Abstract
Brain Computer Interfaces (BCIs) use neural information recorded from the brain for voluntary control of external devices. The development of BCI systems has largely focused on improving functional independence for individuals with severe motor impairments, including providing tools for communication and mobility. In this review, we describe recent advances in intracortical BCI technology and provide potential directions for further research.
Index Terms: Brain Computer Interfaces, ALS, Spinal Cord Injury, Neural Decoding
I. Introduction
BRAIN computer interfaces (BCIs) use neural information recorded from the brain for voluntary control of external devices [1]–[10]. While there are many types of BCIs, all have three components: a sensor to record neural activity or its proxy, a decoder that converts neural activity into a command signal, and an effector such as a computer cursor or robotic arm (Figure 1). The sensor detects and records information. Information sources range from the locally detected electrical discharges of individual neurons, up to aggregate signals reflecting the simultaneous behavior of millions of neurons. Sensor choice impacts whether surgical implantation is required, whether or not the technology is portable or requires a specialized environment, the extent of expert assistance required to run the BCI system, and overall cost.
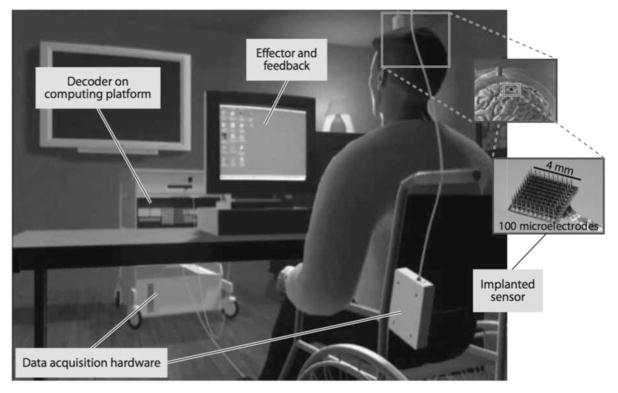
Fig. 1.
Illustration of a closed-loop intracortical BCI setup. The user has an intracortical electrode (Utah array) inserted into the motor cortex acting as the sensor, which then sends real-time voltage signals to the external processing system, converting raw voltages into spikes. The decoder then maps the high-dimensional spike data to low-dimensional output that is fed to the effector as computer cursor control. The real-time cursor control is then provided as feedback to the user, who is then able to modulate further neural activity accordingly.
The decoder is a mathematical algorithm that converts the complex neural data into a signal used to drive the effector. Effective neural decoding is predicated in part on advances in fundamental neuroscience (i.e. understanding the signal and noise characteristics of the sensor output) and logical algorithm choices. Finally, the effector choice reflects the use-case of the BCI. Many BCI systems are being developed to improve functional independence for individuals with severe motor limitations. Early pilot clinical trials are currently investigating the role of intracortical BCIs (iBCIs) for the purpose of restoring communication, such as typing on a computer screen [11]–[17] or as an effector for physical responses, such as controlling a robotic or prosthetic arm [18]–[21] or functional electrical stimulation systems [22], [23].
Other articles in this special edition publication describe BCI sensor technologies such as EEG, fMRI, fNIRS, or ECoG. In this review we provide an introduction to iBCI devices, as pertaining to ongoing feasibility clinical trials. We organize our discussion along each of the BCI components – the sensor, decoder and effector – and conclude by outlining the future directions for the field.
II. Intracortical recording devices as BCI sensors
The BCI sensor choice determines the temporal and spatial resolution of the neural signal [2], [8], [24]. The temporal resolution refers to the timescale over which neural signals are sampled and can be detected. For instance, in its current instantiation, near-infrared spectroscopy requires up to 25 seconds to generate a reliable binary command signal [25] whereas modern intracranial recording devices are able to record high-resolution field potentials [26] and intracortical recording devices are able to record action potentials with sub-millisecond precision. The spatial resolution refers to the volume of neuron-derived information that is aggregated together in the sensor. For instance, EEG recordings reflect changes in huge numbers of changes in neural electrical fields oriented perpendicular to the skull [27], [28], whereas implanted intracortical (or subcortical) recordings are able to focus on individual neuronal action potentials from ensembles of simultaneously recorded neurons.
Since intracortical devices record action potentials, effective decoding is predicated on understanding the functional role of the cortical area being recorded. Accordingly, we begin this section with a discussion of properties of the primary motor cortex relevant for iBCI systems.
A. Neurophysiological foundations of motor-imagery based BCI systems
Cortical regions are demarcated at a centimeter scale and are identified by their location and function [29]. For the purposes of BCI decoding in controlling assistive technologies, many researchers have focused on decoding neural activity from the motor cortex in both non-human primates (NHPs) [30]–[42] and early pilot clinical trials [11]–[15], [18]–[20], [23], [43]–[45].
Decades of basic science research and clinical findings have motivated the use of the motor cortex as a source of neural information for BCIs. It is clear that the primary motor cortex plays a key role in directing voluntary, dexterous movement [2], [7], [46]–[51], although successful decoding can also be achieved from other cortical regions [21], [52], [53]. First, neurons in a small area of primary motor cortex yield a rich variety of signals related to voluntary limb movement. Experiments have demonstrated that neurons modulate activity based on arm position [54], [55], velocity [56] and acceleration [57]; kinetic parameters [58] such as force [59] muscle activation [49], [60]; preparatory activity [44], [48], [61], and even task-related parameters such as instruction cues [62] and movement observation [63]. Importantly, neurons recorded on adjacent electrodes separated by micrometers [64] may display highly variable behavior [50], [65], meaning that recordings from small implantable electrodes may have the potential to sample this rich variety of behavior. Thus, there is tremendous potential to acquire a variety of task-relevant signals for subsequent decoding.
Second, in humans, the hand area of motor cortex is an accessible surgical target. It is easy to identify it pre-operatively using known anatomical landmarks [66], [67], and as part of pre-operative functional MRI protocols [68]–[70].
Third, neural modulation is present in motor cortex despite longstanding downstream injury of pyramidal neurons. This point is non-trivial: destruction of a neuron’s axonal projection triggers degeneration of the axon in a process called Wallerian degeneration, which can lead to cell death [71]. Despite controversies in the non-human primate literature as to the extent of corticospinal destruction resulting from downstream axonal damage [72], [73], recent successes in motor cortex decoding in people with paralysis suggest that sufficient neural information for BCI use [11]–[15], [18]–[20], [43]. Thus, it appears as though motor cortex retains relevant physiological properties despite longstanding damage to the motor system. This includes people with amyotrophic lateral sclerosis (see below), from whom motor cortical recording has provided control over external devices despite disease-related injury or death of pyramidal cells [74].
B. Intracortical recording devices
While there are multiple intracortical recording devices available [75], the intracranial device capable of single unit recordings currently used as part of ongoing clinical trials [2], [19], [21] and for recordings in individuals with epilepsy [76]–[78] is the Utah microarray (formally, Blackrock/NeuroPort array) [64]. Each array is a 4.2 × 4.2mm wafer with 100 shanks, each of which is 1.0 – 1.5mm in length spaced apart by 400μm. At the tip of each shank is a conducting electrode [79]. Extracellular voltage recordings are transmitted from the electrodes to a skull-mounted titanium pedestal via gold/platinum alloy cables. The pedestal has connector pins to which a cable can be attached to connect the device to external amplifiers and then computers for analysis [80].
The process of inserting multiple electrodes simultaneously into the brain is not trivial. While the brain is enveloped in the dura mater, the cortical surface is enveloped by both arachnoid and pial meninges. The pia mater in particular, is intimately attached to the cortical surface. In animal models, unassisted insertion of multiple electrodes on an array can cause cortical deformation, and may even result in cortical injury – thereby damaging the very neurons one is interested in recording [81], [82]. To circumvent this problem, Utah arrays are typically inserted into cortex using a pneumatic insertion device [83]. The pneumatic device attempts to mitigate insertion trauma and ensure the electrodes are inserted at the desired cortical depth, in hopes of optimizing signal quality.
While the standard design of the Utah array allows for a single electrode per shank (with customizable shank lengths), other recording devices have been designed to allow multiple electrode per shank, thereby permitting recordings at various cortical depths simultaneously [84]. Another approach, the Neutrotrophic electrode, contains an insulated gold wire fixed within a glass cone, containing a medium for attracting neurite growth [85].
The interface between brain parenchyma and electrode recording device is complex, and the specific material considerations may dramatically impact recording characteristics [75]. For instance, factors such as material pliability, shank/electrode alloy selection, electrode coating techniques, array insertion techniques, and other factors may impact recording quality [86]–[90].
III. BCI Decoding
We described BCIs as devices that use neural information recorded from the brain for voluntary control of external devices. The process of actually converting the neural information (often high-dimensional) into the output signal (usually low-dimensional) is referred to as neural decoding. We begin by describing how neural features are acquired from raw voltage signals. We then outline how modern neural decoding algorithms transform neural features into the command signals used to control external devices.
