Abstract
Background
Intrapulmonary vascular dilatations (IPVD) frequently are detected in patients with liver disease by the delayed appearance of microbubbles at contrast-enhanced echocardiography. IPVD with an elevated alveolar-arterial (A-a) gradient define hepatopulmonary syndrome (HPS); however, the importance of IPVD in the absence of abnormal gas exchange is unknown. We aimed to determine the clinical impact of IPVD in patients with liver disease.
Methods
We performed a cross-sectional study within the Pulmonary Vascular Complications of Liver Disease 2 Study, a multicenter, prospective cohort study of patients being evaluated for liver transplant. We excluded patients with obstructive or restrictive lung disease, HPS, or intracardiac shunting. We compared patients with and those without IPVD.
Results
Forty-six patients with IPVD and 81 patients without IPVD were included. Patients with IPVD were more likely to have autoimmune hepatitis and less likely to have cryptogenic cirrhosis and hepatocellular carcinoma. Patients with IPVD had higher Child-Pugh scores (6 [interquartile range (IQR), 5-7] vs 5 [IQR, 4-7]; P = .04), possibly higher Model for End-Stage Liver Disease scores (14.5 [IQR, 11.6-15.8] vs 12.2 [IQR, 9.4-15.5]; P = .06), higher PaO2 levels (97.9 [IQR, 92.0-103.0] vs 89.0 [IQR, 82.0-96.9] mm Hg; P < .001), and lower A-a gradients (9.9 [IQR, 6.2-13.5] vs 14.9 [IQR, 9.0-21.8] mm Hg; P < .001). Symptoms and quality of life were similar between the groups.
Conclusions
Autoimmune hepatitis and increased liver disease severity were associated with the presence of IPVD, which was characterized by higher PaO2 levels. Future studies to better characterize IPVD pathogenesis and the relationship of IPVD to HPS are warranted.
Key Words: hepatopulmonary syndrome, intrapulmonary vascular dilatation, liver transplant
Abbreviations: A-a, alveolar-arterial; ABG, arterial blood gas; HCC, hepatocellular carcinoma; HPS, hepatopulmonary syndrome; INR, international normalized ratio; IPVD, intrapulmonary vascular dilatation; IQR, interquartile range; LT, liver transplant; MELD, Model for End-Stage Liver Disease; PPHTN, portopulmonary hypertension
Intrapulmonary vascular dilatations (IPVD), dilatations of the pulmonary precapillary and capillary vessels, are common in patients with advanced liver disease, with an estimated prevalence of 13% to 56%.1, 2, 3, 4, 5, 6, 7 IPVD can lead to abnormal gas exchange and hypoxemia due to ventilation-perfusion mismatch, diffusion limitation, and anatomic shunting.8 The triad of liver disease and/or portal hypertension, IPVD, and abnormal arterial oxygenation (defined as an elevated alveolar-arterial [A-a] gradient ≥ 15 mm Hg [or ≥ 20 mm Hg if age > 64 years]) defines hepatopulmonary syndrome (HPS),9 which is associated with worse quality of life and an increased risk of death.4 IPVD in the setting of portopulmonary hypertension (PPHTN) also may be associated with decreased survival.10
Approximately one-half of patients with liver disease and IPVD, however, have normal gas exchange and do not meet diagnostic criteria for HPS.1, 4 Despite IPVD being a commonly encountered entity, the clinical relevance and implications of isolated IPVD in the absence of gas exchange abnormalities have not been studied previously, to our knowledge. We aimed to determine the associated signs and symptoms and clinical impact of IPVD in a cohort of patients with liver disease and portal hypertension being evaluated for liver transplant (LT).
Methods
Study Sample
The Pulmonary Vascular Complications of Liver Disease 2 Study is a multicenter, prospective cohort study of adult patients with PPHTN or with portal hypertension undergoing evaluation for LT. The only inclusion criterion for cohort assembly was the presence of portal hypertension with or without intrinsic liver disease and undergoing an initial evaluation for LT. We excluded patients with active infection, recent gastrointestinal bleeding (< 2 weeks from date of evaluation), or a history of prior LT or lung transplant. The study sample for this analysis was drawn from 365 enrolled patients undergoing their first LT evaluation at the University of Pennsylvania, Mayo Clinic, and University of Texas at Houston between February 2013 and July 2016. We excluded patients with PPHTN, patients prescribed supplemental oxygen, or patients who failed to complete spirometric and/or arterial blood gas (ABG) sampling, both of which were required by the research protocol.
We excluded patients with obstructive or restrictive ventilatory defects (FEV1/FVC < 0.7 with FEV1 < 80% predicted or FVC < 70% predicted, respectively), definite or indeterminate intracardiac shunting (as defined later), and HPS. As established by the European Respiratory Society Task Force on Pulmonary-Hepatic Vascular Disorders Scientific Committee, HPS was defined as (1) an A-a gradient ≥ 15 mm Hg (or A-a gradient ≥ 20 mm Hg if age > 64 years) and (2) late passage of contrast material at contrast-enhanced transthoracic echocardiography (defined later) in the absence of intracardiac shunting or a significant obstructive or restrictive ventilatory defect at spirometry.8, 9 We performed a subset analysis in which we excluded patients with an A-a gradient ≥ 15 mm Hg (or ≥ 20 mm Hg if age > 64 years) from the control group without IPVD to compare patients with similar oxygenation (because patients with an elevated A-a gradient met criteria for HPS and were excluded from the IPVD group).
Study Procedures
Patients at an LT evaluation clinic were screened for eligibility at each study site. Informed consent was obtained from eligible patients, who then were scheduled for research assessment, which included a history and physical examination (including assessment of dyspnea), quality-of-life assessment with 36-Item Short Form Survey Instrument questionnaires, anthropometric tests, pulse oximetry, a 6-minute walk test, ABG sampling, a spirometric test, and contrast-enhanced echocardiography.
