Key Points
Question
How well does the AJCC Cancer Staging Manual, 8th edition (AJCC 8), tumor (T) classification for head and neck cutaneous squamous cell carcinoma (HNCSCC) stratify disease-related outcomes and how does its performance compare with the 7th edition (AJCC 7)?
Findings
In a cohort of patients with HNCSCC, 17.8% of tumors were classified in AJCC 8 as high tumor categories (T3/T4), and this small subset accounted for 70.4% of the cohort’s poor outcomes. Comparatively via AJCC 7, few tumors (0.7%) and poor outcomes (16.9%) were classified as T3/T4.
Meaning
AJCC 8 demonstrates superior homogeneity and monotonicity compared with AJCC 7.
Abstract
Importance
Previous studies have shown that the AJCC Cancer Staging Manual, 7th edition (AJCC 7), tumor classification for cutaneous squamous cell carcinoma (CSCC) failed to accurately stratify disease-related outcomes. The recently released 8th edition (AJCC 8) features a revised tumor classification for only head and neck CSCC (HNCSCC).
Objective
To compare AJCC 7 and AJCC 8 tumor classifications for HNCSCC and to validate AJCC 8.
Design, Setting, and Participants
This was a 10-year retrospective cohort study (2000-2009) at an academic tertiary care center reviewing 680 primary HNCSCC tumors in 459 patients.
Main Outcomes and Measures
Primary HNCSCC tumors were reviewed for disease-related outcomes (DROs): local recurrence (LR), nodal metastasis (NM), and disease-specific death (DSD). Tumors were stratified by AJCC 7 and AJCC 8 tumor classification. Distinctiveness (outcome differences between categories), homogeneity (outcome similarity within categories), and monotonicity (outcome worsening with increasing categories) were assessed for both classifications.
Results
Most of the 459 patients were white (451 [98.3%]) and male (312 [68.0%]). AJCC 8 high tumor categories (T3/T4) accounted for 121 (17.8%) of total cases but 50 of 71 DROs (70.4%) (22 of 34 of LRs [64.7%], 17 of 24 NMs [70.8%], and 11 of 13 of DSDs [84.6%]). This was a significant improvement over AJCC 7, where only 12 of 71 DROs (16.9%) (4 of 34 LRs [11.8%], 3 of 24 NMs [12.5%], and 5 of 13 DSDs [38.5%]) occurred in T3/T4 categories. However, AJCC 8 T2 and T3 were indistinct, with overlapping 95% CIs for 10-year cumulative incidences of LR, NM, and DSD. The 10-year cumulative incidence of DROs in the 119 AJCC 8 T3 cases were 19.7% (95% CI, 13.0%-29.7%) for LR, 14.1% (95% CI, 9.7%-20.7%) for NM, and 9.3% (95% CI, 6.8%-14.0% for DSD).
Conclusions and Relevance
AJCC 8 demonstrates superior homogeneity and monotonicity compared with AJCC 7. It now may be possible for AJCC 8 HNCSCC T2, T3, and T4 cases to be recorded and tracked by tumor registries because they represented a 23.1% subset in this study, which includes nearly all poor outcomes (85.9%). Further work is needed to validate AJCC 8 with population-level data and to compare AJCC 8 performance against alternative tumor classifications.
This retrospective cohort study compares the tumor classifications for head and neck squamous cell carcinoma using the 7th vs 8th editions of the AJCC Cancer Staging Manual for head amd neck cutaneous csquamous cell carcinomas.
Introduction
Staging is an important tool in the prognostic stratification and clinical management of cancer. Staging systems enable physicians to profile tumor risk and to develop strategic treatment plans. In addition, cancer staging provides a uniform language between physicians around the world. With a shared lexicon, physicians from different settings can compare tumors equitably based on tumor classification.
For cutaneous squamous cell carcinoma (CSCC), the second most common cancer among the white population in the United States, cancer staging is pivotal to identifying rare, high-risk cases from the low-risk majority. Most cases of CSCC carry a favorable prognosis, with 3.0%, 4.0%, and 1.5% risks of local recurrence, nodal metastasis, and death from CSCC, respectively, overall. However, a subset of CSCC has a much higher risk of disease-related outcomes owing to the presence of risk factors, such as perineural or lymphovascular invasion, poorly differentiated histologic characteristics, a diameter of 2 cm or greater, depth beyond the dermis, location on ear or lip, recurrent tumors, and immunocompromised status.