A. Distinguishing between open- and closed-loop systems
To understand BCI decoding, it is important to distinguish between open- or closed- loop decoding. An open-loop system is one where no feedback is provided back to the user. For instance, by recording from several dozen neurons in three motor-related cortical areas in NHPs, the complex movement of 25 joint angles can be faithfully reconstructed for complex reaching and grasping motions [91]. This movement reconstruction maps action potential events from dozens of neurons (high-dimensional) to kinematic reconstructions (low-dimensional). However, the result of the low-dimensional decoder output was not provided as feedback to the NHP at the time of experiment, thus making this an open-loop experiment. By contrast, a closed-loop system is one where the decoded output is provided as feedback to the BCI user, allowing the user to adjust volitional control in real-time. A now-classic example of closed-loop control was performed by Fetz [92]. An electrode was placed into the motor cortex of a NHP and neurons were found that responded to wrist torque; the firing rate of a neuron was used to drive an auditory signal. With practice, the NHP learned to modulate the firing rate of the neuron in real-time to earn a reward. Since the output of the effector (the auditory signal) was provided as feedback in real-time and used to drive further neural behavior, this was a demonstration of closed-loop single-neuron BCI control used to control an external device.
B. From voltages to spikes
Modern microelectrode arrays sample voltages at several thousand times per second and are designed to record single neuron action potentials through each channel. Since action potentials are voltage waves with canonical shapes and wavelengths [71], raw signals are band-pass filtered (typically, 0.5–7500Hz) and action potentials are identified as large sudden changes in the voltage (typically, 3–5 RMS Volts) lasting around 1ms. Due to the characteristic appearance of band-pass filtered signals, action potentials are also known as spikes.
As an alternative to spikes, local field potentials (LFPs) are acquired when raw voltage signals are band-pass filtered in lower frequency ranges (usually in the 0.1 – 200Hz range). LFPs reflect spatially averaged electrical fields generated from groups of neurons simultaneously [26], [93], and are important for coupling different regions of cortex simultaneously, and impact spike timing [94]–[96]. Specific frequency bands are relevant for motor control [97], [98]. LFPs have also been used for neural decoding with intracortical electrodes in NHPs [99]–[103] and in humans [104], [105].
C. Linear neural decoding strategies are predicated on cosine tuning
In what are now classic experiments, NHPs were taught to move a planar manipulandum to one of eight different directions. The spike rate of individual neurons and the direction of limb movement could be described parsimoniously using a sinusoidal curve with a period of 360 degrees [54]. For each neuron, the vector corresponding to the maximum firing rate (the phase offset) is referred to as the neuron’s “preferred direction”. To perform neural decoding using the population vector algorithm (PVA), the firing rate of each neuron is used to scale its preferred direction vector, and the sum of all vectors is used as the low-dimensional output. PVA is a special case of solving a linear regression problem: if the preferred directions are uniformly distributed, then the surface mapping the binned firing rates to the kinematic variables can be solved by solving for the least-squares estimator [106]. Motivated by the sinusoidal tuning properties of motor cortex neurons, the PVA/linear regression algorithm was shown to be successful in NHPs [30], [31], [33], [37], [38], and was modified [107] to allow an individual with tetraplegia to control a robotic limb [18], [19].
Firing rates recorded from a single neuron may be highly variable despite very similar (or even identical) experimental repetitions[108]–[110]. One approach to addressing noise may be smoothing inputs [11], [111] or by using a Weiner filter. Some experimenters have taken the approach of modeling the firing rates as being the “read-out” of a time-evolving hidden variable. This is tantamount to modeling the system as a hidden Markov model, where the observed variables are the firing rates and the hidden variable is modeled as the kinematics of the end-effector. By taking advantage of the cosine-tuning properties of neurons, the Kalman filter provides a principled approach for real-time hidden variable estimation [112], [113]. The Kalman filter has been implemented in early clinical research settings during to provide users with cursor [12]–[16], [43] and robotic limb control [20].
D. Non-linear decoding approaches move away from cosine tuning
The aforementioned decoding approaches -- the PVA, linear decoder, and Kalman -- all rely on binned spike data as input, assuming a rate code. An alternate way of approaching neural decoding is to model precise spike times as important. Rather than modeling neural behavior as a time-evolving rate-function, spike times may be seen as a realization of a point processes with an inhomogeneous (i.e. time-varying) conditional intensity function [35], [114]–[117]. Approaching spike trains in this way allows the experimenter to model how the intensity function varies as a function of: (1) the neuron’s own intrinsic spike history, (2) the behavior of other recorded neurons, and/or (3) external variables of interest [117]. Recent decoding approaches have used point process methods in both NHP [35], [116] and human subjects [117].
Future decoding approaches will undoubtedly take advantage of ongoing developments in the machine learning literature. For instance, recurrent neural networks were used as a decoder in NHPs using high dimensional neural input along with an element of temporal history [118], [119]. Neural decoding has also been done with kernel embedding of neural data [40], [120]–[123].
E. Adapting the user to the decoder vs. the decoder to the user
Two approaches for permitting BCI control have been described [124]. In the wrist torque experiments described above, a NHP learned to modulate firing rates based on sound feedback [92]. Here, the decoder was fixed (mapping firing rate to pitch) and the NHP had to adapt its behavior in order to use the system. Thus, cortical activity adapted to improve decoder use [6]. In fact, NHPs can learn to use sub-optimally parameterized decoders, achieving effective closed-loop control over several days [34], [125]. Moreover, when NHPs are taught to use a particular decoder and the parameters are changed, they are able to adapt to the new parameterizations[38].
Having users adapt their cortical activity to control external devices has been used in a variety of BCI environments [17], [40], [92], [125]–[130]. However, the process of developing reliable control requires days, weeks, or even months to learn to use. By contrast, the second approach -- which has been explored in the iBCI literature in NHPs and humans -- has been to adapt the algorithm to the user. Here, the decoder parameters are computed in a calibration process. One approach to calibration is to record from the cortex while the NHP is performing stereotyped movements [30]–[33], [131]. However, this approach cannot be applied in humans with paralysis.
The state-of-the-art approach to calibration of motor-imagery BCI devices in humans is a three-step process [11]. Users are first asked to attempt, or to imagine, performing an action -- without feedback -- in an open-loop step. Next, a trained technician uses the recorded data to compute decoder parameters. Finally, the user is provided closed-loop control. Decoders built solely from open-loop data yield sub-optimal control in closed-loop decoding, due to context shifts of the neural tuning properties [43], [132], [133]. Accordingly, modern state-of-the-art approaches to decoder calibration in humans require technician supervised closed-loop batch-based calibration sequences [11]–[14], [16], [20], [43], [134]. An alternative to requiring batch-based technician supervision is to perform closed-loop decoder adaptation. Here, the parameters for the decoder are updated as the user is controlling the end-effector in real-time [6], [35], [36], [124], [135]–[137]. Using this approach, NHPs can rapidly gain control of a decoder using a randomly initialized set of decoder parameter settings within minutes [135]. However, not all parameterizations will allow NHP to successfully achieve BCI control, and evidence suggests that low-dimensional features of neural ensembles must be maintained in order for NHPs to be able to learn to use decoders [38], [40].
IV. The BCI effector and its uses in restorative technology
Following the discussion of sensors and decoders, we now turn our attention to discussing the effectors. We focus our discussion for two different disease states in humans the challenges of which stand to be reduced through intracranial recordings.
A. Communication using single neurons by people with locked-in syndrome
The motor system is required for strong, coordinated and fluid voluntary movement; damage occurring at any point in the system may affect motor control. Limb weakness may result in paralysis of the legs (paraplegia) or four limbs (tetraplegia). An even more extensive form of paralysis is referred to as locked-in-syndrome (LIS), wherein individuals lack the ability for most voluntary movements (which usually includes limb, facial movements and speaking), though the individual may remain cognitively normal. One of the most common causes of LIS is amyotrophic lateral sclerosis (ALS). There are as many as 30,000 individuals with ALS in the United States, with as many as 5500 new cases diagnosed each year [138]. Individuals with LIS are dependent on caretakers for all aspects of their wellbeing. Despite the dramatic limitations in motor control, studies indicate that quality of life markers for individuals with ALS consistently demonstrate positive life attitudes [139], [140].
Research in LIS has focused on communication – an identified priority for those living with ALS. A recent Japanese mail-back questionnaire survey was conducted with 37 individuals living with ALS, 29 of whom had undergone tracheostomy for assisted respiratory support [141]. Nearly all respondents described feeling stressed when communicating, and most of the respondents who were already using assisted communication devices complained of difficulties using their present devices. The respondents who were interested in using BCI as a communication tool prioritized conversations with family or caregivers, as well as electronic communication.
BCI technology is a viable approach to providing tools for communication for individuals with ALS [13]–[16]. Kennedy and colleagues were the first to implant electrodes into an individual with ALS [128] using a specialized electrode with two recording wires and a neurotrophic factor [85]. While the first report demonstrated closed-loop control of neurons adjusting auditory signals, users subsequently demonstrated the ability for neurons to drive a computer cursor in one direction[142], and to use a wireless interface driving a one-dimensional signal [143].
