SUMMARY
The immunopathology caused by schistosome helminths varies greatly in humans and among mouse strains. A severe form of parasite egg-induced hepatic granulomatous inflammation, seen in CBA mice, is driven by Th17 cells stimulated by IL-1β and IL-23 produced by dendritic cells that express CD209a (SIGNR5), a C-type lectin receptor (CLR) related to human DC-SIGN. Here, we show that CD209a-deficient CBA mice display decreased Th17 responses and are protected from severe immunopathology. In vitro, CD209a augments the egg-induced IL-1β and IL-23 production initiated by the related CLRs Dectin-2 and Mincle. While Dectin-2 and Mincle trigger an FcRγ-dependent signaling cascade that involves the tyrosine kinase Syk and the trimolecular Card9-Bcl10-Malt1 complex, CD209a promotes the sustained activation of Raf-1. Our findings demonstrate that CD209a drives severe Th17 cell-mediated immunopathology in a helminthic disease based on synergy between DC-SIGN- and Dectin-2-related CLRs.
In Brief
Kalantari et al. demonstrate the role of CD209a (SIGNR5) in the development of Th17 cell-mediated immunopathology in murine schistosomiasis. CD209a drives proinflammatory cytokine production in synergy with Dectin-2 and Mincle, each acting via distinct signaling pathways. These findings denote C-type lectin receptor cross talk resulting in severe helminthic disease.
INTRODUCTION
Schistosomes are trematode helminths that cause widespread disease in tropical regions of the world. Schistosoma mansoni (S. mansoni) is the most frequent causative agent of human schistosomiasis, which collectively affects more than 200 million people and is the second most prevalent parasitic disease after malaria (Hotez and Fenwick, 2009). Infection with S. mansoni results in hepatic and intestinal granulomatous inflammation around deposited parasite eggs. Most individuals develop a mild chronic gastrointestinal form of disease, but a minority of 5%–10% suffers from life-threatening hepatosplenic schistosomiasis, in which there is severe hepatic fibrosis with consequent portal hypertension, portal-systemic shunting, splenomegaly, ascites, and death (Larkin et al., 2012; Pearce and MacDonald, 2002).
A marked variation in disease severity is also observed among mouse strains in an experimental model of schistosomiasis. Typically, CBA/J (CBA) mice develop large, poorly circumscribed hepatic egg granulomas largely mediated by Th17 cells induced by egg-stimulated dendritic cells (DCs) secreting interleukin-1β (IL-1β) and IL-23 (Shainheit et al., 2008, 2011). On the other hand, C57BL/6 (BL/6) mice develop mild pathology with significantly smaller egg granulomas formed in a Th2-dominant cytokine environment (Pearce and MacDonald, 2002). The pathogenic Th17 cells are a highly proinflammatory subset of CD4+ effector T cells that, in addition to their signature cytokine IL-17, also produce IL-22, CSF, CXCL1, CXCL2, and tumor necrosis factor α (TNFα) (Bettelli et al., 2007). Although genetically determined differences in schistosome egg-induced immunopathology among inbred mouse strains have been documented (Cheever et al., 1987), the molecular mechanisms underlying such heterogeneous responses remain to be fully understood. In a previous gene profiling analysis of DCs from high-(CBA) versus low-pathology (BL/6) mice, we found striking differences in the baseline expression of C-type lectin receptors (CLRs), which are a broad family of pattern recognition receptors (PRRs) capable of binding to glycan residues, such as those typically present on schistosome eggs, in a calcium-dependent manner (Ponichtera et al., 2014). In particular, CD209a (SIGNR5) expression was 18-fold higher in CBA versus BL/6 DCs (Ponichtera et al., 2014). Most significantly, silencing of CD209a on CBA DCs, and overexpression on BL/6 bone marrow-derived DCs (BMDCs), suggested that CD209a was necessary for the production of IL-1β and IL-23 by live egg-stimulated BMDCs, and for the consequent development of Th17 cells (Ponichtera et al., 2014).
CD209a is one of seven murine homologs of the human DC-specific ICAM-3-grabbing non-integrin (DC-SIGN, CD209), a type II transmembrane CLR that plays a crucial role in the innate immune sensing and uptake of human pathogens, including the causative agents of HIV, Ebola, tuberculosis, opportunistic fungal infections, and schistosomiasis (reviewed in den Dunnen et al. 2009). DC-SIGN binds to various mannose- and fucose-containing viral, bacterial, fungal, and parasite-derived glycans, and modulates the outcome of immune responses (Cheong et al., 2010; Geijtenbeek and Gringhuis, 2009; Park et al., 2001; Powlesland et al., 2006; van Die et al., 2003). The biological roles of CD209 family members are highly variable as they can promote the establishment of either Th1/Th17 or Th2-biased immune responses in distinct contexts (Anthony et al., 2011; Caparrós et al., 2006; Gringhuis et al., 2014). Among the murine CD209 homologs, CD209b (SIGNR1) has been shown to bind schistosome-derived glycans; however, its deletion has no influence on the immunopathology (Saunders et al., 2009). In contrast, the in vivo impact of CD209a has not been examined.
Although our previous studies suggested a role of CD209a in the severity of egg-induced immunopathology, a distinct CLR, Dectin-2, has been implicated in the recognition of soluble schistosome egg antigens (SEAs) by BMDCs primed with a Toll-like receptor 2 (TLR2) ligand (Ritter et al., 2010). Since cross talk between distinct classes of PRRs has the potential to diversify the innate immune responses of the host following microbial challenge (reviewed in Tan et al. 2014), we postulated that CD209a could synergize with Dectin-2 to drive the inflammatory immune responses initiated by schistosomes in the high-pathology CBA strain. Receptor collaborations among TLRs and CLRs have been amply demonstrated (Ostrop and Lang, 2017). Within the Dectin-2 family, receptor heterodimerization (e.g., between Dectin-2 and Mincle) has been suggested to increase ligand binding and to enhance immune responses (Zhu et al., 2013). However, the cross talk among distinct classes of CLRs has not been examined in any depth, and its investigation in the context of the schistosome infection could provide useful insights into the initiation of inflammatory responses elicited by this and other human pathogens.
To assess the impact of CD209a on the immune responses and immunopathology induced by in vivo infection with S. mansoni, we developed a CD209a-deficient mouse on the CBA (H-2k) background. We now report that such mice exhibit markedly reduced hepatic granulomatous inflammation together with a significantly decreased proinflammatory Th17 response. Furthermore, we demonstrate that CD209a synergizes with Dectin-2 and Mincle to activate proinflammatory signaling cascades that ultimately lead to the induction of pathogenic Th17 responses. These functions require the carbohydrate binding site in CD209a, the gamma subunit of the high-affinity IgE receptor (FcRγ), the spleen tyrosine kinase (Syk), the CBM complex composed of Card9, Bcl10, and Malt-1, as well as, independently, the serine and threonine kinase Raf-1. We conclude that CD209a decisively influences the course of the schistosome infection.
RESULTS
CD209a−/− Mice Develop Reduced Egg-Induced Hepatic Immunopathology
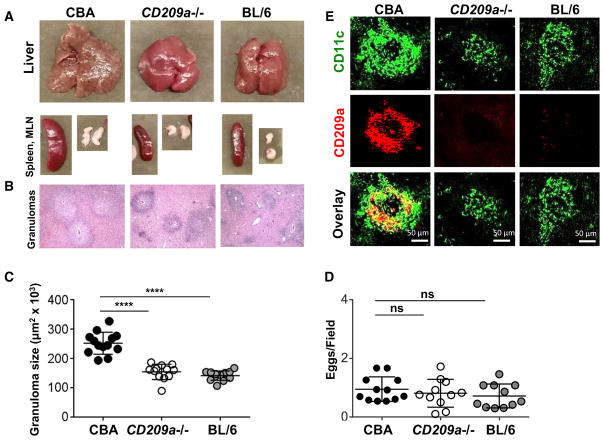
To investigate the role of CD209a in the development of egg-induced hepatic immunopathology in vivo, we backcrossed the CD209a knockout (Cheong et al., 2010) allele to the high-pathology CBA strain. After a 7-week infection with S. mansoni, CD209a−/− livers, granulomatous lesions, spleens, and mesenteric lymph nodes (MLNs) were all markedly smaller than in the CBA counterparts and resembled those typically seen in the BL/6 strain (Figure 1A). On histological examination, the egg granulomas in CD209a−/− and BL/6 livers were smaller and more sharply circumscribed than in the CBA controls (Figure 1B); the significance of these size differences was confirmed by computer-assisted morphometric analysis (Figure 1C). Nevertheless, there were no significant differences in the number of eggs harbored in the livers of all three mouse groups (Figure 1D). Confocal microscopy demonstrated that the large granulomas in the CBA livers contained sizable numbers of CD209a-expressing, CD11c-positive cells, which were absent or markedly reduced in the smaller granulomas from CD209a−/− or BL/6 livers (Figure 1E).
