular cancer in Danish men. Epidemiology. 1996;7:14–19. doi: 10.1097/00001648-199601000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Lee PA, et al. Paternity after unilateral cryptorchidism: A controlled study. Pediatrics. 1996;98:676–679. [PubMed] [Google Scholar]
- 16.Skakkebæk NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: An increasingly common developmental disorder with environmental aspects. Human Reproduction. 2001;16:972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- 17.Nordenvall AS, Frisén L, Nordenström A, Lichtenstein P, Nordenskjöld A. Population based nationwide study of hypospadias in Sweden, 1973 to 2009: Incidence and risk factors. Journal of Urology. 2014;191:783–789. doi: 10.1016/j.juro.2013.09.058. [DOI] [PubMed] [Google Scholar]
- 18.Hutson JM, Thorup JM, Beasley SW. Descent of the testis. 2. Springer International Publishing; 2016. [Google Scholar]
- 19.Abeyaratne MR, Aherne WA, Scott JE. The vanishing testis. Lancet. 1969;2:822–824. doi: 10.1016/s0140-6736(69)92275-2. [DOI] [PubMed] [Google Scholar]
- 20.Kollin C, Karpe B, Hesser U, Granholm T, Ritzén EM. Surgical Treatment of Unilaterally Undescended Testes: Testicular Growth After Randomization to Orchiopexy at Age 9 Months or 3 Years. The Journal of Urology. 2007;178:1589–1593. doi: 10.1016/j.juro.2007.03.173. [DOI] [PubMed] [Google Scholar]
- 21.Kolon TF, et al. Evaluation and treatment of Cryptorchidism: AUA guideline. Journal of Urology. 2014;192:337–345. doi: 10.1016/j.juro.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Jensen MS, et al. Age at cryptorchidism diagnosis and orchiopexy in Denmark: a population based study of 508,964 boys born from 1995–2009. The Journal of Urology. 2011;186:1595–1600. doi: 10.1016/j.juro.2011.03.070. [DOI] [PubMed] [Google Scholar]
- 23.Martin Ritzén E. Undescended testes: A consensus on management. European Journal of Endocrinology. 2008;159:S87–S90. doi: 10.1530/EJE-08-0181. [DOI] [PubMed] [Google Scholar]
- 24.Pettersson A, Richiardi L, Nordenskjold A, Kaijser M, Akre O. Age at Surgery for Undescended Testis and Risk of Testicular Cancer. New England Journal of Medicine. 2007;356:1835–1841. doi: 10.1056/NEJMoa067588. [DOI] [PubMed] [Google Scholar]
- 25.Wood HM, Elder JS. Cryptorchidism and Testicular Cancer: Separating Fact From Fiction. Journal of Urology. 2009;181:452–461. doi: 10.1016/j.juro.2008.10.074. [DOI] [PubMed] [Google Scholar]
- 26.Akre O, Pettersson A, Richiardi L. Risk of contralateral testicular cancer among men with unilaterally undescended testis: A meta analysis. International Journal of Cancer. 2009;124:687–689. doi: 10.1002/ijc.23936. [DOI] [PubMed] [Google Scholar]
- 27.Pottern LM, et al. Testicular cancer risk among young men: Role of cryptorchidism and inguinal hernia. Journal of the National Cancer Institute. 1985;74:377–381. [PubMed] [Google Scholar]
- 28.Hutson JM, Hasthorpe S, Heyns CF. Anatomical and functional aspects of testicular descent and cryptorchidism. Endocrine Reviews. 1997;18:259–280. doi: 10.1210/edrv.18.2.0298. [DOI] [PubMed] [Google Scholar]
- 29.Toppari J, Kaleva M, Virtanen HE. Trends in the incidence of cryptorchidism and hypospadias, and methodological limitations of registry-based data. Human Reproduction Update. 2001;7:282–286. doi: 10.1093/humupd/7.3.282. [DOI] [PubMed] [Google Scholar]
- 30.Chilvers C, Forman D, Pike MC, Fogelman K, Wadsworth MEJ. APPARENT DOUBLING OF FREQUENCY OF UNDESCENDED TESTIS IN ENGLAND AND WALES IN 1962–81. The Lancet. 1984;324:330–332. doi: 10.1016/S0140-6736(84)92697-7. [DOI] [PubMed] [Google Scholar]
- 31.Bonney T, Southwell B, Donnath S, Newgreen D, Hutson J. Orchidopexy trends in the paediatric population of Victoria, 1999–2006. Journal of Pediatric Surgery. 2009;44:427–431. doi: 10.1016/j.jpedsurg.2008.10.099. [DOI] [PubMed] [Google Scholar]
- 32.Capello SA, Giorgi LJ, Jr, Kogan BA. Orchiopexy practice patterns in New York State from 1984 to 2002. J Urol. 2006;176:1180–1183. doi: 10.1016/j.juro.2006.04.052. [DOI] [PubMed] [Google Scholar]
- 33.Richiardi L, Vizzini L, Nordenskjold A, Pettersson A, Akre O. Rates of orchiopexies in Sweden: 1977–1991. Int J Androl. 2009;32:473–478. doi: 10.1111/j.1365-2605.2008.00881.x. [DOI] [PubMed] [Google Scholar]
- 34.Jensen MS, et al. Cryptorchidism and hypospadias in a cohort of 934,538 danish boys: The role of birth weight, gestational age, body dimensions, and fetal growth. American Journal of Epidemiology. 2012;175:917–925. doi: 10.1093/aje/kwr421. [DOI] [PubMed] [Google Scholar]
- 35.Jensen MS, et al. Age at cryptorchidism diagnosis and orchiopexy in Denmark: A population based study of 508,964 boys born from 1995 to 2009. Journal of Urology. 2011;186:1595–1600. doi: 10.1016/j.juro.2011.03.070. [DOI] [PubMed] [Google Scholar]
- 36.Wohlfahrt-Veje C, et al. Acquired cryptorchidism is frequent in infancy and childhood. Int J Androl. 2009;32:423–428. doi: 10.1111/j.1365-2605.2009.00946.x. [DOI] [PubMed] [Google Scholar]
- 37.Hutson J, Li R, Southwell B, Newgreen D, Cousinery M. Regulation of testicular descent. Pediatric Surgery International. 2015;31:317–325. doi: 10.1007/s00383-015-3673-4. [DOI] [PubMed] [Google Scholar]
- 38.Jensen MS, et al. Maternal use of acetaminophen, ibuprofen, and acetylsalicylic acid during pregnancy and risk of cryptorchidism. Epidemiology. 2010;21:779–785. doi: 10.1097/EDE.0b013e3181f20bed. [DOI] [PubMed] [Google Scholar]
- 39.Barteczko KJ, Jacob MI. The testicular descent in human. Origin, development and fate of the gubernaculum Hunteri, processus vaginalis peritonei, and gonadal ligaments. Advances in anatomy, embryology, and cell biology. 2000;156 [PubMed] [Google Scholar]
- 40.Akre O, Lipworth L, Cnattingius S, Sparén P, Ekbom A. Risk factor patterns for cryptorchidism and hypospadias. Epidemiology. 1999;10:364–369. [PubMed] [Google Scholar]
- 41.Sharpe RM, Skakkebaek NE. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? The Lancet. 1993;341:1392–1396. doi: 10.1016/0140-6736(93)90953-E. [DOI] [PubMed] [Google Scholar]
- 42.Bernstein L, et al. Maternal hormone levels in early gestation of cryptorchid males: A case-control study. British Journal of Cancer. 1988;58:379–381. doi: 10.1038/bjc.1988.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGlynn KA, et al. Maternal hormone levels and risk of cryptorchism among populations at high and low risk of testicular germ cell tumors. Cancer Epidemiology Biomarkers and Prevention. 2005;14:1732–1737. doi: 10.1158/1055-9965.EPI-05-0128. [DOI] [PubMed] [Google Scholar]