The first example of reliable multi-dimensional cursor control in humans using a population of neurons in motor cortex was reported in 2006 [11]. Two individuals with paralysis due to cervical spinal cord injury demonstrated the ability to move a 2-dimensional neural cursor, play “pong” (similar to the classic video game but controlled entirely though neural spike trains) and one controlled a rudimentary robotic device. The feasibility of long-term intracortical recording as a communication device was demonstrated when a participant demonstrated successful decoding in an implanted array over 1000 days[13] and subsequently more than five years after implantation [20]. Recently, customized keyboards have been developed to take advantage of the directional cursor control, enabling typing speeds of over 10 words per minute using Internet chat applications[16]. Through this and other advances, it is hoped that intracortical recording will provide the basis for individuals with LIS and ALS to communicate readily [144].
B. Restoration of arm control is a priority for individuals with tetraplegia
There are millions of people worldwide[145] and roughly a quarter-million people in the United States United States[146] who live with a traumatic spinal cord injury (SCI). Younger individuals tend to develop traumatic injuries from high-impact events (e.g. motor vehicle collisions, diving accidents, etc.) whereas older patients develop injuries from low-energy falls, with concomitant degenerative spine disease. Of those who suffer a traumatic SCI, approximately half will develop some degree of tetraplegia[146]. A recent survey of individuals with SCI in the United States identified their specific functional needs[147]. For individuals with tetraplegia, the ability to regain arm and hand function was the most common outcome selected from seven different physiological outcomes. A more recent systematic literature review including 3187 patients also supports this finding[148].
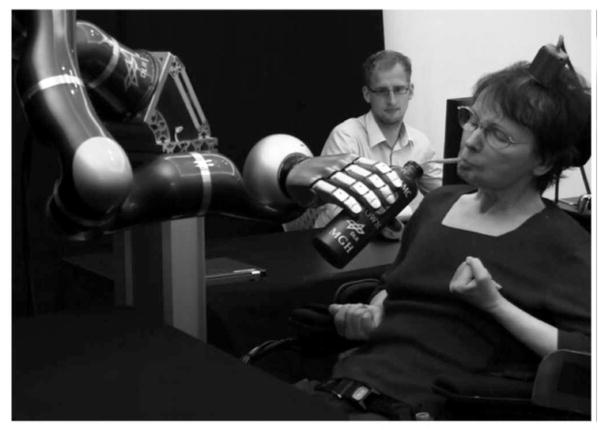
BCI technology is well suited to realize the needs identified in the aforementioned surveys. Several years after the first demonstration of cursor control for communication, implanted electrodes were used to allow an individual who was incompletely locked-in after a brainstem stroke the ability to control a robotic limb using decoded spikes. For the first time in nearly 15 years, the individual was able to use an assistive technology to reach out and grasp a bottle, bring it towards her mouth and drink from it[20] (Figure 3). This research was then independently replicated and expanded when a 52 year-old woman diagnosed with spinocerebellar degeneration was able to control a robotic limb with seven independent degrees of freedom to feed herself [19]; and, when a 32 year-old man with a SCI was able to control a limb using recordings from the posterior parietal cortex[21]. More recently, a brain-spinal interface was demonstrated in NHPs [149], highlighting the potential for intracortical recording to drive restorative approaches for people with paraplegia. Finally, there have been two recent examples of intracortical recording driving a surface FES device in non-human primates [150]–[152] and humans with [22] and an implanted, percutaneous FES system [23], the latter successfully providing reanimation of elbow flexion/extension and hand grasp plus robot-assisted arm elevation in a 53 year-old man with high cervical spinal cord injury.
While the aforementioned demonstrations of robotic and native limb control are at a relatively primitive stage, they are proof of principal that neural information can be used for multi-dimensional limb control. These results demonstrate both the feasibility and potential utility from intracortical recordings in humans.
V. Future directions
Most consumer devices undergo iterative improvements, and this is especially true for successful medical devices [153]. The original deep brain stimulators and cardiac pacemakers began as experimental devices with protruding wires, bulky electronic systems and limited efficacy. Iterative engineering solutions and partnerships between academia and industry allowed these devices to become miniaturized and fully implantable; both devices are now part of the standard of care for their respective disease states and have been implanted in patients around the world.
The development of reliable closed-loop BCIs for people with paralysis benefits from multidisciplinary collaborations across a variety of specialties, including neuroscientists, clinicians, engineers, computer scientists, and applied mathematicians. Keeping in mind that the guiding principles for device development ought to be based on the needs of those who will be using them [141], [147], [148], [154], [155], we highlight several directions for innovation in the near future.
First, much like deep-brain stimulators and cardiac pacemakers, the next generation of devices that will provide robust and reliable decoding of neural information should be fully implantable (i.e. without anything protruding through skin). Recent prototypes have been developed to wireless transmit real-time spike data through a hermetically sealed and fully implanted titanium system[156], [157]. This device has been successfully used in NHPs and is being transitioned toward human medical device manufacturing.
Second, the current devices used for closed-loop control of cursors and robotic limbs all require a dedicated technician to oversee their use [18]–[21]. A major engineering achievement will be the development of a robust and reliable closed-loop algorithms that works 24-hours a day with minimal outside intervention from trained technicians[15].
Third, while cursor control for communication has now been achieved in multiple individuals[15], [16], the ability to control complex robotic arm movements is still limited and has not yet reached the point of being a viable replacement for naturalistic control [18]–[21]. This is, in part, due to algorithmic deficiencies. For instance, algorithmic decoder advancements that incorporate novel neurophysiological insights are more likely to capitalize on the useable signals available through single cell recordings and optimize control [58], [124]. Decoding algorithms may develop strategies to continuously update parameters to deal with system noise [15], [35], [124], [135], [158]. Algorithms may be developed to determine what parameters the brain will be able to adapt to use [38], [40], and what features necessitate recalibration of the decoding parameters[6]. These innovations will also help to further the restoration of native limb movement, by iBCI control FES systems, with the goal of restoring arm/hand function and quality of life to individuals with cervical spinal cord injury or brainstem stroke [22], [23].
Fourth, current cursors and robotic limbs do not provide somatosensory information back to the user. In normal human reaching and grasping, continuous feedback is provided via proprioceptive, visual and tactile information. Closing the motor-sensory loop beyond the currently available visual feedback would likely enable movements closer to that achieved by people without physical disability [58], [159]. In the case of ALS, the sensory system is generally left intact, and devices could be developed in order to mimic proprioceptive information, such as by providing tendon stimulation [71]. By contrast, in complete SCI, sensory information from limb movement is impaired. One approach would be to directly stimulate the cortex in order to mimic proprioceptive information. Early success with intracortical stimulation in humans [160] suggests this may be a viable approach in the future.
Finally, developing clinically viable tools is predicated on demonstrating efficacy. Without being able to demonstrate reproducible, clinically viable outcomes, it will be difficult to bring these devices to those who need them most. With the field of clinical BCI for communication and motor rehabilitation still in its infancy, consensus is required as to how to measure outcomes appropriately [161].
VI. Conclusion
Intracortical brain computer interfaces have the capacity to improve the quality of life for individuals with severe motor deficits. Recent successes in early pilot clinical trials have demonstrated the ability for people with paralysis to control computer cursors and robotic limbs using only the intention to move one’s limb. Through multidisciplinary collaborative efforts, these devices will continue to evolve and we expect we move steadily toward meeting critical and ever-more complex needs for people with paralysis.
Fig. 2.
BCI control of a prosthetic limb. Enrolled in the BrainGate2 pilot clinical trial (https://www.clinicaltrials.gov/ct2/show/NCT00912041), Participant S3 reached out using a prosthetic arm and drank coffee for the first time in over a decade.
Acknowledgments
Support provided in part by the National Institutes of Health: NIDCD (R01DC009899), the Executive Committee on Research (ECOR) of Massachusetts General Hospital, the Rehabilitation Research and Development Service, Office of Research and Development, Department of Veterans Affairs (Merit Review Award B6453R and Center Award N9228-C), Canadian Institute of Health Research (336092), Killam Trust Award Foundation, and the Brown Institute of Brain Science. The contents do not necessarily represent the views of the Department of Veterans Affairs or the United States Government. Caution: The BrainGate Neural Interface System (ClinicalTrials.gov Identifier: NCT00912041) is an investigational device. Limited by federal law to investigational use.
Biographies
David M. Brandman studied biophysics at the University of Brisith Columbia, Vancouver, BC, Canada, and received his medical doctorate from the University of Calgary, AB, Canada. He is a Ph.D. Candidate in Neuroscience at Brown University, Providence, RI, USA. He is currently a senior neurosurgical resident at Dalhousie University, Halifax, NS, Canada.
Sydney S. Cash received the B.S. degree from Yale University, New Haven, CT, USA, and the M.D. and Ph.D. degrees from Columbia University, New York, NY, USA. Afterwards he did an internship in medicine at Massachusetts General Hospital (MGH), Boston, MA, USA, and a Neurology Residency in the combined Massachusetts General Hospital Brigham and Women’s Hospital Partners program, Boston, MA, USA. This was followed by fellowship training in Epilepsy at MGH. He is now Associate Professor in the Department of Neurology and Assistant Director for Research in the Partners Neurology Training Program as well as co-Director of the NeuroTechnology Trials Unit in the department and Clinical Trials Director of the New England Pediatric Device Consortium, Lebanon, NH, USA.
