Abstract
Background
We designed an Internet-Based Monitoring Systems (IBS) survey to facilitate monitoring of asthma symptoms and asthma exacerbations in the Severe Asthma Research Program (SARP). Our objective was to evaluate compliance with the IBS survey tool and to explore how data from an IBS tool can inform understanding of asthma phenotypes.
Methods
We invited adult subjects in the SARP III cohort (N = 528) to complete a monthly IBS asthma control survey. We compared the characteristics of subjects who did and those who did not participate in the IBS survey tool. Among subjects who participated in the IBS (IBS+), we identified participants with low, medium, and high Asthma Control Test (ACT) score variability, and we explored asthma morbidity in these three participant subgroups.
Results
Two hundred fifty-nine subjects participated in the IBS (IBS+) survey. Compared with subjects who did not engage with the IBS (IBS–) survey, IBS+ subjects were older and more likely to be white, college educated, and have an annual household income > $25,000, and have controlled asthma. Among IBS+ participants, the subgroup with the highest ACT score variability was more likely to have severe asthma, with a lower ACT score at baseline and increased asthma-related health-care use (often precipitated by cold and flulike illnesses). Participants with high ACT variability were also characterized by metabolic dysfunction, as evidenced by obesity and hypertension.
Conclusions
Active participation with an Internet-based symptom survey tool in patients with severe asthma is influenced by race, socioeconomic status, and asthma control. Among survey participants, a group with highly variable (labile) asthma control is identifiable as a specific subgroup with unmet treatment needs. The association of asthma lability, increased susceptibility to adverse asthma effects of cold and flulike illnesses, and metabolic dysfunction provides clues for potentially effective intervention strategies.
Key Words: asthma control, Internet-based monitoring, labile asthma, metabolic dysfunction, obesity
Abbreviations: ACT, Asthma Control Test; ATS/ERS, American Thoracic Society/European Respiratory Society; CV, coefficient of variation; IBS, Internet-Based Monitoring Systems; IRR, incident rate ratio; SARP, Severe Asthma Research Program
Internet-based tools that capture information about symptoms and disease behavior facilitate real-time disease monitoring in chronic diseases.1 Internet-Based Monitoring Systems (IBS) have the potential to improve understanding of disease behavior, especially in chronic diseases like asthma that have heterogeneous clinical phenotypes. To date, IBS use in asthma has focused largely on special populations such as children,2 the elderly,3 and pregnant women,4 or have used Internet-based surveys to monitor medication compliance.5 However, few studies have focused on applying IBS to monitor and identify asthma control phenotypes.
Our objective was to evaluate the utility of IBS as a research tool for monitoring asthma control in patients enrolled in the Severe Asthma Research Program-3 (SARP-3), a longitudinal cohort study. We designed an IBS survey tool linked to e-mail reminders, and we collected asthma control data over a 1-year observation period. Our goals were twofold. First, we sought to evaluate participant engagement with the IBS tool and the demographic and clinical characteristics of subgroups who did or did not engage with the tool. Second, we explored the demographic and clinical features of subgroups of patients with asthma with varying degrees of asthma control, with a focus on the subgroup with highly variable (labile) asthma control.
Methods
Study Participants
Data were derived from the SARP III cohort. SARP is an ongoing 3-year longitudinal cohort study consisting of 709 subjects with asthma aged 6-84 years recruited by seven clinical research sites across the United States between November 2012 and February 2015. SARP is focused on severe asthma, and 60% of subjects have American Thoracic Society/European Respiratory Society (ATS/ERS) criteria for severe disease.6 The SARP protocol involves detailed characterization at enrollment, with biannual telephone follow-up visits and annual in-person follow-up visits for 3 years. Asthma control and asthma exacerbations are monitored prospectively using paper questionnaires during biannual encounters. In addition, shortly after the main study protocol was initiated, an IBS survey was developed and deployed to capture data about asthma control and exacerbations on a monthly basis and during exacerbations. All subjects provided written informed consent to participate in the SARP study; subjects were asked to either opt in or opt out of the IBS survey as part of main study consent (ClinicalTrials.gov: NCT01606826). Details of the baseline characterizations have been described recently,7, 8 with key protocol details relevant to this study provided further on. Only subjects > 18 years of age were included in this study. Study procedures were approved by the institutional review board at each institution and an independent data safety monitoring board.
Baseline Assessment
Baseline questionnaires captured information on demographics, socioeconomic status, medical history, and current medications. Clinical disease characterization was performed for all subjects, including a physician-directed history, asthma characterization questionnaire, Asthma Control Test (ACT) score, spirometry, methacholine challenge, CBC count with cell differential, serum IgE measurements, and fractional exhaled nitric oxide levels.
Prospective Follow-up
Follow-up information about interval health-care use for asthma was collected biannually from subjects at 6 months by telephone and at 12 months in person. Questionnaires were administered by trained clinical research coordinators and included questions about asthma-related health-care visits, including unscheduled office visits, ED visits, overnight hospitalizations, and ICU admissions. Subjects were also asked if they had experienced any exacerbations of asthma that necessitated treatment with systemic steroids. “Severe exacerbations” were defined as an exacerbation requiring an ED visit, an overnight hospitalization, or an ICU admission; and “all exacerbations” were defined as those exacerbations that met the severe criteria and also included exacerbations requiring an unscheduled visit to a physician's office or an oral corticosteroid burst, or both.