In 2010, the American Joint Committee on Cancer (AJCC) proposed a revised tumor (T) classification system for CSCC (AJCC Cancer Staging Manual, 7th Edition [AJCC 7]) that incorporated risk factors of tumor thickness greater than 2 mm, perineural invasion (of any size), Clark level of IV or higher, and poorly differentiated histologic characteristics for the first time. While AJCC 7 represented an improvement over the previous AJCC sixth edition, which grouped all nonmelanoma skin cancers together and used only tumor diameter, bone, and intracranial invasion as staging factors, studies have shown that AJCC 7 tumor classification failed to accurately stratify CSCC disease-related outcomes. AJCC 7 tumor classification lacked distinctiveness (outcomes differ between categories), homogeneity (outcomes are similar within categories), and monotonicity (outcomes worsen with increasing categories), which are 3 characteristics set forth by the AJCC to describe an ideal staging system. The recently released AJCC Cancer Staging Manual, 8th Edition (AJCC 8), was implemented in clinical practice in January 2017 and will be implemented by tumor registrars in January 2018 to allow time for training. AJCC 8 features an updated CSCC tumor classification for tumors located on the head or neck only since the system was developed within the AJCC’s head and neck cancer committee.
In AJCC 8, T2 is now restricted to tumors that are 2 cm or larger but less than 4 cm in greatest dimension without any risk factors. AJCC 8 T3 has been expanded to include tumors that are 4 cm or larger in greatest dimension or have 1 or more risk factors. Risk factors for T3 upstaging include deep invasion (defined as invasion beyond the subcutaneous fat or >6 mm), perineural invasion (defined as tumor cells invading the perineural space of nerves lying deeper than the dermis or measuring ≥0.1 mm in caliber, or clinical or radiographic involvement of named nerves), and minor bone invasion. “Poorly differentiated histologic features” was dropped as a risk factor for upstaging tumors in AJCC 8. AJCC 8 T4 is subdivided into T4a, which includes tumors with gross cortical bone and/or marrow invasion, and T4b, which includes tumors with skull base invasion and/or skull base foramen involvement. These changes in AJCC 8 were based primarily on data that have become available after publication of AJCC 7 describing independent prognostic factors in CSCC. The objective of this study was to validate AJCC 8 and to compare AJCC 7 vs AJCC 8 tumor classifications in a large cohort of patients with primary N0 and M0 head and neck CSCC (HNCSCC).
Methods
Data Collection
Data used in the present study included the subset of HNCSCCs from the Brigham and Women’s CSCC cohort study. Data collection procedures have been previously published. In brief, patients with CSCC diagnosed at the Brigham and Women’s Hospital over a 10-year period from January 1, 2000, through December 31, 2009, were identified via the department of pathology electronic database. Pathology reports were reviewed, and cases of noncutaneous SCC, in situ CSCC, and recurrent CSCC were excluded. Tumors located on the trunk and extremities were excluded because these are not staged according to the AJCC 8 staging system for HNCSCC. Tumors located on the eyelid, vulva, penis, and perianal region were also excluded because they have they have their own separate tumor classification systems regardless of histologic subtype. However, tumors of both the vermillion (lipstick region) and cutaneous (hair-bearing) areas of the lip were included because these are included in AJCC 8 HNCSCC staging. The medical records of all patients meeting inclusion criteria were reviewed for demographic information, features of the primary tumor needed for tumor classification (clinical diameter, millimeter and/or anatomic depth of invasion, and presence and location and/or caliber of perineural invasion), and outcomes of interest (local recurrence [LR], nodal metastasis [NM], disease-specific death [DSD], and overall survival [OS]). Slides of tumors with perineural invasion were reviewed to record the diameter of the largest nerve involved. Tumors with missing millimeter depth were assumed to be less than 6 mm deep for staging purposes. Unless otherwise stated in the pathology reports or clinical records, tumors were considered to be confined to the dermis and free of perineural invasion because Brigham and Women’s Hospital dermatopathologists and Mohs surgeons note invasion of subcutaneous fat (and beyond) and perineural invasion when present in CSCC. Tumors were classified according to AJCC 7 and AJCC 8 tumor classifications. The study was approved by the Partners Human Research Committee. Because this study was based on existing data, patient consent was waived.