Figure 1. The Loss of CD209a Results in Marked Decrease in Egg-Induced Granulomatous Inflammation.
CBA, CD209a−/−, and BL/6 mice infected with S. mansoni were sacrificed at 7 weeks post-infection.
(A) Representative images of livers, spleens, and MLNs from infected mice.
(B) Histopathology of liver granulomas (magnification, 100×).
(C) Granuloma size determined by morphometric analysis. Each dot represents average granuloma size of 10–20 granulomas in two sections from individual mice.
(D) Number of eggs per 2.4-mm2 field assessed on H&E-stained liver sections at 100× magnification. An average of 16 fields per liver section was assessed. Data in (C) and (D) represent pooled results from three independent experiments with three to five mice per group.
(E) Liver cryostat sections were stained for CD209a (red) and CD11c (green) and examined by confocal microscopy. Images are representative of three independent experiments. For this and all figures: *p < 0.05, **p < 0.005, ***p < 0.0005, ****p < 0.00005; ns, not significant.
The Absence of CD209a Inhibits Th17 Cell Responses and Enhances Th2 Polarization
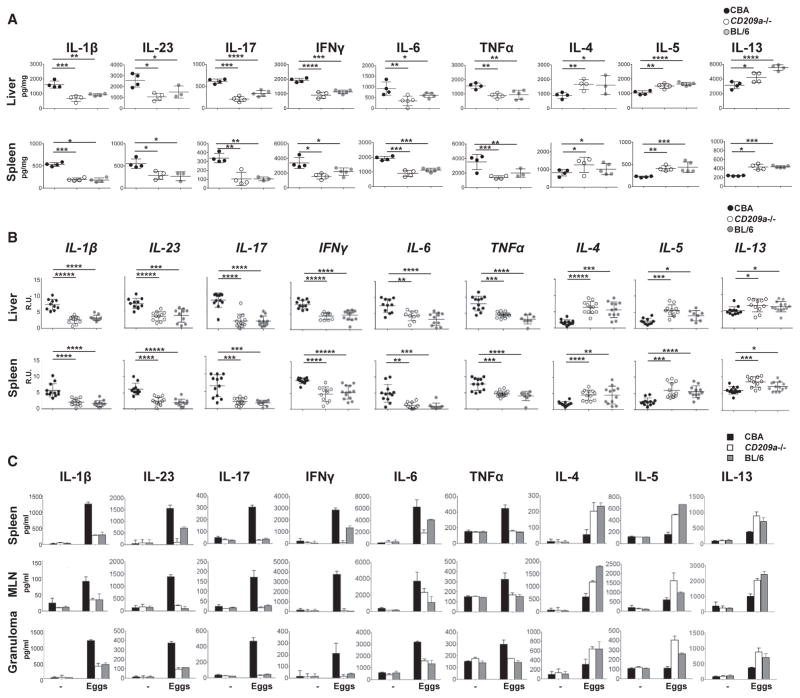
The diminished granulomatous inflammation in CD209a−/− mice was associated with a significant reduction in proinflammatory cytokines (Figure 2A) and their corresponding mRNAs (Figure 2B) in vivo. In lesional liver and spleen homogenates, the loss of CD209a reduced the levels of IL-1β, IL-23, IL-17, interferon γ (IFNγ), IL-6, and TNFα to those observed in BL/6 mice. In contrast, the Th2 cytokines IL-4, IL-5, and IL-13 were significantly higher in CD209a−/− than in CBA mice.
Figure 2. The Loss of CD209a Results in Reduced Th17/Th1 and Enhanced Th2 Cytokine Responses In Vivo and Ex Vivo.
(A–C) Livers and spleens from infected mice were homogenized, and cytokine levels were assessed by ELISA (A) or qPCR (B). Bulk spleen, MLN, and granuloma cells from four to five mice per group were pooled and cultured with live eggs (C). Cytokines in 72-hr supernatants were measured by ELISA. Data in (A) and (C) are representative of one of three independent experiments with similar results. Data in (B) represent pooled results from three independent experiments. Each dot (A and B) and bar (C) represent mean ± SD cytokine levels of triplicate determinations per mouse. R.U., relative units.
Live eggs trigger the hepatic inflammation associated with schistosomiasis and, unlike SEA, activate BMDCs in the absence of TLR-dependent priming signals (Figure S1A). Therefore, to determine how CD209a alters T helper cell responses ex vivo, spleen cells, MLN cells (MLNCs) and granuloma cells were restimulated with live eggs, in the absence of any other stimuli. Consistent with our in vivo results, cultures containing CD209a−/− cells produced significantly less IL-1β, IL-23, IL-17, IFNγ, IL-6, TNFα, and more IL-4, IL-5, and IL-13 than the CBA controls (Figure 2C). Taken together, these findings demonstrate that CD209a promotes the development of Th17 and Th1 responses and represses Th2 polarization during schistosome infection.
CD209a Expression on DCs Enables Live Schistosome Egg-Induced IL-1β, IL-23, and IL-17 Production In Vitro
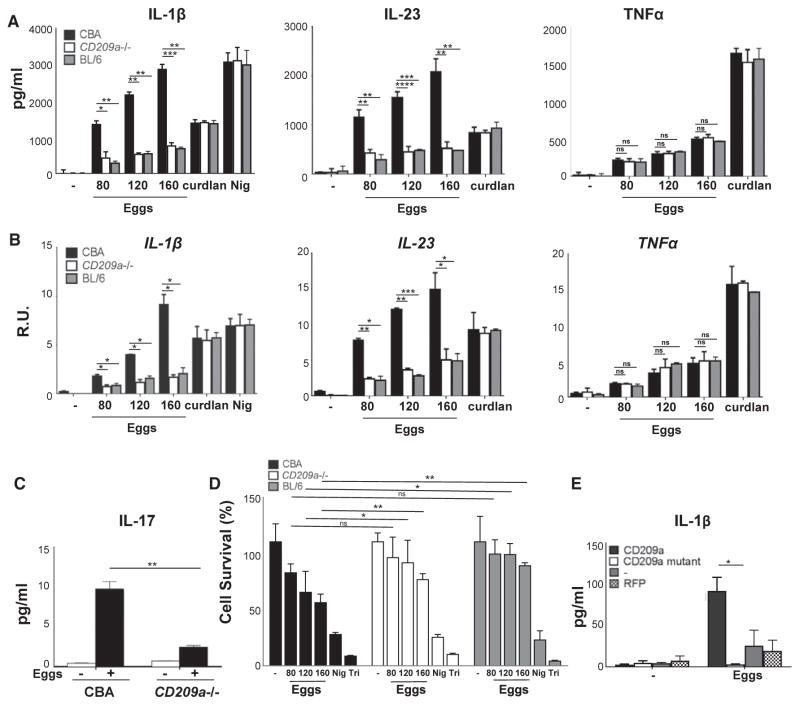
To confirm our in vivo and ex vivo findings, BMDCs were stimulated with live eggs in vitro. CD209a−/− BMDCs produced markedly lower levels of IL-1β and IL-23 than those observed in BL/6 controls (Figure 3A, left and middle panels), while the loss of CD209a had no effect on TNFα (Figure 3A, right panel). The capacity of the CD209a−/− BMDCs to produce IL-1β and IL-23 was not globally impaired, as no differences were observed among the BMDC populations after stimulation with the Dectin-1 agonist curdlan or after lipopolysaccharide (LPS) priming and subsequent treatment with the potassium ionophore nigericin. In all instances, cytokine mRNA levels paralleled the secreted proteins (Figure 3B). Importantly, co-cultures of CD209a−/− DCs with CD4 T cells failed to produce significant levels of IL-17 or IL-1β, demonstrating that CD209a expression is required for the efficient induction of Th17 cells (Figure 3C; Figure S1B). To determine whether live eggs induce changes in cell viability that contribute to the observed alterations in IL-1β, IL-23, and IL-17 production, we assessed cell viability upon egg stimulation using vital probe calcein AM. Higher concentrations of eggs induced BMDC death in a dose-dependent fashion. Cell survival at these higher doses was somewhat enhanced in the absence of CD209a. However, at the doses used in most of our studies, the impact of the eggs on cell viability was negligible (Figure 3D). To test whether ligand recognition is required for the proinflammatory functions of CD209a, we transduced BL/6 BMDCs with either wild-type (WT) CD209a or a mutant form of CD209a with an altered carbohydrate-binding site (R211S/D212G). Although both variants were expressed on the cell surface (Figure S1C), only WT CD209a enabled the production of IL-1β in response to live eggs (Figure 3E), demonstrating that ligand recognition is essential for the promotion of inflammatory responses by CD209a.