Leigh R. Hochberg received the M.D. and Ph.D. degrees in neuroscience from Emory University, Atlanta, GA, USA. He is Associate Professor of Engineering, Brown University; Associate Director, VA Center of Excellence for Neurorestoration and Neurotechnology, Providence VA Medical Center; and Senior Lecturer in Neurology at Harvard Medical School. In addition, he maintains clinical duties on the Stroke and Neurocritical Care Services at the MGH and Brigham and Women’s Hospital, and is on the consultation neurology staff at Spaulding Rehabilitation Hospital. He directs the Laboratory for Restorative Neurotechnology at Brown and MGH and directs the BrainGate pilot clinical trials. His research focuses on the development and testing of implanted neural interfaces to help people with paralysis and other neurologic disorders.
Contributor Information
David M. Brandman, Neuroscience Graduate Program, Brown Institute for Brain Science, Brown University, Providence, RI, USA, and the Department of Surgery (Neurosurgery), Dalhousie University, Halifax, NS, Canada
Sydney S. Cash, Neurotechnology Trials Unit, Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA
Leigh R. Hochberg, Center for Neurorestoration and Neurotechnology, Rehabilitation R&D Service, Department of Veterans Affairs Medical Center, Providence, RI, USA; Brown Institute for Brain Science, Brown University, Providence, RI, USA; Neurotechnology Trials Unit, Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; School of Engineering, Brown University, Providence, RI, USA
References
- 1.Wolpaw JR, Birbaumer N, McFarland DJ, Pfurtscheller G, Vaughan TM. Brain-computer interfaces for communication and control. Clin Neurophysiol. 2002;113(6):767–91. doi: 10.1016/s1388-2457(02)00057-3. [DOI] [PubMed] [Google Scholar]
- 2.Hochberg LR, Donoghue JP. Sensors for Brain-Computer Interfaces. IEEE Eng Med Biol Mag. 2006 Oct;:32–38. doi: 10.1109/memb.2006.1705745. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz AB, Cui XT, Weber DJ, Moran DW. Brain-controlled interfaces: movement restoration with neural prosthetics. Neuron. 2006 Oct;52(1):205–20. doi: 10.1016/j.neuron.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Lebedev MA, Nicolelis MAL. Brain-machine interfaces: past, present and future. Trends Neurosci. 2006;29(9):536–546. doi: 10.1016/j.tins.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Chestek CA, Cunningham JP, Gilja V, Nuyujukian P, Ryu SI, Shenoy KV. Neural Prosthetic Systems: Current Problems and Future Directions. 2009 Annu Int Conf IEEE Eng Med Biol Soc. 2009:3369–3375. doi: 10.1109/IEMBS.2009.5332822. [DOI] [PubMed] [Google Scholar]
- 6.Carmena JM. Advances in Neuroprosthetic Learning and Control. PLoS Biol. 2013;11(5):1–4. doi: 10.1371/journal.pbio.1001561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kao JC, Stavisky SD, Sussillo D, Nuyujukian P, Shenoy KV. Information systems opportunities in brain-machine interface decoders. Proc IEEE. 2014;102(5):666–682. [Google Scholar]
- 8.McFarland DJ, Wolpaw JR. Brain-Computer Interfaces for Communication and Control. Commun ACM. 2011;54(5):60–66. doi: 10.1145/1941487.1941506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaudhary U, Birbaumer N, Ramos-murguialday A. Brain–computer interfaces for communication and rehabilitation. 2016 Aug; doi: 10.1038/nrneurol.2016.113. [DOI] [PubMed] [Google Scholar]
- 10.Green AM, Kalaska JF. Learning to move machines with the mind. Trends Neurosci. 2011 Feb;34(2):61–75. doi: 10.1016/j.tins.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Hochberg LR, Serruya MD, Friehs GM, Mukand Ja, Saleh M, Caplan AH, Branner A, Chen D, Penn RD, Donoghue JP. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006 Jul;442(7099):164–71. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- 12.Kim SP, Simeral JD, Hochberg LR, Donoghue JP, Black MJ. Neural control of computer cursor velocity by decoding motor cortical spiking activity in humans with tetraplegia. J Neural Eng. 2008 Dec;5(4):455–76. doi: 10.1088/1741-2560/5/4/010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simeral JD, Kim SP, Black MJ, Donoghue JP, Hochberg LR. Neural control of cursor trajectory and click by a human with tetraplegia 1000 days after implant of an intracortical microelectrode array. J Neural Eng. 2011 Apr;8(2):25027. doi: 10.1088/1741-2560/8/2/025027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilja V, Pandarinath C, Blabe CH, Nuyujukian P, Simeral JD, Sarma Aa, Sorice BL, Perge Ja, Jarosiewicz B, Hochberg LR, Shenoy KV, Henderson JM. Clinical translation of a high-performance neural prosthesis. Nat Med. 2015;21(10):6–8. doi: 10.1038/nm.3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarosiewicz B, Sarma AA, Bacher D, Masse NY, Simeral JD, Sorice B, Oakley EM, Blabe C, Pandarinath C, Gilja V, Cash SS, Eskandar EN, Friehs GM, Henderson JM, Shenoy KV, Donoghue JP, Hochberg LR. Virtual typing by people with tetraplegia using a self-calibrating intracortical brain-computer interface. Sci Transl Med. 2015;7(313):1–11. doi: 10.1126/scitranslmed.aac7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bacher D, Jarosiewicz B, Masse NY, Stavisky SD, Simeral JD, Newell K, Oakley EM, Cash SS, Friehs G, Hochberg LR. Neural Point-and-Click Communication by a Person With Incomplete Locked-In Syndrome. Neurorehabil Neural Repair. 2015;29(5):462–471. doi: 10.1177/1545968314554624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vansteensel MJ, Pels EGM, Bleichner MG, Branco MP, Denison T, Freudenburg ZV, Gosselaar P, Leinders S, Ottens TH, Van Den Boom MA, Van Rijen PC, Aarnoutse EJ, Ramsey NF. Fully Implanted Brain–Computer Interface in a Locked-In Patient with ALS. N Engl J Med. 2016 doi: 10.1056/NEJMoa1608085. NEJMoa1608085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wodlinger B, Downey JE, Tyler-Kabara EC, Schwartz AB, Boninger ML, Collinger JL. Ten-dimensional anthropomorphic arm control in a human brain-machine interface: difficulties, solutions, and limitations. J Neural Eng. 2015;12(1):16011. doi: 10.1088/1741-2560/12/1/016011. [DOI] [PubMed] [Google Scholar]
- 19.Collinger JL, Wodlinger B, Downey JE, Wang W, Tyler-Kabara EC, Weber DJ, McMorland AJC, Velliste M, Boninger ML, Schwartz AB. High-performance neuroprosthetic control by an individual with tetraplegia. Lancet. 2013 Feb;381(9866):557–64. doi: 10.1016/S0140-6736(12)61816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hochberg LR, Bacher D, Jarosiewicz B, Masse NY, Simeral JD, Vogel J, Haddadin S, Liu J, Cash SS, van der Smagt P, Donoghue JP. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature. 2012 May;485(7398):372–375. doi: 10.1038/nature11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aflalo T, Kellis S, Klaes C, Lee B, Shi Y, Pejsa K, Shanfield K, Hayes-Jackson S, Aisen M, Heck C, Liu C, Andersen R. Decoding motor imagery from the posterior parietal cortex of a tetraplecig human. Science (80- ) 2015;348(6237):906–910. doi: 10.1126/science.aaa5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouton CE, Shaikhouni A, Annetta NV, Bockbrader MA, Friedenberg DA, Nielson DM, Sharma G, Sederberg PB, Glenn BC, Mysiw WJ, Morgan AG, Deogaonkar M, Rezai AR. Restoring cortical control of functional movement in a human with quadriplegia. Nature. 2016;0(7602):1–13. doi: 10.1038/nature17435. [DOI] [PubMed] [Google Scholar]
- 23.Ajiboye AB, Willett FR, Young DR, Memberg W, Walters BC, Sweet JA, Hoyen HA, Keith MW, Peckham PH, Simeral JD, Donoghue JP, Miller JP, Hochberg LR, Kirsch RF. Restoration of reaching and grasping in a person with tetraplegia through brain-controlled muscle stimulation: a proof-of-concept demonstratio. Lancet. 2017 doi: 10.1016/S0140-6736(17)30601-3. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandman DM, Hochberg LR. Brain Computer Interfaces. In: Shephard RK, editor. Neurobionics: The biomedical engineering of neural prostheses. 1. 2016. pp. 231–263. [Google Scholar]