Statistical Analyses
Cox proportional hazards regression with competing risks was performed on primary tumors for each patient with HNCSCC. Death from non-CSCC causes was considered a competing event. Cox proportional hazards regression models were used for the end point of overall death owing to the absence of competing risks. Follow-up time for each end point of interest was defined as the time between CSCC tumor diagnosis and time of outcome occurrence. For tumors that did not result in any poor outcomes, follow-up time was censored on the date of death or date of medical record review if the patient was alive at the time of data collection. The proportional hazards assumption was checked using Schoenfeld residuals.
To evaluate tumor classification distinctiveness, the 10-year cumulative incidences and corresponding 95% CIs of each outcome were compared. Kaplan-Meier curves were used to illustrate the cumulative incidence probabilities of AJCC tumor classification for competing risk end points (LR, NM, and DSD) and cumulative survival probability for OS. Gray test was used to compare cumulative incidence function curves while the log-rank test was used to compare OS curves. To evaluate T-classification homogeneity and monotonicity, the proportion of poor outcomes occurring in low and high AJCC tumor categories was compared, respectively. Statistical analyses were performed using SAS software (version 9.4; SAS Institute Inc). All reported P values were 2 sided with type I error (α) of <.05 considered to be statistically significant.
Results
Most of the 459 patients were white (451 [98.3%]), male (312 [68.0%]), and immunocompetent (380 [82.8%]). The pathology database search yielded a total of 1980 primary CSCC cases. After medical record review, 1152 tumors located on non–head and neck locations and 148 tumors with insufficient primary tumor information were excluded, leaving a total of 680 HNCSCCs in 459 patients. Baseline cohort characteristics of included cases are summarized in Table 1. The median age at diagnosis was 72 years (range, 35-94 years), and median follow-up time was 44 months (range, 3-135 months). The most common treatment modality was excisional surgery (364 [53.5%]) followed by Mohs surgery (243 [35.7%]). A total of 14 patients underwent adjuvant radiation therapy (13 after excisional surgery, 1 after Mohs surgery). Less common treatment modalities included electrodesiccation and curettage (14 [2.1%]), radiation monotherapy (3 [0.4%]), and topical chemotherapy (3 [0.4%]). No treatment information was available for 53 tumors (7.8%).
Table 1. Baseline Cohort Characteristics of 459 Patients and 680 Tumors.
| Characteristic | No. (%) |
|---|---|
| Male sex | 312 (68.0) |
| Median age (range), y | 72 (35-94) |
| Median follow-up time (range), mo | 44 (3-135) |
| White race | 451 (98.3) |
| Immunosuppressed | 79 (17.0) |
| Tumor differentiation | |
| Well | 403 (59.2) |
| Moderate | 147 (21.6) |
| Poor | 130 (19.1) |
| Median clinical tumor diameter (range), cm | 0.8 (0.2-11.0) |
| <2 | 584 (85.9) |
| ≥2 to 4 | 72 (10.6) |
| ≥4 | 10 (1.5) |
| Unknown | 14 (2.1) |
| Perineural invasion, mm | |
| None | 632 (92.9) |
| <0.1 | 19 (3.0) |
| ≥0.1 | 24 (3.5) |
| Present, nerve diameter unknown | 5 (0.7) |
| Median tumor depth (range), mm | 4 (1-60) |
| >2 | 397 (58.3) |
| >6 | 89 (13.1) |
| Unknown | 150 (22.0) |
| Tissue level tumor depth | |
| Dermis | 582 (85.6) |
| Subcutaneous fat | 65 (9.5) |
| Fascia | 1 (0.1) |
| Muscle | 23 (3.0) |
| Soft tissue beyond muscle | 2 (0.3) |
| Cartilage | 2 (0.3) |
| Bone | 5 (0.7) |
About 59% of the tumors (403) were well differentiated, 21.6% (147) were moderately differentiated, and 19.1% (130) were poorly differentiated. The median tumor diameter was 0.8 cm (range, 0.2-11.0 cm) with most tumors less than 2 cm in diameter (85.9%). Only 48 tumors (7.1%) had perineural invasion, of which 24 (50.0%) featured nerve diameters of 0.1 mm or greater. Most tumors (582) were confined to the dermis (85.6%) and only 5 tumors (0.7%) invaded the bone (3 invaded the temporal bone and 2 invaded the skull base).
Table 2 shows the 10-year cumulative incidences of outcomes of interest by AJCC tumor classification. In both AJCC 7 and AJCC 8, most tumors were classified as T1 (AJCC 7, 450 [66.2%]; AJCC 8, 523 [76.9%]). A smaller proportion was classified as T2 (AJCC 7, 225 [33.1%]; AJCC 8, 36 [5.3%]). T3 classification was rare in AJCC 7 (3 [0.4%]) but more common in AJCC 8 (119 [17.5%]). Few tumors were classified as AJCC 7 T4 (2 [0.3%]) or AJCC 8 T4b (2 [0.3%]). No tumors were classified as AJCC 8 T4a.