Clinical data were collected from the medical record and formal patient interviews. Clinical laboratory results obtained closest to the date of the study visit were recorded. Model for End-Stage Liver Disease (MELD) scores were calculated using the following formula: MELD = 10 × [(0.957 × ln[creatinine]) + (0.378 × ln[bilirubin]) + (1.12 × ln[INR])] + 6.43, where “INR” indicates “international normalized ratio.” Body surface area was calculated using the Du Bois and Du Bois formula.11 An unencouraged 6-minute walk test was performed,12 and quality of life was assessed using the 36-Item Short Form Survey Instrument questionnaire. The Modified Borg Dyspnea Scale was used to assess breathlessness before and after the 6-minute walk test.13, 14
Pulse oximetry was performed using a standard professional grade oximeter (Datascope Accutorr Plus Vital Signs Monitor; Datascope Corp, Nonin Pulse Oximeter; Nonin Medical, Inc, Welch Allyn Masimo; Welch Allyn Medical Products, or Protocol Systems Quik Signs; Protocol Systems, Inc [now known as Welch Allen Protocol, Inc]) after the study participant maintained an upright posture for 5 minutes and then was repositioned supine for 5 minutes. ABG sampling was performed with the patient breathing ambient air in a seated position after 10 minutes of rest. The samples were processed in a blood gas analyzer after a 1-point calibration. The A-a gradient was calculated using the following formula: AaPO2 = [(FIO2 × [Patm − PH20]) − (PaCO2/R)] – PaO2, where R is assumed to be 0.8, Patm was the barometric pressure measured in the city on the date of the study visit, PH20 is the partial pressure of water at body temperature.15
Spirometry was performed according to American Thoracic Society-European Respiratory Society recommendations; a minimum of three efforts with no acceptability errors and at least two with repeatability according to American Thoracic Society-European Respiratory Society standards (FVC within 150 mL of largest, FEV1 within 150 mL of largest, and peak flow within 15% of largest) were required.16 Testing was continued until these criteria were met, a total of eight tests were performed, or the patient was unable to continue testing. Sex-, age-, and race-specific prediction equations were used to determine percent predicted based on spirometric reference values derived from the National Health and Nutrition Examination Survey III.17
Contrast-enhanced echocardiography was performed by injecting agitated saline via a peripheral vein during imaging. The apical four-chamber view was the preferred window for image acquisition, although other views were used if the four-chamber view was suboptimal or unavailable. At least 10 continuous cardiac cycles were captured, beginning immediately prior to contrast material injection, to allow accurate assessment of cardiac cycles to determine delay from injection of agitated saline until visualization of contrast material entering the left side of the heart. Identification of microbubbles in either the left atrium or left ventricle after ≥3 cardiac cycles was considered to indicate the presence of IPVD. IPVD severity was categorized as grade I (mild) if few microbubbles were visualized in the left side of the heart without appreciable change in density of the left ventricular cavity, grade II (moderate) if microbubbles were visualized in left side of the heart with less than 50% of comparable density in the right side of the heart, or grade III (severe) if microbubbles were visualized in the left side of the heart with ≥ 50% of comparable density in the right side of the heart. Patients with immediate (< 3 cycles) opacification of the left atrium or ventricle were presumed to have an intracardiac shunt. Doppler flow signal across the atrial septum was presumed to indicate an intracardiac shunt. The Echocardiography Core Laboratory at the Mayo Clinic evaluated all contrast-enhanced echocardiograms obtained at individual study sites, and readers interpreted the studies off-line and were blinded to clinical information.
Statistical Analysis
Categorical variables were summarized by frequencies and proportions, and continuous variables were summarized by means and SDs or medians and interquartile ranges (IQRs), as appropriate. Categorical variables were compared using a χ2 test or Fisher exact test. Continuous variables were compared using a Student t test or Wilcoxon rank sum test. A P value < .05 was considered significant. All data analysis was performed in SAS Version 9.4 (SAS). The institutional review boards at all study sites approved the study (University of Pennsylvania, Office of Regulatory Affairs Protocol 816361; Mayo Clinic Institutional Review Boards Protocol 12-007715; and University of Texas Committee for the Protection of Human Subjects Protocol HSC-MS-12-0481).
Results
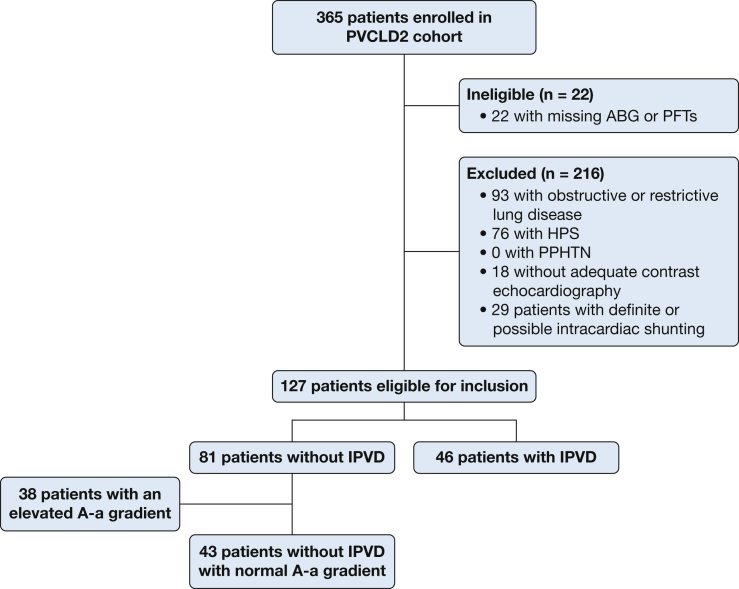
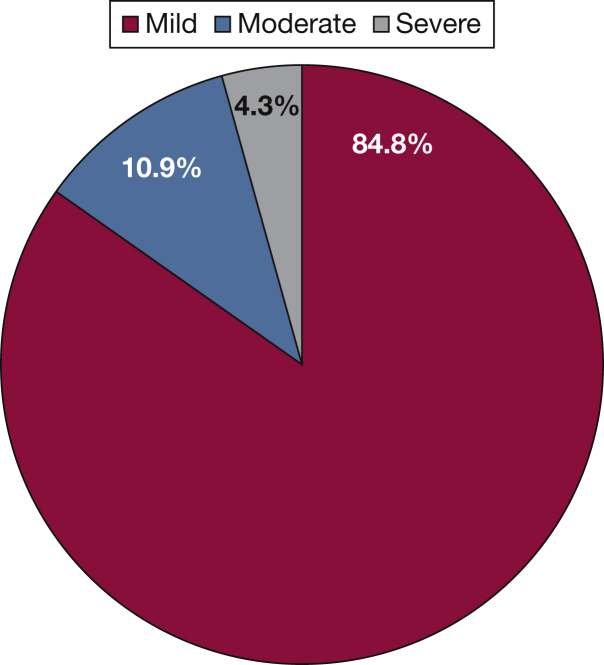
The study cohort included 365 patients (Fig 1). Overall, 57% (n = 209) of the cohort had IPVD. We excluded 93 patients with obstructive or restrictive ventilatory defects (of whom 54% [n = 50] had IPVD), 76 patients with HPS, 18 patients with inadequate contrast-enhanced echocardiograms, and 29 patients with definite or indeterminate intracardiac shunting. All patients with PPHTN were excluded in these initial steps. The final study sample included 46 patients with IPVD and 81 patients without IPVD. The distribution of IPVD severity among patients with IPVD is depicted in Figure 2.
Figure 1.
Selection of study sample. A-a = alveolar-arterial; ABG = arterial blood gas; HPS = hepatopulmonary syndrome; IPVD = intrapulmonary vascular dilatation; PFTs = pulmonary function tests; PPHTN = portopulmonary hypertension; PVCLD2 = Pulmonary Vascular Complications of Liver Disease 2.
Figure 2.
Distribution of intrapulmonary shunt severity among patients with intrapulmonary vascular dilatation: 39 patients (84.8%) had a mild degree of intrapulmonary shunting, five patients (10.9%) had moderate intrapulmonary shunting, and two patients (4.3%) had severe intrapulmonary shunting.
Patient characteristics are summarized in Tables 1 and 2. There was no difference between patients with and those without IPVD in age, sex, or race/ethnicity. Patients with IPVD were more likely to have autoimmune hepatitis and less likely to have cryptogenic cirrhosis and alcoholic cirrhosis. Patients with IPVD were significantly less likely to have hepatocellular carcinoma (HCC). They also had higher Child-Pugh scores and nonsignificantly higher MELD scores, suggesting more severe liver disease, but there was no difference between the groups in complications of liver disease (ascites, encephalopathy, or esophageal varices).