- 44.Burton MH, Davies TW, Raggatt PR. Undescended testis and hormone levels in early pregnancy. Journal of Epidemiology and Community Health. 1987;41:127–129. doi: 10.1136/jech.41.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Key TJA, et al. A case - Control study of cryptorchidism and maternal hormone concentrations in early pregnancy. British Journal of Cancer. 1996;73:698–701. doi: 10.1038/bjc.1996.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davies TW, Williams DRR, Whitaker AH. Risk factors for undescended testis. International Journal of Epidemiology. 1986;15:197–201. doi: 10.1093/ije/15.2.197. [DOI] [PubMed] [Google Scholar]
- 47.Beard CM, Melton LJ, O’Fallon WM, Noller KL, Benson RC. Cryptorchism and maternal estrogen exposure. American Journal of Epidemiology. 1984;120:707–716. doi: 10.1093/oxfordjournals.aje.a113938. [DOI] [PubMed] [Google Scholar]
- 48.Jones ME, Swerdlow AJ, Griffith M, Goldacre MJ. Prenatal risk factors for cryptorchidism: A record linkage study. Paediatric and Perinatal Epidemiology. 1998;12:383–396. doi: 10.1046/j.1365-3016.1998.00144.x. [DOI] [PubMed] [Google Scholar]
- 49.Boyd HA, et al. Maternal serum alpha-fetoprotein level during pregnancy and isolated cryptorchidism in male offspring. American Journal of Epidemiology. 2006;164:478–486. doi: 10.1093/aje/kwj219. [DOI] [PubMed] [Google Scholar]
- 50.Chedane C, Puissant H, Weil D, Rouleau S, Coutant R. Association between altered placental human chorionic gonadotrophin (hCG) production and the occurrence of cryptorchidism: A retrospective study. BMC Pediatrics. 2014;14 doi: 10.1186/1471-2431-14-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mizuno K, et al. Altered expression and localization of estrogen receptors alpha and beta in the testes of a cryptorchid rat model. Urology. 2011;77:251.e251–251.e256. doi: 10.1016/j.urology.2010.06.035. [DOI] [PubMed] [Google Scholar]
- 52.Mizuno K, et al. Influence for testicular development and histological peculiarity in the testes of flutamide-induced cryptorchid rat model. International Journal of Urology. 2007;14:67–72. doi: 10.1111/j.1442-2042.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- 53.Van der Schoot P. Disturbed testicular descent in the rat after prenatal exposure to the antiandrogen flutamide. Journal of Reproduction and Fertility. 1992;96:483–496. doi: 10.1530/jrf.0.0960483. [DOI] [PubMed] [Google Scholar]
- 54.Abbo O, et al. Male infants with hypospadias and/or cryptorchidism show a lower 2D/4D digit ratio than normal boys. Archives of Disease in Childhood. 2015;100:643–647. doi: 10.1136/archdischild-2014-306454. [DOI] [PubMed] [Google Scholar]
- 55.Fénichel P, et al. Cord blood Insulin-like peptide 3 (INSL3) but not testosterone is reduced in idiopathic cryptorchidism. Clinical Endocrinology. 2015;82:242–247. doi: 10.1111/cen.12500. [DOI] [PubMed] [Google Scholar]
- 56.Chevalier N, et al. A negative correlation between insulin-like peptide 3 and bisphenol A in human cord blood suggests an effect of endocrine disruptors on testicular descent during fetal development. Human Reproduction. 2015;30:447–453. doi: 10.1093/humrep/deu340. [DOI] [PubMed] [Google Scholar]
- 57.Scott HM, Mason JI, Sharpe RM. Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr Rev. 2009;30:883–925. doi: 10.1210/er.2009-0016. [DOI] [PubMed] [Google Scholar]
- 58.Zimmermann S, et al. Targeted disruption of the Insl3 gene causes bilateral cryptorchidism. Molecular endocrinology (Baltimore, Md) 1999;13:681–691. doi: 10.1210/mend.13.5.0272. [DOI] [PubMed] [Google Scholar]
- 59.Bay K, Andersson AM. Human testicular insulin-like factor 3: in relation to development, reproductive hormones and andrological disorders. Int J Androl. 2011;34:97–109. doi: 10.1111/j.1365-2605.2010.01074.x. [DOI] [PubMed] [Google Scholar]
- 60.Toft G, et al. Perfluorooctane sulfonate concentrations in amniotic fluid, biomarkers of fetal leydig cell function, and cryptorchidism and hypospadias in Danish boys (1980–1996) Environmental Health Perspectives. 2016;124:151–156. doi: 10.1289/ehp.1409288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klemetti R, et al. Children born after assisted fertilization have an increased rate of major congenital anomalies. Fertility and Sterility. 2005;84:1300–1307. doi: 10.1016/j.fertnstert.2005.03.085. [DOI] [PubMed] [Google Scholar]
- 62.Pinborg A, Henningsen AKA, Malchau SS, Loft A. Congenital anomalies after assisted reproductive technology. Fertility and Sterility. 2013;99:327–332. doi: 10.1016/j.fertnstert.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 63.Funke S, et al. Male reproductive tract abnormalities: More common after assisted reproduction? Early Human Development. 2010;86:547–550. doi: 10.1016/j.earlhumdev.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 64.Bang JK, et al. Does infertility treatment increase male reproductive tract disorder? Urology. 2013;81:644–648. doi: 10.1016/j.urology.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 65.Fedder J, Loft A, Parner ET, Rasmussen S, Pinborg A. Neonatal outcome and congenital malformations in children born after ICSI with testicular or epididymal sperm: A controlled national cohort study. Human Reproduction. 2013;28:230–240. doi: 10.1093/humrep/des377. [DOI] [PubMed] [Google Scholar]
- 66.Kermani RM, et al. Congenital anomalies in infants conceived by assisted reproductive techniques. Archives of Iranian Medicine. 2012;15:228–231. [PubMed] [Google Scholar]
- 67.Yan J, et al. Birth defects after assisted reproductive technologies in China: Analysis of 15,405 offspring in seven centers (2004 to 2008) Fertility and Sterility. 2011;95:458–460. doi: 10.1016/j.fertnstert.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 68.Damgaard IN, et al. Risk factors for congenital cryptorchidism in a prospective birth cohort study. PLoS ONE. 2008;3 doi: 10.1371/journal.pone.0003051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hansen M, Kurinczuk JJ, De Klerk N, Burton P, Bower C. Assisted reproductive technology and major birth defects in Western Australia. Obstetrics and Gynecology. 2012;120:852–863. doi: 10.1097/AOG.0b013e318269c282. [DOI] [PubMed] [Google Scholar]
- 70.Halliday JL, et al. Increased risk of blastogenesis birth defects, arising in the first 4 weeks of pregnancy, after assisted reproductive technologies. Human reproduction (Oxford, England) 2010;25:59–65. doi: 10.1093/humrep/dep364. [DOI] [PubMed] [Google Scholar]