- 25.Gallegos-Ayala G, Furdea A, Takano K, Ruf CA, Flor H, Birbaumer N. Brain communication in a completely locked-in patient using bedside near-infrared spectroscopy. Neurology. 2014;82(21):1930–1932. doi: 10.1212/WNL.0000000000000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buzsáki G, Anastassiou Ca, Koch C. The origin of extracellular fields and currents--EEG, ECoG, LFP and spikes. Nat Rev Neurosci. 2012;13(6):407–20. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srinivasan R, Winter WR, Nunez PL. Source analysis of EEG oscillations using high-resolution EEG and MEG. Prog Brain Res. 2006;159:29–42. doi: 10.1016/S0079-6123(06)59003-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tao JX, Baldwin M, Hawes-Ebersole S, Ebersole JS. Cortical substrates of scalp EEG epileptiform discharges. J Clin Neurophysiol. 2007 Apr;24(2):96–100. doi: 10.1097/WNP.0b013e31803ecdaf. [DOI] [PubMed] [Google Scholar]
- 29.Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937 Dec;60(4):389–443. [Google Scholar]
- 30.Serruya MD, Hatsopoulos NG, Paninski L, Fellows MR, Donoghue JP. Instant neural control of a movement signal. Nature. 2002 Mar;416:141–142. doi: 10.1038/416141a. [DOI] [PubMed] [Google Scholar]
- 31.Velliste M, Perel S, Spalding MC, Whitford AS, Schwartz AB. Cortical control of a prosthetic arm for self-feeding. Nature. 2008 Jun;453(7198):1098–101. doi: 10.1038/nature06996. [DOI] [PubMed] [Google Scholar]
- 32.Wessberg J, Stambaugh CR, Kralik JD, Beck PD, Laubach M, Chapin JK, Kim J, Biggs SJ, Srinivasan Ma, Nicolelis Ma. Real-time prediction of hand trajectory by ensembles of cortical neurons in primates. Nature. 2000 Nov;408(6810):361–5. doi: 10.1038/35042582. [DOI] [PubMed] [Google Scholar]
- 33.Taylor DM, Tillery SIH, Schwartz AB, Craggs M, Wolpaw JR, Chapin JK, Moxon KA, Markowitz RS, Nicholelis MAL, Wessberg J, Georgopoulos AP, Kettner RE, Schwartz AB, Shoham S, Halgren E, Maynard EM, Normann RA, Kennedy PR, Bakay RAE, Moore MM, Adams K, Goldwaithe J, Williams JC, Rennaker RL, Kipke DR. Direct cortical control of 3D neuroprosthetic devices. Science. 2002;296(5574):1829–32. doi: 10.1126/science.1070291. [DOI] [PubMed] [Google Scholar]
- 34.Ganguly K, Carmena JM. Emergence of a stable cortical map for neuroprosthetic control. PLoS Biol. 2009;7(7) doi: 10.1371/journal.pbio.1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shanechi MM, Orsborn AL, Carmena JM. Robust Brain-Machine Interface Design Using Optimal Feedback Control Modeling and Adaptive Point Process Filtering. PLoS Comput Biol. 2016;12(4):1–29. doi: 10.1371/journal.pcbi.1004730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orsborn AL, Moorman HG, Overduin SA, Shanechi MM, Dimitrov DF, Carmena JM. Closed-loop decoder adaptation shapes neural plasticity for skillful neuroprosthetic control. Neuron. 2014;82(6):1380–1393. doi: 10.1016/j.neuron.2014.04.048. [DOI] [PubMed] [Google Scholar]
- 37.Carmena JM, Lebedev MA, Crist RE, O’Doherty JE, Santucci DM, Dimitrov DF, Patil PG, Henriquez CS, Nicolelis MAL. Learning to control a brain-machine interface for reaching and grasping by primates. PLoS Biol. 2003;1(2):193–208. doi: 10.1371/journal.pbio.0000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jarosiewicz B, Chase SM, Fraser GW, Velliste M, Kass RE, Schwartz AB. Functional network reorganization during learning in a brain-computer interface paradigm. Proc Natl Acad Sci U S A. 2008;105(49):19486–91. doi: 10.1073/pnas.0808113105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kao JC, Nuyujukian P, Ryu SI, Churchland MM, Cunningham JP, Shenoy KV. Single-trial dynamics of motor cortex and their applications to brain-machine interfaces. Nat Commun. 2015 May;6:7759. doi: 10.1038/ncomms8759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sadtler PT, Quick KM, Golub MD, Chase SM, Ryu SI, Tyler-Kabara EC, Yu BM, Batista AP. Neural constraints on learning. Nature. 2014;512(7515):423–426. doi: 10.1038/nature13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nuyujukian P, Fan JM, Kao JC, Ryu SI, Shenoy KV. A high-performance keyboard neural prosthesis enabled by task optimization. IEEE Trans Biomed Eng. 2015;62(1):21–29. doi: 10.1109/TBME.2014.2354697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santhanam G, Ryu SI, Yu BM, Afshar A, Shenoy KV. A high-performance brain–computer interface. Nature. 2006;442(7099):195–198. doi: 10.1038/nature04968. [DOI] [PubMed] [Google Scholar]
- 43.Jarosiewicz B, Masse NY, Bacher D, Cash SS, Eskandar E, Friehs G, Donoghue JP, Hochberg LR. Advantages of closed-loop calibration in intracortical brain-computer interfaces for people with tetraplegia. J Neural Eng. 2013;10(4):46012. doi: 10.1088/1741-2560/10/4/046012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pandarinath C, Gilja V, Blabe CH, Nuyujukian P, Sarma AA, Sorice BL, Eskandar EN, Hochberg LR, Henderson JM, Shenoy KV. Neural population dynamics in human motor cortex during movements in people with ALS. Elife. 2015;4:e07436. doi: 10.7554/eLife.07436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Willett FR, Pandarinath C, Jarosiewicz B, Murphy BA, Memberg WD, Blabe CH, Saab J, Walter BL, Sweet JA, Miller JP, Henderson JM, Shenoy KV, Simeral JD, Hochberg LR, Kirsch RF, Ajiboye AB. Feedback control policies employed by people using intracortical brain-computer interfaces. J Neural Eng. 2017 Feb;14(1):16001. doi: 10.1088/1741-2560/14/1/016001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rizzolatti G, Luppino G, Matelli M. The organization of the cortical motor system: New concepts. Electroencephalogr Clin Neurophysiol. 1998;106(4):283–296. doi: 10.1016/s0013-4694(98)00022-4. [DOI] [PubMed] [Google Scholar]
- 47.Cheney PD, Hill-Karrer J, Belhaj-Saïf a, McKiernan BJ, Park MC, Marcario JK. Cortical motor areas and their properties: implications for neuroprosthetics. Prog Brain Res. 2000 Jan;128:135–60. doi: 10.1016/s0079-6123(00)28013-8. [DOI] [PubMed] [Google Scholar]
- 48.Shenoy KV, Sahani M, Churchland MM. Cortical Control of Arm Movements: A Dynamical Systems Perspective. Annu Rev Neurosci. 2013;36(1):337–359. doi: 10.1146/annurev-neuro-062111-150509. [DOI] [PubMed] [Google Scholar]
- 49.Lemon RN. Descending pathways in motor control. Annu Rev Neurosci. 2008;31(Cm):195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- 50.Kaas JH, Stepniewska I, Gharbawie O. Cortical networks subserving upper limb movements in primates. European Journal of Physical and Rehabilitation Medicine. 2012;48(2):299–306. [PMC free article] [PubMed] [Google Scholar]
- 51.Kalaska JF. From intention to action: motor cortex and the control of reaching movements. Adv Exp Med Biol. 2009;629:139–178. doi: 10.1007/978-0-387-77064-2_8. [DOI] [PubMed] [Google Scholar]
- 52.Musallam S, Corneil BD, Greger B, Scherberger H, Andersen RA. Cognitive control signals for neural prosthetics. Science. 2004;305(5681):258–62. doi: 10.1126/science.1097938. [DOI] [PubMed] [Google Scholar]
- 53.Mulliken GH, Musallam S, Andersen RA. Decoding Trajectories from Posterior Parietal Cortex Ensembles. J Neurosci. 2008;28(48):12913–12926. doi: 10.1523/JNEUROSCI.1463-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Georgopoulos A, Kalaska J, Caminiti R, Massey J. On the relations between the direction of two-dimensional arm movements and cell discharge in primate motor cortex. J Neurosci. 1982;2(11):1527–1537. doi: 10.1523/JNEUROSCI.02-11-01527.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Georgopoulos aP, Kettner RE, Schwartz aB. Primate motor cortex and free arm movements to visual targets in three-dimensional space. II. Coding of the direction of movement by a neuronal population. J Neurosci. 1988 Aug;8(8):2928–37. doi: 10.1523/JNEUROSCI.08-08-02928.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwartz AB. Direct cortical representation of drawing. Science. 1994;265(5171):540–542. doi: 10.1126/science.8036499. [DOI] [PubMed] [Google Scholar]
- 57.Paninski L, Fellows MR, Hatsopoulos NG, John P, Wang W, Sudre GP, Xu Y, Kass RE, Collinger JL, Alan D, Bagic AI, Weber DJ. Spatiotemporal Tuning of Motor Cortical Neurons for Hand Position and Velocity Spatiotemporal Tuning of Motor Cortical Neurons for Hand Position and Velocity. J Neurophysiol. 2004 Sep;:515–532. doi: 10.1152/jn.00587.2002. 2003. [DOI] [PubMed] [Google Scholar]
- 58.Bensmaia SJ, Miller LE. Restoring sensorimotor function through intracortical interfaces: progress and looming challenges. Nat Rev Neurosci. 2014;15(5):313–325. doi: 10.1038/nrn3724. [DOI] [PubMed] [Google Scholar]