Table 1.
Patient Demographic Characteristics, Comorbidities, and Liver Disease Characteristics
| Characteristic | No. of Patients | No IPVD | No. of Patients | IPVD | P Value |
|---|---|---|---|---|---|
| Age, median (IQR), y | 81 | 58 (53-62) | 46 | 57 (48-63) | .51 |
| Male, No. (%) | 81 | 62 (76.5) | 46 | 30 (65.2) | .17 |
| Race/ethnicity, No. (%) | 81 | 46 | .41 | ||
| Non-Hispanic white | 52 (64.2) | 29 (63.0) | |||
| Hispanic | 17 (21.0) | 9 (19.6) | |||
| Black | 9 (11.1) | 3 (6.5) | |||
| Other | 3 (3.7) | 5 (10.9) | |||
| Liver disease cause, No. (%)a | 81 | 46 | |||
| Hepatitis C | 36 (44.4) | 21 (45.7) | .90 | ||
| Alcoholic cirrhosis | 32 (39.5) | 11 (23.9) | .07 | ||
| NAFLD | 14 (17.3) | 6 (13.0) | .53 | ||
| Autoimmune hepatitis | 0 (0.0) | 6 (13.0) | .002 | ||
| Primary biliary cirrhosis | 2 (2.5) | 2 (4.3) | .62 | ||
| Primary sclerosing cholangitis | 5 (6.2) | 3 (6.5) | > .99 | ||
| Cryptogenic cirrhosis | 10 (12.3) | 0 (0.0) | .01 | ||
| Liver disease severity, median (IQR) | |||||
| MELD score | 80 | 12.2 (9.4-15.5) | 45 | 14.5 (11.6-15.8) | .06 |
| Child-Pugh score | 81 | 5 (4-7) | 46 | 6 (5-7) | .04 |
| Complications of liver disease, No. (%) | 81 | 46 | |||
| Ascites | 48 (59.3) | 30 (65.2) | .51 | ||
| Encephalopathy | 39 (48.1) | 24 (52.2) | .66 | ||
| Esophageal varices | 50 (61.7) | 32 (69.6) | .37 | ||
| Hepatic hydrothorax | 8 (9.9) | 4 (8.7) | > .99 | ||
| Prior TIPS, No. (%) | 81 | 5 (6.2) | 46 | 2 (4.3) | > .99 |
| Comorbidities, No. (%) | 81 | 46 | |||
| Hepatocellular carcinoma | 35 (43.2) | 10 (21.7) | .02 | ||
| Hypertension | 46 (56.8) | 19 (41.3) | .09 | ||
| Hyperlipidemia | 17 (21.0) | 6 (13.0) | .26 | ||
| Congestive heart failure | 2 (2.5) | 3 (6.5) | .35 | ||
| Diabetes mellitus | 33 (40.7) | 16 (34.8) | .51 | ||
| Hypothyroidism | 12 (14.8) | 6 (13.0) | .78 | ||
| OSA | 15 (18.5) | 4 (8.7) | .20 | ||
| Venous thromboembolism | 4 (4.9) | 0 (0.0) | .30 | ||
| Interstitial lung disease | 3 (3.7) | 0 (0.0) | .55 | ||
| Asthma | 2 (2.5) | 2 (4.3) | .62 | ||
| Smoking history, No. (%) | |||||
| Current or former smoker | 81 | 48 (59.3) | 46 | 23 (50.0) | .31 |
| Current smoker (last 30 d) | 81 | 11 (13.6) | 46 | 2 (4.3) | .10 |
Data are presented as mean (SD) for normally distributed continuous variables, median (IQR) for nonnormally distributed continuous variables, or No. (%). IPVD = intrapulmonary vascular dilatation; IQR = interquartile range; MELD = Model for End-Stage Liver Disease; NAFLD = nonalcoholic fatty liver disease; TIPS = transjugular intrahepatic portosystemic shunt.
Patients may have had more than one cause of liver disease.
Table 2.
Symptoms, Physical Examination Results, Laboratory Test Results, and Other Test Results
| Characteristic | No. of Patients | No IPVD | No. of Patients | IPVD | P Value |
|---|---|---|---|---|---|
| Symptoms | |||||
| Dyspnea, No. (%) | 81 | 15 (18.5) | 46 | 11 (23.9) | .47 |
| Borg score baseline, median (IQR) | 69 | 0 (0-0.5) | 41 | 0 (0-0.5) | .64 |
| Borg score after exercise, median (IQR) | 69 | 2 (0.5-3.0) | 41 | 2 (0.5-3) | .99 |
| Platypnea, No. (%) | 81 | 0 (0.0) | 46 | 2 (4.3) | .13 |
| Orthopnea, No. (%) | 81 | 2 (2.5) | 46 | 2 (4.3) | .62 |
| Edema, No. (%) | 81 | 33 (40.7) | 46 | 24 (52.2) | .21 |
| Palpitations, No. (%) | 81 | 5 (6.2) | 46 | 1 (2.2) | .42 |
| Syncope, No. (%) | 81 | 2 (2.5) | 46 | 0 (0.0) | .53 |
| Angina, No. (%) | 81 | 4 (4.9) | 46 | 2 (4.3) | > .99 |
| WHO functional class, No. (%) | 81 | 46 | .84 | ||
| 1 | 34 (42.0) | 19 (41.3) | |||
| 2 | 36 (44.4) | 19 (41.3) | |||
| 3 | 11 (13.6) | 8 (17.4) | |||
| Physical examination results | |||||
| Weight, median (IQR), kg | 81 | 86.1 (75.0-101.0) | 46 | 85.1 (73.2-99.1) | .54 |
| Height, median (IQR), cm | 81 | 173.0 (165.1-178.2) | 46 | 170.5 (165.0-178.8) | .40 |
| BMI, median (IQR), kg/m2 | 81 | 28.7 (25.4-35.6) | 46 | 28.7 (25.0-33.3) | .85 |
| Body surface area, median (IQR), m2 | 81 | 2.0 (1.9-2.2) | 46 | 2.0 (1.8-2.2) | .49 |
| Systolic BP, mean (SD), mm Hg | 81 | 122.6 (17.6) | 46 | 122.5 (17.3) | .99 |
| Diastolic BP, mean (SD), mm Hg | 81 | 70.3 (12.0) | 46 | 69.4 (10.0) | .65 |
| Respiratory rate, median (IQR) | 81 | 18 (13-18) | 45 | 18 (14-18) | .78 |
| Heart rate, mean (SD) | 79 | 66.0 (14.1) | 46 | 66.3 (10.6) | .88 |
| Seated oxygen saturation, median (IQR), % | 81 | 98 (97-99) | 46 | 99 (97-99) | .20 |
| Supine oxygen saturation, median (IQR), % | 81 | 98 (97-99) | 45 | 99 (97-100) | .32 |
| Clubbing, No. (%) | 81 | 4 (4.9) | 46 | 1 (2.2) | .65 |
| Spider angioma, No. (%) | 80 | 22 (27.5) | 46 | 13 (28.3) | .93 |
| Asterixis, No. (%) | 81 | 3 (3.7) | 46 | 2 (4.3) | > .99 |
| Jaundice, No. (%) | 81 | 16 (19.8) | 46 | 14 (30.4) | .17 |
| Laboratory test results, median (IQR) | |||||
| Sodium, mEq/L | 81 | 139 (135-140) | 46 | 140 (137-141) | .25 |
| BUN | 75 | 15 (12-20) | 43 | 16 (10-22) | .97 |
| Creatinine, mg/dL | 81 | 1.0 (1.0-1.2) | 46 | 1.0 (1.0-1.1) | .04 |
| Glucose | 81 | 103 (87-138) | 46 | 100(85-132) | .79 |
