Abstract
Inactivated influenza vaccines are not approved for use in infants less than 6 months of age due to poor immunogenicity in that population. While the live attenuated influenza vaccine has the potential to be more immunogenic, it is not an option for infants and other vulnerable populations, including the elderly and immunocompromised individuals due to safety concerns. In an effort to improve the immunogenicity of the inactivated vaccine for use in vulnerable populations, we have used an approach of chemically crosslinking the Toll-like receptor (TLR) 7/8 agonist R848 directly to virus particles. We have reported previously that an R848-conjugated, inactivated vaccine is more effective at inducing adaptive immune responses and protecting against lung pathology in influenza challenged neonatal African green monkeys than is the unmodified counterpart. In the current study, we describe a second generation vaccine that utilizes an amide-sulfhydryl crosslinker with different spacer chemistry and length to couple R848 to virions. The new vaccine has significantly enhanced immunostimulatory activity for murine macrophages and importantly for monocyte derived human dendritic cells. Demonstration of the significant differences in stimulatory activity afforded by modest changes in linker impacts our fundamental view of the design of TLR agonist-antigen vaccines.
Keywords: influenza, vaccine, Toll-like receptor, R848, adjuvant, dendritic cell
INTRODUCTION
Inactivated vaccines against viral and bacterial pathogens are generally not as immunogenic as their live, attenuated counterparts. Nevertheless, they remain an important part of the armament of vaccines currently in use due to their relative safety, especially in vulnerable populations such as infants, the elderly, and other immunocompromised individuals. The most highly utilized adjuvant alum can significantly increase the immunogenicity of killed and component vaccines [1]; however, its propensity to elicit Th2 responses, limited ability to promote CD8+ T cell responses, and capacity to stimulate local inflammation [2] provide strong rationale for the development of new adjuvants. In recent years, it has been demonstrated in a variety of systems that small molecule Toll-like receptor (TLR) agonists are effective adjuvants (for review see [3, 4]). TLR belong to a family of pattern recognition receptors that bind molecules derived from viral, bacterial, and fungal pathogens as well as endogenous molecules that signal danger [5, 6]. TLR are broadly distributed on immune cells and their engagement promotes robust cellular activation/maturation following microbial infection. Thus, multiple cell types including T cells, B cells, and dendritic cells (DC) can be modulated by these ligands. The majority of TLR are expressed at the cell surface, but a subset (TLR3, TLR7, TLR8, and TLR9) are contained within endosomes [7]. This subset of TLR recognize nucleic acids structures; i.e. dsRNA (TLR3), ssRNA (TLR7 and TLR8), and unmethylated CpG (TLR9).
Complications from influenza virus infection kill approximately 20–30,000 individuals per year in the U.S. alone, and most of these deaths occur in immunocompromised individuals including the very young and the elderly. The increased susceptibility to severe disease in these groups is compounded by the poor immunogenicity of the inactivated vaccine [8, 9]. With this knowledge, we have focused our efforts on developing a safe and effective influenza vaccine for these difficult-to-vaccinate, vulnerable populations. To this end, we have explored the TLR7/8 agonist resiquimod (R848) as an adjuvant for an inactivated influenza vaccine. In experimental settings, R848 (or its closely related analog 3M-012) has been shown to promote multiple arms of the adaptive immune response including antibody, CD8+ T cells, and Th1 responses [10–14]. In addition, R848 engagement of TLR8 has been reported to inhibit the suppressive activity of Tregs [15]. Thus, R848 has immunoregulatory properties that make it very attractive as an adjuvant. TLR7 has been reported to play an important role in the immune response to influenza virus [16–18]. While the role of TLR8 is relatively unknown, given the demonstrated TLR8 signaling mediated by influenza viral RNA [19], it seems likely that this sensor contributes to immunity following infection.
We have employed the principle of physically associating adjuvant and antigen given the studies showing physical association of TLR agonists with antigen promotes more robust adaptive immune responses in comparison to a mixture of adjuvant and antigen (for review see [4]). Association has been achieved by a variety of approaches including chemical linkage and synthesis of fusion proteins [4, 11, 20–22]. The proposed advantage of direct linkage lies in targeting of the immunostimulatory component and the antigen to the same cell resulting in optimal maturation of the antigen presenting cell.
We have stably conjugated R848 to influenza virus particles followed by formalin-inactivation. This approach has the advantage that all viral proteins present in the vaccine can serve as potential targets for the immune response. Our published studies show that R848-conjugated inactivated influenza virus is a potent inducer of antibody and cell mediated immunity in a nonhuman primate neonate model [23]. Here we describe a second generation construct that has markedly improved potency for maturation of human DC.
MATERIALS and METHODS
Virus
Purified Influenza virus strain A/PR/8/34 was purchased from Charles River Avian Vaccine Services. For inactivation, virus was treated with 0.74% formaldehyde for 1 hr at 37°C, followed by extensive dialysis (20K MWCO). Recombinant vaccinia virus (WR strain) was inactivated by the same method.
Antibodies and reagents
Antibodies for flow cytometry were as follows: APC-labeled anti-human CD11c, clone S-HCL-3 (BD Biosciences); PerCP/Cy5.5-labeled anti-human CD40, clone 5C3 (Biolegend); APC-H7-labeled anti-human CD80, clone L307.4 (BD Biosciences); BV510-labeled anti-human CD86, clone 2331; PE-labeled anti-mouse CD40, clone 3/23 (BD Biosciences). Accutase cell detachment solution was from BD Biosciences. The TLR agonists Imiquimod (R837, TLR7-specific) and TL8-506 (TLR8-specific) were from InvivoGen. R848 (TLR7/TLR8 agonist) was synthesized in house to introduce a primary amino group in place of the hydroxyl group of native R848. Compounds ML2-102-1, ML2-103-1, ML2-104-1, MAL-PEG2, MAL-PEG8, and MAL-PEG16-1 were produced by PharmAgra Labs (Brevard, NC).
Preparation of virus conjugates
R848-conjugated influenza virus was prepared by linking a derivative of R848 which is modified to contain a primary amine, directly to virions in a 2-step process using an amide to sulfhydryl crosslinker, SM(PEG)4 (Thermo Scientific) as previously described [23]. This protocol was also used to generate R848-conjugated, inactivated vaccinia virus (IVV). Preparation of R848-conjugated influenza virus using a 1-step method was the same as for the 2-step method, except that the first step, covalent linkage of R848 to linker was completed by commercial production of R848 modified with 5 different amide-sulfhydryl crosslinker moieties. Free thiol content of live virus particles was measured before and after the conjugation protocol using a Measure-iT Thiol assay kit (Thermo Fisher Scientific #M30550) according to manufacturer’s instructions. A reduction of virus-associated free-thiols served as an indirect measure of the amount of R848 that had covalently bound to virus particles.