Figure 3. Egg-Induced IL-1β and IL-17 Production Is Markedly Inhibited in CD209a−/− Cells.
BMDCs were cultured for 24 hr with the indicated numbers of live eggs, curdlan, or LPS plus nigericin (Nig).
(A) Cytokines in supernatants were measured by ELISA.
(B) Cytokine mRNA levels were assessed by qRT-PCR and normalized to Gapdh.
(C) BMDC-T cell co-cultures were stimulated with 80 live eggs, and IL-17A in 72-hr supernatants was measured by ELISA.
(D) BMDCs were cultured for 24 hr with the indicated numbers of live eggs, LPS plus Nig, or Triton X-100 (Tri) for 10 min. Cell survival was measured using calcein AM. The medium was set to 100%.
(E) BL/6 BMDCs were transduced with lentiviruses expressing, CD209a, a carbohydrate binding site mutant of CD209a (R211D/S212G) or RFP control. The cells were stimulated with 80 live eggs, and IL-1β was measured by ELISA. Bars represent the mean ± SD cytokine levels of three biological replicates from one representative experiment of three with similar results.
IL-1β and IL-23 Jointly Elicit Maximal IL-17 Production from Egg-Stimulated T Cells
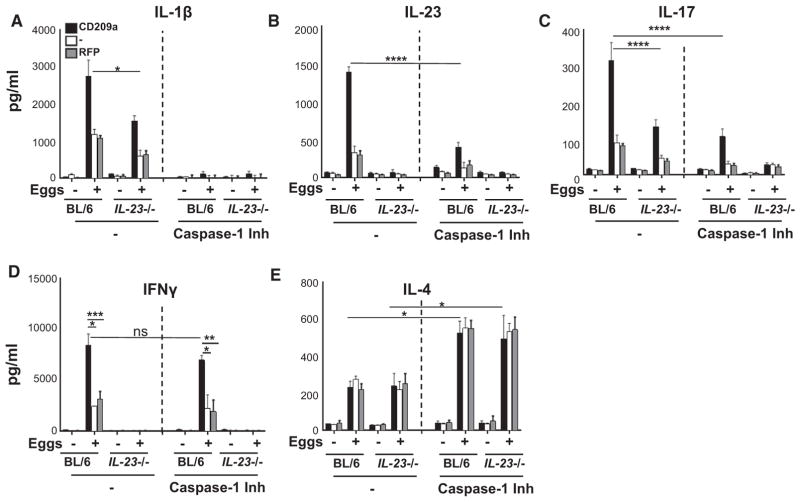
Previous work from our laboratory identified IL-1β and IL-23 as prerequisites for Th17 cell differentiation in response to schistosome eggs (Shainheit et al., 2008, 2011). To test the extent to which these cytokines implement the proinflammatory functions of CD209a, we used IL-23 p19−/− BMDCs, which lack IL-23, and/or blocked IL-1β production with the caspase-1 inhibitor ZYVAD-FMK. Each cytokine enhanced the production of the other, although IL-23 showed a greater dependence on IL-1β (Figures 4A and 4B; Figure S2A). Moreover, although low levels of IL-17 were retained in the absence of either IL-1β or IL-23, the loss of both cytokines abrogated IL-17 production (Figure 4C). Importantly, IL-1β, IL-23, and IL-17 were all significantly elevated upon expression of CD209a. CD209a also enhanced IFNγ production, which was not affected by IL-1β, but was dependent on IL-23 (Figure 4D). Last, CD209a expression had no effect on the production of IL-4, which was independent of IL-23, but was enhanced in the absence of IL-1β (Figure 4E).
Figure 4. CD209a Augments IL-17 Production via IL-1β and IL-23.
(A–E) BL/6 and IL-23p19−/− (IL-23−/− in figure) BMDCs were transduced with CD209a or control RFP. Some BMDCs were pretreated with the caspase-1 inhibitor ZYVAD-FMK (50 μM) for 1 hr before establishing co-cultures with T cells in the presence or absence of 80 live eggs. IL-1β (A), IL-23 (B), IL-17 (C), IFNγ (D), and IL-4 (E) in 72-hr supernatants were measured by ELISA. Bars are as described in Figure 3.
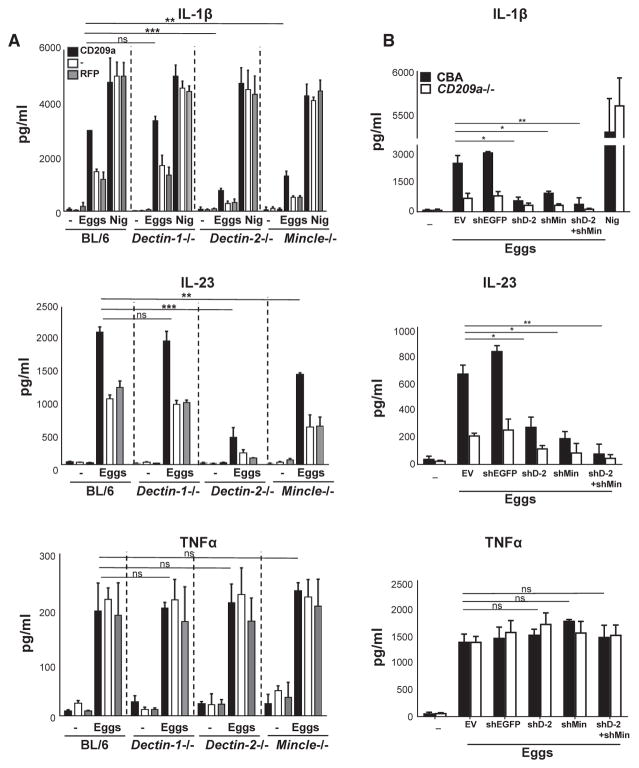
DC Expression of Both CD209a and Dectin-2/Mincle Enables Robust IL-1β and IL-23 Production
Dectin-2 has been implicated in the production of IL-1β by TLR-primed, SEA-triggered BL/6 BMDCs (Ritter et al., 2010). Dectin-2, with Dectin-3 and Mincle, defines a subgroup of type-II transmembrane CLRs that is more closely related to CD209a than to Dectin-1 and that is expressed by both BL/6 and CBA BMDCs (Herrero et al., 2016; Ponichtera et al., 2014; Strasser et al., 2012). To determine whether this subgroup of CLRs contributes to the proinflammatory cytokine responses induced by CD209a, we stimulated CD209a-transduced BMDCs from WT, Dectin-1−/−, Dectin-2−/−, Dectin-3−/−, and Mincle−/− mice with live eggs. Dectin-1 was not required for the production of IL-1β, IL-23, or TNFα irrespective of CD209a, although its absence abrogated curdlan-dependent cytokine responses (Figure 5A; Figures S2B and S3A). In contrast, the loss of Dectin-2 or Mincle significantly impaired IL-1β and IL-23 production, with differences that were most pronounced in CD209a-expressing BMDCs. Despite the impact of Dectin-2 and Mincle on IL-1β and IL-23, their loss had no effect on TNFα production (Figure 5A; Figure S2B). Last, the absence of Dectin-3 had no effect on any of these cytokines (Figures S2C and S3B).
Figure 5. CD209a Induces IL-1β and IL-23 Production in Conjunction with Dectin-2 and Mincle.
(A and B) BMDCs from BL/6, Dectin-1−/−, Dectin-2−/−, and Mincle−/− were transduced with CD209a or control RFP (A); CBA and CD209a−/− BMDCs were transduced with empty vector (EV), control shRNA against eGFP (shEGFP), shDectin-2 (shD-2), and shMincle (shMin) (B). Cells were cultured with 80 live eggs or LPS plus Nig as indicated. Cytokines in 24-hr supernatants were measured by ELISA. Bars are as described in Figure 3.