| Platelets, 1,000/μL | 80 | 93 (64-130) | 46 | 89 (59-135) | .51 |
| Hemoglobin | 81 | 12.3 (11.1-13.6) | 46 | 12.4 (10.4-13.5) | .66 |
| INR | 80 | 1.2 (1.1-1.4) | 45 | 1.4 (1.2-1.5) | .008 |
| Albumin, g/dL | 80 | 3.4 (2.8-3.8) | 46 | 3.1 (2.7-3.4) | .07 |
| Total bilirubin, mg/dL | 80 | 1.2 (1.0-2.4) | 46 | 2.0 (1.5-3.9) | .004 |
| Direct bilirubin, mg/dL | 79 | 0.5 (0.2-0.9) | 46 | 0.8 (0.4-1.4) | .02 |
| Alanine aminotransferase | 80 | 49 (27-73) | 46 | 51 (27-74) | .82 |
| Aspartate aminotransferase | 80 | 54 (36-87) | 46 | 67 (39-96) | .43 |
| Alkaline phosphatase | 80 | 147 (109-203) | 46 | 147 (115-183) | .86 |
| Arterial blood gas | 81 | 46 | |||
| pH, mean (SD) | 7.44 (0.04) | 7.44 (0.04) | .66 | ||
| PaCO2, mean (SD), mm Hg | 36.0 (4.6) | 34.2 (4.8) | .047 | ||
| PaO2, median (IQR), mm Hg | 89.0 (82.0-96.9) | 97.9 (92.0-103.0) | < .001 | ||
| A-a gradient, median (IQR), mm Hg | 14.9 (9.0-21.8) | 9.9 (6.2-13.5) | < .001 | ||
| Spirometric results, mean (SD) | 81 | 46 | |||
| FVC, L | 3.80 (0.92) | 3.70 (0.86) | .52 | ||
| FVC, % predicted | 89.2 (12.0) | 89.3 (12.9) | .98 | ||
| FEV1, L | 2.95 (0.63) | 2.91 (0.63) | .74 | ||
| FEV1, % predicted | 90.7 (11.2) | 91.0 (11.7) | .90 | ||
| FEV1/FVC | 0.78 (0.06) | 0.79 (0.06) | .32 | ||
| FEV1/FVC, % predicted | 78.4 (5.6) | 79.5 (6.4) | .32 | ||
| Echocardiographic results | |||||
| Left atrial volume, mean (SD), mL | 69 | 71.0 (19.4) | 41 | 77.9 (23.4) | .09 |
| LV end systolic volume, median (IQR), mL | 54 | 61.5 (53.0-80.0) | 35 | 62.0 (46.0-82.0) | .73 |
| LV end diastolic volume, median (IQR), mL | 54 | 162.5 (135.0-204.0) | 35 | 160.0 (129.0-196.0) | .92 |
| RV systolic area, median (IQR), cm2 | 31 | 10.9 (9.1-13.6) | 21 | 11.9 (7.5-13.0) | .72 |
| RV diastolic area, median (IQR), cm2 | 36 | 21.7 (16.5-26.5) | 26 | 21.0 (14.6-24.7) | .34 |
| Stroke volume, mean (SD), mL | 74 | 87.7 (26.6) | 43 | 91.8 (22.5) | .39 |
| Stroke volume index, mean (SD), mL/m2 | 74 | 43.7 (11.7) | 43 | 46.1 (10.5) | .27 |
| Cardiac output, median (IQR), L/min | 74 | 5.5 (4.5-6.5) | 43 | 6.2 (4.5-6.9) | .20 |
| Cardiac index, mean (SD), L/min/m2 | 74 | 2.8 (0.7) | 43 | 3.0 (0.7) | .15 |
| Ejection fraction, mean (SD), % | 81 | 62.3 (6.2) | 46 | 63.4 (6.7) | .39 |
| Right atrial pressure, median (IQR), mm Hg | 81 | 5 (5-10) | 46 | 5 (5-10) | .90 |
| RVSP, median (IQR), mm Hg | 50 | 28 (24-34) | 34 | 30 (24-37) | .38 |
| TAPSE, median (IQR), mm | 59 | 25 (21-29) | 39 | 26 (24-32) | .10 |
| Visual RV function, No. (%) | 81 | 46 | .30 | ||
| Normal | 76 (93.8) | 46 (100.0) | |||
| Mild dysfunction | 4 (4.9) | 0 (0.0) | |||
| Indeterminate | 1 (1.2) | 0 (0.0) | |||
| Exercise capacity | 69 | 41 | |||
| 6-min walk distance, mean (SD), m | 432.4 (88.5) | 414.7 (109.9) | .36 | ||
| Quality of life (SF-36) | 80 | 46 | |||
| Physical component summary, mean (SD) | 37.3 (10.3) | 34.8 (11.3) | .21 | ||
| Physical role, median (IQR) | 50 (25-75) | 44 (25-75) | .37 | ||
| Physical functioning, median (IQR) | 65 (38-85) | 50 (35-75) | .30 | ||
| Bodily pain, median (IQR) | 52 (37-74) | 51 (41-74) | .89 | ||
| General health perception, median (IQR) | 40 (25-67) | 36 (22-52) | .19 | ||
| Mental component summary, median (IQR) | 48.8 (39.6-55.0) | 49.7 (40.3-56.6) | .63 | ||
| Emotional role, median (IQR) | 67 (42-100) | 67 (50-100) | .39 | ||
| Social functioning, median (IQR) | 63 (38-94) | 63 (50-88) | .98 | ||
| Mental health, median (IQR) | 75 (58-90) | 75 (60-85) | .93 | ||
| Vitality, median (IQR) | 44 (25-66) | 38 (25-56) | .56 |
Data are presented as mean (SD) for normally distributed continuous variables, median (IQR) for nonnormally distributed continuous variables, or No. (%). Percentages may not sum to 100% because of rounding. A-a = alveolar-arterial; INR = international normalized ratio; LV = left ventricular; RV = right ventricular; RVSP = right ventricular systolic pressure; SF-36 = 36-Item Short Form Survey Instrument; TAPSE = tricuspid annular plane systolic excursion; WHO = World Health Organization. See Table 1 legend for expansion of other abbreviations.
There was no significant difference between the groups in symptoms or physical examination characteristics. Patients with IPVD had a higher INR and serum bilirubin level and lower creatinine level. Counterintuitively, patients with IPVD had a higher PaO2 level, a lower A-a gradient, and a lower PaCO2 level, despite no difference in spirometric test results (Table 2). The differences in PaO2 level, A-a gradient, and PaCO2 level persisted when patients with a pleural effusion at chest imaging (five patients with IPVD and nine patients without IPVD) were excluded from the analysis. Among patients with IPVD, there was no difference in oxygenation between patients with mild IPVD (n = 39) vs those with moderate to severe IPVD (n = 7) (Table 3). Patients with and those without IPVD had similar echocardiographic parameters, 6-minute walk distance, and quality of life (Table 2).