Asthma IBS Platform
Self-reported information characterizing exacerbations and periods of worsening symptoms were collected using the asthma IBS tool. The asthma IBS platform was built using online survey software (Qualtrics, Provo, UT) with branching logic that presented the user with more or fewer questions based on previous answers. All subjects who opted in to participate in the IBS survey had their e-mail addresses registered, and a link to the survey was sent at the start of each month. This link was personalized to each participant and could be used multiple times throughout each month to provide real-time updates about worsening asthma control and exacerbations. These instructions were repeated in the e-mail reminder sent each month. The survey contained 26 questions (e-Table 1) about asthma control and exacerbation details, including when symptoms began, identifiable triggers (e-Fig 1), and any related changes to therapy. All participants were asked a minimum of eight questions as part of the survey, including the five questions from the ACT.9 The ACT was reproduced electronically with permission (Quality Metrics Inc.). Participants did not receive additional compensation for participating in the IBS survey. The survey was compatible with desktop computers and smart phones. In addition, one participant elected to complete the survey through monthly telephone interviews. Survey data and SARP questionnaire data captured through December 31, 2015 were included in this analysis.
Statistical Analysis
Statistical analyses were performed using JMP 12 software package (SAS Institute) and Stata, version 12.0 (StataCorp LLC), and P values < .05 were considered statistically significant. Two group comparisons between participants who engaged with the IBS platform and those who did not were made using the Student t test for continuous variables meeting the distributional assumption for the t test, the Wilcoxon rank-sum test for continuous variables not meeting those assumptions, and a Fisher exact test for categorical variables. We calculated kappa statistics to quantify agreement between methods to monitor asthma exacerbations between the biannual SARP follow-up asthma exacerbation questionnaire and the IBS tool.
Poisson regression models using robust error variance10 were constructed to identify the clinical characteristics associated with IBS nonengagement. The binary outcome variable in these models was IBS nonengagement (0 = IBS engagement or IBS+, 1 = IBS nonengagement or IBS–). The predictor variables in these models included all variables that were significantly different between IBS– and IBS+ subjects in an initial unadjusted bivariate analysis (P < .05). Results of the final model are presented as incident rate ratios (IRRs) and 95% CIs.
Participants who were enrolled in the platform for at least 12 months and completed at least two surveys in their first year of platform enrollment were included in a secondary analysis to associate variability of asthma control with baseline characteristics and prospective monitoring of exacerbations and periods of worsening asthma control. The coefficient of variation (CV) of the ACT score was calculated for each participant, and the cohort was divided into tertiles based on the CV; tertile 1 was composed of those with the least amount of ACT score variation, and tertile 3 was composed of those with the greatest amount of ACT score variation. Baseline characteristics were then compared across tertiles. Nonparametric tests for trend were used to test differences across ACT score tertiles for discrete variables,11 and analysis of variance testing was used for continuous variables.
Results
Engagement With the IBS Platform
Monthly monitoring of asthma control
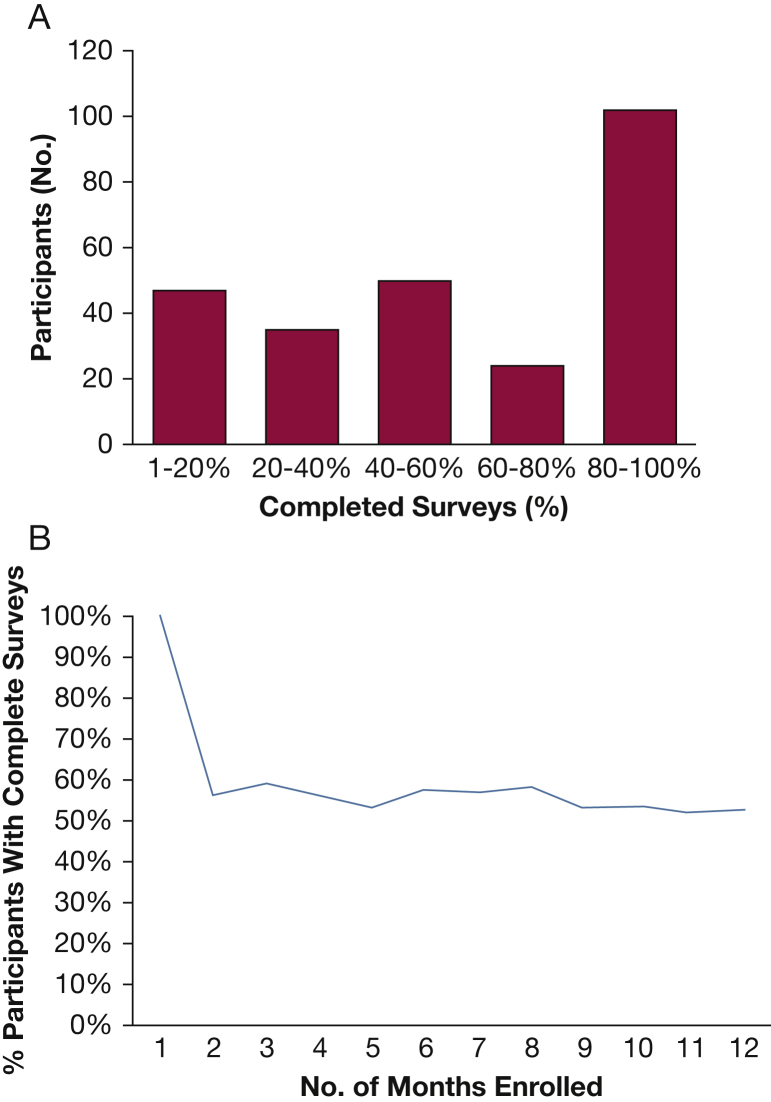
Among SARP subjects, 528 adults (18 years and older) were eligible to participate in IBS-based tracking of asthma symptoms. Of these 528 subjects, 269 (51%) did not log on to the platform at all (IBS–), and 259 (49%) logged on at least once (IBS+). Of these 259 IBS+ participants, 202 (78%) completed surveys in at least two of the first 12 months of platform enrollment, and many (n = 102) of these subjects completed the surveys more than 80% of the time (Fig 1A). Following completion of the first survey, adherence to the platform remained stable during the following year (Fig 1B).