To confirm that Dectin-2 and Mincle play similar roles in CD209a-dependent cytokine production in CBA-derived cells, we silenced Dectin-2 and Mincle using lentivirally encoded small hairpin RNAs (shRNAs) in WT and CD209a−/− CBA-derived BMDCs. The suppression of the mRNAs for both targets was highly effective, as documented by qRT-PCR (Figures S2G and S2H). In these cells, the loss of Dectin-2 and Mincle caused a marked drop in egg-stimulated IL-1β and IL-23 production (Figure 5B). The effects of these knockdowns were proportionally greater in the CD209a-expressing WT BMDCs. In addition, the simultaneous elimination of Dectin-2 and Mincle virtually eliminated IL-1β and IL-23 production in CBA-derived WT and CD209a−/− BMDCs, but had no effect on TNFα (Figure 5B). These findings demonstrate that highest levels of IL-1β and IL-23 production require CD209a to act in conjunction with Dectin-2 and Mincle.
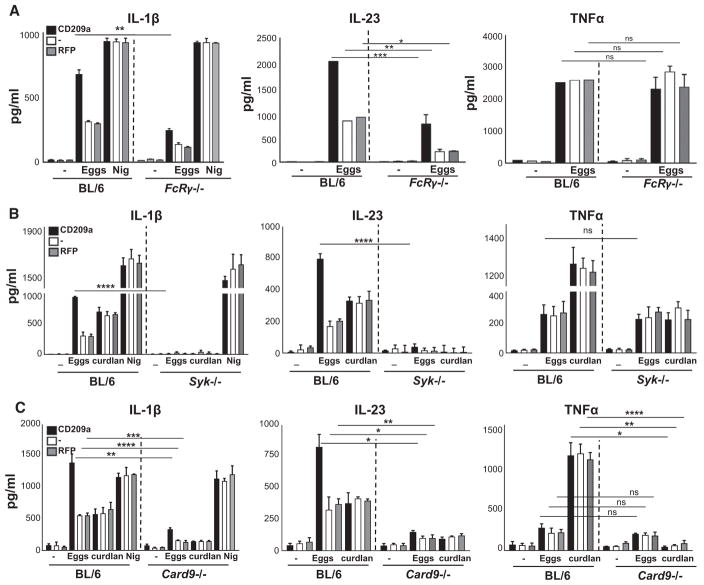
Dectin-2/Mincle Signaling Involves FcRγ, Syk, and the CBM Complex
Since Dectin-2 and Mincle have been reported to signal via FcRγ (Ritter et al., 2010; Sancho and Reis e Sousa, 2012), we examined egg-induced cytokine responses in FcRγ-deficient BMDCs. The absence of FcRγ resulted in a marked drop in IL-1β and IL-23, particularly from BMDCs expressing CD209a, but had no effect on the production of TNFα (Figure 6A; Figure S2D). Given the requirement of the FcRγ subunit, which participates in multimeric receptor complexes with Dectin-2 and Mincle, we reasoned that the effects of CD209a would also require immunoreceptor tyrosine-based activation motif (ITAM)-dependent pathways downstream of this shared receptor subunit. The most proximal effector of the FcRγ subunit in DCs is Syk. By treating CBA BMDCs with increasing doses of the Syk inhibitor piceatannol, we determined that Syk is essential for the production of IL-1β and IL-23 in response to eggs or curdlan (Figure S4A). Consistent with our pharmacological data, the loss of Syk dramatically reduced the ability of CD209a-expressing BMDCs to produce IL-1β and IL-23 (Figure 6B, left and center panels; Figure S2E). In contrast, although curdlan-induced TNFα required Syk, its production in response to live eggs was Syk independent (LeibundGut-Landmann et al., 2007) (Figure 6B, right panel). Of note, the loss of Syk also eliminated CD209a-dependent increases in IL-17 production by egg-stimulated co-cultures of BMDCs and activated CD4 T cells (Figure S4B). To clarify whether the signals initiated by CD209a intersect with those initiated by Dectin-2 at the level of Syk, we examined Syk phosphorylation in CBA BMDCs treated with biotinylated antibodies (Abs) targeting CD209a and/or Dectin-2, and cross-linked the relevant receptors using streptavidin. Under these conditions, Dectin-2 ligation rapidly resulted in the phosphorylation of Syk on Y352, which is a hallmark of Syk activation. In contrast, CD209a ligation had no obvious impact on Syk phosphorylation whether crosslinked independently, or in conjunction with Dectin-2 (Figure S4C).
Figure 6. CD209a-Exacerbated IL-1β and IL-23 Production Involves the Activation of FcRγ, Syk, and CBM.
(A–C) BMDCs from BL/6 and FcRγ−/− (A), BL/6 and Syk−/− (B), and BL/6 and Card9−/− (C) mice were transduced with CD209a or control RFP. The cells were cultured with 80 live eggs or LPS plus Nig as indicated. Cytokines in 24-hr supernatants were measured by ELISA. Bars are as described in Figure 3.
FcRγ-coupled ITAM-coupled receptors respond to fungal glycans by triggering the assembly of oligomeric scaffolds known as CBM complexes (Saijo et al., 2010). In DCs, CBM complexes are formed when Card9 seeds the assembly of polymers containing the adaptor Bcl10 and the paracaspase Malt1. Therefore, we examined whether Card9 and Malt1 contribute to the enhancement of egg-induced cytokine responses by CD209a. Curdlan was included as a control because it is known to induce CBM complex formation and because curdlan-induced responses are CD209a independent (Gringhuis et al., 2014; Jia et al., 2014). Egg-induced IL-1β and IL-23 were markedly lower in CD209a-transduced Card9−/− BMDCs, whereas TNFα was not significantly affected (Figure 6C; Figure S2F). In contrast, the loss of Card9 decreased the curdlan-induced production of all three cytokines (Jia et al., 2014). Similarly, the Malt1 inhibitor Z-VRPR-FMK blocked the egg-induced production of IL-1β and IL-23 by CBA BMDCs and blocked IL-1β, IL-23, and TNFα production in response to curdlan (Figure S4D). Collectively, these findings demonstrate that the CD209a-dependent pathways that augment egg-induced IL-1β and IL-23 production require the concurrent delivery of signals via ITAM-coupled pathways involving Syk and the CBM complex.
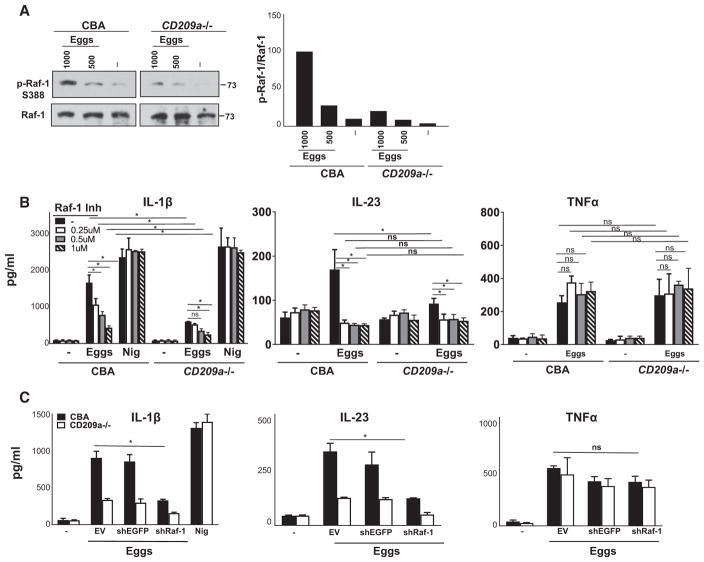
CD209a Amplifies IL-1β and IL-23 Production by Triggering the Sustained Activation of Raf-1
Pathogen interaction with DC-SIGN results in the phosphorylation of Raf-1 (Geijtenbeek et al., 2009) as well as in phosphorylation, acetylation, and enhanced activation of the p65 subunit of nuclear factor κB (NF-κB), which is essential for the production of IL-23 and pro-IL-1β (Geijtenbeek and Gringhuis, 2009). Here, we show that in CBA BMDCs, a 24-hr exposure to live eggs stimulated pronounced Raf-1 activation (Figure 7A, left), which was notably weaker in CD209a−/− BMDCs (Figure 7A, right; also see adjacent densitometry). These findings indicate that CD209a plays a crucial role in the activation of Raf-1 in egg-stimulated BMDCs. The pharmacological inhibition of Raf-1 confirmed that Raf-1 plays a crucial role in the production of IL-1β and IL-23 (Figure 7B). Moreover, blocking the expression of Raf-1 in CBA and CD209a−/− BMDCs with lentivirally encoded shRNA (Figure S2I) similarly decreased IL-1β and IL-23 production without affecting TNFα (Figure 7C). These data confirm that Raf-1 plays an essential role in the CD209a-dependent augmentation of proinflammatory cytokine production by BMDCs stimulated with live eggs.