Table 3.
Gas Exchange in Patients With Mild IPVD vs Gas Exchange in Patients With Moderate to Severe IPVD
| Parameter | Mild IPVD (n = 39) | Moderate to Severe IPVD (n = 7) | P Value |
|---|---|---|---|
| pH | 7.44 (7.42-7.46) | 7.44 (7.40-7.47) | .76 |
| PaCO2, mm Hg | 34.0 (31.6-36.0) | 34.0 (30.0-37.0) | .99 |
| PaO2, mm Hg | 97.7 (92.0-103.0) | 99.0 (87.4-105.0) | .99 |
| A-a gradient, mm Hg | 10.0 (5.5-13.5) | 9.7 (9.0-14.6) | .90 |
Data are presented as median (IQR). See Table 2 legend for expansion of abbreviations.
We performed a subset analysis after excluding patients with an elevated A-a gradient in the group without IPVD (n = 38). The associations between autoimmune hepatitis, HCC, and liver disease severity were similar. Patients with IPVD had significantly lower PaCO2 levels, and there still appeared to be a higher PaO2 level, which was no longer statistically significant (with a smaller sample size). There was possibly increased dyspnea in the IPVD group (23.9% vs 9.3%; P = .09), and patients with IPVD in the subset analysis had a significantly higher cardiac output, cardiac index, left ventricular stroke volume, and left atrial volume (Tables 4, 5).
Table 4.
Demographic Characteristics, Comorbidities, and Liver Disease Characteristics of Patients With and Those Without IPVD, Excluding Patients With an Elevated A-a Gradient From the Group Without IPVD
| Characteristic | No. of Patients | No IPVD | No. of Patients | IPVD | P Value |
|---|---|---|---|---|---|
| Age, median (IQR), y | 43 | 57 (52-64) | 46 | 57 (48-63) | .58 |
| Male, No. (%) | 43 | 35 (81.4) | 46 | 30 (65.2) | .09 |
| Race/ethnicity, No. (%) | 43 | 46 | .40 | ||
| Non-Hispanic white | 25 (58.1) | 29 (63.0) | |||
| Hispanic | 9 (20.9) | 9 (19.6) | |||
| Black | 7 (16.3) | 3 (6.5) | |||
| Other | 2 (4.7) | 5 (10.9) | |||
| Liver disease cause, No. (%)a | 43 | 46 | |||
| Hepatitis C | 21 (48.8) | 21 (45.7) | .76 | ||
| Alcoholic cirrhosis | 17 (39.5) | 11 (23.9) | .11 | ||
| NAFLD | 5 (11.6) | 6 (13.0) | .84 | ||
| Autoimmune hepatitis | 0 (0.0) | 6 (13.0) | .03 | ||
| Primary biliary cirrhosis | 1 (2.3) | 2 (4.3) | > .99 | ||
| Primary sclerosing cholangitis | 4 (9.3) | 3 (6.5) | .71 | ||
| Cryptogenic cirrhosis | 4 (9.3) | 0 (0.0) | .05 | ||
| Liver disease severity, median (IQR) | |||||
| MELD score | 43 | 12.1 (9.4-15.0) | 45 | 14.5 (11.6-15.8) | .06 |
| Child-Pugh score | 43 | 5 (3-7) | 46 | 6 (5-7) | .03 |
| Complications of liver disease, No. (%) | 43 | 46 | |||
| Ascites | 25 (58.1) | 30 (65.2) | .49 | ||
| Encephalopathy | 21 (48.8) | 24 (52.2) | .75 | ||
| Esophageal varices | 22 (51.2) | 32 (69.6) | .08 | ||
| Hepatic hydrothorax | 4 (9.3) | 4 (8.7) | > .99 | ||
| Prior TIPS, No. (%) | 43 | 4 (9.3) | 46 | 2 (4.3) | .42 |
| Comorbidities, No. (%) | 43 | 46 | |||
| Hepatocellular carcinoma | 21 (48.8) | 10 (21.7) | .007 | ||
| Hypertension | 22 (51.2) | 19 (41.3) | .35 | ||
| Hyperlipidemia | 7 (16.3) | 6 (13.0) | .67 | ||
| Congestive heart failure | 0 (0.0) | 3 (6.5) | .24 | ||
| Diabetes mellitus | 13 (30.2) | 16 (34.8) | .65 | ||
| Hypothyroidism | 8 (18.6) | 6 (13.0) | .47 | ||
| OSA | 7 (16.3) | 4 (8.7) | .34 | ||
| Venous thromboembolism | 1 (2.3) | 0 (0.0) | .48 | ||
| Interstitial lung disease | 1 (2.3) | 0 (0.0) | .48 | ||
| Asthma | 1 (2.3) | 2 (4.3) | > .99 | ||
| Smoking history, No. (%) | |||||
| Current or former smoker | 43 | 26 (60.5) | 46 | 23 (50.0) | .32 |
| Current smoker (last 30 d) | 43 | 6 (14.0) | 46 | 2 (4.3) | .11 |
Data are presented as mean ± SD for normally distributed continuous variables, median (IQR) for nonnormally distributed continuous variables, or No. (%). See Table 1 legend for expansion of abbreviations.
Patients may have had more than one cause of liver disease.
Table 5.