Figure 1.
Survey adherence among patients who participated in the Internet-based Monitoring Systems (IBS+) survey. A, The percentage of completed surveys (x-axis) among the 259 IBS+ participants. Few subjects completed < 20% of the available surveys, and the majority of subjects completed > 40% of the available surveys. B, Following completion of the first survey, the percentage of participants who completed surveys remained stable over the course of the following year.
Reporting of asthma exacerbations
Among the 259 IBS+ participants, 104 (40%) reported an exacerbation of asthma using the tool at least once during platform enrollment. Of these, the majority (70 subjects [67%]) reported exacerbations only as part of their monthly reporting, whereas 21 (20%) reported exacerbations using real-time reporting only; 13 subjects (13%) sometimes used monthly reporting and other times used real-time reporting to document exacerbations.
IBS Users vs Nonusers
In comparing the demographic features of IBS+ participants and IBS– participants, we found that IBS+ participants were older and more likely to be white, had received at least some college education, and had an annual household income > $25,000 (Table 1). In comparing the clinical features of IBS+ and IBS– participants, we found that IBS– participants more frequently had uncontrolled asthma, as defined by any of the following criteria: two or more steroid courses in the past year, an asthma-related hospitalization or ICU admission in the past year, need for mechanical ventilation in the past year, an FEV1 < 80% predicted, or current ACT < 20.6 Compared with IBS+ participants, IBS– participants demonstrated no difference in FEV1 % predicted values, no difference in the frequency of subjects with severe asthma, and no difference in the frequency of asthma-associated hospitalizations or ED visits in the previous year (Table 1). IBS– participants did have a higher frequency of missing in-person and telephone visits with SARP study staff compared with IBS+ participants (49 [19%] vs 20 [8%]; P < .0001).
Table 1.
Demographic Features and Asthma Control and Compliance Variables of Participants Who Did or Did Not Engage With the Internet-Based Monitoring Tool (IBS+ and IBS– Subgroups)
| Characteristics (as Measured at Baseline) | IBS– (n = 269) |
IBS+ (n = 259) |
P Value |
|---|---|---|---|
| Mean ± SD or No. (%) or Median (IQR) | |||
| Age, y | 45.9 ± 13.5 | 49.1 ± 14.2 | .008 |
| Female sex | 178 (66) | 176 (68) | .75 |
| Race/ethnicity | < .001 | ||
| Asian | 9 (3) | 11 (4) | |
| Black | 94 (35) | 39 (15) | |
| White, non-Hispanic | 143 (53) | 194 (75) | |
| American Indian/Alaskan Native | 1 (0) | 1 (0) | |
| Other/more than one | 22 (8) | 14 (5) | |
| Household annual income < $25,000a | 54 (20) | 34 (14) | .001 |
| BMI, kg/m2 | 33.0 ± 8.2 | 32.0 ± 8.5 | .06 |
| Some college education or more | 209 (79) | 241 (93) | < .0001 |
| Baseline FEV1 % predicted | 72.1 ± 19.5 | 72.8 ± 19.9 | .66 |
| Adult-onset of asthma, ≥ 18 y | 174 (65) | 153 (59) | .18 |
| Uncontrolled asthmab | 256 (95) | 233 (90) | .02 |
| Severe asthma | 158 (59) | 157 (61) | .66 |
| Asthma-related ED visit past 12 m | 66 (25) | 60 (23) | .71 |
| Asthma-related hospitalization past 12 m | 26 (10) | 30 (12) | .47 |
| ACT score | 17 (13-21) | 18 (14-21) | .28 |
| MARS score | 23 (20-24) | 23 (21-24) | .26 |
ACT = Asthma Control Test; IBS+ = patients who participated in the Internet-based Monitoring Systems survey; IBS– = patients who did not participate in the Internet-based Monitoring Systems survey; MARS = Medication Adherence Report Scale.
Seventy-eight participants did not have information about household income.
Uncontrolled asthma defined as one or more of the following: (1) two or more steroid courses in the past year, (2) an asthma-related hospitalization or ICU admission in the past year, (3) need for mechanical ventilation in the past year, (4) FEV1 < 80% predicted, or (5) current ACT score < 20.