Human monocyte-derived dendritic cells
Monocyte-derived dendritic cells (DC) were differentiated from CD14+ human peripheral blood mononuclear cells (PMBC) as previously described using 10 ng/ml each recombinant human GMCSF and IL-4 [24]. DC purity (>95%) was determined on Day 7 by CD11c expression (flow cytometry) and morphology (cytocentrifugation and Diff-Quik staining).
Stimulation of RAW 264.7 cells or human DC
Inactivated virus preparations or TLR7/8 agonists were added to cells and cultured for 24h. In the case of human DC, the cells were used on day 7 of culture. Activation was assessed by measuring the expression of costimulatory molecules (CD40, CD80, CD86) by flow cytometry, and by production of cytokines by ELISA (TNFα for RAW 264.7) or by inflammatory cytometric bead array (IL-12p70, TNFα, IL-10, IL-6, IL-1β, IL-8 for human DC; BD Biosciences). Samples were acquired using a BD FACS Canto II instrument. Analysis was performed using FlowJo software.
Alexafluor-680 (AF680) labeling of vaccine conjugates and measurement of association with DC
Vaccine conjugates were fluorescently labeled with AF680 NHS ester (Thermo Fisher #A37574) as follows: 50 μg of iPR8-SM(PEG)4-R848 or iPR8-GMBS-R848 (50 μl of 1 mg/ml stock in PBS, pH 7.4) was mixed with 2.5 μg AF680 stock (2.5 μl of 1 mg/ml stock in DMSO), yielding 0.05 μg of AF680/μg conjugate protein. After 1 hr with vigorous shaking at 23°C, the conjugates were extensively dialyzed (20K MWCO Slide-A Lyzer MINI dialysis units) to remove unincorporated AF680. The protein concentration was measured by the BCA protein assay, and the fluorescence units per μg of conjugate determined by measuring the AF680 signal using a POLARstar Omega instrument (BMG Labtech). To measure relative cell association of the two conjugates, human monocyte-derived DC were prepared as for activation experiments. AF680-labeled IPR8-SM(PEG)4-R848 or AF680-labeled IPR8-GMBS-R848 were diluted in DC culture medium and incubated with cells for 1 hr at 37°C. The cells were then extensively washed at 4°C to remove AF680-labeled conjugate that had not been bound or internalized, and stained for CD11c. Data was acquired using a BD Fortessa flow cytometer and analyzed using Diva software. Mean fluorescent intensities (MFI) were normalized to fluorescence units/μg protein for each conjugate.
Statistics
Experiments were performed at least 3 times and significance was determined by unpaired Student’s t test using GraphPad Prism software.
RESULTS
R848-conjugated PR8 activates RAW 264.7 macrophages
We developed an approach to enhance the stimulatory capacity of inactivated influenza virus through conjugation with the TLR7/8 agonist R848 (Fig. 1a). An amine derivative of R848 (R848-NH2, indicated by arrow) was synthesized to allow for amide bond formation with the reactive N-hydroxysuccinimide (NHS) ester group of the heterobifunctional crosslinker, NHS-PEG4-maleimide (SM(PEG)4). Conditions were optimized to drive maximum coupling of R848 to the linker in the first step. In the second step, R848-SM(PEG)4 was incubated with purified PR8 influenza virus. While we had originally pre-treated the virus with the reducing agent TCEP to maximize free thiol groups [23], we found in subsequent experiments that this did not result in additional stimulatory capacity and thus could be omitted (data not shown). The final step was formalin-inactivation of the R848-virus conjugate. The vaccine prepared in this manner is referred to as IPR8-SM(PEG)4-R848.
Fig. 1. Construction of IPR8-SM(PEG)4-R848 conjugate vaccine and biological activity on RAW 264.7 cells.
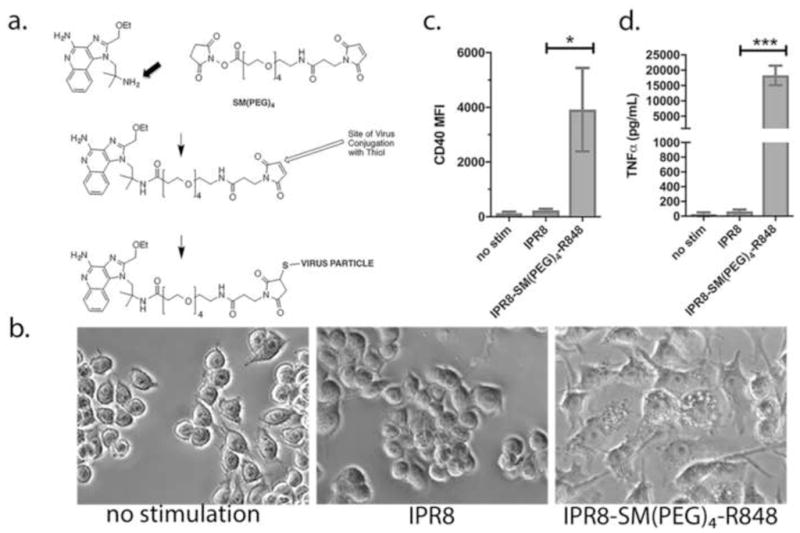
(a) An amine-derivative of R848 (substitution of native OH group with NH2 group is indicated by an arrow) was synthesized to allow for amide bond formation with the reactive NHS-ester end of the amide-sulfhydryl crosslinker, SM(PEG)4. in step 1. Purified PR8 influenza virus was linked via free thiols with the maleimide end of the SM(PEG)4 crosslinker in step 2. To measure biological activity, RAW 264.7 cells were treated with 10 μg/ml IPR8-SM(PEG)4-R848 or unconjugated IPR8 (without the R848 modification). (b) Morphology of cells 24 hrs after addition of IPR8 or IPR8-SM(PEG)4-R848, or no stimulation as a control. As a quantitative measure of activation, CD40 (c) and TNFα (d) production were measured at 24 hrs. Results represent the mean ± sd from 3 experiments. p = 0.01 for CD40 and p = 0.0006 for TNFα, IPR8 vs IPR8-R848-SM(PEG)4 as determined by unpaired Student’s t test.