Symptoms, Physical Examination Results, Laboratory Test Results, and Other Test Results in Patients With and Those Without IPVD, Excluding Patients With an Elevated A-a Gradient From the Group Without IPVD
| Characteristic | No. of Patients | No IPVD | No. of Patients | IPVD | P Value |
|---|---|---|---|---|---|
| Symptoms | |||||
| Dyspnea, No. (%) | 43 | 4 (9.3) | 46 | 11 (23.9) | .09 |
| Borg score baseline, median (IQR) | 36 | 0 (0-1) | 41 | 0 (0-0.5) | .78 |
| Borg score after exercise, median (IQR) | 36 | 1.5 (0.5-3) | 41 | 2 (0.5-3) | .79 |
| Platypnea, No. (%) | 43 | 0 (0.0) | 46 | 2 (4.3) | .49 |
| Orthopnea, No. (%) | 43 | 0 (0.0) | 46 | 2 (4.3) | .49 |
| Edema, No. (%) | 43 | 18 (41.9) | 46 | 24 (52.2) | .33 |
| Palpitations, No. (%) | 43 | 2 (4.7) | 46 | 1 (2.2) | .61 |
| Syncope, No. (%) | 43 | 2 (4.7) | 46 | 0 (0.0) | .23 |
| Angina, No. (%) | 43 | 1 (2.3) | 46 | 2 (4.3) | > .99 |
| WHO functional class, No. (%) | 43 | 46 | .67 | ||
| 1 | 21 (48.8) | 19 (41.3) | |||
| 2 | 17 (39.5) | 19 (41.3) | |||
| 3 | 5 (11.6) | 8 (17.4) | |||
| Physical examination results | |||||
| Weight, median (IQR), kg | 43 | 82.1 (72.1-96.0) | 46 | 85.1 (73.2-99.1) | .70 |
| Height, median (IQR), cm | 43 | 174.4 (165.1-178.2) | 46 | 170.5 (165.0-178.8) | .45 |
| BMI, median (IQR), kg/m2 | 43 | 26.9 (24.8-33.1) | 46 | 28.7 (25.0-33.3) | .47 |
| Body surface area, median (IQR), m2 | 43 | 1.9 (1.8-2.1) | 46 | 2.0 (1.8-2.2) | > .99 |
| Systolic BP, mean (SD), mm Hg | 43 | 124.1 (16.9) | 46 | 122.5 (17.3) | .66 |
| Diastolic BP, mean (SD), mm Hg | 43 | 72.7 (11.9) | 46 | 69.4 (10.0) | .16 |
| Respiratory rate median (IQR), | 43 | 18 (13-18) | 45 | 18 (14-18) | .92 |
| Heart rate, mean (SD) | 42 | 63.1 (12.7) | 46 | 66.3 (10.6) | .21 |
| Seated oxygen saturation, median (IQR), % | 43 | 98 (97-99) | 46 | 99 (97-99) | .94 |
| Supine oxygen saturation, median (IQR), % | 43 | 99 (98-100) | 45 | 99 (97-100) | .62 |
| Clubbing, No. (%) | 43 | 2 (4.7) | 46 | 1 (2.2) | .61 |
| Spider angioma, No. (%) | 42 | 10 (23.8) | 46 | 13 (28.3) | .64 |
| Asterixis, No. (%) | 43 | 2 (4.7) | 46 | 2 (4.3) | > .99 |
| Jaundice, No. (%) | 43 | 8 (18.6) | 46 | 14 (30.4) | .20 |
| Laboratory test results, median (IQR) | |||||
| Sodium, mEq/L | 43 | 139 (136-141) | 46 | 140 (137-141) | .43 |
| BUN | 42 | 15 (12-21) | 43 | 16 (10-22) | .79 |
| Creatinine, mg/dL | 43 | 1.1 (1.0-1.3) | 46 | 1.0 (1.0-1.1) | .02 |
| Glucose | 43 | 100 (81-131) | 46 | 100 (85-132) | .48 |
| Platelets, 1,000/μL | 43 | 96 (64-164) | 46 | 89 (59-135) | .18 |
| Hemoglobin | 43 | 12.5 (11.1-13.6) | 46 | 12.4 (10.4-13.5) | .62 |
| INR | 43 | 1.2 (1.1-1.4) | 45 | 1.4 (1.2-1.5) | .01 |
| Albumin, g/dL | 43 | 3.5 (2.8-4.0) | 46 | 3.1 (2.7-3.4) | .07 |
| Total bilirubin, mg/dL | 43 | 1.1 (1.0-2.1) | 46 | 2.0 (1.5-3.9) | .008 |
| Direct bilirubin, mg/dL | 42 | 0.5 (0.2-0.9) | 46 | 0.8 (0.4-1.4) | .06 |
| Alanine aminotransferase | 43 | 46 (27-76) | 46 | 51 (27-74) | .83 |
| Aspartate aminotransferase | 43 | 54 (35-84) | 46 | 67 (39-96) | .39 |
| Alkaline phosphatase | 43 | 150 (106-234) | 46 | 147 (115-183) | .61 |
| Arterial blood gas | 43 | 46 | |||
| pH, mean (SD) | 7.42 (0.04) | 7.44 (0.04) | .10 | ||
| PaCO2, mean (SD), mm Hg | 36.6 (4.3) | 34.2 (4.8) | .02 | ||
| PaO2, median (IQR), mm Hg | 94 (90-101) | 98 (92-103) | .10 | ||
| A-a gradient, median (IQR), mm Hg | 9.4 (4.6-12.5) | 9.9 (6.2-13.5) | .70 | ||
| Spirometric results, mean (SD) | 43 | 46 | |||
| FVC, L | 3.83 (0.92) | 3.70 (0.86) | .49 | ||
| FVC, % predicted | 89.9 (13.8) | 89.3 (12.9) | .82 | ||
| FEV1, L | 2.96 (0.59) | 2.91 (0.63) | .73 | ||
| FEV1, % predicted | 91.0 (11.1) | 91.0 (11.7) | .99 | ||
| FEV1/FVC | 0.78 (0.06) | 0.79 (0.06) | .37 | ||
| FEV1/FVC, % predicted | 78.3 (6.1) | 79.5 (6.4) | .38 | ||
| Echocardiographic results | |||||
| Left atrial volume, median (IQR), mL | 38 | 64 (54-79) | 41 | 71 (62-92) | .04 |
| LV end systolic volume, median (IQR), mL | 31 | 61.0 (55.0-84.0) | 35 | 62.0 (46.0-82.0) | .59 |
| LV end diastolic volume, median (IQR), mL | 31 | 156.0 (135.0-209.0) | 35 | 160.0 (129.0-196.0) | .72 |
| RV systolic area, median (IQR), cm2 | 16 | 11.1 (10.0-16.1) | 21 | 11.9 (7.5-13.0) | .30 |
| RV diastolic area, median (IQR), cm2 | 20 | 21.8 (18.4-27.0) | 26 | 21.0 (14.6-24.7) | .40 |
| Stroke volume, mean (SD), mL | 40 | 81.7 (20.6) | 43 | 91.8 (22.5) | .04 |
| Stroke volume index, median (IQR), mL/m2 | 40 | 42.4 (36.1-47.9) | 43 | 42.7 (39.0-55.0) | .08 |
| Cardiac output, median (IQR), L/min | 40 | 5.2 (3.8-5.9) | 43 | 6.2 (4.5-6.9) | .004 |
| Cardiac index, mean (SD), L/min/m2 | 40 | 2.6 (2.1-2.9) | 43 | 3.0 (2.4-3.6) | .004 |
| Ejection fraction, mean (SD) % | 43 | 61.5 (7.2) | 46 | 63.4 (6.7) | .21 |
| Right atrial pressure, median (IQR), mm Hg | 43 | 5 (5-10) | 46 | 5 (5-10) | .85 |
| RVSP, median (IQR), mm Hg | 26 | 27 (24-34) | 34 | 30 (24-37) | .38 |
| TAPSE, median (IQR), mm | 37 | 25 (22-29) | 39 | 26 (24-32) | .15 |
| Visual RV function, No. (%) | 43 | 46 | .11 | ||
| Normal | 40 (93.0) | 46 (100.0) | |||
| Mild dysfunction | 3 (7.0) | 0 (0.0) | |||
| Exercise capacity | 36 | 41 | |||
| 6-min walk distance, mean (SD), m | 441.1 (80.2) | 414.7 (109.9) | .24 | ||
| Quality of life (SF-36), median (IQR) | 43 | 46 | |||
| Physical component summary | 38.6 (30.9-45.9) | 32.7 (26.8-40.8) | .11 | ||
| Physical functioning | 65 (30-85) | 50 (35-75) | .25 | ||
| Physical role | 56 (38-75) | 44 (25-75) | .14 | ||
| Bodily pain | 51 (41-72) | 51 (41-74) | .93 | ||
| General health | 52 (25-77) | 36 (22-52) | .10 | ||
| Mental component summary | 49.4 (41.6-54.7) | 49.7 (40.3-56.6) | .93 | ||
| Emotional role | 67 (50-92) | 67 (50-100) | .45 | ||
| Social functioning | 63 (38-100) | 63 (50-88) | .76 | ||
| Mental health | 75 (60-90) | 75 (60-85) | .54 | ||
| Vitality | 50 (31-69) | 38 (25-56) | .09 |
Data are presented as mean (SD) for normally distributed continuous variables, median (IQR) for nonnormally distributed continuous variables, or No. (%). Percentages may not sum to 100% because of rounding. See Table 2 legend for expansion of abbreviations.