- 59.Humphrey DR, Schmidt EM, Thompson WD. Predicting measures of motor performance from multiple cortical spike trains. Science. 1970;170(959):758–762. doi: 10.1126/science.170.3959.758. [DOI] [PubMed] [Google Scholar]
- 60.Pohlmeyer Ea, Solla Sa, Perreault EJ, Miller LE. Prediction of upper limb muscle activity from motor cortical discharge during reaching. J Neural Eng. 2007;4:369–379. doi: 10.1088/1741-2560/4/4/003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Churchland MM, Cunningham JP, Kaufman MT, Foster JD, Nuyujukian P, Ryu SI, Shenoy KV. Neural Population Dynamics During Reaching. Nature. 2012;487(7405):1–20. doi: 10.1038/nature11129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rao NG, Donoghue JP. Cue to action processing in motor cortex populations. J Neurophysiol. 2014;111(2):441–53. doi: 10.1152/jn.00274.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kilner JM, Lemon RN. What we know currently about mirror neurons. Curr Biol. 2013;23(23):R1057–R1062. doi: 10.1016/j.cub.2013.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maynard EM, Nordhausen CT, Normann RA. The Utah intracortical Electrode Array: a recording structure for potential brain-computer interfaces. Electroencephalogr Clin Neurophysiol. 1997 Mar;102(3):228–239. doi: 10.1016/s0013-4694(96)95176-0. [DOI] [PubMed] [Google Scholar]
- 65.Georgopoulos AP, Merchant H, Naselaris T, Amirikian B. Mapping of the preferred direction in the motor cortex. Proc Natl Acad Sci USA. 2007;104(26):11068–11072. doi: 10.1073/pnas.0611597104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ahdab R, Ayache SS, Farhat WH, Mylius V, Schmidt S, Brugières P, Lefaucheur JP. Reappraisal of the anatomical landmarks of motor and premotor cortical regions for image-guided brain navigation in TMS practice. Hum Brain Mapp. 2014;35(5):2435–2447. doi: 10.1002/hbm.22339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120(1):141–157. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]
- 68.Cramer SC, Lastra L, Lacourse MG, Cohen MJ. Brain motor system function after chronic, complete spinal cord injury. Brain. 2005;128(12):2941–2950. doi: 10.1093/brain/awh648. [DOI] [PubMed] [Google Scholar]
- 69.Shoham S, Halgren E, Maynard EM, Normann Ra. Motor-cortical activity in tetraplegics. Nature. 2001;413(6858):793. doi: 10.1038/35101651. [DOI] [PubMed] [Google Scholar]
- 70.Sabbah P, De SS, Leveque C, Gay S, Pfefer F, Nioche C, Sarrazin JL, Barouti H, Tadie M, Cordoliani YS. Sensorimotor cortical activity in patients with complete spinal cord injury: a functional magnetic resonance imaging study. J Neurotrauma. 2002;19(1):53–60. doi: 10.1089/089771502753460231. [DOI] [PubMed] [Google Scholar]
- 71.Kandel E. Principles of Neural Science. 5. McGraw-Hill Education; 2013. [Google Scholar]
- 72.Sanes JN, Donoghue JP. Plasticity and Primary Motor Cortex. Annu Rev Neurosci. 2000:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- 73.Wannier T, Schmidlin E, Bloch J, Rouiller EM. A unilateral section of the corticospinal tract at cervical level in primate does not lead to measurable cell loss in motor cortex. J Neurotrauma. 2005;22(6):703–17. doi: 10.1089/neu.2005.22.703. [DOI] [PubMed] [Google Scholar]
- 74.Lawyer TJ, Netsky MG. Amyotrophic lateral sclerosis. AMA Arch Neurol Psychiatry. 1953 Feb;69(2):171–192. doi: 10.1001/archneurpsyc.1953.02320260029002. [DOI] [PubMed] [Google Scholar]
- 75.Homer ML, Nurmikko AV, Donoghue JP, Hochberg LR. Sensors and decoding for intracortical brain computer interfaces. Annu Rev Biomed Eng. 2013 Jan;15:383–405. doi: 10.1146/annurev-bioeng-071910-124640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Truccolo W, Ahmed OJ, Harrison MT, Eskandar EN, Cosgrove GR, Madsen JR, Blum AS, Potter NS, Hochberg LR, Cash SS. Neuronal Ensemble Synchrony during Human Focal Seizures. J Neurosci. 2014;34(30):9927–9944. doi: 10.1523/JNEUROSCI.4567-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cash SS, Hochberg LR. The emergence of single neurons in clinical neurology. Neuron. 2015;86(1):79–91. doi: 10.1016/j.neuron.2015.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wagner FB, Truccolo W, Wang J, Nurmikko AV. Spatiotemporal dynamics of optogenetically induced and spontaneous seizure transitions in primary generalized epilepsy. J Neurophysiol. 2015 Apr;113(7):2321–2341. doi: 10.1152/jn.01040.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jiang J, Willett FR, Taylor DM. Relationship between microelectrode array impedance and chronic recording quality of single units and local field potentials. Conf Proc … Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Conf. 2014;2014:3045–3048. doi: 10.1109/EMBC.2014.6944265. [DOI] [PubMed] [Google Scholar]
- 80.Barrese JC, Rao N, Paroo K, Triebwasser C, Vargas-Irwin C, Franquemont L, Donoghue JP. Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates. J Neural Eng. 2013;10(6):66014. doi: 10.1088/1741-2560/10/6/066014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rennaker RL, Street S, Ruyle aM, Sloan aM. A comparison of chronic multi-channel cortical implantation techniques: manual versus mechanical insertion. J Neurosci Methods. 2005 Mar;142(2):169–76. doi: 10.1016/j.jneumeth.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 82.Bjornsson CS, Oh SJ, Al-Kofahi Ya, Lim YJ, Smith KL, Turner JN, De S, Roysam B, Shain W, Kim SJ. Effects of insertion conditions on tissue strain and vascular damage during neuroprosthetic device insertion. J Neural Eng. 2006 Sep;3(3):196–207. doi: 10.1088/1741-2560/3/3/002. [DOI] [PubMed] [Google Scholar]
- 83.Rousche PJ, Normann Ra. A method for pneumatically inserting an array of penetrating electrodes into cortical tissue. Ann Biomed Eng. 1992 Jan;20(4):413–22. doi: 10.1007/BF02368133. [DOI] [PubMed] [Google Scholar]
- 84.Kipke DR, Vetter RJ, Williams JC, Hetke JF, Science C, Arbor A. Feasibility of Long-term Spike Recording in Cerebral Cortex Using Silicon-substrate Intracortical Microelectrode Arrays. Electr Eng. 2003;11(2):1–13. doi: 10.1109/TNSRE.2003.814443. [DOI] [PubMed] [Google Scholar]
- 85.Kennedy PR. The cone electrode: a long-term electrode that records from neurites grown onto its recording surface. J Neurosci Methods. 1989;29(3):181–193. doi: 10.1016/0165-0270(89)90142-8. [DOI] [PubMed] [Google Scholar]
- 86.Prasad A, Xue QS, Sankar V, Nishida T, Shaw G, Streit WJ, Sanchez JC. Comprehensive characterization and failure modes of tungsten microwire arrays in chronic neural implants. J Neural Eng. 2012;9(5):56015. doi: 10.1088/1741-2560/9/5/056015. [DOI] [PubMed] [Google Scholar]
- 87.Sommakia S, Lee HC, Gaire J, Otto KJ. Materials approaches for modulating neural tissue responses to implanted microelectrodes through mechanical and biochemical means. Curr Opin Solid State Mater Sci. 2014 Dec;18(6):319–328. doi: 10.1016/j.cossms.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kozai TDY, Jaquins-Gerstl AS, Vazquez AL, Michael AC, Cui XT. Brain tissue responses to neural implants impact signal sensitivity and intervention strategies. ACS Chem Neurosci. 2015;6(1):48–67. doi: 10.1021/cn500256e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Green R, Abidian MR. Conducting Polymers for Neural Prosthetic and Neural Interface Applications. Adv Mater. 2015 Dec;27(46):7620–7637. doi: 10.1002/adma.201501810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jorfi M, Skousen JL, Weder C, Capadona JR. Progress towards biocompatible intracortical microelectrodes for neural interfacing applications. J Neural Eng. 2015 Feb;12(1):11001. doi: 10.1088/1741-2560/12/1/011001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vargas-Irwin CE, Shakhnarovich G, Yadollahpour P, Mislow JMK, Black MJ, Donoghue JP. Decoding Complete Reach and Grasp Actions from Local Primary Motor Cortex Populations. J Neurosci. 2010;30(29):9659–9669. doi: 10.1523/JNEUROSCI.5443-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fetz EE. Operant Conditioning of Cortical Unit Activity. 1969;163(3870):955–958. doi: 10.1126/science.163.3870.955. [DOI] [PubMed] [Google Scholar]
- 93.Buzsaki G. Rhythms of the Brain. Oxford University Press; 2006. [Google Scholar]