The stimulatory capacity of IPR8-SM(PEG)4-R848 as compared to IPR8 was measured using the murine RAW264.7 macrophage cell line, which is known to be responsive to TLR7 engagement (e.g. [25]). At 24h after the addition of 10 μg/ml IPR8-SM(PEG)4-R848 or IPR8, morphological changes consistent with activation (spreading, firm adherence and vacuolization) were observed in cells treated with the conjugated virus (Fig. 1b). Cells treated with the same concentration of non-conjugated virus were morphologically similar to that of non-stimulated controls. Cytokine and costimulatory molecule production were measured as quantitative criteria for activation (Fig. 1 c, d). IPR8 alone had modest stimulatory capability as measured by CD40 and TNFα production, while IPR8-SM(PEG)4-R848 induced significant increases in CD40 and TNFα. Therefore, R848 linkage confers robust macrophage stimulatory capacity to inactivated PR8 influenza virus.
The conjugation methodology developed for influenza virus is applicable to vaccinia virus
We next determined whether the same R848 conjugation methodology might be useful for other enveloped viruses. To address this possibility, the same method was used to conjugate R848 to vaccinia virus. As demonstrated for influenza virus, R848 conjugated vaccinia virus (IVV-SM(PEG)4-R848) showed high stimulatory capacity for RAW264.7 cells, while IVV induced minimal responses as measured by CD40 (Fig. 2a) and TNFα (Fig. 2b) production. Thus, this approach may be broadly applicable for conjugating R848 to virions.
Fig. 2. Conjugation methodology is applicable to vaccinia virus.
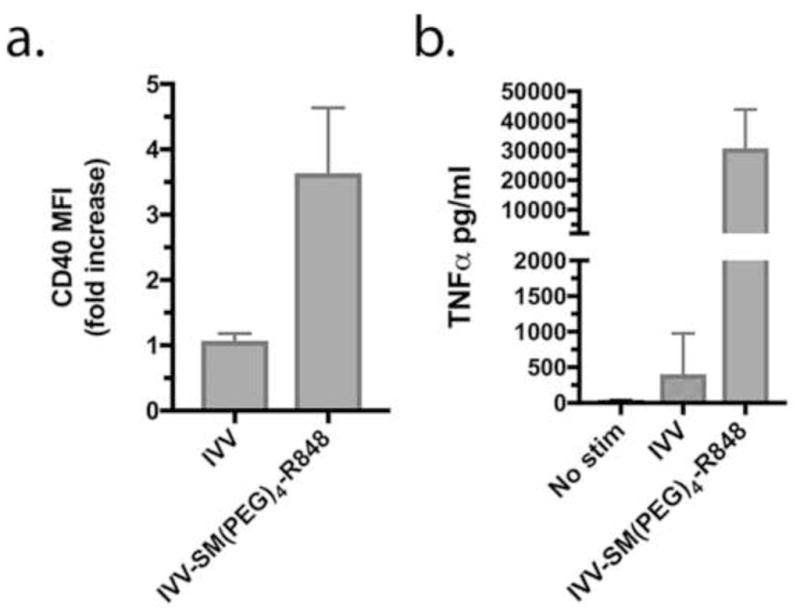
R848 was conjugated to vaccinia virus (VV) by the same method used for influenza virus. Biological activity was measured by CD40 (a) and TNFα (b) production 24 hrs after treatment of RAW 264.7 cells with IVV (inactivated VV, not conjugated) or IVV-SM(PEG)4-R848. Results represent mean±SD from 3 experiments. p = 0.01 for CD40 and TNFα, IVV vs IVV-SM(PEG)4-R848, as determined by unpaired Student’s t test.
The crosslinker utilized for conjugation impacts the stimulatory capacity of R848-virus conjugates
The SM(PEG)4 crosslinker was initially chosen for its properties of stability, flexibility, low immunogenicity and low tendency to aggregate [26]. To determine if the specific linker used for conjugation was a factor in determining stimulatory activity of the R848-virus conjugate, a series of R848-linker compounds, shown in Table 1, were produced commercially. The linkers were chosen to vary the distance between R848 and the virus, a function of spacer length (5.9 – 65.3Å), while retaining the amide to sulfhydryl bonding chemistry. The length of the SM(PEG)4 spacer in the current vaccine is 24.6Å. Commercial production of purified R848-linker compounds allowed for conjugation to PR8 in a single step. As shown in Table 1, the R848-linker compounds all induced TNFα production by RAW264.7 cells. As controls, unstimulated RAW264.7 cells produced <10 pg/ml TNFα, and R848-NH2 without a linker attached produced 6,867±1,611 pg/ml TNFα.
Table 1. Biological activity of R848 covalently coupled to the NHS ester end of 7 different amide-sulfhydryl crosslinkers.
R848-linker compounds were produced commercially with the exception of the R848-SM(PEG)4 compound (indicated by a star), which was produced in-house and was used to construct the original IPR8 vaccine (IPR8-SM(PEG)4-R848). Raw264 cells were stimulated with 10 μM R848-linker compounds for 24 hrs and TNFα production was measured. Controls: no stimulation: 6.7±5.7 pg/ml; R848.NH2: 6867±1611pg/ml. Results shown are the mean±SD from 3 independent experiments. Differences in TNFα production of R848-linker compounds as compared to SM(PEG)4-R848 or as compared to R848-NH2 were not significant by one-way ANOVA analysis with Dunnett’s multiple comparisons test.
| R848-linker compound | spacer arm | length | TNFα (pg/ml) | Spacer arm structure |
|---|---|---|---|---|
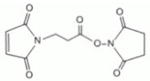
| ML2-102-1 | BMPS | 5.9Å | 9481 ± 3440 |
|
| ML2-103-1 | GMBS | 7.3Å | 8970 ± 3065 |

|
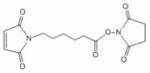
| ML2-104-1 | EMCS | 9.4Å | 4595 ± 1566 |
|
| MAL-PEG2-1 | SM(PEG)2 | 17.6Å | 7985 ± 2639 |
|
| MAL-PEG4-1* | SM(PEG)4 | 24.6Å | 7236 ± 1526 |
|
| MAL-PEG8-1 | SM(PEG)8 | 39.2Å | 5938 ± 2706 |
|
| MAL-PEG16-1 | SM(PEG)16 | 65.3Å | 5906 ± 2437 |
|
Having established that the R848-linker compounds had biological activity, the compounds were linked to PR8, and the resulting virus conjugates inactivated and tested for biological activity. As shown in Figure 3, the vaccine conjugates varied in their capacity to activate RAW264.7 cells. The IPR8 conjugate generated with one of the R848-linker compounds in the panel, ML2-103-1 (R848-GMBS), had the most activity, inducing approximately 70-fold and 30-fold more CD40 than unconjugated IPR8 at doses of 10 μg/ml and 1 μg/ml, respectively. In comparison, the conjugate made with SM(PEG)4 (using the 2-step method) induced approximately 20-fold and 8-fold more CD40 relative to IPR8 (Fig. 3a). TNFα activity showed a similar trend (Fig. 3b). These results suggest that crosslinker choice impacts conjugate activity, and in particular that IPR8-ML2-103-1, in which a GMBS crosslinker was used to couple R848 to virus particles, was superior to the current conjugate which utilizes an SM(PEG)4 crosslinker.