Discussion
In this study, we found that IPVD in the setting of normal gas exchange was common in patients being evaluated for LT and was associated with autoimmune hepatitis and laboratory markers of increased liver disease severity but was not associated with symptoms, exercise capacity, or quality of life. We also found that IPVD paradoxically was associated with a higher PaO2 level, lower A-a gradient, and lower PaCO2 level compared with those in the control subjects with liver disease without IPVD. To our knowledge, this is the first study to describe the manifestations of isolated IPVD in advanced liver disease in a prospective study and to demonstrate that IPVD are associated with specific patient factors. When we focused only on patients with normal gas exchange, we also found that patients with IPVD had a higher cardiac output, cardiac index, and left ventricular stroke volume, suggestive of a more hyperdynamic state, and may have had more dyspnea.
Patients with IPVD had increased liver disease severity as assessed by the Child-Pugh score and possibly the MELD score, with higher bilirubin and INR levels. This finding differs from those of a prior study that described similar MELD scores in patients with and those without IPVD.18 However, this prior retrospective study included patients with HPS in the IPVD group and was limited to patients who underwent contrast-enhanced echocardiography for clinical suspicion of HPS.18 In contrast, our study performed standardized echocardiography with blinded interpretation by a research core laboratory, thereby minimizing selection bias and measurement error.
Dyspnea is common in end-stage liver disease and can be multifactorial, related to cardiac or respiratory disease, ascites, or other causes.19 We sought to determine whether IPVD alone were associated with clinical signs or symptoms, such as dyspnea. We excluded patients with obstructive or restrictive ventilatory defects and PPHTN to minimize the impact of concomitant lung disease. We did not find an association between IPVD and symptoms in our main analysis, but our results may have been biased toward the null by including patients with gas exchange abnormalities in the control group but not in the IPVD group. In our subset analysis, there was a trend toward increased dyspnea in patients with IPVD, but this did not reach statistical significance. Patients with IPVD also had a higher cardiac output and stroke volume, suggesting that IPVD may be associated with a hyperdynamic state.
We were surprised to find that patients with IPVD had a higher PaO2 level and lower A-a gradient than did patients without IPVD. We were concerned that these differences were due to the nature of our exclusion criteria (ie, only excluding patients with an abnormal A-a gradient from the IPVD group because they met criteria for HPS), so we performed a subset analysis as described. Abnormal gas exchange was surprisingly common (46.9%; 38 of 81) in patients without IPVD, restrictive or obstructive lung disease, or PPHTN. The cause of abnormal gas exchange in these patients was not evident but could be related to atelectasis or pleural effusions. In our subset analysis, patients with IPVD still had a somewhat higher PaO2 level and a significantly lower PaCO2 level. The reasons for this are not clear, but differences in gas exchange may be related to increased hyperventilation in the IPVD group given the lower PaCO2 level. Alternatively, the higher PaO2 level in patients with IPVD may be related to the higher cardiac output observed in these patients. Assuming there was a stable arterial-venous oxygen difference, a higher cardiac output could lead to an increase in mixed venous oxygen saturation and PaO2 level.20
Mechanisms for the pathogenesis of IPVD in liver disease are not known. Given the possible association with hyperventilation and a hyperdynamic state, potential mechanisms include increasing levels of circulating vasodilatory factors, such as nitric oxide,21 a greater pro-angiogenic milieu in the setting of advancing liver disease, or higher circulating levels of progesterone and sex hormones. Previous studies have described an association between higher progesterone and estradiol levels and hyperventilation, as well as pulmonary vasodilatation, in patients with cirrhosis,22, 23 thus supporting the hypothesis that sex hormones may play a role in IPVD pathogenesis. Given the higher prevalence of autoimmune hepatitis in patients with IPVD, it is also possible that immunologic pathways play an important role. Clinical studies and experimental models suggest that nitric oxide, endothelin-1, intravascular monocyte accumulation, altered estrogen signaling, and increased vascular endothelial growth factor-mediated angiogenesis are involved in the pathogenesis of HPS, but it is not known whether these same pathways play a role in the pathogenesis of IPVD.9, 24, 25
We identified several important distinctions between IPVD and HPS. In contrast to our findings in patients with IPVD, HPS has not been associated with autoimmune hepatitis or higher bilirubin or INR levels.4 HCC also was described recently as an independent risk factor for HPS, but we found that patients with IPVD were less likely to have HCC.26 Our data suggest that IPVD may be a distinct entity from HPS, especially because gas exchange was better in patients with IPVD than in control subjects. Development of HPS in patients with IPVD may require a “second hit”, such as impairment in ventilation-perfusion matching,27 for the development of abnormal arterial oxygenation.
Our study had several limitations. First, a large proportion of the study sample had obstructive or restrictive lung disease, HPS, intracardiac shunting, or abnormal gas exchange, reflecting their high prevalence in this population of patients with advanced liver disease. We excluded these patients to prevent misclassification of our phenotypes of interest, but residual confounding was still possible. Our study also did not define the mechanism of IPVD pathogenesis in patients with liver disease, but future studies of genetic or plasma biomarker predictors of IPVD should be performed. Lastly, we did not have duplicate testing or longitudinal assessments to determine the clinical course of IPVD across time or the impact of IPVD on outcomes, such as mortality.
Conclusions
In summary, IPVD in patients with liver disease was associated with autoimmune hepatitis, laboratory markers of increased liver disease severity, a higher PaO2 level, and a reduced PaCO2 level. Our study results suggest that IPVD in the absence of abnormal arterial oxygenation are a distinct entity that warrants further study either as a precursor to HPS or as its own disease phenotype. Future studies to characterize the mechanism of IPVD in patients with liver disease and to determine whether IPVD in the absence of gas exchange abnormalities portend the emergence of HPS are warranted.
Acknowledgments
Author contributions: H. M. D. is the guarantor of the content of this manuscript. H. M. D. and M. S. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. H. M. D. and S. M. K. contributed to the drafting of the manuscript. The remainder of the authors contributed substantially to the study concept and design, data acquisition and interpretation, and critical revision of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: J. K. O. is the director of the Echocardiography Core Laboratory. None declared (H. M. D., M. J. K., K. A. F., K. K., M. P., T. S., M. S., G. L., C. D. M., P. D. S., M. B. F., S. M. K.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
FUNDING/SUPPORT: This study was funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health [Grants R01HL113988 and K24 HL103844].