- 71.Zhu JL, Basso O, Obel C, Bille C, Olsen J. Infertility, infertility treatment, and congenital malformations: Danish national birth cohort. British Medical Journal. 2006;333:679–681. doi: 10.1136/bmj.38919.495718.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Simpson JL. Birth defects and assisted reproductive technologies. Seminars in fetal & neonatal medicine. 2014;19:177–182. doi: 10.1016/j.siny.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 73.Massaro PA, Maclellan DL, Anderson PA, Romao RLP. Does intracytoplasmic sperm injection pose an increased risk of genitourinary congenital malformations in offspring compared to in vitro fertilization? A systematic review and meta-analysis. Journal of Urology. 2015;193:1837–1842. doi: 10.1016/j.juro.2014.10.113. [DOI] [PubMed] [Google Scholar]
- 74.Källén B, Finnström O, Nygren KG, Olausson PO. In vitro fertilization (IVF) in Sweden: Risk for congenital malformations after different IVF methods. Birth Defects Research Part A - Clinical and Molecular Teratology. 2005;73:162–169. doi: 10.1002/bdra.20107. [DOI] [PubMed] [Google Scholar]
- 75.Pierik FH, Burdorf A, Deddens JA, Juttmann RE, Weber RFA. Maternal and paternal risk factors for cryptorchidism and hypospadias: A case-control study in newborn boys. Environmental Health Perspectives. 2004;112:1570–1576. doi: 10.1289/ehp.7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McBride ML, Van Den Steen N, Lamb CW, Gallagher RP. Maternal and gestational factors in cryptorchidism. International Journal of Epidemiology. 1991;20:964–970. doi: 10.1093/ije/20.4.964. [DOI] [PubMed] [Google Scholar]
- 77.Mavrogenis S, Urbán R, Czeizel AE. Characteristics of boys with the so-called true undescended testis diagnosed at the third postnatal month - A population-based case-control study. Journal of Maternal-Fetal and Neonatal Medicine. 2015;28:1152–1157. doi: 10.3109/14767058.2014.947569. [DOI] [PubMed] [Google Scholar]
- 78.Mori M, Davies TW, Tsukamoto T, Kumamoto Y, Fukuda K. Maternal and other factors of cryptorchidism--a case-control study in Japan. Kurume Medical Journal. 1992;39:53–60. doi: 10.2739/kurumemedj.39.53. [DOI] [PubMed] [Google Scholar]
- 79.Kurahashi N, et al. Parental and neonatal risk factors for cryptorchidism. Medical Science Monitor. 2005;11:CR274–CR283. [PubMed] [Google Scholar]
- 80.Zakaria M, Azab S, El Baz M, Fawaz L, Bahagat A. Cryptorchidism in Egyptian neonates. Journal of Pediatric Urology. 2013;9:815–819. doi: 10.1016/j.jpurol.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 81.Berkowitz GS, Lapinski RH, Godbold JH, Dolgin SE, Holzman IR. Maternal and neonatal risk factors for cryptorchidism. Epidemiology. 1995;6:127–131. doi: 10.1097/00001648-199503000-00007. [DOI] [PubMed] [Google Scholar]
- 82.McGlynn KA, Graubard BI, Klebanoff MA, Longnecker MP. Risk factors for cryptorchism among populations at differing risks of testicular cancer. International Journal of Epidemiology. 2006;35:787–795. doi: 10.1093/ije/dyl024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Møller H, Skakkebæk NE. Testicular cancer and cryptorchidism in relation to prenatal factors: Case-control studies in Denmark. Cancer Causes and Control. 1997;8:904–912. doi: 10.1023/A:1018472530653. [DOI] [PubMed] [Google Scholar]
- 84.Swerdlow AJ, Wood KH, Smith PG. A case-control study of the aetiology of cryptorchidism. Journal of Epidemiology and Community Health. 1983;37:238–244. doi: 10.1136/jech.37.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Csermely G, Susánszky É, Czeizel AE. Association of young and advanced age of pregnant women with the risk of isolated congenital abnormalities in Hungary - A population-based case-matched control study. Journal of Maternal-Fetal and Neonatal Medicine. 2015;28:436–442. doi: 10.3109/14767058.2014.918946. [DOI] [PubMed] [Google Scholar]
- 86.Weidner IS, Møller H, Jensen TK, SkakkebÆk NE. Risk factors for cryptorchidism and hypospadias. Journal of Urology. 1999;161:1606–1609. doi: 10.1016/S0022-5347(05)68992-6. [DOI] [PubMed] [Google Scholar]
- 87.Hjertkvist M, Damber JE, Bergh A. Cryptorchidism: A registry based study in Sweden on some factors of possible aetiological importance. Journal of Epidemiology and Community Health. 1989;43:324–329. doi: 10.1136/jech.43.4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mayr JM, Lawrenz K, Berghold A. Undescended testicles: An epidemiological review. Acta Paediatrica, International Journal of Paediatrics. 1999;88:1089–1093. doi: 10.1080/08035259950168144. [DOI] [PubMed] [Google Scholar]
- 89.Biggs ML, Baer A, Critchlow CW. Maternal, delivery, and perinatal characteristics associated with cryptorchidism: A population-based case-control study among births in Washington State. Epidemiology. 2002;13:197–204. doi: 10.1097/00001648-200203000-00015. [DOI] [PubMed] [Google Scholar]
- 90.Emanuele N, Emanuele MA. The endocrine system alcohol alters critical hormonal balance. Alcohol Research and Health. 1997;21:53–64. [PMC free article] [PubMed] [Google Scholar]
- 91.Emanuele MA, Emanuele N. Alcohol and the male reproductive system. Alcohol Research and Health. 2001;25:282–287. [PMC free article] [PubMed] [Google Scholar]
- 92.Brouwers MM, et al. Risk factors for undescended testis. Journal of Pediatric Urology. 2012;8:59–66. doi: 10.1016/j.jpurol.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 93.Giordano F, et al. Maternal diet and the risk of hypospadias and cryptorchidism in the offspring. Paediatric and Perinatal Epidemiology. 2008;22:249–260. doi: 10.1111/j.1365-3016.2007.00918.x. [DOI] [PubMed] [Google Scholar]
- 94.Berkowitz GS, Lapinski RH. Risk factors for cryptorchidism: A nested case-control study. Paediatric and Perinatal Epidemiology. 1996;10:39–51. doi: 10.1111/j.1365-3016.1996.tb00024.x. [DOI] [PubMed] [Google Scholar]
- 95.Mongraw-Chaffin ML, Cohn BA, Cohen RD, Christianson RE. Maternal smoking, alcohol consumption, and caffeine consumption during pregnancy in relation to a son’s risk of persistent cryptorchidism: A prospective study in the child health and development studies cohort, 1959–1967. American Journal of Epidemiology. 2008;167:257–261. doi: 10.1093/aje/kwm311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jensen MS, Bonde JP, Olsen J. Prenatal alcohol exposure and cryptorchidism. Acta Paediatrica, International Journal of Paediatrics. 2007;96:1681–1685. doi: 10.1111/j.1651-2227.2007.00506.x. [DOI] [PubMed] [Google Scholar]
- 97.Damgaard IN, et al. Cryptorchidism and maternal alcohol consumption during pregnancy. Environmental Health Perspectives. 2007;115:272–277. doi: 10.1289/ehp.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Carbone P, et al. The possible role of endocrine disrupting chemicals in the aetiology of cryptorchidism and hypospadias: A population-based case-control study in rural Sicily. International Journal of Andrology. 2007;30:3–13. doi: 10.1111/j.1365-2605.2006.00703.x. [DOI] [PubMed] [Google Scholar]