Figure 7. Raf-1 Activation Is Required for CD209a-Mediated IL-1β and IL-23 Production.
(A) Left panel: Immunoblot analysis of 2 × 106 CBA and CD209a−/− BMDCs plated in six-well plates and stimulated with 500 or 1,000 live eggs per well for 24 hr. Cell lysates were used for western blot analysis using Abs against phosphorylated Raf-1 (p-Raf-1-S338) and total Raf-1. Results are representative of three independent experiments. At right is ratio of p-Raf-1 expression over total Raf-1 as assessed by densitometry. The ratio related to CBA BMDCs stimulated with 1,000 eggs was set to 100.
(B) CBA and CD209a−/− BMDCs were treated with various concentrations of Raf-1 inhibitor (Inh) GW5074 or DMSO for 1 hr and then cultured with 80 live eggs or LPS plus Nig.
(C) BMDCs from CBA and CD209a−/− mice were transduced with EV, shEGFP, or shRNA against Raf-1 (shRaf-1). In (B) and (C), cells were stimulated with 80 live eggs, and cytokines in 24-hr supernatants were assessed by ELISA. Bars are as described in Figure 3.
DISCUSSION
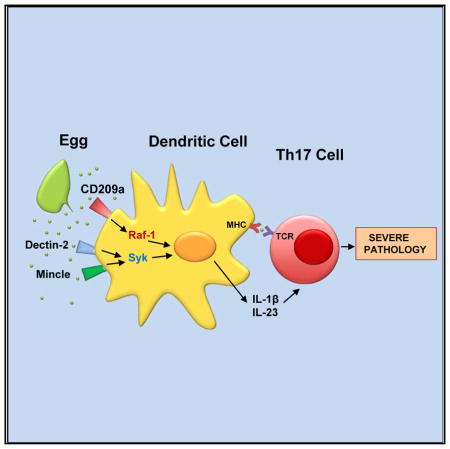
In this study, we determined that CD209a is necessary for the development of severe, Th17-mediated immunopathology in a murine model of schistosome infection. Specifically, the loss of CD209a in CBA mice decreases the severity of hepatic granulomatous inflammation induced by parasite eggs, limits the production of IL-1β and IL-23 by egg-stimulated DCs, and reduces the induction of pathogenic Th17 cells. The loss of CD209a also causes an increase in the Th2 cytokines IL-4 and IL-5, which are typically dominant in BL/6 mice. Analogous changes in cytokine production are also observed in vitro, in BMDCs challenged with live eggs. We chose to use live eggs, in contrast to the more commonly used SEA, because they are the drivers of inflammation in the context of natural infection (Ponichtera et al., 2014; Shainheit et al., 2011) and are per se capable of triggering IL-1β production by DCs. We also found that CD209a-dependent IL-1β and IL-23 mutually reinforce one another to direct the differentiation of pathogenic Th17 cells in our in vitro mixed cultures. Overall, these findings suggest that CD209a plays its decisive role in the exacerbation of egg-induced immunopathology through its impact on DC function.
Murine CD209a is a member of an extended family of CD209 paralogs, which are type II transmembrane receptors that recognize mannosylated or fucosylated carbohydrate ligands via related C-type lectin-like domains (CTLDs). CD209a is one of seven murine paralogs that can be distinguished by their structural features, carbohydrate-binding specificities, and dissimilar patterns of expression (Garcia-Vallejo and van Kooyk, 2013). Within this family, the CTLD is separated from the transmembrane domain by repeat units that have been implicated in receptor multimerization (Park et al., 2001; Powlesland et al., 2006). Whereas the human homologs DC-SIGN (CD209) and DC-SIGNR (CLEC4M) each possess a long “neck” comprising eight repeats, the murine paralogs possess shorter neck regions, with only CD209b retaining multiple repeat units. Although human DC-SIGN transmits signals via its cytoplasmic tail, this region is highly variable among the murine CD209 paralogs, with only CD209a (SIGNR5), CD209b (SIGNR1), CD209d (SIGNR3), and CD209f (SIGNR8) retaining full-length cytoplasmic tails, and only CD209a and CD209b retaining a juxtamembrane G-C-x-x-H motif conserved across multiple species. This structural diversity has made it difficult to identify true functional homologs for DC-SIGN and DC-SIGNR. However, the expression pattern of CD209a most closely matches that of human DC-SIGN (Cheong et al., 2010).
Schistosome eggs are rich in fucose and mannose-containing glycoproteins that can be sensed by a number of CLRs. Human CLRs, including DC-SIGN, DC-SIGNR, and the more distantly related macrophage galactose-type lectin (MGL) and mannose receptor (MRC1), can bind to schistosome-derived carbohydrate epitopes, including Lewis X (Lex), LDN, and LDN-F and GalNAcβ1–4[Fucα1–3]GlcNAc (Lac-diNAc-Fucose) (reviewed in Prasanphanich et al. 2013). Murine CD209b has been shown to bind to egg-derived ligands but has not been implicated in immunopathology (Saunders et al., 2009; van Die et al., 2003). At present, the identity of the egg-derived glycan(s) promoting Th17-biased immune responses via CD209a remains unknown, although our mutagenesis study suggests that the relevant ligand(s) engages CD209a directly.
The CD209 receptor family falls within a larger group of CLRs that includes the ITAM-coupled innate immune receptors Dectin-2 (Clec4n), Dectin-3/MCL (Clec4d), and Mincle (Clec4e). These receptors are expressed on murine DCs and have been implicated in the recognition of glycoprotein and glycolipid structures associated with diverse bacterial and eukaryotic pathogens (reviewed in Sancho and Reis e Sousa, 2012). Our findings indicate that CD209a cooperates with Dectin-2, Mincle, and the ITAM-containing FcRγ (Fcer1g) subunit to maximize the production of Th17-biasing proinflammatory cytokines. In contrast, Dectin-3 is not required to support CD209a-dependent cytokine production. Although a more distantly related type I transmembrane CLR, Dectin-1 (Clec7a), is capable of inducing CD209a-independent inflammatory responses (Drummond and Brown, 2011), this receptor does not drive schistosome-induced immunopathology in the absence of CD209a and does not support egg-induced cytokine production in the absence of FcRγ. Our implication of Dectin-2 and FcRγ in egg-induced inflammatory responses is consistent with a previous report in which both proteins were shown to contribute to IL-1β production in DCs primed with the TLR2 agonist Pam3Cys and then exposed to SEA (Ritter et al., 2010). However, these studies employed the low-pathology BL/6 strain, in which CD209a is poorly expressed, and thus were unable to capture the crucial role of CD209a in the exacerbation of immunopathology.
It is now well established that Dectin-2 and Mincle contribute to the Th17 responses induced by bacterial and fungal pathogens via the FcRγ subunit, the tyrosine kinase Syk, and the CBM signalosome (Hardison and Brown, 2012; Kerrigan and Brown, 2011). However, our study significantly implicates the CBM signalosome in the recognition of a parasitic helminth. Typically, this complex promotes the activation of canonical NF-κB isoforms, and via the activity of Malt1 preferentially activates c-Rel, which plays a critical role in the induction of both IL-1β and IL-23 (Gringhuis et al., 2011). Based on our Malt1 inhibitor studies, it is likely that Dectin-2/Mincle-inducedCBM complexes direct the egg-induced production of IL-1β and IL-23 via the nuclear translocation of both p65/p50 and c-Rel/p50 heterodimers.
A remarkable feature of DC-SIGN signaling is that the same receptor activates distinct signaling pathways depending on the nature of the triggering ligand (Gringhuis et al., 2007, 2009a, 2010). Mannosylated ligands act via a DC-SIGN-associated signalosome that results in the activation of Raf-1 with consequent enhancement of proinflammatory cytokine production (Geijtenbeek and Gringhuis, 2016; Gringhuis et al., 2009b). In contrast, fucosylated ligands promote the dissociation of the Raf-1 signalosome and favor the assembly of an alternative DC-SIGN-associated complex, which supports a Th2 cytokine profile (Gringhuis et al., 2014). Although this phenomenon is now well established, the mechanisms that enable mannosylated and fucosylated ligands to differentially impact DC-SIGN function are not well understood. Of the nine human and murine DC-SIGN paralogs, only DC-SIGNR, CD209a, and CD209f (SIGNR8) display a strong bias toward the recognition of mannosylated, rather than fucosylated ligands. Therefore, CD209a may be intrinsically biased toward the induction of proinflammatory signals via a receptor-associated Raf-1 signalosome. Consistent with this hypothesis, we observed sustained Raf-1 phosphorylation in egg-stimulated CBA-derived BMDCs, and reduced levels of phospho Raf-1 in BMDCs derived from BL/6 or CD209a−/− mice. Furthermore, we found that either the pharmacological inhibition of Raf-1 or the shRNA-mediated suppression of Raf-1 eliminated the CD209a-dependent augmentation of egg-induced IL-1β and IL-23 production.