References
- 1.Abrams G.A., Jaffe C.C., Hoffer P.B., Binder H.J., Fallon M.B. Diagnostic utility of contrast echocardiography and lung perfusion scan in patients with hepatopulmonary syndrome. Gastroenterology. 1995;109(4):1283–1288. doi: 10.1016/0016-5085(95)90589-8. [DOI] [PubMed] [Google Scholar]
- 2.Krowka M.J., Tajik A.J., Dickson E.R., Wiesner R.H., Cortese D.A. Intrapulmonary vascular dilatations (IPVD) in liver transplant candidates: screening by two-dimensional contrast-enhanced echocardiography. Chest. 1990;97(5):1165–1170. doi: 10.1378/chest.97.5.1165. [DOI] [PubMed] [Google Scholar]
- 3.Hopkins W.E., Waggoner A.D., Barzilai B. Frequency and significance of intrapulmonary right-to-left shunting in end-stage hepatic disease. Am J Cardiol. 1992;70(4):516–519. doi: 10.1016/0002-9149(92)91200-n. [DOI] [PubMed] [Google Scholar]
- 4.Fallon M.B., Krowka M.J., Brown R.S. Impact of hepatopulmonary syndrome on quality of life and survival in liver transplant candidates. Gastroenterology. 2008;135(4):1168–1175. doi: 10.1053/j.gastro.2008.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anand A.C., Mukherjee D., Rao K.S., Seth A.K. Hepatopulmonary syndrome: prevalence and clinical profile. Indian J Gastroenterol. 2001;20(1):24–27. [PubMed] [Google Scholar]
- 6.Santa-Cruz R.A., Pearson M.D., Cohen M.G. Clinical predictors and characteristics of patients with chronic liver disease and intrapulmonary shunts. Clin Cardiol. 2005;28(9):437–441. doi: 10.1002/clc.4960280910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lenci I., Alvior A., Manzia T.M., Toti L., Neuberger J., Steeds R. Saline contrast echocardiography in patients with hepatopulmonary syndrome awaiting liver transplantation. J Am Soc Echocardiogr. 2009;22(1):89–94. doi: 10.1016/j.echo.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 8.Rodríguez-Roisin R., Krowka M.J., Hervé P., Fallon M.B., and the ERS Task Force Pulmonary-Hepatic Vascular Disorders (PHD) Scientific Committee Pulmonary-hepatic vascular disorders (PHD) Eur Respir J. 2004;24(5):861–880. doi: 10.1183/09031936.04.00010904. [DOI] [PubMed] [Google Scholar]
- 9.Krowka M.J., Fallon M.B., Kawut S.M. International Liver Transplant Society practice guidelines: diagnosis and management of hepatopulmonary syndrome and portopulmonary hypertension. Transplantation. 2016;100(7):1440–1452. doi: 10.1097/TP.0000000000001229. [DOI] [PubMed] [Google Scholar]
- 10.Fussner L.A., Iyer V.N., Cartin-Ceba R., Lin G., Watt K.D., Krowka M.J. Intrapulmonary vascular dilatations are common in portopulmonary hypertension and may be associated with decreased survival. Liver Transpl. 2015;21(11):1355–1364. doi: 10.1002/lt.24198. [DOI] [PubMed] [Google Scholar]
- 11.Du Bois D., Du Bois E.F. A formula to estimate the approximate surface area if height and weight be known: 1916. Nutrition. 1989;5(5):303–311. [PubMed] [Google Scholar]
- 12.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 13.Borg G.A. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. [PubMed] [Google Scholar]
- 14.Mahler D.A., Rosiello R.A., Harver A., Lentine T., McGovern J.F., Daubenspeck J.A. Comparison of clinical dyspnea ratings and psychophysical measurements of respiratory sensation in obstructive airway disease. Am Rev Respir Dis. 1987;135(6):1229–1233. doi: 10.1164/arrd.1987.135.6.1229. [DOI] [PubMed] [Google Scholar]
- 15.Crapo R.O., Jensen R.L., Hegewald M., Tashkin D.P. Arterial blood gas reference values for sea level and an altitude of 1,400 meters. Am J Respir Crit Care Med. 1999;160(5 pt 1):1525–1531. doi: 10.1164/ajrccm.160.5.9806006. [DOI] [PubMed] [Google Scholar]
- 16.Hankinson J.L., Bang K.M. Acceptability and reproducibility criteria of the American Thoracic Society as observed in a sample of the general population. Am Rev Respir Dis. 1991;143(3):516–521. doi: 10.1164/ajrccm/143.3.516. [DOI] [PubMed] [Google Scholar]
- 17.Hankinson J.L., Odencrantz J.R., Fedan K.B. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal P.D., Hughes P.J., Runo J.R., Ibrisim D., Lucey M.R., Said A. The clinical significance of intrapulmonary vascular dilations in liver transplant candidates. Clin Transplant. 2013;27(1):148–153. doi: 10.1111/ctr.12033. [DOI] [PubMed] [Google Scholar]
- 19.Kaltsakas G., Antoniou E., Palamidas A.F. Dyspnea and respiratory muscle strength in end-stage liver disease. World J Hepatol. 2013;5(2):56–63. doi: 10.4254/wjh.v5.i2.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez-Roisin R. Nonpulmonary influences on gas exchange. Compr Physiol. 2014;4(4):1455–1494. doi: 10.1002/cphy.c100001. [DOI] [PubMed] [Google Scholar]
- 21.Martin P.Y., Gines P., Schrier R.W. Nitric oxide as a mediator of hemodynamic abnormalities and sodium and water retention in cirrhosis. N Engl J Med. 1998;339(8):533–541. doi: 10.1056/NEJM199808203390807. [DOI] [PubMed] [Google Scholar]
- 22.Aller R., Moya J.L., Avila S. Implications of estradiol and progesterone in pulmonary vasodilatation in cirrhotic patients. J Endocrinol Invest. 2002;25(1):4–10. doi: 10.1007/BF03343954. [DOI] [PubMed] [Google Scholar]
- 23.Lustik S.J., Chhibber A.K., Kolano J.W. The hyperventilation of cirrhosis: progesterone and estradiol effects. Hepatology. 1997;25(1):55–58. doi: 10.1002/hep.510250110. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J., Fallon M.B. Hepatopulmonary syndrome: update on pathogenesis and clinical features. Nat Rev Gastroenterol Hepatol. 2012;9(9):539–549. doi: 10.1038/nrgastro.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts K.E., Kawut S.M., Krowka M.J., and the Pulmonary Vascular Complications of Liver Disease Study Group Genetic risk factors for hepatopulmonary syndrome in patients with advanced liver disease. Gastroenterology. 2010;139(1) doi: 10.1053/j.gastro.2010.03.044. 130.e24-139.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soulaidopoulos S., Goulis I., Giannakoulas G. Hepatopulmonary syndrome is associated with the presence of hepatocellular carcinoma in patients with decompensated cirrhosis. Ann Gastroenterol. 2017;30(2):225–231. doi: 10.20524/aog.2016.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez-Roisin R., Roca J., Agusti A.G., Mastai R., Wagner P.D., Bosch J. Gas exchange and pulmonary vascular reactivity in patients with liver cirrhosis. Am Rev Respir Dis. 1987;135(5):1085–1092. doi: 10.1164/arrd.1987.135.5.1085. [DOI] [PubMed] [Google Scholar]