- 99.Strandberg-Larsen K, Jensen MS, Ramlau-Hansen CH, Grønbæk M, Olsen J. Alcohol binge drinking during pregnancy and cryptorchidism. Human Reproduction. 2009;24:3211–3219. doi: 10.1093/humrep/dep325. [DOI] [PubMed] [Google Scholar]
- 100.Zhang L, et al. Maternal gestational smoking, diabetes, alcohol drinking, pre-pregnancy obesity and the risk of cryptorchidism: A systematic review and meta-analysis of observational studies. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0119006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cnattingius S. The epidemiology of smoking during pregnancy: Smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine and Tobacco Research. 2004;6:S125–S140. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- 102.Werler M. Maternal smoking and undescended testes: Reaching a tipping point. Epidemiology. 2007;18:197–198. doi: 10.1097/01.ede.0000254697.64626.1f. [DOI] [PubMed] [Google Scholar]
- 103.Windham GC, Mitchell P, Anderson M, Lasley BL. Cigarette Smoking and Effects on Hormone Function in Premenopausal Women. Environmental Health Perspectives. 2005;113:1285–1290. doi: 10.1289/ehp.7899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang J, Savitz DA, Schwingl PJ, Cai WW. A case-control study of paternal smoking and birth defects. International Journal of Epidemiology. 1992;21:273–278. doi: 10.1093/ije/21.2.273. [DOI] [PubMed] [Google Scholar]
- 105.Jensen MS, Toft G, Thulstrup AM, Bonde JP, Olsen J. Cryptorchidism according to maternal gestational smoking. Epidemiology. 2007;18:220–225. doi: 10.1097/01.ede.0000254061.90686.9f. [DOI] [PubMed] [Google Scholar]
- 106.Hackshaw A, Rodeck C, Boniface S. Maternal smoking in pregnancy and birth defects: A systematic review based on 173 687 malformed cases and 11.7 million controls. Human Reproduction Update. 2011;17:589–604. doi: 10.1093/humupd/dmr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thorup J, Cortes D, Petersen BL. The Incidence of Bilateral Cryptorchidism is Increased and the Fertility Potential is Reduced in Sons Born to Mothers who Have Smoked During Pregnancy. Journal of Urology. 2006;176:734–737. doi: 10.1016/j.juro.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 108.Gaspari L, et al. Prenatal environmental risk factors for genital malformations in a population of 1442 French male newborns: A nested casecontrol study. Human Reproduction. 2011;26:3155–3162. doi: 10.1093/humrep/der283. [DOI] [PubMed] [Google Scholar]
- 109.Office on Smoking and Health. A report of the Surgeon General. US Department of Health and Human Services; Washington, DC: 1980. The health consequences of smoking for women. [Google Scholar]
- 110.Mavrogenis S, Urban R, Czeizel AE, Ács N. Possible association of maternal factors with the higher risk of isolated true undescended testis: A population-based case-control study. Congenital Anomalies. 2014;54:178–183. doi: 10.1111/cga.12061. [DOI] [PubMed] [Google Scholar]
- 111.Palmer JR, et al. Urogenital abnormalities in men exposed to diethylstilbestrol in utero: A cohort study. Environmental Health: A Global Access Science Source. 2009;8 doi: 10.1186/1476-069X-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Townsend CL, Willey BA, Cortina-Borja M, Peckham CS, Tookey PA. Antiretroviral therapy and congenital abnormalities in infants born to HIV-infected women in the UK and Ireland, 1990–2007. AIDS. 2009;23:519–524. doi: 10.1097/QAD.0b013e328326ca8e. [DOI] [PubMed] [Google Scholar]
- 113.Ács N, Bánhidy F, Puhó EH, Czeizel AE. No association between vulvovaginitis-bacterial vaginosis, related drug treatments of pregnant women, and congenital abnormalities in their offspring - A population-based case-control study. Central European Journal of Medicine. 2008;3:332–340. doi: 10.2478/s11536-008-0027-9. [DOI] [Google Scholar]
- 114.Czeizel AE, Kazy Z, Puhó E. A population-based case-control teratological study of oral nystatin treatment during pregnancy. Scandinavian Journal of Infectious Diseases. 2003;35:830–835. doi: 10.1080/00365540310017069. [DOI] [PubMed] [Google Scholar]
- 115.Louik C, Lin AE, Werler MM, Hernández-Díaz S, Mitchell AA. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. New England Journal of Medicine. 2007;356:2675–2683. doi: 10.1056/NEJMoa067407. [DOI] [PubMed] [Google Scholar]
- 116.Ács N, Bánhidy F, Puhó EH, Czeizel AE. Senna treatment in pregnant women and congenital abnormalities in their offspring-A population-based case-control study. Reproductive Toxicology. 2009;28:100–104. doi: 10.1016/j.reprotox.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 117.Bártfai Z, Somoskövi A, Puhó EH, Czeizel AE. No teratogenic effect of prenoxdiazine: A population-based case-control study. Congenital Anomalies. 2007;47:16–21. doi: 10.1111/j.1741-4520.2006.00136.x. [DOI] [PubMed] [Google Scholar]
- 118.Kjær D, et al. Use of phenytoin, phenobarbital, or diazepam during pregnancy and risk of congenital abnormalities: A case-time-control study. Pharmacoepidemiology and Drug Safety. 2007;16:181–188. doi: 10.1002/pds.1288. [DOI] [PubMed] [Google Scholar]
- 119.Dessens AB, et al. Association of prenatal phenobarbital and phenytoin exposure with genital anomalies and menstrual disorders. Teratology. 2001;64:181–188. doi: 10.1002/tera.1063. [DOI] [PubMed] [Google Scholar]
- 120.Diav-Citrin O, et al. Pregnancy outcome after in utero exposure to valproate: Evidence of dose relationship in teratogenic effect. CNS Drugs. 2008;22:325–334. doi: 10.2165/00023210-200822040-00004. [DOI] [PubMed] [Google Scholar]
- 121.Mazaud-Guittot S, et al. Paracetamol, aspirin, and indomethacin induce endocrine disturbances in the human fetal testis capable of interfering with testicular descent. Journal of Clinical Endocrinology and Metabolism. 2013;98:E1757–E1767. doi: 10.1210/jc.2013-2531. [DOI] [PubMed] [Google Scholar]
- 122.Snijder CA, et al. Intrauterine exposure to mild analgesics during pregnancy and the occurrence of cryptorchidism and hypospadia in the offspring: The Generation R Study. Human Reproduction. 2012;27:1191–1201. doi: 10.1093/humrep/der474. [DOI] [PubMed] [Google Scholar]
- 123.Kristensen DM, et al. Intrauterine exposure to mild analgesics is a risk factor for development of male reproductive disorders in human and rat. Human Reproduction. 2011;26:235–244. doi: 10.1093/humrep/deq323. [DOI] [PubMed] [Google Scholar]
- 124.Jensen MS, et al. Maternal use of acetaminophen, ibuprofen, and acetylsalicylic acid during pregnancy and risk of cryptorchidism. Epidemiology. 2010;21:779–785. doi: 10.1097/EDE.0b013e3181f20bed. [DOI] [PubMed] [Google Scholar]