Table 2. Evaluation of the Seventh and Eighth Editions of the AJCC Cancer Staging Manual (AJCC 7 and AJCC 8) Tumor Classification System Distinctivenessa.
| Tumor Category | Tumors, No. (%) (n = 680) | LR | NM | DSD | OS | ||||
|---|---|---|---|---|---|---|---|---|---|
| Events, No. | 10-y CIN (95% CI) | Events, No. | 10-y CIN (95% CI) | Events, No. | 10-y CIN (95% CI) | Events, No. | 10-y CIN (95% CI) | ||
| AJCC 7 T1 | 450 (66.2) | 4 | 1.0 (0.4-2.5) | 2 | 0.5 (0.1-1.7) | 0 | NA | 163 | 47.1 (40.1-53.4) |
| AJCC 7 T2 | 225 (33.1) | 26 | 13.1 (9.2-18.8) | 19 | 9.2 (6.1-13.9) | 8 | 5.4 (2.6-11.2) | 88 | 40.6 (29.5-51.4) |
| AJCC 7 T3 | 3 (0.4) | 2 | 90.2 (81.4-100) | 2 | 77.3 (38.1-100) | 3 | 98.5 (92.1-100) | 3 | 0 |
| AJCC 7 T4 | 2 (0.3) | 2 | 74.5 (66.5-83.5) | 1 | 42.7 (12.9-100) | 2 | 88.0 (68.9-100) | 2 | 0 |
| AJCC 8 T1 | 523 (76.9) | 7 | 1.1 (0.4-2.6) | 3 | 0.4 (0-1.9) | 0 | No events | 188 | 46.1 (39.2-52.6) |
| AJCC 8 T2 | 36 (5.3) | 5 | 15.8 (7.5-33.0) | 4 | 12.2 (5.4-27.6) | 2 | 7.6 (4.4-13.1) | 15 | 40.2 (18.1-61.2) |
| AJCC 8 T3 | 119 (17.5) | 20 | 19.7 (13.0-29.7) | 16 | 14.1 (9.7-20.7) | 9 | 9.3 (6.8-14.0) | 51 | 41.6 (29.2-53.5) |
| AJCC 8 T4a | 0 | 0 | NA | 0 | NA | 0 | NA | 0 | NA |
| AJCC 8 T4b | 2 (0.3) | 2 | 73.6 (66.8-81.0) | 1 | 42.6 (12.6-100) | 2 | 82.2 (68.3-91.5) | 2 | 0 |
Abbreviations: CIN, cumulative incidence; DSD, disease-specific death; LR, local recurrence; NA, not applicable; NM, nodal metastasis; OS, overall survival.
AJCC 7, tumor classification: T1, tumor ≤2 cm in greatest dimension with <2 high-risk factors; T2, tumor >2 cm in greatest dimension or with ≥2 high-risk factors; T3, tumor with invasion of orbit, or of maxilla, mandible, or temporal bones; T4, tumor with invasion of other bone or direct perineural invasion of skull base; AJCC 7 high-risk factors: >2 mm thickness, Clark level of ≥IV, perineural invasion, primary site ear, primary site non–hair-bearing (vermillion) lip, or poorly differentiated histologic characteristics; AJCC 8, tumor classification: T1, tumor <2 cm in greatest dimension; T2, tumor ≥2 cm in size, but <4 cm in greatest dimension; T3, tumor ≥4 cm in greatest dimension or minor bone erosion or perineural invasion or deep invasion (deep invasion defined as invasion beyond the subcutaneous fat or >6 mm (as measured from the granular layer of adjacent normal epidermis to the base of the tumor), perineural invasion defined as tumor cells within the nerve sheath of a nerve lying deeper than the dermis or measuring ≥0.1 mm in caliber or presenting with clinical or radiographic involvement of named nerves without skull base invasion or transgression); T4a, tumor with gross cortical bone/marrow invasion; T4b, tumor with skull base invasion and/or skull base foramen involvement.