Fig. 3. Variation in biological activity among IPR8-R848 conjugates constructed with different crosslinkers.
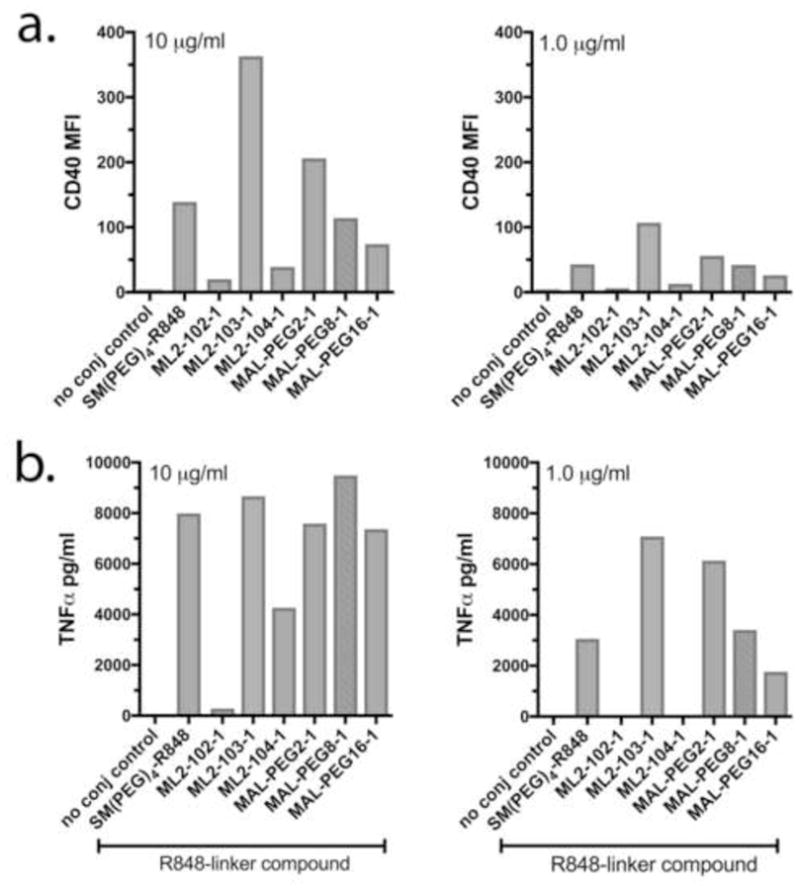
RAW 264.7 cells were stimulated with 10 ug/ml or 1 ug/ml IPR8-SM(PEG)4-R848 made by the 2-step protocol (current vaccine, positive control) or IPR8-R848 conjugates constructed in 1 step using commercially produced R848-linker compounds (chemical structures indicated in Table 1). The x axis indicates the R848-linker compound that was used to prepare the virus conjugates. IPR8 served as the negative (no conjugate) control. Results shown are from 1 of 2 independent screens that measured CD40 (a) and TNFα (b).
To confirm that the GMBS crosslinker produces a more biologically active conjugate relative to the SM(PEG)4 crosslinker, and that enhanced activity was not simply a function of using commercially-prepared R848-linker compounds, we constructed IPR8-GMBS-R848 in parallel with IPR8-SM(PEG)4-R848 by our 2-step method. The biological activities of the two conjugates, along with IPR8-ML2-103-1, made in 1 step with commercially produced R848-GMBS, were tested. A representative histogram depicting CD40 production after stimulation with 1.1 μg/ml conjugates is shown in Fig. 4a, and compiled CD40 data from multiple dose-response experiments is shown in Fig. 4b. Both IPR8-GMBS-R848 and IPR8-ML2-103-1 had significantly increased biological activity relative to IPR8-SM(PEG)4-R848 over a range of doses (p < 0.05 for the 1.1 and 0.4 μg/ml doses, GMBS vs. SM(PEG)4 conjugates; p < 0.007 for the 10, 3.3, 1.1 and 0.4 μg/ml doses, ML2-103-1 vs SM(PEG)4 conjugates). The activity of IPR8-GMBS-R848 as compared to IPR8-ML2-103-1 was not significantly different (p > 0.05, all doses). Enhanced activity of the GMBS conjugate over the SM(PEG)4 conjugate was also shown by TNFα production over a range of doses (Fig. 4c; p< 0.005 for GMBS vs SM(PEG)4 conjugates at all but the 2 highest doses). Similar TNFα results were obtained for IPR8-ML2-103-1 conjugate as compared to the IPR8-SM(PEG)4 conjugate in 2 independent experiments (data not shown).
Fig. 4. Biological activity of IPR8-GMBS-R848 conjugates made by 2-step and 1-step method.
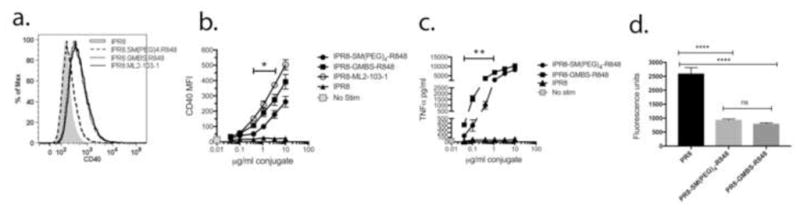
RAW 264.7 cells were treated with IPR8-SM(PEG)4-R848, IPR8-GMBS-R848, IPR8-ML2-103-1 or IPR8 for 24 hrs. CD40 and TNFα production were measured as indicators of activation. (a) Representative histogram showing CD40 production after a 24 hr treatment with IPR8 conjugates or IPR8 alone at a dose of 1.1 μg/ml. Data shown in (b) CD40 and (c) TNFα production depict the mean ± SEM from at least 3 experiments. The gray square depicts no stimulation, in which no TNFα was detected. Statistical significance was determined by unpaired Student’s t test. * p<0.05 for the indicated doses. ** p< 0.005 for the indicated doses. (d) Quantitation of live virus-associated free thiols before and after conjugation of R848. A commercial fluorescence-based assay for free thiol content was used as an indirect measure of the amount of R848 bound to PR8 before and after completing the conjugation protocol. Fluorescence units were normalized to protein content of unconjugated PR8, PR8-SM(PEG)4-R848 and PR8-GMBS-R848 virus conjugates. For the unconjugated PR8 control, DMSO vehicle was added in place of R848-linker compounds and subject to the same protocol steps as for R848-conjugated PR8. The mean±SD from 3 independent experiments is shown. There was no statistical difference (ns, p>0.05) between PR8-SM(PEG)4-R848 and PR8-GMBS-R848 by one-way ANOVA with Tukey’s multiple comparisons post-test. Differences between PR8 and each R848 conjugate were significant (****p < 0.0001).