- 94.Canolty RT, Ganguly K, Carmena JM. Task-dependent changes in cross-level coupling between single neurons and oscillatory activity in multiscale networks. PLoS Comput Biol. 2012;8(12):e1002809. doi: 10.1371/journal.pcbi.1002809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zanos S, Zanos TP, Marmarelis VZ, Ojemann GA, Fetz EE. Relationships between spike-free local field potentials and spike timing in human temporal cortex. J Neurophysiol. 2012 Apr;107(7):1808–1821. doi: 10.1152/jn.00663.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Murthy VN, Fetz EE. Synchronization of neurons during local field potential oscillations in sensorimotor cortex of awake monkeys. J Neurophysiol. 1996 Dec;76(6):3968–3982. doi: 10.1152/jn.1996.76.6.3968. [DOI] [PubMed] [Google Scholar]
- 97.Khanna P, Carmena JM. Neural oscillations: beta band activity across motor networks. Curr Opin Neurobiol. 2015 Jun;32:60–67. doi: 10.1016/j.conb.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 98.Rule ME, Vargas-Irwin C, Donoghue JP, Truccolo W. Contribution of LFP dynamics to single-neuron spiking variability in motor cortex during movement execution. Front Sys Neurosci. 2015 Jun;9:89. doi: 10.3389/fnsys.2015.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Flint RD, Lindberg EW, Jordan LR, Miller LE, Slutzky MW. Accurate decoding of reaching movements from field potentials in the absence of spikes. J Neural Eng. 2012 Aug;9(4):46006. doi: 10.1088/1741-2560/9/4/046006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhuang J, Truccolo W, Vargas-Irwin C, Donoghue JP. Reconstructing grasping motions from high-frequency local field potentials in primary motor cortex. 2010 Annu Int Conf IEEE Eng Med Biol Soc EMBC’10. 2010:4347–4350. doi: 10.1109/IEMBS.2010.5626228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bansal AK, Truccolo W, Vargas-Irwin CE, Donoghue JP. Decoding 3D reach and grasp from hybrid signals in motor and premotor cortices: spikes, multiunit activity, and local field potentials. J Neurophysiol. 2012;107(5):1337–55. doi: 10.1152/jn.00781.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stavisky SD, Kao JC, Nuyujukian P, Ryu SI, Shenoy KV. A high performing brain-machine interface driven by low-frequency local field potentials alone and together with spikes. J Neural Eng. 2015 Jun;12(3):36009. doi: 10.1088/1741-2560/12/3/036009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Spinks RL, Kraskov A, Brochier T, Umilta MA, Lemon RN. Selectivity for grasp in local field potential and single neuron activity recorded simultaneously from M1 and F5 in the awake macaque monkey. J Neurosci. 2008 Oct;28(43):10961–10971. doi: 10.1523/JNEUROSCI.1956-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ajiboye AB, Simeral JD, Donoghue JP, Hochberg LR, Kirch RD. Prediction of Imagined Single-Joint Movements in a Person with High Level Tetraplegia. IEEE Trans Biomed Eng. 2012;59(10):2755–2765. doi: 10.1109/TBME.2012.2209882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Perge Ja, Zhang S, Malik WQ, Homer ML, Cash S, Friehs G, Eskandar EN, Donoghue JP, Hochberg LR. Reliability of directional information in unsorted spikes and local field potentials recorded in human motor cortex. J Neural Eng. 2014;11(4):46007. doi: 10.1088/1741-2560/11/4/046007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kass RE, Brown EN, Robert E, Statistical ENB. Statistical Issues in the Analysis of Neuronal Data. 2005:8–25. doi: 10.1152/jn.00648.2004. [DOI] [PubMed] [Google Scholar]
- 107.Salinas E, Abbott LF. Vector reconstruction from firing rates. J Comput Neurosci. 1994;1(1–2):89–107. doi: 10.1007/BF00962720. [DOI] [PubMed] [Google Scholar]
- 108.Shadlen MN, Newsome WT. The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. J Neurosci. 1998;18(10):3870–96. doi: 10.1523/JNEUROSCI.18-10-03870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stein RB, Gossen ER, Jones KE. Neuronal variability: noise or part of the signal? Nat Rev Neurosci. 2005 May;6(5):389–397. doi: 10.1038/nrn1668. [DOI] [PubMed] [Google Scholar]
- 110.Faisal AA, Selen LPJ, Wolpert DM. Noise in the nervous system. Nat Rev Neurosci. 2008 Apr;9:292–303. doi: 10.1038/nrn2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Golub MD, Yu BM, Chase SM. Internal models engaged by brain-computer interface control. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:1327–1330. doi: 10.1109/EMBC.2012.6346182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu W, Black MJ, Gao Y, Bienenstock E, Serruya M, Shaikhouni A, Donoghue JP. Neural Decoding of Cursor Motion Using a Kalman Filter. Adv Neural Inf Process Syst 15 Proc 2002 Conf. 2003;(1):133–140. [Google Scholar]
- 113.Wu W, Gao Y, Bienenstock E, Donoghue JP, Black MJ, Biemenstock E, Donoghue JP, Black MJ. Bayesian population decoding of motor cortical activity using a Kalman filter. Neural Comput. 2005;18(1):80–118. doi: 10.1162/089976606774841585. [DOI] [PubMed] [Google Scholar]
- 114.Brown EN, Kass RE, Mitra P. Multiple neural spike train data analysis: state-of-the-art and future challenges. Nat Neurosci. 2004;7(5):456–461. doi: 10.1038/nn1228. [DOI] [PubMed] [Google Scholar]
- 115.Brown EN, Barbieri R, Ventura V, Kass RE, Frank LM. The time-rescaling theorem and its application to neural spike train data analysis. Neural Comput. 2002;14(2):325–46. doi: 10.1162/08997660252741149. [DOI] [PubMed] [Google Scholar]
- 116.Shanechi MM, Orsborn A, Moorman H, Gowda S, Carmena JM. High-performance brain-machine interface enabled by an adaptive optimal feedback-controlled point process decoder. Conf Proc … Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Conf. 2014;2014:6493–6496. doi: 10.1109/EMBC.2014.6945115. [DOI] [PubMed] [Google Scholar]
- 117.Truccolo W, Friehs GM, Donoghue JP, Hochberg LR. Primary motor cortex tuning to intended movement kinematics in humans with tetraplegia. J Neurosci. 2008 Jan;28(5):1163–78. doi: 10.1523/JNEUROSCI.4415-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sussillo D, Churchland MM, Kaufman MT, Shenoy KV. A neural network that finds a naturalistic solution for the production of muscle activity. Nat Neurosci. 2015;18(7):1025–33. doi: 10.1038/nn.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Burrow M, Dugger J, Humphrey D. Cortical control of a robot using a time-delay neural network. Int Conf Rehabil Robot. 1997 [Google Scholar]
- 120.Brockmeier AJ, Choi JS, Kriminger EG, Francis JT, Principe JC. Neural Decoding with Kernel-Based Metric Learning. Neural Comput. 2014 Jun;26(6):1080–1107. doi: 10.1162/NECO_a_00591. [DOI] [PubMed] [Google Scholar]
- 121.Shpigelman L, Lalazar H, Vaadia E. Kernel-ARMA for Hand Tracking and Brain-Machine Interfacing During 3D Motor Control. Neural Inf Process Syst. 2008:1489–1496. [Google Scholar]
- 122.Li L, Brockmeier AJ, Choi JS, Francis JT, Sanchez JC, Principe JC. A tensor-product-kernel framework for multiscale neural activity decoding and control. Comput Intell Neurosci. 2014;2014 doi: 10.1155/2014/870160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yu B, Cunningham J, Santhanam G, Ryu S, Shenoy K, Sahani M. Gaussian-Process Factor Analysis for Low-Dimensional Single-Trial Analysis of Neural Population Activity (vol 102, pg 614, 2009) J Neurophysiol. 2009 Apr;102:614–635. doi: 10.1152/jn.90941.2008. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shenoy K, Carmena J. Combining decoder design and neural adaptation in brain-machine interfaces. Neuron. 2014;84(4):665–680. doi: 10.1016/j.neuron.2014.08.038. [DOI] [PubMed] [Google Scholar]
- 125.Ganguly K, Carmena JM. Neural correlates of skill acquisition with a cortical brain-machine interface. J Mot Behav. 2010 Feb;42:355–360. doi: 10.1080/00222895.2010.526457. [DOI] [PubMed] [Google Scholar]
- 126.Birbaumer N, Ghanayim N, Hinterberger T, Iversen I, Kotchoubey B, Kübler A, Perelmouter J, Taub E, Flor H. A spelling device for the paralysed. Nature. 1999;398(6725):297–298. doi: 10.1038/18581. [DOI] [PubMed] [Google Scholar]
- 127.Ganguly K, Carmena JM. Emergence of a Stable Cortical Map for Neuroprosthetic Control. PLoS Biol. 2009;7(7):1–13. doi: 10.1371/journal.pbio.1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kennedy PR, Bakay Ra. Restoration of neural output from a paralyzed patient by a direct brain connection. Neuroreport. 1998 Jun;9(8):1707–11. doi: 10.1097/00001756-199806010-00007. [DOI] [PubMed] [Google Scholar]