Using Poisson regression models, we found that age, race, educational attainment, and asthma control were significant independent predictors of IBS nonuse (Table 2). Attending college and older age were predictive of use, whereas uncontrolled asthma was predictive of nonuse. Subjects identifying as black were more likely than those identifying as white (reference) to not engage with the IBS platform. No other specific racial/ethnic group was significantly more or less likely to engage with the IBS platform compared with the reference group (Table 2).
Table 2.
Race, Education, and Poor Asthma Control Predict Nonengagement With the IBS Tool
| Outcome | IRR | 95% CI | P Value |
|---|---|---|---|
| Race/ethnicitya | |||
| White | Reference | ||
| Black | 1.40 | 1.11-1.76 | .004 |
| Asian | 1.30 | 0.76-2.2 | .35 |
| More than one | 1.40 | 0.97-2.00 | .07 |
| Some college education | 0.70 | 0.55-0.87 | .002 |
| Income < $25,000 | 1.18 | 0.93-1.48 | .17 |
| Uncontrolled asthma | 1.62 | 1.00-2.63 | .05 |
| Age, 10-y increaseb | 0.93 | 0.86-1.00 | .05 |
IRR = incident rate ratio.
Two American Indian/Alaskan Natives omitted due to limited data.
Age reflects the change in IRR for each 10-year increase in age.
Comparing Exacerbations Recorded Using IBS and Exacerbations Recorded in the SARP Protocol Using Biannual Questionnaires
IBS defined asthma exacerbations as self-reported periods of worsening symptoms. These self-reported exacerbations could be recorded in real time or at the end of the monthly recording period. The SARP protocol recorded asthma exacerbations at 6-month intervals during telephone calls and in-person visits. The SARP protocol defined an asthma exacerbation as an increase in the use of systemic corticosteroids lasting 3 days or more. We compared the agreement between exacerbations reported using the IBS tool and exacerbations recorded through the biannual SARP exacerbation questionnaire. We found that agreement between the biannual SARP follow-up and the IBS data regarding reporting of exacerbations was fair (kappa = 0.56). Specifically, 78% of participant reports of asthma exacerbations in the previous year were congruent in the SARP questionnaire data and the IBS data; 12% of participants reported an exacerbation using the IBS tool but did not report an exacerbation using the SARP questionnaire, whereas 9% reported an exacerbation using the SARP questionnaire but did not report one using the IBS tool.
Variability of Asthma Control Over Time
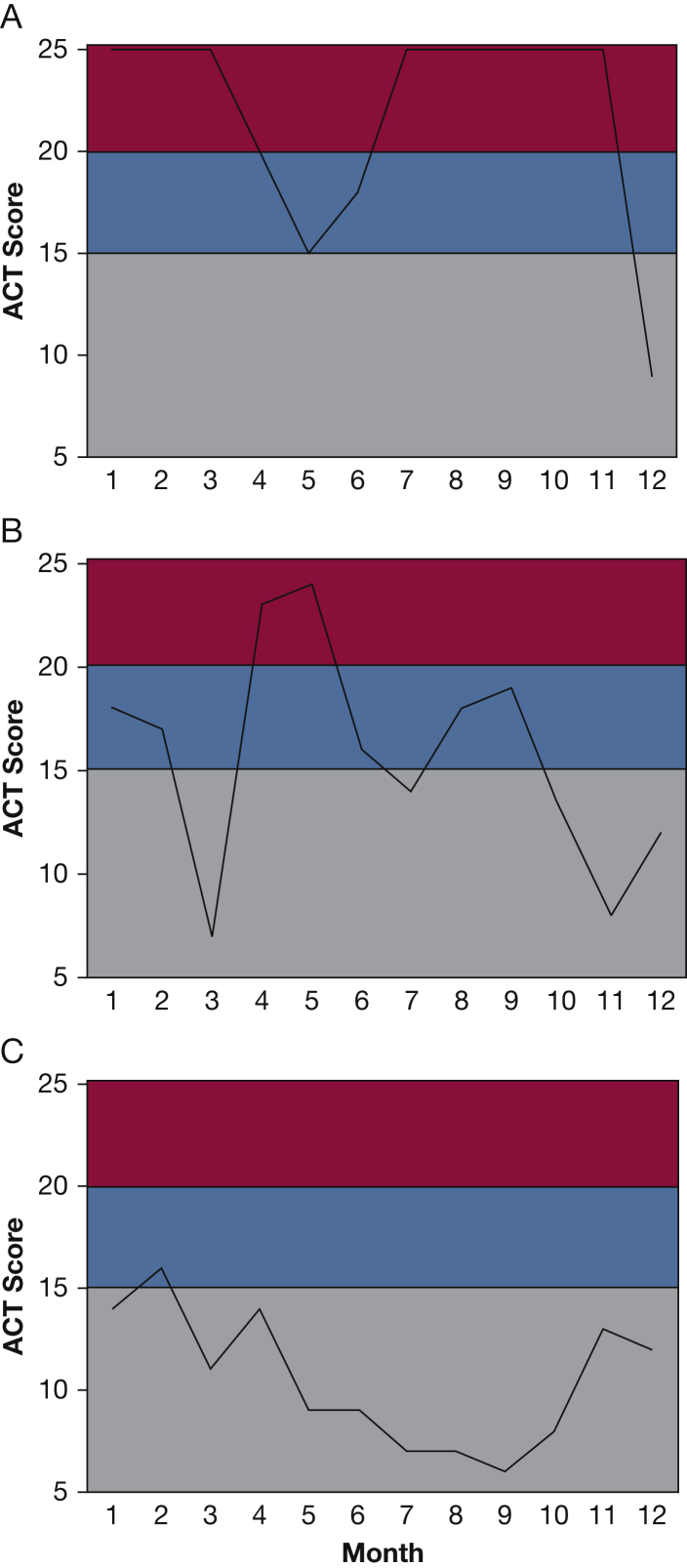
In examining ACT scores over time in different participants, we noted that some participants had ACT scores that varied widely from month to month (Fig 2). To quantify this variability or lability in asthma control, we focused on a subgroup of the 202 IBS users who completed surveys in at least two of the first 12 months of platform enrollment. For this purpose, we excluded 27 participants who did not have the opportunity to complete 12 months of surveys (either because they did not remain enrolled in SARP or because they opted in to receive surveys < 12 months prior to the close of data collection) and an additional 30 participants who completed only one monthly survey during the first year of platform enrollment. Among the remaining 202 participants, the average number of monthly survey completions was eight (range, two to 12), and we set out to analyze the data for variability in asthma control in these patients, with an emphasis on the subgroup with highly variable symptoms. Because the survey data included the five questions from the Asthma Control Test,9 it allowed an ACT score to be generated from each survey completion. We used multiple ACT scores from each participant in the first year of platform enrollment to calculate the CV for their ACT scores. We found that the IBS captured significantly more ACT variability compared with the SARP biannual ACT questionnaires (e-Fig 2). We then examined the summary ACT CV data for the cohort as tertiles representing low, medium, and high levels of variability in the ACT score.