To address the possibility that increased biological activity of iPR8-GMBS-R848 as compared to iPR8-SM(PEG)4-R848 was due to a greater amount of R848 binding to virus when the GMBS linker was used, a fluorescence assay for free thiol content was performed on virus particles before and after the conjugation protocol. As shown in Fig. 4d, a similar reduction in free thiols was noted for the two conjugates relative to the pre-conjugated PR8. The data in Fig. 4 provide evidence that the crosslinker used to couple R848 to influenza virus particles impacts the biological activity of the resulting vaccine conjugate.
IPR8-GMBS-R848 results in both qualitative and quantitative increases in maturation of human dendritic cells compared to IPR8-SM(PEG)4-R848
Results indicating that IPR8-GMBS-R848 is more effective at activating RAW 264.7 cells than is the current SM(PEG)4 vaccine conjugate prompted us to test the relative activities of the conjugates on human dendritic cells (DC). For this experiment, DC were differentiated from human peripheral blood CD14+ mononuclear cells from 3 healthy donors. A representative dot plot demonstrating that cells were >95% CD11c+ and had veiled morphology typical of conventional DC is shown in Fig. 5a. As shown in Fig 5b, DC from all 3 donors produced TNFα in response to the TLR7/8 agonist R848, and also responded to the TLR8-specific agonist TL8-506. However, the TLR7-specific agonist R837 had minimal stimulatory activity relative to R848 and TL8-506 (Fig. 5b). This finding is consistent with previous reports of preferential expression and/or response through TLR8 over TLR7 in human monocyte-derived DC [27–29].
Fig. 5. Human monocyte-derived DC respond preferentially to TLR8-specific agonist.
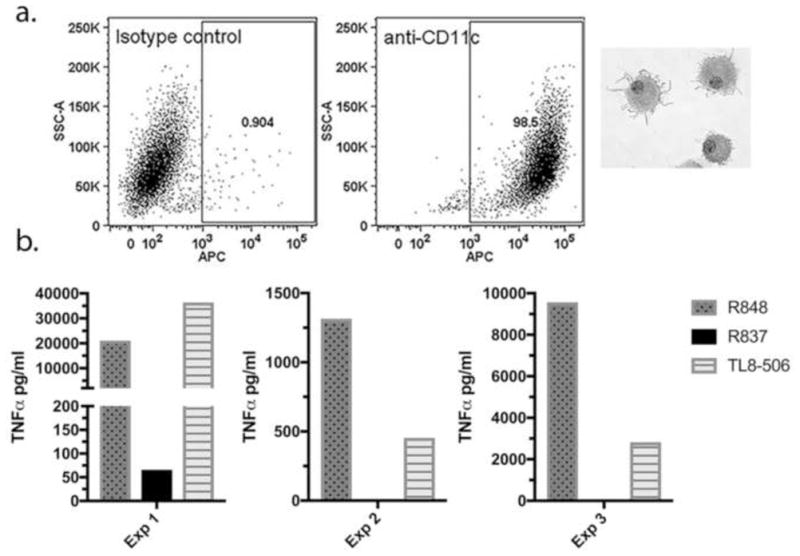
(a) Human CD14+ monocytes propagated for 7 days as described in Materials and Methods were >95% CD11c+ and had typical veiled morphology of conventional DC after cytocentrifugation and staining with Diff-Quick. Representative dot plots depicting anti-CD11c staining and the isotype control from 1 of 3 donors are shown. (b) On day 7 cells were treated for 24 hrs with 10 μM R848 (TLR7/8 agonist), R837 (TLR7-specific agonist) or TL8-506 (TLR8-specific agonist) and TNFα in supernatants was measured by ELISA. Data from 3 experiments using different donors is shown.
To assess the response to vaccine conjugates, DC were treated with IPR8-GMBS-R848, IPR8-SM(PEG)4-R848, or IPR8 (10 - 0.03 μg/ml). As measures of activation, CD40, CD80 and CD86 (Fig. 6a) and inflammatory cytokine production (Fig. 6b) were assessed. As shown in Fig. 6a, IPR8-SM(PEG)4-R848 had a modest capacity to increase costimulatory molecule production on human DC. In contrast, IPR8-GMBS-R848 induced CD40 and CD80 production well above the unstimulated level in all 3 donors. With regard to CD86 production, clearly enhanced activity of the vaccine conjugate made with the GMBS linker as compared to the SM(PEG)4 linker was observed for 1 of 3 donors. Non-conjugated vaccine (IPR8) induced little to no costimulatory molecule production.
Fig. 6. Stimulation of human monocyte-derived DC with IPR8 conjugates.
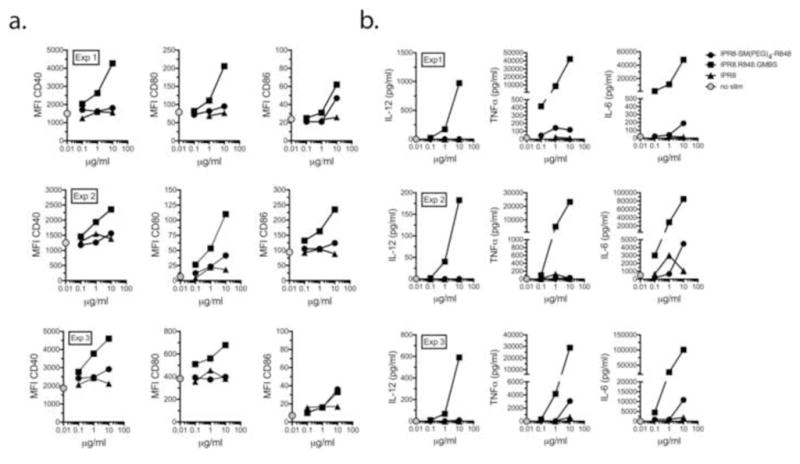
Human DC were cultured with IPR8, IPR8-SM(PEG)4-R848 or IPR8-GMBS-R848 for 24 hrs at the indicated concentrations. (a) Cells were stained for CD40, CD80 and CD86 and analyzed by flow cytometry or (b) cytokines (IL-12p70, TNFα and IL-6) were measured by cytometric bead array. Three experiments were performed (indicated as Exp 1, Exp 2, and Exp 3) using different donors. The gray circle indicates the unstimulated control.