- 129.Rockstroh B, Birbaumer N, Elbert T, Lutzenberger W. Operant control of EEG and event-related and slow brain potentials. Biofeedback Self Regul. 1984;9(2):139–60. doi: 10.1007/BF00998830. [DOI] [PubMed] [Google Scholar]
- 130.Elbert T, Rockstroh B, Lutzenberger W, Birbaumer N. Biofeedback of slow cortical potentials. I. Electroencephalogr Clin Neurophysiol. 1980;48(3):293–301. doi: 10.1016/0013-4694(80)90265-5. [DOI] [PubMed] [Google Scholar]
- 131.Gilja V, Nuyujukian P, Chestek CA, Cunningham JP, Yu BM, Fan JM, Ryu SI, Shenoy KV. A brain machine interface control algorithm designed from a feedback control perspective. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:1318–1322. doi: 10.1109/EMBC.2012.6346180. [DOI] [PubMed] [Google Scholar]
- 132.Koyama S, Chase SM, Whitford AS, Velliste M, Schwartz AB, Kass RE. Comparison of brain-computer interface decoding algorithms in open-loop and closed-loop control. J Comput Neurosci. 2010 Aug;29(1–2):73–87. doi: 10.1007/s10827-009-0196-9. [DOI] [PubMed] [Google Scholar]
- 133.Chase SM, Schwartz AB, Kass RE. Bias, optimal linear estimation, and the differences between open-loop simulation and closed-loop performance of spiking-based brain-computer interface algorithms. Neural Networks. 2009;22(9):1203–1213. doi: 10.1016/j.neunet.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Masse NY, Jarosiewicz B, Simeral JD, Bacher D, Stavisky SD, Cash SS, Oakley EM, Berhanu E, Eskandar E, Friehs G, Hochberg LR, Donoghue JP. Non-causal spike filtering improves decoding of movement intention for intracortical BCIs. J Neurosci Methods. 2015;244:94–103. doi: 10.1016/j.jneumeth.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Orsborn AL, Dangi S, Moorman HG, Carmena JM. Closed-loop decoder adaptation on intermediate time-scales facilitates rapid BMI performance improvements independent of decoder initialization conditions. IEEE Trans Neural Syst Rehabil Eng. 2012;20(4):468–477. doi: 10.1109/TNSRE.2012.2185066. [DOI] [PubMed] [Google Scholar]
- 136.Orsborn AL, Dangi S, Moorman HG, Carmena JM. Exploring time-scales of closed-loop decoder adaptation in brain-machine interfaces. Proc Annu Int Conf IEEE Eng Med Biol Soc EMBS. 2011:5436–5439. doi: 10.1109/IEMBS.2011.6091387. [DOI] [PubMed] [Google Scholar]
- 137.Dangi S, Gowda S, Heliot R, Carmena JM. Adaptive Kalman filtering for closed-loop Brain-Machine Interface systems. 2011 5th Int IEEE/EMBS Conf Neural Eng NER 2011. 2011:609–612. [Google Scholar]
- 138.A. ALS. About ALS. 2014. [Google Scholar]
- 139.Lulé D, Häcker S, Ludolph A, Birbaumer N, Kübler A. Depression and quality of life in patients with amyotrophic lateral sclerosis. Dtsch Arztebl Int. 2008 Jun;105(23):397–403. doi: 10.3238/arztebl.2008.0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lulé D, Zickler C, Häcker S, Bruno Ma, Demertzi a, Pellas F, Laureys S, Kübler a. Life can be worth living in locked-in syndrome. Prog Brain Res. 2009 Jan;177(9):339–51. doi: 10.1016/S0079-6123(09)17723-3. [DOI] [PubMed] [Google Scholar]
- 141.Kageyama Y, Hirata M, Yanagisawa T, Shimokawa T, Sawada J, Morris S, Mizushima N, Kishima H, Sakura O, Yoshimine T. Severely affected ALS patients have broad and high expectations for brain-machine interfaces. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(7–8):513–519. doi: 10.3109/21678421.2014.951943. [DOI] [PubMed] [Google Scholar]
- 142.Kennedy PR, Bakay Ra, Moore MM, Adams K, Goldwaithe J. Direct control of a computer from the human central nervous system. IEEE Trans Rehabil Eng. 2000;8(2):198–202. doi: 10.1109/86.847815. [DOI] [PubMed] [Google Scholar]
- 143.Kennedy PR, Kirby MT, Moore MM, King B, Mallory A. Computer control using human intracortical local field potentials. IEEE Trans Neural Syst Rehabil Eng. 2004;12(3):339–44. doi: 10.1109/TNSRE.2004.834629. [DOI] [PubMed] [Google Scholar]
- 144.Murguialday AR, Hill J, Bensch M, Martens S, Halder S, Nijboer F, Schoelkopf B, Birbaumer N, Gharabaghi A. Transition from the locked in to the completely locked-in state: A physiological analysis. Clin Neurophysiol. 2011;122(5):925–933. doi: 10.1016/j.clinph.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 145.Oudega M, Perez Ma. Corticospinal reorganization after spinal cord injury. J Physiol. 2012 Aug;590(Pt 16):3647–63. doi: 10.1113/jphysiol.2012.233189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.National Spinal Cord Injury Statistical Center. The 2012 Annual Statistical Report for the Spinal Cord Injury Model Systems. Nscisc. 2012:21. [Google Scholar]
- 147.Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004 Oct;21(10):1371–83. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- 148.Simpson La, Eng JJ, Hsieh JTC, Wolfe DL. The health and life priorities of individuals with spinal cord injury: a systematic review. J Neurotrauma. 2012 May;29(8):1548–55. doi: 10.1089/neu.2011.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Capogrosso M, Milekovic T, Borton D, Wagner F, Martin Moraud E, Mignardot J-B, Buse N, Gandar J, Barraud Q, Xing D, Rey E, Duis S, Jianzhong Y, DKWK, Li Q, Detemple P, Denison T, Micera S, Bezard E, Bloch J, Courtine G. A brain-spinal interface alleviating gait deficits after spinal cord injury in primates. Nature. 2016;(7628):284–288. doi: 10.1038/nature20118. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Moritz CT, Perlmutter SI, Fetz EE. Direct control of paralyzed muscles by cortical neurons. Nature. 2008 Dec;456(7222):639–642. doi: 10.1038/nature07418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ethier C, Oby ER, Bauman MJ, Miller LE. Restoration of grasp following paralysis through brain-controlled stimulation of muscles. Nature. 2012 May;485(7398):368–371. doi: 10.1038/nature10987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ethier C, Miller LE. Brain-controlled muscle stimulation for the restoration of motor function. Neurobiol Dis. 2015 Nov;83:180–190. doi: 10.1016/j.nbd.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shepherd RK, editor. Neurobionics: The biomedical engineering of neural prostheses. 1. Wiley-Blackwell; 2016. [Google Scholar]
- 154.Blabe CH, Gilja V, Chestek Ca, Shenoy KV, Anderson KD, Henderson JM. Assessment of brain-machine interfaces from the perspective of people with paralysis. J Neural Eng. 2015;12(4):43002. doi: 10.1088/1741-2560/12/4/043002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lahr J, Schwartz C, Heimbach B, Aertsen A, Rickert J, Ball T. Invasive brain-machine interfaces: A survey of paralyzed patients’ attitudes, knowledge and methods of information retrieval. J Neural Eng. 2015;12(4):43001. doi: 10.1088/1741-2560/12/4/043001. [DOI] [PubMed] [Google Scholar]
- 156.Yin M, Borton DA, Aceros J, Patterson WR, Nurmikko AV. A 100-channel hermetically sealed implantable device for chronic wireless neurosensing applications. IEEE Trans Biomed Circuits Syst. 2013;7(2):115–128. doi: 10.1109/TBCAS.2013.2255874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Borton DA, Yin M, Aceros J, Nurmikko A. An Implantable Wireless Neural Interface for Recording Cortical Circuit Dynamics in Moving Primates. J Neural Eng. 2013 Apr;10(2):26010. doi: 10.1088/1741-2560/10/2/026010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Perge JA, Homer ML, Malik WQ, Cash S, Eskandar E, Friehs G, Donoghue JP, Hochberg LR. Intra-day signal instabilities affect decoding performance in an intracortical neural interface system. J Neural Eng. 2013;10(3):36004. doi: 10.1088/1741-2560/10/3/036004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Scott SH. NEWS & VIEWS Converting thoughts into action. 2006 Jul;442:9–11. doi: 10.1038/442141a. [DOI] [PubMed] [Google Scholar]
- 160.Flesher SN, Collinger JL, Foldes ST, Weiss JM, Downey JE, Tyler-Kabara EC, Bensmaia SJ, Schwartz AB, Boninger ML, Gaunt RA. Intracortical microstimulation of human somatosensory cortex. Sci Transl Medicne. 2016:1–11. doi: 10.1126/scitranslmed.aaf8083. [DOI] [PubMed] [Google Scholar]
- 161.Thompson DE, Quitadamo LR, Mainardi L, Laghari KurR, Gao S, Kindermans P-J, Simeral JD, Fazel-Rezai R, Matteucci M, Falk TH, Bianchi L, Chestek CA, Huggins JE. Performance Measurement for Brain-Computer or Brain-Machine Interfaces: A Tutorial. J Neural Eng. 2014 Jun;11(3):35001. doi: 10.1088/1741-2560/11/3/035001. [DOI] [PMC free article] [PubMed] [Google Scholar]