- 125.Philippat C, et al. Analgesics During Pregnancy and Undescended Testis. Epidemiology. 2011;22:747–749. doi: 10.1097/EDE.0b013e318225bf33. [DOI] [PubMed] [Google Scholar]
- 126.Rebordosa C, et al. Acetaminophen use during pregnancy: effects on risk for congenital abnormalities. American Journal of Obstetrics and Gynecology. 2008;198:178.e171–178.e177. doi: 10.1016/j.ajog.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 127.Møbjerg Kristensen D, Main KM, Jensen TK, Skakkebæk NE, Toppari J. Reply: Analgesic use and its effect on male reproduction. Human Reproduction. 2011;26:2259–2260. doi: 10.1093/humrep/der152. [DOI] [PubMed] [Google Scholar]
- 128.Gurney J, Shaw C, Stanley J, Signal V, Sarfati D. Cannabis exposure and risk of testicular cancer: a systematic review and meta-analysis. BMC Cancer. 2015;15:1–10. doi: 10.1186/s12885-015-1905-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lacson JCA, et al. Population-based case-control study of recreational drug use and testis cancer risk confirms an association between marijuana use and nonseminoma risk. Cancer. 2012;118:5374–5383. doi: 10.1002/cncr.27554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Trabert B, Sigurdson AJ, Sweeney AM, Strom SS, McGlynn KA. Marijuana use and testicular germ cell tumors. Cancer. 2011;117:848–853. doi: 10.1002/cncr.25499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Daling JR, et al. Association of marijuana use and the incidence of testicular germ cell tumors. Cancer. 2009;115:1215–1223. doi: 10.1002/cncr.24159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shono T, Shima Y, Kondo T, Suita S. In utero exposure to mono-N-butyl phthalate impairs insulin-like factor 3 gene expression and the transabdominal phase of testicular descent in fetal rats. Journal of Pediatric Surgery. 2005;40:1861–1864. doi: 10.1016/j.jpedsurg.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 133.Shono T, Suita S, Kai H, Yamaguchi Y. The Effect of a Prenatal Androgen Disruptor, Vinclozolin, on Gubernacular Migration and Testicular Descent in Rats. Journal of Pediatric Surgery. 2004;39:213–216. doi: 10.1016/j.jpedsurg.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 134.Saillenfait AM, Gallissot F, Sabaté JP. Differential developmental toxicities of di-n-hexyl phthalate and dicyclohexyl phthalate administered orally to rats. Journal of Applied Toxicology. 2009;29:510–521. doi: 10.1002/jat.1436. [DOI] [PubMed] [Google Scholar]
- 135.Drake AJ, et al. Glucocorticoids amplify dibutyl phthalate-induced disruption of testosterone production and male reproductive development. Endocrinology. 2009;150:5055–5064. doi: 10.1210/en.2009-0700. [DOI] [PubMed] [Google Scholar]
- 136.Gray LE, Jr, et al. Effects of environmental antiandrogens on reproductive development in experimental animals. Human Reproduction Update. 2001;7:248–264. doi: 10.1093/humupd/7.3.248. [DOI] [PubMed] [Google Scholar]
- 137.McKinnell C, et al. Expression of insulin-like factor 3 protein in the rat testis during fetal and postnatal development and in relation to cryptorchidism induced by in utero exposure to Di (n-butyl) phthalate. Endocrinology. 2005;146:4536–4544. doi: 10.1210/en.2005-0676. [DOI] [PubMed] [Google Scholar]
- 138.Jørgensen KT, et al. Risk of cryptorchidism among sons of horticultural workers and farmers in Denmark. Scandinavian Journal of Work, Environment and Health. 2014;40:323–330. doi: 10.5271/sjweh.3399. [DOI] [PubMed] [Google Scholar]
- 139.Weidner IS, Møller H, Jensen TK, Skakkebæk NE. Cryptorchidism and hypospadias in sons of gardeners and farmers. Environmental Health Perspectives. 1998;106:793–796. doi: 10.1289/ehp.98106793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gabel P, et al. The risk of cryptorchidism among sons of women working in horticulture in Denmark: A cohort study. Environmental Health: A Global Access Science Source. 2011;10 doi: 10.1186/1476-069X-10-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kristensen P, Irgens LM, Andersen A, Bye AS, Sundheim L. Birth defects among offspring of Norwegian farmers, 1967–1991. Epidemiology. 1997;8:537–544. doi: 10.1097/00001648-199709000-00011. [DOI] [PubMed] [Google Scholar]
- 142.Morales-Surez-Varela MM, et al. Parental occupational exposure to endocrine disrupting chemicals and male genital malformations: A study in the danish national birth cohort study. Environmental Health: A Global Access Science Source. 2011;10 doi: 10.1186/1476-069X-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vaktskjold A, et al. Genital malformations in newborns of female nickel-refinery workers. Scandinavian Journal of Work, Environment and Health. 2006;32:41–50. doi: 10.5271/sjweh.975. [DOI] [PubMed] [Google Scholar]
- 144.Jørgensen KT, et al. Risk of cryptorchidism and hypospadias among boys of maternal hairdressers - A Danish population-based cohort study. Scandinavian Journal of Work, Environment and Health. 2013;39:302–309. doi: 10.5271/sjweh.3330. [DOI] [PubMed] [Google Scholar]
- 145.Carran M, Shaw IC. New Zealand Malayan war veterans’ exposure to dibutylphthalate is associated with an increased incidence of cryptorchidism, hypospadias and breast cancer in their children. New Zealand Medical Journal. 2012;125:52–63. [PubMed] [Google Scholar]
- 146.Bornman R, de Jager C, Worku Z, Farias P, Reif S. DDT and urogenital malformations in newborn boys in a malarial area. BJU international. 2010;106:405–411. doi: 10.1111/j.1464-410X.2009.09003.x. [DOI] [PubMed] [Google Scholar]
- 147.Carbone P, et al. Cryptorchidism and hypospadias in the Sicilian district of Ragusa and the use of pesticides. Reproductive Toxicology. 2006;22:8–12. doi: 10.1016/j.reprotox.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 148.García-Rodríguez J, et al. Exposure to pesticides and cryptorchidism: Geographical evidence of a possible association. Environmental Health Perspectives. 1996;104:1090–1095. doi: 10.1289/ehp.104-1469503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Agopian AJ, Lupo PJ, Canfield MA, Langlois PH. Case-Control Study of Maternal Residential Atrazine Exposure and Male Genital Malformations. American Journal of Medical Genetics, Part A. 2013;161:977–982. doi: 10.1002/ajmg.a.35815. [DOI] [PubMed] [Google Scholar]
- 150.Czeizel AE, Hegedüs S, Tímár L. Congenital abnormalities and indicators of germinal mutations in the vicinity of an acrylonitrile producing factory. Mutation Research - Fundamental and Molecular Mechanisms of Mutagenesis. 1999;427:105–123. doi: 10.1016/S0027-5107(99)00090-1. [DOI] [PubMed] [Google Scholar]