The enhanced biological activity of IPR8-GMBS-R848 as compared to IPR8-SM(PEG)4-R848 was even more apparent when cytokines were measured (Fig. 6b). IPR8-SM(PEG) 4-R848 induced modest levels of pro-inflammatory cytokines relative to IPR8 (Fig. 6b). Notably, there was no IL-12p70 detected in the supernatants of any of the donors as a result of IPR8-SM(PEG)4-R848 stimulation. In sharp contrast, IL-12p70, TNFα and IL-6 were all highly induced in DC treated with IPR8-GMBS-R848 (Fig. 6b). Similar results, indicating an enhanced response of DC from all 3 donors to the GMBS as compared to the SM(PEG)4 vaccine was observed for IL-1β, IL-8 and IL-10 (data not shown). These results reveal a striking difference in the DC-activating potential of vaccine conjugates prepared using the GMBS as compared to SM(PEG)4 crosslinker, with the former imparting substantially greater biological activity to IPR8-R848 vaccine conjugates. Collectively the data indicate that the crosslinker choice within the amide to sulfhydryl family has a substantial impact on the immunostimulatory capability of R848-virus conjugates.
The increased stimulatory capacity of IPR8-GMBS-R848 is not correlated with increased association of the vaccine with DC
One possibility to explain the increased stimulatory activity of IPR8-GMBS-R848 was increased interaction with the DC. To assess this possibility the vaccine was labeled with AF680 to allow measurement of its association with the cell. Two independently produced SM(PEG)4 or GMBS-linked vaccines were tested using DC from two donors. DC were cultured in the presence of titrated amounts of the vaccine for 1 hour. Following extensive washing, the associated vaccine was measured by flow cytometry. The intensity of the DC-associated AF680 signal was similar for the two vaccines (Fig. 7). These data suggest that increased association of the GMBS linked vaccine is not responsible for the increased stimulatory activity.
Fig. 7. The increased stimulatory capacity of IPR8-GMBS-R848 is not the result of increased association with DC.
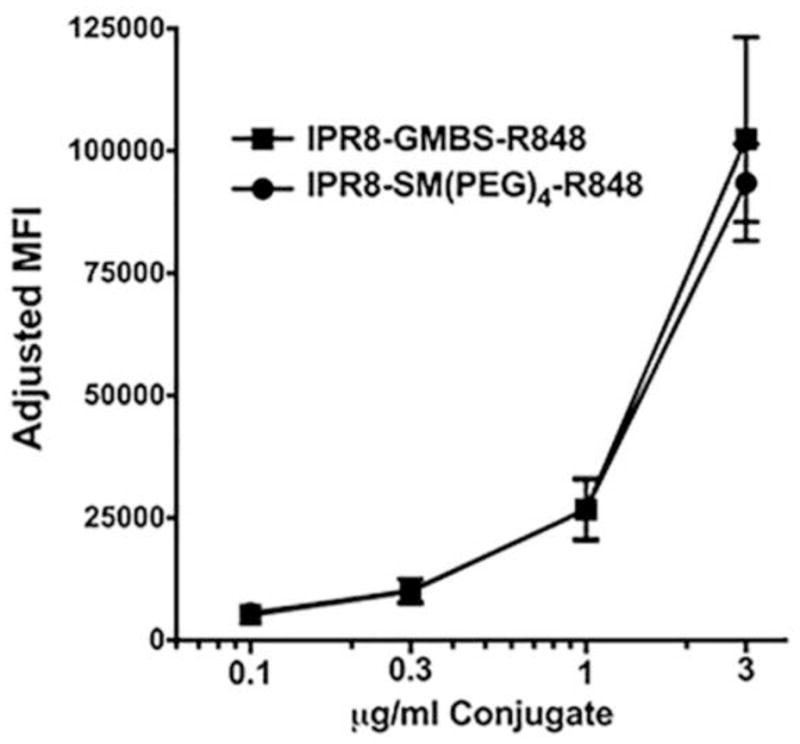
DC differentiated from two donors were cultured in the presence of titrated amounts (3, 1.1, 0.3, 0.1 μg/ml) of AF680-labeled IPR8-GMBS-R848 or IPR8-SM(PEG)4-R848 for 1 hour. Cells were then washed and AF680 intensity on CD11c+ cells determined by flow cytometric analysis. Data are the average of results using 2 independently produced IPR8-GMBS-R848 or IPR8-SM(PEG)4 vaccine conjugates tested on DC generated from two donors.
DISCUSSION
In this study we have demonstrated an approach to increase the immunostimulatory activity of inactivated influenza virus for the purpose of developing a safe and immunogenic vaccine for use in vulnerable populations such as neonates and the elderly. We had previously published results indicating that formalin-inactivated PR8 influenza virus, which we modified by covalent linkage of the TLR7/8 agonist R848 using an SM(PEG)4 crosslinker, is more immunogenic in neonatal nonhuman primates than is unmodified inactivated PR8 virus [23]. Here, we extended these promising results by improving the vaccine platform through identification of an alternative linker that results in a construct that activates antigen presenting cells more effectively than does the original. The differential activation potential was noted most strikingly in human DC, a cell type with key roles in the generation of an adaptive immune response. Based on results with TLR7 and TLR8-specific agonists, our data support a model wherein stimulation by R848 and R848-virus conjugates is mediated largely through TLR8 in human monocyte-derived DC.