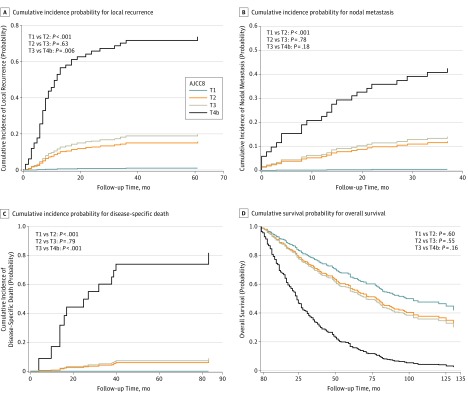
The 95% CIs overlapped substantially for AJCC 7 T3 and T4 tumors and AJCC 8 T2 and T3 tumors for all end points of interest, indicating that these categories are not distinct. In both classification systems, there was also overlap in the 95% CIs for T1 and T2 for the end point of OS. However, there was no overlap for AJCC 7 and AJCC 8 T1 and T2 for the end points of LR, NM, and DSD, indicating that these categories are distinct for both classification systems. Because no tumors were classified as AJCC 8 T4a, AJCC 8 T3 and T4b tumors were compared instead. No overlap was observed for AJCC 8 T3 and T4b tumors for the end points of LR and DSD. The Figure shows Kaplan-Meier curves for the cumulative incidence of poor outcomes by AJCC 8 tumor classification. The curves for AJCC 7 tumor classification are included in the eFigure in the Supplement.
Figure. Kaplan-Meier Competing Risk Probability Curves by the AJCC Cancer Staging Manual, Eighth Edition (AJCC 8), Tumor Classification.
A, Cumulative incidence probability for local recurrence. B, Cumulative incidence probability for nodal metastasis. C, Cumulative incidence probability for disease-specific death. D, Cumulative survival probability for overall survival.
Table 3 shows the results of homogeneity and monotonicity evaluation for AJCC 7 and AJCC 8 tumor classification. Homogeneity was evaluated by comparing the proportion of poor outcomes in low AJCC tumor categories (T1/T2), while monotonicity was evaluated by comparing the proportion of poor outcomes in high tumor categories (AJCC 7 T3/T4 and AJCC 8 T3/T4b). About 83.1% of poor outcomes were clustered in low AJCC 7 tumor categories (LR, 30 of 34 [88.2%]; NM, 21 of 24 [87.5%]; and DSD, 8 of 13 [61.5%]), while only 29.6% of poor outcomes occurred in low AJCC 8 tumor categories (LR, 12 of 34 [35.3]%; NM, 7 of 24 [29.2%]; and DSD, 2 of 13 [15.3%]). Conversely, only 16.9% of poor outcomes occurred in high AJCC 7 tumor categories (LR, 4 of 34 [11.8%]; NM, 3 of 24 [12.5%]; and DSD, 5 of 13 [38.5%]), while 70.4% of the poor outcomes occurred in high AJCC 8 tumor categories (LR, 22 of 34 [64.7%]; NM, 17 of 24 [70.8%]; and DSD, 11 of 13 [84.6%]). AJCC 8 tumor classification therefore showed superior homogeneity and monotonicity compared with AJCC 7 tumor classification (McNemar P < .001 for all end points of interest).
Table 3. Evaluation of the Seventh and Eighth Editions of the AJCC Cancer Staging Manual (AJCC 7 and AJCC 8) Tumor Classification System Homogeneity and Monotonicity.
| Tumor Classification | LR | NM | DSD | Overall Events |
|---|---|---|---|---|
| Homogeneity: Proportion of Poor Outcomes Occurring in Low Tumor Categories, No. (%) | ||||
| AJCC 7 T1/T2 | 30 of 34 (88.2) | 21 of 24 (87.5) | 8 of 13 (61.5) | 59 of 71 (83.1) |
| AJCC 8 T1/T2 | 12 of 34 (35.3) | 7 of 24 (29.2) | 2 of 13 (15.3) | 21 of 71 (29.6) |
| Monotonicity: Proportion of Poor Outcomes Occurring in High Tumor Categories, No. (%) | ||||
| AJCC 7 T3/T4 | 4 of 34 (11.8) | 3 of 24 (12.5) | 5 of 13 (38.5) | 12 of 71 (16.9) |
| AJCC 8 T3/T4a/T4b | 22 of 34 (64.7) | 17 of 24 (70.8) | 11 of 13 (84.6) | 50 of 71 (70.4) |
Abbreviations: DSD, disease-specific death; LR, local recurrence; NM, nodal metastasis.