- 151.Chul Kim S, Kyoung Kwon S, Pyo Hong Y. Trends in the incidence of cryptorchidism and hypospadias of registry-based data in Korea: A comparison between industrialized areas of petrochemical estates and a non-industrialized area. Asian Journal of Andrology. 2011;13:715–718. doi: 10.1038/aja.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Waliszewski SM, et al. Persistent organochlorine pesticides levels in blood serum lipids in women bearing babies with undescended testis. Bulletin of Environmental Contamination and Toxicology. 2005;75:952–959. doi: 10.1007/s00128-005-0842-5. [DOI] [PubMed] [Google Scholar]
- 153.Trabert B, Longnecker MP, Brock JW, Klebanoff MA, McGlynn KA. Maternal pregnancy levels of trans-nonachlor and oxychlordane and prevalence of cryptorchidism and hypospadias in boys. Environmental Health Perspectives. 2012;120:478–482. doi: 10.1289/ehp.1103936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Small CM, et al. Maternal exposure to a brominated flame retardant and genitourinary conditions in male offspring. Environmental Health Perspectives. 2009;117:1175–1179. doi: 10.1289/ehp.0800058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bhatia R, et al. Organochlorine pesticides and male genital anomalies in the child health and development studies. Environmental Health Perspectives. 2005;113:220–224. doi: 10.1289/ehp.7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pierik FH, Klebanoff MA, Brock JW, Longnecker MP. Maternal pregnancy serum level of heptachlor epoxide, hexachlorobenzene, and β-hexachlorocyclohexane and risk of cryptorchidism in offspring. Environmental Research. 2007;105:364–369. doi: 10.1016/j.envres.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.McGlynn KA, et al. Maternal pregnancy levels of polychlorinated biphenyls and risk of hypospadias and cryptorchidism in male offspring. Environmental Health Perspectives. 2009;117:1472–1476. doi: 10.1289/ehp.0800389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Longnecker MP, et al. Maternal serum level of 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene and risk of cryptorchidism, hypospadias, and polythelia among male offspring. American Journal of Epidemiology. 2002;155:313–322. doi: 10.1093/aje/155.4.313. [DOI] [PubMed] [Google Scholar]
- 159.Main KM, et al. Flame retardants in placenta and breast milk and cryptorchildism in newborn boys. Environmental Health Perspectives. 2007;115:1519–1526. doi: 10.1289/ehp.9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Virtanen HE, et al. Associations between congenital cryptorchidism in newborn boys and levels of dioxins and PCBs in placenta. International Journal of Andrology. 2012;35:283–293. doi: 10.1111/j.1365-2605.2011.01233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shen H, et al. Infant exposure to persistent organochlorine compounds is higher in Denmark than in Finland. WIT Transactions on Ecology and the Environment. 2008;110:45–52. doi: 10.2495/ETOX080051. [DOI] [Google Scholar]
- 162.Shen H, et al. Concentrations of persistent organochlorine compounds in human milk and placenta are higher in Denmark than in Finland. Human reproduction (Oxford, England) 2008;23:201–210. doi: 10.1093/humrep/dem199. [DOI] [PubMed] [Google Scholar]
- 163.Main KM, et al. Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in infants three months of age. Environmental Health Perspectives. 2006;114:270–276. doi: 10.1289/ehp.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Krysiak-Baltyn K, et al. Association between chemical pattern in breast milk and congenital cryptorchidism: Modelling of complex human exposures. International Journal of Andrology. 2012;35:294–302. doi: 10.1111/j.1365-2605.2012.01268.x. [DOI] [PubMed] [Google Scholar]
- 165.Damgaard IN, et al. Persistent pesticides in human breast milk and cryptorchidism. Environmental Health Perspectives. 2006;114:1133–1138. doi: 10.1289/ehp.8741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Brucker-Davis F, et al. Cord blood thyroid tests in boys born with and without cryptorchidism: Correlations with birth parameters and in utero xenobiotics exposure. Thyroid. 2011;21:1133–1141. doi: 10.1089/thy.2010.0459. [DOI] [PubMed] [Google Scholar]
- 167.Arrebola JP, et al. A novel biomarker for anti-androgenic activity in placenta reveals risks of urogenital malformations. Reproduction. 2015;149:605–613. doi: 10.1530/REP-14-0525. [DOI] [PubMed] [Google Scholar]
- 168.Jensen DV, et al. No association between exposure to perfluorinated compounds and congenital cryptorchidism: A nested case-control study Among 215 Boys from Denmark and Finland. Reproduction. 2014;147:411–417. doi: 10.1530/REP-13-0444. [DOI] [PubMed] [Google Scholar]
- 169.Rantakokko P, et al. Association of placenta organotin concentrations with congenital cryptorchidism and reproductive hormone levels in 280 newborn boys from Denmark and Finland. Human Reproduction. 2013;28:1647–1660. doi: 10.1093/humrep/det040. [DOI] [PubMed] [Google Scholar]
- 170.Fernandez MF, et al. Human exposure to endocrine-disrupting chemicals and prenatal risk factors for cryptorchidism and hypospadias: A nested case-control study. Environmental Health Perspectives. 2007;115:8–14. doi: 10.1289/ehp.9351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fernández MF, et al. Bisphenol A and other phenols in human placenta from children with cryptorchidism or hypospadias. Reproductive Toxicology. 2016;59:89–95. doi: 10.1016/j.reprotox.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 172.Fénichel P, et al. Unconjugated bisphenol A cord blood levels in boys with descended or undescended testes. Human Reproduction. 2012;27:983–990. doi: 10.1093/humrep/der451. [DOI] [PubMed] [Google Scholar]
- 173.Koskenniemi JJ, et al. Association between levels of persistent organic pollutants in adipose tissue and cryptorchidism in early childhood: a case-control study. Environmental Health: A Global Access Science Source. 2015;14 doi: 10.1186/s12940-015-0065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hosie S, Loff S, Witt K, Niessen K, Waag KL. Is there a correlation between organochlorine compounds and undescended testes? European Journal of Pediatric Surgery. 2000;10:304–309. doi: 10.1055/s-2008-1072381. [DOI] [PubMed] [Google Scholar]