To our knowledge, the approach of making a vaccine consisting of inactivated, intact virus particles with a covalently-linked small molecule TLR agonist/adjuvant to increase immunogenicity has not been reported for influenza virus prior to our studies [23]. However, approaches to enhance immunogenicity by attachment or association of virus-derived peptide antigens with various types of particles, sometimes in combination with TLR agonists and other adjuvants, have been reported [30–34]. In exploring strategies to further increase the potency of R848-conjugated inactivated influenza virus, we reasoned that the choice of methodology for linking adjuvant and antigen could influence immunogenicity of the fusion product. In particular, crosslinker chemistry could cause bioactive regions of fusion partners to be more or less accessible to relevant receptors, proteases, or signaling molecules present in the responding cells (e.g. [35]). The original crosslinker that we chose from the PEG spacer family of NHS-maleimide heterobifunctional crosslinkers turned out to be less effective at inducing DC maturation than a crosslinker with the same reactive groups but different spacer arm length and chemical properties. The GMBS crosslinker has an aliphatic spacer of 7.3Å as compared to the PEG-based hydrophilic spacer of 24.6Å. An indirect evaluation of R848 conjugation to virus (measured by free thiol groups on the virus before and after conjugation) indicated that both SM(PEG)4-R848 and GMBS-R848 were linked to a similar extent. Thus, although we do not have an assay to directly measure the amount of R848 bound to the virus, we do not favor the hypothesis that an increase in the amount of virus-associated R848 when GMBS is used as the crosslinker is responsible for the greater biological activity. Further, our data do not support the hypothesis that a higher amount of association of IPR8-GMBS-R848 with DC accounts for the superior activity. Additional possibilities include an alternative presentation of R848 to TLR7/8 in the endosome as a result of the distinct spacer arms or differences in trafficking and/or the fate of the particles in intracellular vesicles. Further studies will be required to address these possibilities.
In humans, TLR7 is expressed primarily by plasmacytoid DC (pDC), while myeloid DC predominantly express TLR8 [5, 36, 37]. Our data with human monocyte-derived DC are in keeping with this given the increased production of IL-12p70 by IPR8-GMBS-R848 compared to IPR8-SM(PEG)4-R848, since TLR8, but not TLR7 engagement leads to the production of biologically active IL-12p70 [29, 38]. In contrast, TLR7 has been shown to be a stronger inducer of IFNα [38], a finding consistent with its expression on PDC [39]. Interestingly, in a study by Kerdine-Römer and colleagues, DC differentiated from cord blood were found to express both TLR7 and TLR8. The cytokines produced by these cells depended on whether they were stimulated with the TLR7 ligand imiquimod or the TLR8 ligand 3M002 [29]. Activity on murine RAW264.7 cells, which express functional TLR7 but likely not TLR8 [40], suggests that the R848-virus conjugates reported here also engage TLR7. Whether IPR8-GMBS-R848 is a more potent inducer of type I IFN via TLR7 engagement in pDC or other human cells remains to be determined.
In summary, we propose IPR8-GMBS-R848 as a potentially superior vaccine based on the increased stimulatory capacity for human DC. Our data support a model wherein this enhanced signal is mediated through TLR8. The potential for higher level costimulatory molecule expression and production of Th1-promoting cytokines should be a highly beneficial component of driving effective adaptive responses in immune impaired individuals.
HIGHLIGHTS.
Inactivated flu virus modified with GMBS-R848 is a potent activator of human DC
The activity of an R848-influenza conjugate is greatly impacted by crosslinker choice
Poor vaccine responsiveness may be overcome with high DC stimulatory capacity
Acknowledgments
This work was supported by the National Institutes of Health grant R01 AI098339 (M.A.A.-M.). We thank Jason Grayson for helpful comments and Melissa Stanley for assistance with manuscript preparation.
Footnotes
CONFLICT OF INTEREST
The authors have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.He P, Zou Y, Hu Z. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum Vaccin Immunother. 2015;11:477–88. doi: 10.1080/21645515.2014.1004026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sivakumar SM, Safhi MM, Kannadasan M, Sukumaran N. Vaccine adjuvants - Current status and prospects on controlled release adjuvancity. Saudi Pharm J. 2011;19:197–206. doi: 10.1016/j.jsps.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maisonneuve C, Bertholet S, Philpott DJ, De Gregorio E. Unleashing the potential of NOD- and Toll-like agonists as vaccine adjuvants. Proc Natl Acad Sci USA. 2014;111:12294–9. doi: 10.1073/pnas.1400478111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujita Y, Taguchi H. Overview and outlook of Toll-like receptor ligand-antigen conjugate vaccines. Ther Deliv. 2012;3:749–60. doi: 10.4155/tde.12.52. [DOI] [PubMed] [Google Scholar]
- 5.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. NatImmunol. 2004;5:987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 6.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Microbes Infect. 2004;6:1382–7. doi: 10.1016/j.micinf.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 7.Chaturvedi A, Pierce SK. How location governs toll-like receptor signaling. Traffic. 2009;10:621–8. doi: 10.1111/j.1600-0854.2009.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groothuis JR, Levin MJ, Rabalais GP, Meiklejohn G, Lauer BA. Immunization of high-risk infants younger than 18 months of age with split-product influenza vaccine. Pediatrics. 1991;87:823–8. [PubMed] [Google Scholar]
- 9.Halasa NB, Gerber MA, Chen Q, Wright PF, Edwards KM. Safety and immunogenicity of trivalent inactivated influenza vaccine in infants. J Infect Dis. 2008;197:1448–54. doi: 10.1086/587643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma R, Du JL, Huang J, Wu CY. Additive effects of CpG ODN and R-848 as adjuvants on augmenting immune responses to HBsAg vaccination. Biochem Biophys Res Commun. 2007;361:537–42. doi: 10.1016/j.bbrc.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 11.Wille-Reece U, Flynn BJ, Lore K, Koup RA, Kedl RM, Mattapallil JJ, et al. HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proc Natl Acad Sci U S A. 2005;102:15190–4. doi: 10.1073/pnas.0507484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomai MA, Miller RL, Lipson KE, Kieper WC, Zarraga IE, Vasilakos JP. Resiquimod and other immune response modifiers as vaccine adjuvants. Expert Rev Vaccines. 2007;6:835–47. doi: 10.1586/14760584.6.5.835. [DOI] [PubMed] [Google Scholar]