Figure 2.
Examples of participants with high ACT variability. A, Participant who had many months of good control with episodic drops in control. B, Participant who fluctuated dramatically across months. C, Participant with high variability but no periods of good control. ACT = Asthma Control Test.
Patients with asthma in the highest ACT CV tertile did not differ from the lower tertiles regarding age, race/ethnicity, income, or educational attainment, but their baseline questionnaire data showed that they were more likely to be women and reported more ED visits for asthma in the previous 12 months (Table 3). In addition, they reported more hospitalizations (including ICU care) for asthma during their lifetimes (Table 3). Furthermore, patients with asthma in the highest ACT CV tertile had lower baseline ACT scores and several features of metabolic dysfunction, including higher BMI, greater frequency of hypertension, and a trend toward higher numbers of WBC counts (Table 3). Notably, blood and airway measures of type 2 inflammation (eosinophils, exhaled nitric oxide) did not differ among patients with asthma in the three ACT CV tertiles (Table 3) nor did outcomes of atopy (total IgE).
Table 3.
Demographics and Characteristics of Asthma and Asthma-Related Inflammation Associated With Variability of Control Over Time
| Characteristics (as Measured at Baseline) | ACT CV Tertile 1 0%-10.6% (n = 68) |
ACT CV Tertile 2 10.7%-21.6% (n = 67) |
ACT CV Tertile 3 21.7%-67.3% (n = 67) |
P Valuea |
|---|---|---|---|---|
| Mean ± SD or No. (%) or Median (IQR) | ||||
| Age, y | 48.5 ± 14.3 | 49.2 ± 13.5 | 50.5 ± 12.5 | .44 |
| Age of asthma onset, y | 15 (6-33) | 14 (5-32) | 12 (4-23) | .31 |
| Female sex, No. (%) | 45 (67) | 44 (65) | 54 (81) | .01 |
| Race/ethnicity, No. (%) | .45 | |||
| Asian | 4 (6) | 2 (3) | 3 (4) | |
| Black | 14 (21) | 7 (10) | 7 (10) | |
| White | 48 (71) | 54 (81) | 52 (78) | |
| Mixed/other | 2 (3) | 4 (6) | 5 (7) | |
| BMI, kg/m2 | 30.2 ± 7.7 | 31.0 ± 6.9 | 34.6 ± 9.9 | .004 |
| Nasal polyps, No. (%) | 11 (16) | 18 (27) | 19 (28) | .12 |
| Chronic rhinosinusitis, No. (%) | 21 (31) | 33 (49) | 39 (58) | .001 |
| GERD, No. (%) | 25 (37) | 32 (48) | 41 (61) | .006 |
| Hypertension, No. (%) | 17 (25) | 23 (34) | 29 (43) | .03 |
| Diabetes, No. (%) | 6 (9) | 9 (13) | 9 (13) | .30 |
| ATS/ERS severe category, No. (%) | .02 | |||
| Mild | 19 (28) | 7 (10) | 11 (16) | |
| Moderate | 16 (24) | 17 (25) | 10 (15) | |
| Severe | 33 (49) | 43 (64) | 46 (69) | |
| Baseline ACT | 20 (17-22) | 18 (14-21) | 15 (12-20) | < .0001 |
| Asthma-related ED visit in past 12 m, No. (%) | 4 (6) | 20 (30) | 22 (33) | < .001 |
| Asthma-related hospitalization in past 12 m, No. (%) | 3 (4) | 11 (16) | 9 (13) | .10 |
| Lifetime history of asthma-related ICU stay, No. (%) | 5 (7) | 13 (19) | 13 (19) | .05 |
| Baseline FEV1 % | 77.1 ± 22.6 | 70.0 ± 20.7 | 73.5 ± 18.6 | .30 |
| Baseline FVC % | 88.2 ± 17.5 | 81.7 ± 17.6 | 85.3 ± 16.0 | .29 |
| Exhaled nitric oxide, ppb | 18.5 (13-31) | 22 (13-39) | 26 (14-42) | .11 |
| IgE, IU/mL | 150 (38-267) | 145 (36-378) | 98 (27-281) | .40 |
| WBC count, ×106/L | 6.7 (5.6-8.0) | 7.0 (5.7-8.3) | 7.6 (6.0-9.9) | .07 |
| Peripheral blood neutrophils, ×106/L | 3,966 (2,910-5,370) | 4,008 (3,097-5,106) | 4,402 (3,294-5,964) | .12 |
| Peripheral blood eosinophils, ×106/L | 181 (125-347) | 244 (144-378) | 251 (124-456) | .25 |
CV = coefficient of variation, IgE = immunoglobulin E; ppb = parts per billion. See Table 1 legend for expansion of other abbreviations.