- 175.Saiyed H, et al. Effect of endosulfan on male reproductive development. Environmental Health Perspectives. 2003;111:1958–1962. doi: 10.1289/ehp.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Komarowska MD, et al. Serum Bisphenol A Level in Boys with Cryptorchidism: A Step to Male Infertility? International Journal of Endocrinology. 2015;2015 doi: 10.1155/2015/973154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Brucker-Davis F, et al. Cryptorchidism at birth in Nice area (France) is associated with higher prenatal exposure to PCBs and DDE, as assessed by colostrum concentrations. Human Reproduction. 2008;23:1708–1718. doi: 10.1093/humrep/den186. [DOI] [PubMed] [Google Scholar]
- 178.Chevrier C, et al. Maternal urinary phthalates and phenols and male genital anomalies. Epidemiology. 2012;23:353–356. doi: 10.1097/EDE.0b013e318246073e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jackson MB, Swerdlow AJ. Seasonal variations in cryptorchidism. Journal of Epidemiology and Community Health. 1986;40:210–213. doi: 10.1136/jech.40.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kaleva M, et al. Circannual rhythm in the incidence of cryptorchidism in Finland. International Journal of Andrology. 2005;28:53–57. doi: 10.1111/j.1365-2605.2005.00510.x. [DOI] [PubMed] [Google Scholar]
- 181.Mamoulakis C, et al. Cryptorchidism: Seasonal variations in Greece do not support the theory of light. Andrologia. 2002;34:194–203. doi: 10.1046/j.1439-0272.2002.00492.x. [DOI] [PubMed] [Google Scholar]
- 182.Brantsæter AL, et al. Organic food consumption during pregnancy and hypospadias and cryptorchidism at birth: The Norwegian mother and child cohort study (MoBa) Environmental Health Perspectives. 2016;124:357–364. doi: 10.1289/ehp.1409518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Fulgoni VL, 3rd, Keast DR, Lieberman HR. Trends in intake and sources of caffeine in the diets of US adults: 2001–2010. The American journal of clinical nutrition. 2015;101:1081–1087. doi: 10.3945/ajcn.113.080077. [DOI] [PubMed] [Google Scholar]
- 184.Ralis ZA. Birth trauma to muscles in babies born by breech delivery and its possible fatal consequences. Arch Dis Child. 1975;50:4–13. doi: 10.1136/adc.50.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dunn PM. Testicular birth trauma. Arch Dis Child. 1975;50:744–745. [Google Scholar]
- 186.Tiwary CM. Testicular damage following breech delivery. Clin Res. 1973;21 [Google Scholar]
- 187.Depue RH. Maternal and gestational factors affecting the risk of cryptorchidism and inguinal hernia. International Journal of Epidemiology. 1984;13:311–318. doi: 10.1093/ije/13.3.311. [DOI] [PubMed] [Google Scholar]
- 188.Depue RH. Cryptorchidism, and epidemiologic study with emphasis on the relationship to central nervous system dysfunction. Teratology. 1988;37:301–305. doi: 10.1002/tera.1420370403. [DOI] [PubMed] [Google Scholar]
- 189.Gurney J, Sarfati D, Stanley J, Studd R. Do ethnic patterns in cryptorchidism reflect those found in testicular cancer? Journal of Urology. 2013;190:1852–1857. doi: 10.1016/j.juro.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 190.Jain VG, Singal AK. Shorter anogenital distance correlates with undescended testis: A detailed genital anthropometric analysis in human newborns. Human Reproduction. 2013;28:2343–2349. doi: 10.1093/humrep/det286. [DOI] [PubMed] [Google Scholar]
- 191.Kramer MS. Determinants of low birth weight: Methodological assessment and meta-analysis. Bulletin of the World Health Organization. 1987;65:663–737. [PMC free article] [PubMed] [Google Scholar]
- 192.Kayode GA, et al. Contextual risk factors for low birth weight: A multilevel analysis. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0109333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Jensen MS, et al. Cryptorchidism concordance in monozygotic and dizygotic twin brothers, full brothers, and half-brothers. Fertility and Sterility. 2010;93:124–129. doi: 10.1016/j.fertnstert.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 194.Schnack TH, et al. Familial aggregation of cryptorchidism--a nationwide cohort study. American journal of epidemiology. 2008;167:1453–1457. doi: 10.1093/aje/kwn081. [DOI] [PubMed] [Google Scholar]
- 195.Nef S, Parada LF. Cryptorchidism in mice mutant for Insl3. Nature Genetics. 1999;22:295–299. doi: 10.1038/10364. [DOI] [PubMed] [Google Scholar]
- 196.Elert A, Jahn K, Heidenreich A, Hofmann R. Population-based investigation of familial undescended testis and its association with other urogenital anomalies. Journal of Pediatric Urology. 2005;1:403–407. doi: 10.1016/j.jpurol.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 197.Barthold JS, et al. Pathway analysis supports association of nonsyndromic cryptorchidism with genetic loci linked to cytoskeleton-dependent functions. Human Reproduction. 2015;30:2439–2451. doi: 10.1093/humrep/dev180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.El Houate B, et al. Novel mutations involving the INSL3 gene associated with cryptorchidism. J Urol. 2007;177:1947–1951. doi: 10.1016/j.juro.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 199.Ferlin A, et al. Mutations in INSL3 and RXFP2 genes in cryptorchid boys. Annals of the New York Academy of Sciences. 2009;1160:213–214. doi: 10.1111/j.1749-6632.2008.03784.x. [DOI] [PubMed] [Google Scholar]
- 200.Ferlin A, et al. Mutational screening of NR5A1 gene encoding steroidogenic factor 1 in cryptorchidism and male factor infertility and functional analysis of seven undescribed mutations. Fertility and Sterility. 2015;104:163–169.e161. doi: 10.1016/j.fertnstert.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 201.Qin XY, et al. Association of variants in genes involved in environmental chemical metabolism and risk of cryptorchidism and hypospadias. Journal of Human Genetics. 2012;57:434–441. doi: 10.1038/jhg.2012.48. [DOI] [PubMed] [Google Scholar]
- 202.Gurney J, Sarfati D, Stanley J. Obscure etiology, unusual disparity: the epidemiology of testicular cancer in New Zealand. Cancer Causes and Control. 2015;26:561–569. doi: 10.1007/s10552-015-0533-4. [DOI] [PubMed] [Google Scholar]
- 203.Sarfati D, Shaw C, Blakely T, Atkinson J, Stanley J. Ethnic and socioeconomic trends in testicular cancer. International Journal of Cancer. 2010;128:1683–1691. doi: 10.1002/ijc.25486. [DOI] [PubMed] [Google Scholar]
- 204.Bay K, Main KM, Toppari J, Skakkebaek NE. Testicular descent: INSL3, testosterone, genes and the intrauterine milieu. Nature Reviews Urology. 2011;8:187–196. doi: 10.1038/nrurol.2011.23. [DOI] [PubMed] [Google Scholar]
- 205.Zimmermann S, et al. Targeted disruption of the Insl3 gene causes bilateral cryptorchidism. Molecular Endocrinology. 1999;13:681–691. doi: 10.1210/mend.13.5.0272. [DOI] [PubMed] [Google Scholar]