The FcRγ-coupled CLRs Dectin-2 and Mincle have also been variably implicated in the induction of either Th17 or Th2 responses, whereas the more distantly related hemi-ITAM-containing CLR Dectin-1 consistently gives rise to Th17 responses (Geijtenbeek and Gringhuis, 2016; Tjota et al., 2014; Wüthrich et al., 2015). Although cytokine production by both groups of receptors is Syk dependent, Dectin-1 is unusual in that it simultaneously employs a Syk- and CBM-dependent pathway that activates multiple NF-κB isoforms, and a Syk-independent pathway that activates Raf-1 to promote p65 phosphorylation and acetylation (Gringhuis et al., 2009b). In contrast, Dectin-2 and Mincle both generate Syk-dependent CBM complexes, and potently activate c-Rel, but have not been reported to trigger the alterative Raf-1 pathway (Gringhuis et al., 2011). In conjunction with our findings, these observations suggest that the optimal induction of Th17-biasing cytokines requires the concurrent activation of both the CBM-dependent and Raf-1 dependent pathways, and that ligands that do not fully engage both of these pathways will be unable to support Th17 differentiation and will deviate toward the production of Th2-biasing cytokines. This model suggests that the differential responses mediated by DC-SIGN family members are determined by the simultaneous co-engagement of additional pattern receptors, rather than ligand-specific conformational changes in the receptor. In keeping with this model, it is clear that TLR-dependent signals modulate the DC-SIGN-associated signalosome during the recognition of fucosylated ligands, so as to favor the production of Th2-biasing cytokines. It is equally plausible that mannose-binding receptors impinge on DC-SIGN to favor the formation of the Raf-1 signalosome that enables Th17-biased cytokine production in response to mannosylated ligands. This mechanism of signal integration is more in keeping with the paradigm of antigen receptor costimulation and is far more likely to tolerate the changes in receptor neck structure that have accompanied the diversification of the murine DC-SIGN family. As one example, the mycobacterial product mannosylated lipoarabinomannan (ManLAM) has been shown to bind both human DC-SIGN and murine CD209d (SIGNR3) (Gringhuis et al., 2009a). Because the anti-mycobacterial function of CD209d requires the activity of Syk, it was postulated that SIGNR3 engages Syk directly. However, this conclusion is at odds with our current study, in which the direct engagement of CD209a fails to induce the phosphorylation of Syk. A possible resolution of this discrepancy lies in the subsequent discovery that the Syk-coupled CLR Dectin-2 is also a receptor for ManLAM (Yonekawa et al., 2014). By analogy with this study, the production of protective cytokines in response to mycobacterial ManLAM may require synergy between a CD209d-dependent pathway that drives Raf-1 activation, and a parallel Dectin-2-dependent pathway that drives Syk activation and CBM complex formation. These findings emphasize that our understanding of the cross talk between CLRs and other PRRs is at an early stage, and that much additional work will be required to elaborate how various receptor pairings shape the development of immunity and immunopathology.
In conclusion, we have provided direct evidence of cross talk between the FcRγ-coupled CLRs Dectin-2 and Mincle and a DC-SIGN-related receptor. Specifically, we show that live schistosome eggs are recognized by at least two DC-expressed CLRs, including CD209a and a multimeric receptor that includes either Dectin-2 or Mincle and FcRγ. Although ITAM-coupled CLRs play an indispensable role in the recognition of schistosome-derived ligands, CD209a is crucial for the initiation of the Th17-biased immune responses that drive severe immunopathology in the CBA mouse. The induction of highly inflammatory responses by schistosome egg-exposed DCs involves both the Syk-dependent activation of the CBM complex and a Raf-1-dependent pathway that in humans is involved in recognition of mannosylated ligands by DC-SIGN. Although no correlations with DC-SIGN or other CLR polymorphisms have yet been established, our studies suggest that such polymorphisms and their downstream effectors could contribute to the heterogeneous responses of human populations to schistosome infection.
EXPERIMENTAL PROCEDURES
Mice, Parasites, and Infection
Five- to six-week-old female CBA/J and C57BL/6 mice were purchased from The Jackson Laboratory, and Swiss Webster mice and FcRγ−/− mice from Taconic Biosciences. CD209a−/− mice were provided by C.C. (Institute de Recherches Cliniques de Montreal) and were backcrossed to the CBA (H2k) background for more than ten generations. Dectin-1−/−, Dectin-2−/−, Mincle−/−, Card9−/−, and Dectin3−/− and BL/6×129 mice were bred at the University of Wisconsin-Madison. Mice with targeted deletion of Syk in CD11c+ cells were provided by J. Weinstock (Tufts Medical Center) and IL-23 p19−/− mice by V. Kuchroo (Harvard Medical School). All mice were maintained at the Tufts University School of Medicine Animal Facility in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care guidelines under Institutional Animal Care and Use Committee (IACUC)-approved protocol number B2015-62. Mice were infected by intraperitoneal (i.p.) injection with 85 cercariae of S. mansoni (Puerto Rico strain) shed from infected Biomphalaria glabrata snails. The snails were provided by the National Institute of Allergy and Infectious Diseases (NIAID) Schistosomiasis Resource Center of the Biomedical Research Institute (Rockville, MD) through NIH-NIAID Contract HHSN272201000005I for distribution through BEI Resources. After 7 weeks of infection, at the time of peak granulomatous inflammation, the mice were sacrificed and examined for immunopathology and various immunological parameters. Live schistosome eggs were isolated from livers of infected Swiss Webster mice as previously described (Shainheit et al., 2008). SEA was obtained as previously described (Rutitzky et al., 2008).
Reagents
RPMI 1640 medium was obtained from Lonza, fetal bovine serum (FBS) from Atlanta Biologicals, glutamine and penicillin-streptomycin from Gibco, and recombinant murine granulocyte-macrophage colony-stimulating factor (GM-CSF) from PeproTech. The Syk inhibitor Piceatannol was obtained from Tocris Biosciences, the Malt1 inhibitor Z-VRPR-FMK was from Santa Cruz, the caspase-1 inhibitor ZYVAD-FMK was from APEx Bio, and the Raf-1 inhibitor GW5074 was from Sigma. Nigericin, Red Blood Cell Lysing Buffer, and 2-mercaptoethanol were from Sigma, and curdlan, LPS, and calcein AM were from Invivogen.
Cells, Cell Cultures, and Cell Stimulations
Restimulation of Bulk Lymphoid Cells
Spleen cells, MLNs, and granuloma cells from 7-week-infected CBA, CD209a−/−, and BL/6 mice were prepared as previously described (Rutitzky et al., 2008). In total, 2 × 105 cells were plated and stimulated with 80 live eggs for 72 hr.
DC Cultures
BMDCs were generated as described previously (Ponichtera et al., 2014). In total, 2 × 105 BMDCs were plated and stimulated with 80 live eggs, curdlan (100 μg/mL), LPS (100 ng/mL), or LPS (100 ng/mL) plus SEA (15 μg/mL) for 24 hr. Nigericin (10 μM) was added to LPS-primed cultures 1 hr before the collection of cell supernatants.
DC-T Cell Co-cultures
CD4+ T cells were prepared from normal BL/6 spleens using a CD4+ T Cell Isolation Kit II for mouse (Miltenyi Biotec, Cambridge, MA) in accordance with the manufacturer’s instructions. Purified CD4+ T cells (2 × 105) were cultured with 105 syngeneic BMDCs and stimulated with 80 live eggs plus 8 × 104 anti-CD3/CD28-coated beads (Gibco Dynal Dynabeads) for 72 hr.