Statistical comparison done using a nonparametric test for trend.
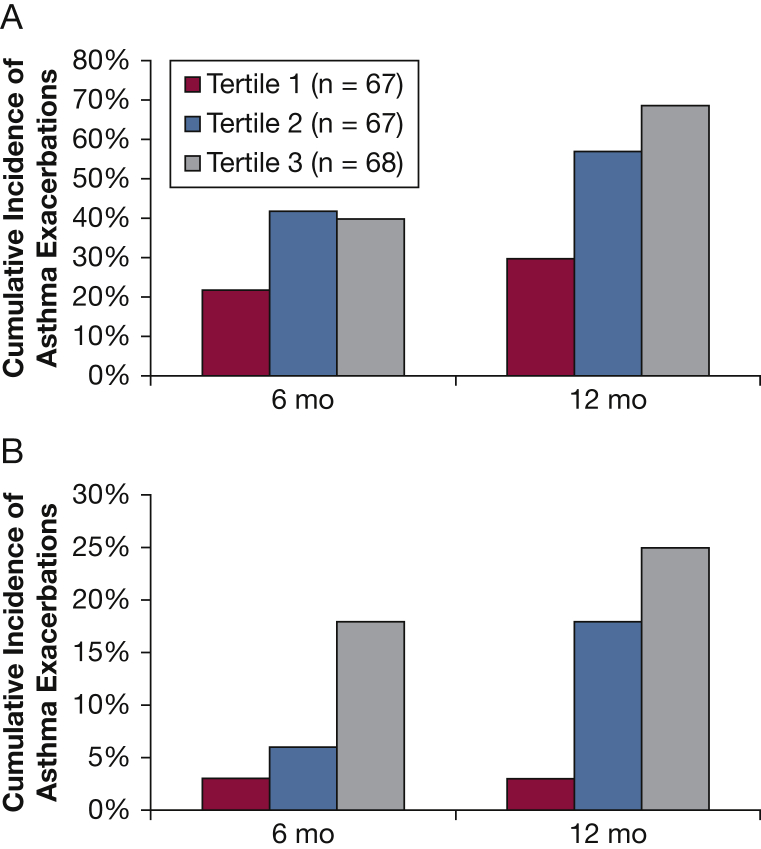
In examining asthma exacerbation data, we found that patients with asthma in the highest ACT CV tertile had a higher cumulative incidence of experiencing an asthma exacerbation at 12-month follow-up. In addition, subjects with asthma and high ACT variability also experienced a higher cumulative incidence of a severe exacerbation by 12 months of follow-up (Fig 3). Not surprisingly, the patients with asthma in the highest ACT CV tertile had more periods of worsening asthma symptoms (Table 4), regardless of whether these worsening symptoms met the threshold for a clinical exacerbation. When responding to questions in the IBS survey about perceived triggers of worsening asthma symptoms, patients with asthma in the highest ACT CV tertile more frequently identified cold and flulike illness as the precipitating cause (Table 4, e-Fig 1). In contrast, patients with asthma in the lowest ACT CV tertile more commonly identified weather changes as a precipitating cause (Table 4).
Figure 3.
Cumulative incidence of asthma exacerbations at 6-month and 12-month follow-up stratified by Asthma Control Test (ACT) variability tertiles. Subjects with high ACT variability (tertile 3) experienced a higher incidence of severe and nonsevere asthma exacerbations at the 12-month follow up. A, Cumulative incidence of all asthma exacerbations, as defined by any of the following: (1) unscheduled visit to a physician's office, (2) oral corticosteroid burst, (3) ED visit, or (4) hospital admission. B, Cumulative incidence of severe asthma exacerbations, limited to one of the following: (1) ED visit or (2) hospital admission.
Table 4.