- 13.Vasilakos JP, Tomai MA. The use of Toll-like receptor 7/8 agonists as vaccine adjuvants. Expert Rev Vaccines. 2013;12:809–19. doi: 10.1586/14760584.2013.811208. [DOI] [PubMed] [Google Scholar]
- 14.Levy O, Suter EE, Miller RL, Wessels MR. Unique efficacy of Toll-like receptor 8 agonists in activating human neonatal antigen-presenting cells. Blood. 2006;108:1284–90. doi: 10.1182/blood-2005-12-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W, Fu T, et al. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309:1380–4. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- 16.Heer AK, Shamshiev A, Donda A, Uematsu S, Akira S, Kopf M, et al. TLR signaling fine- tunes anti-influenza B cell responses without regulating effector T cell responses. J Immunol. 2007;178:2182–91. doi: 10.4049/jimmunol.178.4.2182. [DOI] [PubMed] [Google Scholar]
- 17.Jeisy-Scott V, Kim JH, Davis WG, Cao W, Katz JM, Sambhara S. TLR7 recognition is dispensable for influenza virus A infection but important for the induction of hemagglutinin- specific antibodies in response to the 2009 pandemic split vaccine in mice. J Virol. 2012;86:10988–98. doi: 10.1128/JVI.01064-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koyama S, Ishii KJ, Kumar H, Tanimoto T, Coban C, Uematsu S, et al. Differential role of TLR- and RLR-signaling in the immune responses to influenza A virus infection and vaccination. J Immunol. 2007;179:4711–20. doi: 10.4049/jimmunol.179.7.4711. [DOI] [PubMed] [Google Scholar]
- 19.Wang JP, Bowen GN, Padden C, Cerny A, Finberg RW, Newburger PE, et al. Toll-like receptor-mediated activation of neutrophils by influenza A virus. Blood. 2008;112:2028–34. doi: 10.1182/blood-2008-01-132860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wille-Reece U, Flynn BJ, Lore K, Koup RA, Miles AP, Saul A, et al. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J ExpMed. 2006;203:1249–58. doi: 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wille-Reece U, Wu CY, Flynn BJ, Kedl RM, Seder RA. Immunization with HIV-1 Gag protein conjugated to a TLR7/8 agonist results in the generation of HIV-1 Gag-specific Th1 and CD8+ T cell responses. J Immunol. 2005;174:7676–83. doi: 10.4049/jimmunol.174.12.7676. [DOI] [PubMed] [Google Scholar]
- 22.Oh JZ, Kurche JS, Burchill MA, Kedl RM. TLR7 enables cross-presentation by multiple dendritic cell subsets through a type I IFN-dependent pathway. Blood. 2011;118:3028–38. doi: 10.1182/blood-2011-04-348839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holbrook BC, Kim JR, Blevins LK, Jorgensen MJ, Kock ND, D’Agostino RB, Jr, et al. A novel R848-conjugated inactivated influenza virus vaccine is efficacious and safe in a neonate nonhuman primate model. J Immunol. 2016;197:555–64. doi: 10.4049/jimmunol.1600497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arimilli S, Johnson JB, Clark KM, Graff AH, Alexander-Miller MA, Mizel SB, et al. Engineered expression of the TLR5 ligand flagellin enhances paramyxovirus activation of human dendritic cell function. J Virol. 2008;82:10975–85. doi: 10.1128/JVI.01288-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma F, Zhang J, Zhang J, Zhang C. The TLR7 agonists imiquimod and gardiquimod improve DC-based immunotherapy for melanoma in mice. Cell Mol Immunol. 2010;7:381–8. doi: 10.1038/cmi.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pasut G. Polymers for protein conjugation. Polymers. 2014;6:160–78. [Google Scholar]
- 27.Colak E, Leslie A, Zausmer K, Khatamzas E, Kubarenko AV, Pichulik T, et al. RNA and imidazoquinolines are sensed by distinct TLR7/8 ectodomain sites resulting in functionally disparate signaling events. J Immunol. 2014;192:5963–73. doi: 10.4049/jimmunol.1303058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, et al. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–7. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 29.Larange A, Antonios D, Pallardy M, Kerdine-Romer S. TLR7 and TLR8 agonists trigger different signaling pathways for human dendritic cell maturation. J Leukoc Biol. 2009;85:673–83. doi: 10.1189/jlb.0808504. [DOI] [PubMed] [Google Scholar]
- 30.Song JM, Van Rooijen N, Bozja J, Compans RW, Kang SM. Vaccination inducing broad and improved cross protection against multiple subtypes of influenza A virus. Proc Natl Acad Sci U S A. 2011;108:757–61. doi: 10.1073/pnas.1012199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leibl H, Tomasits R, Eibl MM, Mannhalter JW. Adjuvant/carrier activity of inactivated tick-borne encephalitis virus. Vaccine. 1998;16:340–5. doi: 10.1016/s0264-410x(97)80911-5. [DOI] [PubMed] [Google Scholar]
- 32.Deng L, Kim JR, Chang TZ, Zhang H, Mohan T, Champion JA, et al. Protein nanoparticle vaccine based on flagellin carrier fused to influenza conserved epitopes confers full protection against influenza A virus challenge. Virology. 2017;509:82–9. doi: 10.1016/j.virol.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kapadia CH, Tian S, Perry JL, Luft JC, DeSimone JM. Reduction sensitive PEG hydrogels for codelivery of antigen and adjuvant to induce potent CTLs. Mol Pharm. 2016;13:3381–94. doi: 10.1021/acs.molpharmaceut.6b00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hao J, Kwissa M, Pulendran B, Murthy N. Peptide crosslinked micelles: a new strategy for the design and synthesis of peptide vaccines. Int J Nanomedicine. 2006;1:97–103. doi: 10.2147/nano.2006.1.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan M, Hayashi T, Mathewson RD, Yao S, Gray C, Tawatao RI, et al. Synthesis and characterization of PEGylated toll like receptor 7 ligands. Bioconjug Chem. 2011;22:445–54. doi: 10.1021/bc1004813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Makni-Maalej K, Marzaioli V, Boussetta T, Belambri SA, Gougerot-Pocidalo MA, Hurtado-Nedelec M, et al. TLR8, but not TLR7, induces the priming of the NADPH oxidase activation in human neutrophils. J Leukoc Biol. 2015;97:1081–7. doi: 10.1189/jlb.2A1214-623R. [DOI] [PubMed] [Google Scholar]
- 37.Kabelitz D. Expression and function of Toll-like receptors in T lymphocytes. Curr Opin Immunol. 2007;19:39–45. doi: 10.1016/j.coi.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 38.Gorden KB, Gorski KS, Gibson SJ, Kedl RM, Kieper WC, Qiu X, et al. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J Immunol. 2005;174:1259–68. doi: 10.4049/jimmunol.174.3.1259. [DOI] [PubMed] [Google Scholar]
- 39.Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–7. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 40.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. NatImmunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]