Table 4 shows the number of tumors that were upgraded and downgraded by using AJCC 8 tumor classification compared with AJCC 7 tumor classification and the number of poor outcomes associated with such changes in tumor classification. Of the 101 tumors that were downgraded from AJCC 7 T2 to AJCC 8 T1, there were only 4 LRs, 2 NMs, and no DSDs. Similarly, of the 8 tumors that were upgraded from AJCC 7 T1 to AJCC 8 T2, there was only 1 LR, 1 NM, and no DSDs. A total of 20 tumors were upgraded from AJCC 7 T1 to AJCC 8 T3 primarily owing to the greater than 6-mm depth, high-risk factor defining tumors as T3 in AJCC 8 tumor classification. However, there were no poor outcomes among these tumors, indicating that they were inappropriately upgraded.
Table 4. Number of Tumors of 680 Upgraded and Downgraded Using the AJCC Cancer Staging Manual, Eighth Edition (AJCC 8) Tumor Classification System.
| Changes From AJCC 7 to AJCC 8 | Tumors, No. | Disease-Related Outcomes, No. | ||
|---|---|---|---|---|
| LR | NM | DSD | ||
| Upgrading | ||||
| T1→T2 | 8 | 1 | 1 | 0 |
| T1→T3 | 20 | 0 | 0 | 0 |
| T2→T3 | 96 | 18 | 14 | 6 |
| Downgrading | ||||
| T2→T1 | 101 | 4 | 2 | 0 |
Abbreviations: DSD, disease-specific death; LR, local recurrence; NM, nodal metastasis.
Among 96 tumors that were upgraded from AJCC 7 T2 to AJCC 8 T3 there were 18 LRs, 14 NMs, and 6 DSDs. These data indicate that tumors upgraded from AJCC 7 T2 to AJCC 8 T3 have an elevated risk of poor outcomes and are therefore appropriately upgraded overall. Among the 96 T2 to T3 upgraded cases, 71 had 1 risk factor only. Of these 71 cases, the sole risk factor was greater than 6 mm in depth in 42 cases (5 LRs, 4 NMs, 2 DSDs), invasion beyond the subcutaneous fat in 14 cases (4 LRs, 3 NMs, 0 DSDs), perineural invasion of nerves deeper than the dermis or at least 0.1 mm in caliber in 12 cases (3 LRs, 2 NM, 0 DSDs), and diameter of at least 4 cm in 3 cases (1 LR, 1 NM, 1 DSD). A total of 41 of these 71 cases were poorly differentiated, and they accounted for 9 LRs, 10 NMs, and 3 DSDs, while the remaining 30 cases were not poorly differentiated, and they accounted for 4 LRs, 0 NMs, and 0 DSDs.
Discussion
To our knowledge, this study is the first to evaluate the AJCC 8 tumor classification in a cohort of patients with HNCSCC. The results herein suggest that AJCC 8 is a significant improvement over AJCC 7. In AJCC 7, T3 and T4 classifications were reserved for rare tumors that invaded the bone; thus, most poor outcomes were clustered in low tumor categories. In this study’s cohort, only 5 of 680 tumors (0.7%) were in high tumor categories (T3 and T4) according to AJCC 7, and these tumors accounted for only 16.9% of the cohort’s poor outcomes (11.8% of all LRs, 12.5% of all NMs, and 38.5% of all CSCC deaths). Comparatively, via AJCC 8, more tumors (17.8%) were in high tumor categories and this small subset accounted for 70.4% of poor outcomes (64.7% of all LRs, 70.8% of all NMs, and 84.6% of all CSCC deaths), most of which occurred in T3 cases.
AJCC 8 was created with the advantage of being able to use data from several CSCC cohort studies that emerged since the creation of AJCC 7. Risk factors from these studies were incorporated into AJCC 8 tumor classification with a marked expansion of criteria for T3. In AJCC 8, tumor size greater than 4 cm or the presence of another risk factor (eg, minor bone erosion, large caliber or subdermal perineural invasion, or deep invasion past fat or >6 mm) classifies a tumor as T3. In the present study, 96 tumors were upgraded from AJCC 7 T2 to AJCC 8 T3, which shifted classification of most tumors causing LR, NM, and DSD from T2 to T3. The change in inclusion criteria for AJCC 8 T3 resulted in superior homogeneity and monotonicity, with greater separation of low- and high-risk tumors.