Self-Reported Triggers of Worsening Asthma Symptoms Among Participants Who Reported at Least One Episode of Worsening Asthma
| Asthma Trigger | ACT CV Tertile 1 0%-10.6% (n = 68) |
ACT CV Tertile 2 10.7%-21.6% (n = 67) |
ACT CV Tertile 3 21.7%-67.3% (n = 67) |
P Value |
|---|---|---|---|---|
| ≥ 1 episode of worsening asthma, No. (%) | 51 (75) | 64 (96) | 66 (99) | < .0001 |
| Triggers of worsening asthma, No. (%) | ||||
| Illness | 33 (65) | 39 (61) | 54 (82) | .03 |
| Airborne irritants | 16 (31) | 24 (38) | 31 (47) | .08 |
| Emotion | 10 (20) | 21 (33) | 27 (41) | .02 |
| Allergens | 32 (63) | 41 (64) | 39 (59) | .66 |
| Physical exertion | 14 (27) | 18 (28) | 23 (35) | .37 |
| Tobacco smoke | 2 (4) | 9 (14) | 12 (18) | .02 |
| Weather changes | 18 (35) | 15 (23) | 12 (18) | .04 |
| Unknown | 6 (12) | 16 (25) | 20 (30) | .02 |
| Other | 2 (4) | 6 (9) | 7 (11) | .20 |
See Table 1 legend for expansion of abbreviations.
Discussion
In this study, we evaluated the utility of a monthly IBS platform to capture ACT data in a cohort of patients with asthma (many with severe disease) over a 1-year period. We found that half of the cohort engaged with the IBS platform and half did not, and predictors of engagement included multiple measures associated with higher socioeconomic status. Among patients with asthma who participated, we found that a subgroup had highly variable ACT scores, and these patients with labile asthma were characterized by obesity, metabolic dysfunction, and loss in asthma control precipitated by cold and flulike illness.
Roughly half of our cohort did not engage with the IBS platform, and although there was a higher rate of noncompliance with SARP visits among those who did not engage with the IBS survey, the vast majority of the IBS– participants were still able to adhere to other SARP activities (81%). Among the demographic factors associated with nonengagement were black race and a lack of higher education. There are potential methods of increasing participant adherence to IBS. First, our study did not compensate participants for completing the survey, and it is possible that a payment incentive could have improved engagement. In addition, the platform contained minimal interfacing with medical staff and did not provide any feedback on survey responses. Other Internet-based monitoring tools have incorporated feedback features12 such as text messaging, and it is possible that these could have improved engagement with our IBS tool. Finally, recent data suggest that up to 15% of American adults do not use the Internet,13 and it is possible that a lack of access to the Internet prohibited some subjects from participating in the IBS survey.
Internet-based technologies are a powerful tool to monitor asthma control, but it will be important to continue to explore the utility of these technologies and ensure that the data generated are generalizable. Our findings suggest that race and educational attainment significantly influence the use of Internet-based monitoring tools. Our findings also indicate that participants with increased disease morbidity are at higher risk for nonuse. The finding that patients with uncontrolled asthma are less likely to use IBS is concerning, because a primary objective of IBS is to characterize asthma exacerbations among a population with a higher proportion of subjects with severe asthma. Furthermore, nonadherence would limit the ability of IBS to serve as a recruitment tool to identify participants with exacerbation-prone disease. Nevertheless, the IBS survey did capture data in half of all adult enrolled participants. Additionally, 40% of IBS+ participants provided data about recent exacerbations. Although the agreement between the exacerbations reported using IBS and those reported at live encounters with SARP staff was good, the IBS tool captured data about each exacerbation, including triggers of exacerbation, which would not have been practical given recall limitations at the live biannual encounters. Given that the IBS tool used here contained minimal interaction with medical staff and lacked any additional compensation, we consider that the adherence was good and that the IBS tool is practical for real-time sensing of exacerbations of asthma in research.
Through the use of IBS, we identified a subset of patients with asthma with highly variable ACT scores. Using less frequent monitoring systems, such as 6-month or annual questionnaires, missed identifying these patients with labile asthma. Participants with labile asthma more frequently met ATS/ERS criteria for severe asthma and experienced more exacerbations during the 12-month follow-up period. Furthermore, the IBS data allowed us to characterize asthma exacerbation triggers and show that cold and flulike illnesses were common causes of worsening asthma control. Conversely, neither low lung function nor degree of bronchodilator reversibility was a clinical feature of “patients with labile asthma.” Thus, labile asthma seems to relate to factors associated with susceptibility to viral illnesses rather than factors associated with excessive airway narrowing or smooth muscle dysfunction.
Compared with patients with asthma who have minimal or moderate ACT score variability, patients with asthma with high ACT score variability were also characterized by obesity, hypertension, and a trend toward higher WBC counts, but they did not have prominent systemic or airway measures of type 2 inflammation. Our findings are consistent with studies that identify diabetes as a risk factor for the development of respiratory tract infections,14 and our recent data that interleukin-6-associated systemic inflammation is associated with more severe asthma phenotypes.7
Conclusions
We demonstrate that an IBS tool is effective for monitoring asthma control in patients with severe disease, but that demographic factors such as race and education influence engagement with the tool. The use of the IBS tool in the SARP-3 cohort identifies a subgroup of patients with asthma who have highly variable asthma control and are characterized by metabolic dysfunction and increased susceptibility to loss of asthma control triggered by cold and flulike illnesses. IBS has the potential to identify patients who are at risk for the development of asthma exacerbations, and IBS could enable providers to target asthma interventions, including higher inhaled corticosteroid doses, to prevent future exacerbations in these patients.