- 206.Hutson JM, Balic A, Nation T, Southwell B. Cryptorchidism. Seminars in pediatric surgery. 2010;19:215–224. doi: 10.1053/j.sempedsurg.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 207.Hughes IA, Acerini CL. Factors controlling testis descent. European Journal of Endocrinology. 2008;159:S75–S82. doi: 10.1530/EJE-08-0458. [DOI] [PubMed] [Google Scholar]
- 208.Virtanen HE, et al. Mild gestational diabetes as a risk factor for congenital cryptorchidism. Journal of Clinical Endocrinology and Metabolism. 2006;91:4862–4865. doi: 10.1210/jc.2006-1420. [DOI] [PubMed] [Google Scholar]
- 209.Nielsen GL, et al. Risk of specific congenital abnormalities in offspring of women with diabetes. Diabetic Medicine. 2005;22:693–696. doi: 10.1111/j.1464-5491.2005.01477.x. [DOI] [PubMed] [Google Scholar]
- 210.Bánhidy F, Ács N, Puhó EH, Czeizel AE. Congenital abnormalities in the offspring of pregnant women with type 1, type 2 and gestational diabetes mellitus: A population-based case-control study. Congenital Anomalies. 2010;50:115–121. doi: 10.1111/j.1741-4520.2010.00275.x. [DOI] [PubMed] [Google Scholar]
- 211.Trabert B, et al. Gestational diabetes and the risk of cryptorchidism and hypospadias. Epidemiology. 2014;25:152–153. doi: 10.1097/EDE.0000000000000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Agopian AJ, Langlois PH, Ramakrishnan A, Canfield MA. Epidemiologic features of male genital malformations and subtypes in Texas. American journal of medical genetics. Part A. 2014;164a:943–949. doi: 10.1002/ajmg.a.36389. [DOI] [PubMed] [Google Scholar]
- 213.Klebanoff MA, Mills JL. Is vomiting during pregnancy teratogenic? British Medical Journal. 1986;292:724–726. doi: 10.1136/bmj.292.6522.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Czeizel AE, Puhó E, Ács N, Bánhidy F. Inverse association between severe nausea and vomiting in pregnancy and some congenital abnormalities. American Journal of Medical Genetics. 2006;140 A:453–462. doi: 10.1002/ajmg.a.31097. [DOI] [PubMed] [Google Scholar]
- 215.Zhang J, Olshan A, Cai WW. Birth defects in relation to threatened abortion. Epidemiology. 1994;5:341–344. doi: 10.1097/00001648-199405000-00014. [DOI] [PubMed] [Google Scholar]
- 216.Zhang J, Cai WW. Association of the common cold in the first trimester of pregnancy with birth defects. Pediatrics. 1993;92:559–563. [PubMed] [Google Scholar]
- 217.Ingstrup KG, et al. Maternal bereavement and cryptorchidism in offspring. Epidemiology (Cambridge, Mass) 2015;26:100–105. doi: 10.1097/EDE.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 218.Shono T, Suita S. Disturbed pituitary-testicular axis inhibits testicular descent in the prenatal rat. BJU international. 2003;92:641–643. doi: 10.1046/j.1464-410X.2003.04436.x. [DOI] [PubMed] [Google Scholar]
- 219.Adams SV, Hastert TA, Huang Y, Starr JR. No Association between Maternal Pre-pregnancy Obesity and Risk of Hypospadias or Cryptorchidism in Male Newborns. Birth defects research. Part A, Clinical and molecular teratology. 2011;91:241–248. doi: 10.1002/bdra.20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Aschim EL, et al. Linkage between cryptorchidism, hypospadias, and GGN repeat length in the androgen receptor gene. Journal of Clinical Endocrinology and Metabolism. 2004;89:5105–5109. doi: 10.1210/jc.2004-0293. [DOI] [PubMed] [Google Scholar]
- 221.Zhou B, et al. The variations in the AXIN1 gene and susceptibility to cryptorchidism. Journal of Pediatric Urology. 2015;11:132.e131–132.e135. doi: 10.1016/j.jpurol.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 222.Tang KF, Zheng JZ, Xing JP. Molecular analysis of SNP12 in estrogen receptor α Gene in hypospadiac or cryptorchid patients from northwestern China. Urologia Internationalis. 2011;87:359–362. doi: 10.1159/000330902. [DOI] [PubMed] [Google Scholar]
- 223.Yoshida R, et al. Association of cryptorchidism with a specific haplotype of the estrogen receptor α gene: Implication for the susceptibility to estrogenic environmental endocrine disruptors. Journal of Clinical Endocrinology and Metabolism. 2005;90:4716–4721. doi: 10.1210/jc.2005-0211. [DOI] [PubMed] [Google Scholar]
- 224.Galan JJ, et al. Molecular analysis of estrogen receptor alpha gene AGATA haplotype and SNP12 in European populations: Potential protective effect for cryptorchidism and lack of association with male infertility. Human Reproduction. 2007;22:444–449. doi: 10.1093/humrep/del391. [DOI] [PubMed] [Google Scholar]
- 225.Kolon TF, et al. Analysis of homeobox gene HOXA10 mutations in cryptorchidism. Journal of Urology. 1999;161:275–280. doi: 10.1016/S0022-5347(01)62132-3. [DOI] [PubMed] [Google Scholar]
- 226.Foresta C, Ferlin A. Role of INSL3 and LGR8 in cryptorchidism and testicular functions. Reproductive biomedicine online. 2004;9:294–298. doi: 10.1016/s1472-6483(10)62144-x. [DOI] [PubMed] [Google Scholar]
- 227.Gorlov IP, et al. Mutations of the GREAT gene cause cryptorchidism. Human Molecular Genetics. 2002;11:2309–2318. doi: 10.1093/hmg/11.19.2309. [DOI] [PubMed] [Google Scholar]
- 228.Salemi M, et al. Expression of Phosphodiesterase 4B cAMP-Specific Gene in Subjects With Cryptorchidism and Down’s Syndrome. Journal of Clinical Laboratory Analysis. 2015 doi: 10.1002/jcla.21835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ars E, et al. Further insights into the role of t222p variant of rxfp2 in non-syndromic cryptorchidism in two mediterranean populations. International Journal of Andrology. 2011;34:333–338. doi: 10.1111/j.1365-2605.2010.01088.x. [DOI] [PubMed] [Google Scholar]
- 230.Wada Y, Okada M, Fukami M, Sasagawa I, Ogata T. Association of cryptorchidism with Gly146Ala polymorphism in the gene for steroidogenic factor-1. Fertility and Sterility. 2006;85:787–790. doi: 10.1016/j.fertnstert.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 231.Salemi M, et al. SPAG5 mRNA is over-expressed in peripheral blood leukocytes of patients with Down’s syndrome and cryptorchidism. Neurological Sciences. 2013;34:549–551. doi: 10.1007/s10072-012-1152-4. [DOI] [PubMed] [Google Scholar]
- 232.Salemi M, et al. Expression of STRBP mRNA in patients with cryptorchidism and Down’s syndrome. Journal of Endocrinological Investigation. 2012;35:5–7. doi: 10.1007/BF03345414. [DOI] [PubMed] [Google Scholar]
- 233.Chew G, Hutson JM. Incidence of cryptorchidism and ascending testes in Trisomy 21: A 10 year retrospective review. Pediatric Surgery International. 2004;20:744–747. doi: 10.1007/s00383-004-1290-8. [DOI] [PubMed] [Google Scholar]
- 234.Barthold JS, et al. Altered infant feeding patterns in boys with acquired nonsyndromic cryptorchidism. Birth Defects Research Part A - Clinical and Molecular Teratology. 2012;94:900–907. doi: 10.1002/bdra.23075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