While AJCC 8 represents an improvement over AJCC 7, further refinement is required in future editions. AJCC 8 T4 remains rarely used because very few tumors meet the rare inclusion criteria at the time of primary tumor presentation (0 cases were classified as T4a in this cohort, whereas only 2 cases were classified as T4b). However, head and neck surgeons feel bone invasion is an important prognostic factor that affects surgical planning and morbidity, particularly in recurrent disease. A larger issue is that 95% CIs overlapped for AJCC 8 T2 and T3 for all end points of interest, indicating that these categories are indistinct. Because the risk of disease-related outcomes is similar between AJCC 8 T2 and T3, clinicians should recognize that some T2 tumors may still develop poor outcomes and may benefit from adjuvant therapy or nodal staging, when appropriate. Most of the failures that occurred in AJCC 8 T2 (2 of 5 LRs [40.0%], 2 of 4 NMs [50.0%], and all [2 of 2] DSDs [100%]) were cases of poorly differentiated tumors. A low percentage of overall failures (10 of 71 [14.1]%) occurred in AJCC 8 T1 tumors (7 LRs, 3 NMs, and 0 DSDs). As in AJCC 8 T2 tumors, most of the failures that occurred in AJCC 8 T1 (3 LRs and 2 NMs that occurred in T1 cases) were cases of poorly differentiated tumors.
Although poor differentiation was acknowledged as a risk factor by the AJCC 8 HNCSCC subcommittee based on available data, it was considered to be too inconsistent in its definition and application in the United States and elsewhere to be a reliable risk factor for inclusion in the tumor classification system. Similarly, although 2 or more risk factors were found to be more highly predictive of poor outcomes than a single factor in 2 studies, the data regarding how risk changes with accumulation of risk factors were felt by the AJCC HNCSCC subcommittee to be insufficient for incorporation into AJCC staging at this time. Because risk factors often occur together, this decision may have little impact in most cases. However, among the 96 cases that were upgraded from AJCC 7 T1 or T2 to AJCC 8 T3, 71 were upgraded based on a single risk factor. Of these, only cases that were poorly differentiated had an elevated risk of poor outcomes (9 LRs, 10 NMs, and 3 DSDs in the 41 cases that were poorly differentiated vs only 4 LRs and no NM or DSD in the 30 cases that were well or moderately differentiated). This may indicate that AJCC 8 T3 cases that do well (and are therefore being inappropriately upgraded to T3) will be well and moderately differentiated HNCSCCs upgraded to T3 based on a sole risk factor. These findings highlight the prognostic importance of differentiation.
Depth of invasion is another criterion which changed in AJCC 8. AJCC 7 used 2-mm depth, Clark level IV, and bone invasion as criterion for upstaging. AJCC 8 relies on 2 studies that respectively showed 6-mm depth and depth beyond subcutaneous fat to have prognostic value. Millimeter depth is measured from the granular layer of adjacent normal epidermis to the base of the tumor. It is as yet unknown which of these methods of ascertainment of depth will have the greatest prognostic reliability. Efforts may be undertaken within the pathology and Mohs community to increase reliability and reproducibility of differentiation grading and depth recording across centers so that depth ascertainment may be optimized and differentiation may be incorporated into future AJCC staging for CSCC. In addition, future studies are encouraged to compare AJCC 8 performance vs alternative classification systems, such as the Brigham and Women’s Hospital tumor classification system for CSCC, which includes poor differentiation as a risk factor.
Despite its imperfections, the improvements within AJCC 8 whereby T3 now seems to capture most HNCSCC poor outcomes may prove highly beneficial. It now may be possible for AJCC 8 CSCC T2, T3, and T4 cases to be systematically recorded and tracked by tumor registries because they represented a 23.1% subset in this study, which includes nearly all poor outcomes (85.9%). Such a subset may be feasible for registrars to track, whereas CSCC cases in general are too numerous for tracking by registries (eg, CSCC is excluded from the SEER registry for this reason). Such efforts will be greatly aided if clinicians consistently report clinical tumor diameter and pathologists report staging risk factors consistently in a synoptic fashion. Such population-based registry data are sorely needed to validate and refine CSCC tumor classification so that a clinically effective tumor classification system can be built.