ELISA and Quantitative RT-PCR
Cytokine protein measurements were performed in culture supernatants and tissue homogenates for IL-1β, IL-23, IL-17A, TNFα, IFNγ, IL-6, IL-4, IL-5, and IL-13 using ELISA kits from R&D Systems in accordance with the manufacturer’s instructions. Spleens and livers from 7-week-infected mice were homogenized, and total protein concentrations were determined using a BCA protein assay kit (Thermo Scientific). RNA was obtained from cultured cells and spleen and liver homogenates using TRIzol Reagent (Ambion) or PureLink RNA kit (Ambion) and measured for the same cytokines. cDNA was synthesized with a High Capacity cDNA RT Kit (Applied Biosystems) and TaqMan probes for Il1b (MM00434228), Il23a (MM00518984), TNF (MM00443258), ifng (MM1422581), Il6 (MM00446191), Il4 (MM-00445259), Il5 (MM00439646), Il13 (MM99999190), Cd209a (MM00460067), Clec4e (MM01183703), Clec4n (MM00490934), Raf1 (MM00466513), and Gapdh (4351309) were used in combination with TaqMan Gene Expression Master Mix (all from Applied Biosystems). cDNA was synthesized with a iScript Adv cDNA Kit for Gapdh and Il17a. For SYBR Green reactions for Gapdh, primer sequences were as follows: forward, 5′-TGGATTTCGCATTGGTC-3′; reverse, 5′-TTTGCACTGGTACGTGTTGAT-3′. Il17a primer sequences were as follows: forward, 5′-CCTGGACTGTGAGCATGGATA-3′; reverse, 5′-GTAAGGGGCGT CATCAGGAC-3′.
Liver Pathology and Immunofluorescence Studies
Liver samples obtained from infected mice were fixed in 10% buffered formalin, processed, and sectioned by routine histopathologic technique, stained with H&E, and examined by light microscopy. Blinded morphometric analysis was performed using Image-Pro Plus software as previously described (Rutitzky et al., 2008) to measure the size of individual hepatic granulomas with visible central eggs. Immunofluorescence stains on tissue cryostat sections were performed as previously described (Ponichtera et al., 2014).
CD209a Mutagenesis, Lentivirus Production, and Lentiviral Transduction
The open reading frame sequence for CD209a (Cheong et al., 2010; Park et al., 2001) was inserted into a self-inactivating lentiviral plasmid (pLK4.EFv6.IRES.Puro) as described previously (Ponichtera et al., 2014). CD209a mutants were generated using QuikChange site-directed mutagenesis (Agilent). A portion of the sugar-binding site of CD209a was converted from RD (residues 211–212) to SG using primers: 5′-GAAGACTGTGCAGAGTTCTCCGGTGACGGCTGGAATGACACC-3′; 5′-GGT GTCATTCCAGCCGTCACCGGAGAACTCTGCACAGTCTTC-3′ (reverse). Constructs were verified by sequencing. Lentiviral vectors were packaged using the packaging vector psPAX2 and the VSV-G pseudotyping vector pMD2.G, both of which were gifts from Didier Trono (Addgene plasmids 12259 and 12260). Viral particles were produced in HEK293T cells using GeneJuice Transfection Reagent (Novagen). Lentiviruses expressing CD209a, CD209a mutant, or the red fluorescent protein TagRFP-Turbo (abbreviated RFP) were used to infect BMDCs, as previously described (Ponichtera et al., 2014). Transduced BMDCs were analyzed using a BD LSRII flow cytometer (BD Biosciences) and an APC-labeled Ab targeting CD209a (BD Pharmingen).
RNA Interference
BMDCs were transduced with lentiviruses containing lentiviral TRC shRNA expression plasmids targeting Raf-1, Clec4e, and Clec4n and EGFP (Dharmacon). The production of viral particles and transduction of BMDCs was conducted as described above.
Western Blotting
BMDCs were washed, lysed, and prepared with Laemmli Buffer (Boston BioProducts). Samples were run on an SDS-PAGE gel and transferred to a nitrocellulose membrane (Bio-Rad), which was blocked in 5% BSA. The activation of Raf-1 and Syk was detected with Ab specific for phospho-Raf-1 Ser338 (9427) and phospho-Syk PY352 (2701), respectively. Total protein expression was detected with Abs specific for Raf-1 (9422) and ERK (all from Cell Signaling). Biotinylated CD209a (Novus) and biotinylated Dectin-2 (R&D Systems) Abs were used for the crosslinking experiment.
Statistical Analysis
Student’s t test was applied to statistically analyze differences between groups using Microsoft Excel 2016 for Windows. p values < 0.05 were considered significant.
Supplementary Material
Highlights.
CD209a (SIGNR5) deletion causes a marked drop of schistosome egg-induced immunopathology
CD209a synergizes with Dectin-2 and Mincle to activate the pathogenic Th17 cell response
CD209a activates Raf-1, whereas Dectin-2 and Mincle signal via FcRγ/Syk/CBM
The enhanced helminth pathology is a product of C-type lectin receptor cross talk
Acknowledgments
We deeply regret the passing of our esteemed colleague and co-author Cheolho Cheong and dedicate this paper to his memory. We thank Teunis Geijtenbeek for valuable insights into CLR signaling. This work was supported by NIAID Grant R01 AI018919 to M.J.S.
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and four figures and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.01.001.
AUTHOR CONTRIBUTIONS
P.K. designed and performed the experiments with help from Y.M., E.A.M., L.D.J., and H.E.P. C.C. provided the CD209a−/− BL/6 mice and performed the tissue immunostains. M.A.W. provided mice and expert advice. S.C.B., M.C.S., J.M.R., and Y.M. designed and assembled the viral vectors and CD209a mutants. M.J.S. and S.C.B. oversaw the project. P.K. and M.J.S. wrote the manuscript.
DECLARATION OF INTERESTS
The authors declare no competing interests.
References
- Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 2011;475:110–113. doi: 10.1038/nature10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- Caparrós E, Munoz P, Sierra-Filardi E, Serrano-Gómez D, Puig-Kröger A, Rodríguez-Fernández JL, Mellado M, Sancho J, Zubiaur M, Corbí AL. DC-SIGN ligation on dendritic cells results in ERK and PI3K activation and modulates cytokine production. Blood. 2006;107:3950–3958. doi: 10.1182/blood-2005-03-1252. [DOI] [PubMed] [Google Scholar]
- Cheever AW, Duvall RH, Hallack TA, Jr, Minker RG, Malley JD, Malley KG. Variation of hepatic fibrosis and granuloma size among mouse strains infected with Schistosoma mansoni. Am J Trop Med Hyg. 1987;37:85–97. doi: 10.4269/ajtmh.1987.37.85. [DOI] [PubMed] [Google Scholar]
- Cheong C, Matos I, Choi JH, Dandamudi DB, Shrestha E, Longhi MP, Jeffrey KL, Anthony RM, Kluger C, Nchinda G, et al. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell. 2010;143:416–429. doi: 10.1016/j.cell.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Dunnen J, Gringhuis SI, Geijtenbeek TB. Innate signaling by the C-type lectin DC-SIGN dictates immune responses. Cancer Immunol Immunother. 2009;58:1149–1157. doi: 10.1007/s00262-008-0615-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond RA, Brown GD. The role of Dectin-1 in the host defence against fungal infections. Curr Opin Microbiol. 2011;14:392–399. doi: 10.1016/j.mib.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Garcia-Vallejo JJ, van Kooyk Y. The physiological role of DC-SIGN: a tale of mice and men. Trends Immunol. 2013;34:482–486. doi: 10.1016/j.it.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009;9:465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek TB, Gringhuis SI. C-type lectin receptors in the control of T helper cell differentiation. Nat Rev Immunol. 2016;16:433–448. doi: 10.1038/nri.2016.55. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, den Dunnen J, Gringhuis SI. Pathogen recognition by DC-SIGN shapes adaptive immunity. Future Microbiol. 2009;4:879–890. doi: 10.2217/fmb.09.51. [DOI] [PubMed] [Google Scholar]
- Gringhuis SI, den Dunnen J, Litjens M, van Het Hof B, van Kooyk Y, Geijtenbeek TB. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-kappaB. Immunity. 2007;26:605–616. doi: 10.1016/j.immuni.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Geijtenbeek TB. Carbohydrate-specific signaling through the DC-SIGN signalosome tailors immunity to Mycobacterium tuberculosis, HIV-1 and Helicobacter pylori. Nat Immunol. 2009a;10:1081–1088. doi: 10.1038/ni.1778. [DOI] [PubMed] [Google Scholar]
- Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Wevers B, Bruijns SC, Geijtenbeek TB. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-kappaB activation through Raf-1 and Syk. Nat Immunol. 2009b;10:203–213. doi: 10.1038/ni.1692. [DOI] [PubMed] [Google Scholar]
- Gringhuis SI, van der Vlist M, van den Berg LM, den Dunnen J, Litjens M, Geijtenbeek TB. HIV-1 exploits innate signaling by TLR8 and DC-SIGN for productive infection of dendritic cells. Nat Immunol. 2010;11:419–426. doi: 10.1038/ni.1858. [DOI] [PubMed] [Google Scholar]
- Gringhuis SI, Wevers BA, Kaptein TM, van Capel TM, Theelen B, Boekhout T, de Jong EC, Geijtenbeek TB. Selective C-Rel activation via Malt1 controls anti-fungal T(H)-17 immunity by dectin-1 and dectin-2. PLoS Pathog. 2011;7:e1001259. doi: 10.1371/journal.ppat.1001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gringhuis SI, Kaptein TM, Wevers BA, Mesman AW, Geijtenbeek TB. Fucose-specific DC-SIGN signalling directs T helper cell type-2 responses via IKKε- and CYLD-dependent Bcl3 activation. Nat Commun. 2014;5:3898. doi: 10.1038/ncomms4898. [DOI] [PubMed] [Google Scholar]
- Hardison SE, Brown GD. C-type lectin receptors orchestrate antifungal immunity. Nat Immunol. 2012;13:817–822. doi: 10.1038/ni.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero J, Muffato M, Beal K, Fitzgerald S, Gordon L, Pignatelli M, Vilella AJ, Searle SM, Amode R, Brent S, et al. Ensembl comparative genomics resources. Database (Oxford) 2016;2016:bav096. doi: 10.1093/database/bav096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez PJ, Fenwick A. Schistosomiasis in Africa: an emerging tragedy in our new global health decade. PLoS Negl Trop Dis. 2009;3:e485. doi: 10.1371/journal.pntd.0000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia XM, Tang B, Zhu LL, Liu YH, Zhao XQ, Gorjestani S, Hsu YM, Yang L, Guan JH, Xu GT, Lin X. CARD9 mediates Dectin-1-induced ERK activation by linking Ras-GRF1 to H-Ras for antifungal immunity. J Exp Med. 2014;211:2307–2321. doi: 10.1084/jem.20132349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrigan AM, Brown GD. Syk-coupled C-type lectins in immunity. Trends Immunol. 2011;32:151–156. doi: 10.1016/j.it.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin BM, Smith PM, Ponichtera HE, Shainheit MG, Rutitzky LI, Stadecker MJ. Induction and regulation of pathogenic Th17 cell responses in schistosomiasis. Semin Immunopathol. 2012;34:873–888. doi: 10.1007/s00281-012-0341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, Reis e Sousa C. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- Ostrop J, Lang R. Contact, collaboration, and conflict: signal integration of Syk-coupled C-type lectin receptors. J Immunol. 2017;198:1403–1414. doi: 10.4049/jimmunol.1601665. [DOI] [PubMed] [Google Scholar]
- Park CG, Takahara K, Umemoto E, Yashima Y, Matsubara K, Matsuda Y, Clausen BE, Inaba K, Steinman RM. Five mouse homologues of the human dendritic cell C-type lectin, DC-SIGN. Int Immunol. 2001;13:1283–1290. doi: 10.1093/intimm/13.10.1283. [DOI] [PubMed] [Google Scholar]
- Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- Ponichtera HE, Shainheit MG, Liu BC, Raychowdhury R, Larkin BM, Russo JM, Salantes DB, Lai CQ, Parnell LD, Yun TJ, et al. CD209a expression on dendritic cells is critical for the development of pathogenic Th17 cell responses in murine schistosomiasis. J Immunol. 2014;192:4655–4665. doi: 10.4049/jimmunol.1400121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powlesland AS, Ward EM, Sadhu SK, Guo Y, Taylor ME, Drickamer K. Widely divergent biochemical properties of the complete set of mouse DC-SIGN-related proteins. J Biol Chem. 2006;281:20440–20449. doi: 10.1074/jbc.M601925200. [DOI] [PubMed] [Google Scholar]
- Prasanphanich NS, Mickum ML, Heimburg-Molinaro J, Cummings RD. Glycoconjugates in host-helminth interactions. Front Immunol. 2013;4:240. doi: 10.3389/fimmu.2013.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter M, Gross O, Kays S, Ruland J, Nimmerjahn F, Saijo S, Tschopp J, Layland LE, Prazeres da Costa C. Schistosoma mansoni triggers Dectin-2, which activates the Nlrp3 inflammasome and alters adaptive immune responses. Proc Natl Acad Sci USA. 2010;107:20459–20464. doi: 10.1073/pnas.1010337107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutitzky LI, Bazzone L, Shainheit MG, Joyce-Shaikh B, Cua DJ, Stadecker MJ. IL-23 is required for the development of severe egg-induced immunopathology in schistosomiasis and for lesional expression of IL-17. J Immunol. 2008;180:2486–2495. doi: 10.4049/jimmunol.180.4.2486. [DOI] [PubMed] [Google Scholar]
- Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, Fujikado N, Kusaka T, Kubo S, Chung SH, et al. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010;32:681–691. doi: 10.1016/j.immuni.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Sancho D, Reis e Sousa C. Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu Rev Immunol. 2012;30:491–529. doi: 10.1146/annurev-immunol-031210-101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders SP, Walsh CM, Barlow JL, Mangan NE, Taylor PR, McKenzie AN, Smith P, Fallon PG. The C-type lectin SIGNR1 binds Schistosoma mansoni antigens in vitro, but SIGNR1-deficient mice have normal responses during schistosome infection. Infect Immun. 2009;77:399–404. doi: 10.1128/IAI.00762-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shainheit MG, Smith PM, Bazzone LE, Wang AC, Rutitzky LI, Stadecker MJ. Dendritic cell IL-23 and IL-1 production in response to schistosome eggs induces Th17 cells in a mouse strain prone to severe immunopathology. J Immunol. 2008;181:8559–8567. doi: 10.4049/jimmunol.181.12.8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shainheit MG, Lasocki KW, Finger E, Larkin BM, Smith PM, Sharpe AH, Dinarello CA, Rutitzky LI, Stadecker MJ. The pathogenic Th17 cell response to major schistosome egg antigen is sequentially dependent on IL-23 and IL-1β. J Immunol. 2011;187:5328–5335. doi: 10.4049/jimmunol.1101445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser D, Neumann K, Bergmann H, Marakalala MJ, Guler R, Rojowska A, Hopfner KP, Brombacher F, Urlaub H, Baier G, et al. Syk kinase-coupled C-type lectin receptors engage protein kinase C-σ to elicit Card9 adaptor-mediated innate immunity. Immunity. 2012;36:32–42. doi: 10.1016/j.immuni.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan RS, Ho B, Leung BP, Ding JL. TLR cross-talk confers specificity to innate immunity. Int Rev Immunol. 2014;33:443–453. doi: 10.3109/08830185.2014.921164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjota MY, Hrusch CL, Blaine KM, Williams JW, Barrett NA, Sperling AI. Signaling through FcRgamma-associated receptors on dendritic cells drives IL-33-dependent TH2-type responses. J Allergy Clin Immunol. 2014;134:706–713e8. doi: 10.1016/j.jaci.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Die I, van Vliet SJ, Nyame AK, Cummings RD, Bank CM, Appelmelk B, Geijtenbeek TB, van Kooyk Y. The dendritic cell-specific C-type lectin DC-SIGN is a receptor for Schistosoma mansoni egg antigens and recognizes the glycan antigen Lewis x. Glycobiology. 2003;13:471–478. doi: 10.1093/glycob/cwg052. [DOI] [PubMed] [Google Scholar]
- Wüthrich M, Wang H, Li M, Lerksuthirat T, Hardison SE, Brown GD, Klein B. Fonsecaea pedrosoi-induced Th17-cell differentiation in mice is fostered by Dectin-2 and suppressed by Mincle recognition. Eur J Immunol. 2015;45:2542–2552. doi: 10.1002/eji.201545591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonekawa A, Saijo S, Hoshino Y, Miyake Y, Ishikawa E, Suzukawa M, Inoue H, Tanaka M, Yoneyama M, Oh-Hora M, et al. Dectin-2 is a direct receptor for mannose-capped lipoarabinomannan of mycobacteria. Immunity. 2014;41:402–413. doi: 10.1016/j.immuni.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Zhu LL, Zhao XQ, Jiang C, You Y, Chen XP, Jiang YY, Jia XM, Lin X. C-type lectin receptors Dectin-3 and Dectin-2 form a heterodimeric pattern-recognition receptor for host defense against fungal infection. Immunity. 2013;39:324–334. doi: 10.1016/j.immuni.2013.05.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.