Acknowledgments
Author contributions: K. W. M. and M. C. P. are the guarantors of the paper, taking responsibility for the integrity of the work as a whole from inception to published article. K. W. M., J. V. F., and M. C. P. conceived and designed the study. L. C. D., E. R. B., M. C., B. G., E. I., N. N. J., D. T. M., S. P., B. R. P., and S. E. W. made substantial contributions to the design and analysis of this study. K. W. M. and M. C. P. conducted the data analysis. K. W. M. and M. C. P. prepared the first draft of the manuscript, and all authors revised the draft critically for important intellectual content. All authors gave final approval for the manuscript version to be published.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: L. C. D. has consulted for GSK. E. R. B. has undertaken clinical trials through his employer Wake Forest School of Medicine and the University of Arizona and AstraZeneca, Medimmune, Boehringer Ingelheim, Cephalon/Teva, Genentech, GSK, Johnson & Johnson (Janssen), Novartis, and Sanofi Regeneron. He has also served as a paid consultant for AstraZeneca, Medimmune, Boehringer Ingelheim, GSK, Novartis, and Sanofi Regeneron outside the submitted work. M. C. currently receives pharmaceutical grant monies from Medimmune, Teva, GSK, Boehringer Ingelheim, Invion, Gilead, and Sanofi Aventis. Royalties are received from Elsevier. E. I. reports receiving consulting fees from AstraZeneca, Bird Rock Bio, Novartis, Nuvelution Pharmaceuticals, Philips Respironics, Regeneron Pharmaceuticals, TEVA Specialty Pharmaceuticals, and Vitaeris, Inc.; travel grant support from Research in Real Life (RIRL) and TEVA Specialty Pharmaceuticals; Deputy Editor fees from the American Thoracic Society; DSMB Member for Novartis with no compensation, and grant support paid to his institution from Genentech, Sanofi, and the National Institutes of Health (NIH). D. T. M. reports grant support from the NIH. J. V. F. has acted as a paid consultant to Sanofi Genzyme and Boehringer Ingelheim and has received grant monies from the NIH. M. C. P. has acted as a paid consultant to Merck, has participated in speaking activities at Genentech and Amgen, and reports grant support from the NIH. None declared (K. W. M., B. G., N. N. J., S. P., B. R. P., S. E. W.).
Other contributions: We thank Patricia Noel, PhD, and Robert Smith, PhD (Division of Lung Diseases, National Heart, Lung, and Blood Institute, Bethesda, MD) for their support and leadership of the Severe Asthma Research Program, and all the volunteers who participated in these studies.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Figures and e-Table can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This study was funded by the National Institutes of Health [Grants PO1 HL107201, R01 HL080414, K23 HL138303, U19AI077439, U10 HL109146, U10 HL109164, U10 HL109172, U10 HL109086, U10 HL109250, U10 HL109168, U10HL109257, and U10 HL109152] and by grants from the Parker B. Francis Foundation.
Supplementary Data
References
- 1.Paolotti D., Carnahan A., Colizza V. Web-based participatory surveillance of infectious diseases: the Influenzanet participatory surveillance experience. Clin Microbiol Infect. 2014;20(1):17–21. doi: 10.1111/1469-0691.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Troullos E., Baird L., Jayawardena S. Common cold symptoms in children: results of an Internet-based surveillance program. J Med Internet Res. 2014;16(6):e144. doi: 10.2196/jmir.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valdes E.G., Sadeq N.A., Harrison Bush A.L., Morgan D., Andel R. Regular cognitive self-monitoring in community-dwelling older adults using an Internet-based tool. J Clin Exp Neuropsychol. 2016;38(9):1026–1037. doi: 10.1080/13803395.2016.1186155. [DOI] [PubMed] [Google Scholar]
- 4.Loubet P., Guerrisi C., Turbelin C. First nationwide web-based surveillance system for influenza-like illness in pregnant women: participation and representativeness of the French G-GrippeNet cohort. BMC Public Health. 2016;16:253. doi: 10.1186/s12889-016-2899-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Price D., Fletcher M., van der Molen T. Asthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med. 2014;24:14009. doi: 10.1038/npjpcrm.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung K.F., Wenzel S.E., Brozek J.L. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 7.Peters M.C., McGrath K.W., Hawkins G.A. Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: a cross-sectional analysis of two cohorts. Lancet Respir Med. 2016;4(7):574–584. doi: 10.1016/S2213-2600(16)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denlinger L.C., Phillips B.R., Ramratnam S. Inflammatory and co-morbid features of patients with severe asthma and frequent exacerbations. Am J Respir Crit Care Med. 2017;195(3):302–313. doi: 10.1164/rccm.201602-0419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nathan R.A., Sorkness C.A., Kosinski M. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 11.Cuzick J. A Wilcoxon-type test for trend. Stat Med. 1985;4(1):87–90. doi: 10.1002/sim.4780040112. [DOI] [PubMed] [Google Scholar]
- 12.van der Meer V., Bakker M.J., van den Hout W.B. Internet-based self-management plus education compared with usual care in asthma: a randomized trial. Ann Intern Med. 2009;151(2):110–120. doi: 10.7326/0003-4819-151-2-200907210-00008. [DOI] [PubMed] [Google Scholar]
- 13.Pew Research Center: Internet and Technology. Americans’ Internet access: 2000-2015. http://www.pewinternet.org/2015/06/26/americans-internet-access-2000-2015/. Accessed December 2, 2017.
- 14.Casqueiro J., Casqueiro J., Alves C. Infections in patients with diabetes mellitus: a review of pathogenesis. Indian J Endocrinol Metab. 2012;16(suppl 1):S27–S36. doi: 10.4103/2230-8210.94253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.