Limitations
This study is subject to a few limitations. First, this study was based on data from an academic medical center, which may not represent the population at large of patients with CSCC and the institution may treat CSCCs differently than elsewhere, thus having a unique impact on outcomes. However, uniform reporting of risk factors should make risks of poor outcomes relatively similar among primary CSCCs with similar risk profiles, regardless of treating physicians or institution. While it would be ideal for AJCC 8 to be validated using population-level data, such data are presently unavailable. Thus, the cohort in this study, which represents the largest cohort available of primary HNCSCC, provide the best available current data with which to validate AJCC tumor classification. The present study did not validate N and M classifications of AJCC 8 given the rarity of primary CSCCs presenting with regional or distant metastases. This will need to be the subject of larger population-based studies. Finally, because AJCC 8 was just released, its validation required a retrospective application of AJCC 8 guidelines. Larger, prospective studies may represent the ideal method to validate AJCC 8, yet such studies may not be available for several years. Meanwhile, a retrospective study design is justified.
Conclusions
AJCC 8 demonstrates improved homogeneity and monotonicity compared with AJCC 7. The upper categories of AJCC 8 (T3 and T4a/b) demonstrate a strong ability to stratify tumors with a significant risk of disease-related poor outcomes. AJCC 8 T2, T3, and T4 together comprise a 23.1% subset of CSCC containing almost all poor outcomes which merits population-based validation to refine AJCC CSCC staging.
eFigure. Kaplan-Meier curves by AJCC 7 classification
References
- 1.Asare EA, Washington MK, Gress DM, Gershenwald JE, Greene FL. Improving the quality of cancer staging. CA Cancer J Clin. 2015;65(4):261-263. [DOI] [PubMed] [Google Scholar]
- 2.Greene FL, Sobin LH. The staging of cancer: a retrospective and prospective appraisal. CA Cancer J Clin. 2008;58(3):180-190. [DOI] [PubMed] [Google Scholar]
- 3.Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151(10):1081-1086. [DOI] [PubMed] [Google Scholar]
- 4.Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68(6):957-966. [DOI] [PubMed] [Google Scholar]
- 5.Brantsch KD, Meisner C, Schönfisch B, et al. . Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol. 2008;9(8):713-720. [DOI] [PubMed] [Google Scholar]
- 6.Mourouzis C, Boynton A, Grant J, et al. . Cutaneous head and neck SCCs and risk of nodal metastasis: UK experience. J Craniomaxillofac Surg. 2009;37(8):443-447. [DOI] [PubMed] [Google Scholar]
- 7.Schmults CD, Karia PS, Carter JB, Han J, Qureshi AA. Factors predictive of recurrence and death from cutaneous squamous cell carcinoma: a 10-year, single-institution cohort study. JAMA Dermatol. 2013;149(5):541-547. [DOI] [PubMed] [Google Scholar]
- 8.Brougham ND, Tan ST. The incidence and risk factors of metastasis for cutaneous squamous cell carcinoma: implications on the T-classification system. J Surg Oncol. 2014;110(7):876-882. [DOI] [PubMed] [Google Scholar]
- 9.Carter JB, Johnson MM, Chua TL, Karia PS, Schmults CD. Outcomes of primary cutaneous squamous cell carcinoma with perineural invasion: an 11-year cohort study. JAMA Dermatol. 2013;149(1):35-41. [DOI] [PubMed] [Google Scholar]
- 10.Thompson AK, Kelley BF, Prokop LJ, Murad MH, Baum CL. Risk Factors for cutaneous squamous cell carcinoma recurrence, metastasis, and disease-specific death: a systematic review and meta-analysis. JAMA Dermatol. 2016;152(4):419-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds. AJCC Cancer Staging Manual. 7th ed New York, NY: Springer; 2010. [Google Scholar]
- 12.Greene FL, Page DL, Fleming ID, et al. , eds. AJCC Cancer Staging Manual. 6th ed New York, NY: Springer; 2002. [Google Scholar]
- 13.Farasat S, Yu SS, Neel VA, et al. . A new American Joint Committee on Cancer staging system for cutaneous squamous cell carcinoma: creation and rationale for inclusion of tumor (T) characteristics. J Am Acad Dermatol. 2011;64(6):1051-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, et al. . Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol. 2013;149(4):402-410. [DOI] [PubMed] [Google Scholar]
- 15.Karia PS, Jambusaria-Pahlajani A, Harrington DP, Murphy GF, Qureshi AA, Schmults CD. Evaluation of American Joint Committee on Cancer, International Union Against Cancer, and Brigham and Women’s Hospital tumor staging for cutaneous squamous cell carcinoma. J Clin Oncol. 2014;32(4):327-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amin MB, Edge S, Greene F, et al. , eds. AJCC Cancer Staging Manual. 8th ed New York, NY: Springer; 2017. [Google Scholar]
- 17.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. doi: 10.2307/2670170 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Kaplan-Meier curves by AJCC 7 classification