Abstract
Study Objective
To describe the current epidemiology of bacteremia in febrile infants ≤ 60 days-old in the Pediatric Emergency Care Applied Research Network (PECARN).
Methods
We conducted a planned secondary analysis of a prospective observational study of febrile infants ≤ 60 days-old presenting to any of 26 PECARN emergency departments (EDs) (2008 –2013) who had blood cultures obtained. We excluded infants with significant comorbidities or critically-ill appearances. The primary outcome was prevalence of bacteremia.
Results
Of 7,335 screened infants, 4,778 (65.1%) had blood cultures and were enrolled. Of these, 84 had bacteremia (1.8%; 95% CI 1.4–2.2%). The prevalence of bacteremia in infants ≤ 28 days-old (47/1,515) was 3.1% (95% CI 2.3–4.1%) and in infants 29–60 days-old (37/3,246) was 1.1% (95% CI 0.8–1.6%). Prevalence differed by week of age for infants ≤ 28 days (0–7 days: 4/156, 2.6%), (8–14 days: 19/356, 5.3%), (15–21 days: 15/449, 3.3%), (22–28 days: 9/554, 1.6%). The most common pathogens were Escherichia coli (39.3%; 95% CI 29.5–50.0%) and group B streptococcus (23.8%; 95% CI 16.0–33.9%). Bacterial meningitis occurred in 19/1515 infants ≤ 28 days-old (1.3%; 95% CI 0.8–2.0%) and 5/3,246 infants 29–60 days-old (0.2%; 95% CI 0.1–0.4%). Of 84 infants with bacteremia, 36 (42.9%; 95% CI 32.8–53.5%) had urinary tract infections (Escherichia coli 83%); 11 (13.1%; 95%CI 7.5–21.9%) had bacterial meningitis.
Conclusion
The prevalence of bacteremia and meningitis among febrile infants ≤ 28 days-old is high and exceeds that observed in infants 29–60 days old. Escherichia coli and group B streptococcus are the most common bacterial pathogens.
Background
In young infants, fever can be the only sign of serious bacterial infections. Although higher rates have been reported in the past, current United States data suggest that 2% of infants younger than 2 months old presenting with fever with no source have bacteremia, and 0.3–0.4% have bacterial meningitis.1–4 This decrease from previous reports is likely a result of group B streptococcus peripartum antibiotic prophylaxis and herd immunity resulting from Streptococcus pneumoniae immunization.1–6 Many febrile infants undergo comprehensive evaluations including cultures of the blood, urine and often cerebrospinal fluid followed by use of empiric broad spectrum antibiotics and inpatient hospitalization.7
Among infants older than 28 days, the medical literature reports variation in management in both emergency department (ED) and office settings. As bacteremia and bacterial meningitis rates have decreased in the past decade, some clinicians are more selectively using laboratory testing to screen for bacterial illnesses in young febrile infants.4–6, 8 Although bacteremia and bacterial meningitis are relatively uncommon, missed diagnosis can have serious long-term sequelae. Additional up-to-date information about the prevalence and epidemiology of bacteremia and bacterial meningitis among young, febrile infants will help to inform clinical evaluation and decision-making.
The age of the infant appears to be a potential contributing factor to the prevalence of bacterial infection and to decisions about laboratory testing. The purpose of this study was to describe the epidemiology of bacteremia stratified by week of age in febrile infants 60 days of age and younger from a geographically-diverse sample of previously healthy infants treated in United States pediatric EDs. As a secondary aim, we report the epidemiology of associated bacterial meningitis and urinary tract infections.
Methods
Study Design and Setting
This was a planned secondary analysis of a prospective observational study that enrolled infants ≤60 days old with temperatures ≥ 38° C and who had blood cultures performed as part of standard clinical care.9 The study was conducted in 26 EDs participating in the Pediatric Emergency Care Applied Research Network (PECARN) (children’s hospitals (18) and academic medical centers (8)) between December 2008 and May 2013. 9,10 The study was approved by the institutional review board at all sites and we obtained written informed consent for all infants.
Selection of Participants
We included infants ≤60 days old with temperatures ≥ 38° C (measured at home, in the clinic, or in the ED) and who had blood cultures performed as part of standard clinical care and were enrolled in the parent RNA Biosignatures study.9 For the parent RNA study, staff enrolled a convenience sample of eligible infants at various times of day and there were no processes to account for all eligible patients. Infants who were critically ill, as well as those with congenital heart disease, prematurity (≤ 36 weeks gestation), inherited or acquired immunodeficiency, indwelling devices or catheters, and/or receipt of antibiotics in the preceding 48 hours were excluded. 9,10 All clinical care including laboratory testing in addition to the blood culture, antibiotics, and disposition was at the discretion of the treating providers.
Methods of Measurement
Trained staff collected the following data at the time of enrollment: age and sex, qualifying temperature (location home, clinic, or ED), Yale Observation Scale score (YOS), laboratory data (CBC count with differential, urinalysis, cerebrospinal fluid (CSF) studies, viral studies), imaging reports, and study site, visit date, and disposition. We abstracted bacterial cultures (blood, urine, and cerebrospinal fluid) from the medical record.
Outcome Measures
Our primary outcomes were bacteremia, defined as growth of pathogenic bacteria in the blood culture. We also evaluated for concomitant bacterial meningitis, defined as growth of pathogenic bacteria in the CSF, and concomitant urinary tract infection (defined below). All concurrent bacterial infections were by definition associated with the same organism, and were assumed to be indicative of systemic dissemination of the same pathogen. Growth of multiple bacteria or those not commonly considered pathogens (e.g. coagulase-negative staphylococcus, diphtheroids, bacillus species) were categorized as contaminants. The three study principal investigators (pediatric emergency and pediatric infectious disease physicians) classified bacterial growth as pathogens or contaminants by consensus. In the parent study of RNA biosignatures, there were 13 patients from whom the blood cultures could not be categorized definitively. For example, growth of multiple organisms where one could have been a pathogen, or positive Gram stain without growth on culture. Although these most likely reflected contaminants, these 13 patients were excluded from the parent study RNA analysis and this sub analysis. We defined urinary tract infection in a catheter specimen as culture growth of pathogenic bacteria ≥50,000 colony forming units (cfu)/ml or ≥10,000 cfu/ml associated with a positive urinalysis (>5 white blood cells per high power field, positive nitrate, or leukocyte esterase) and in a suprapubic aspiration specimen as ≥1000 cfu/ml. We contacted the family of each enrolled infant who did not have a lumbar puncture completed in the ED and who was discharged to home 8–30 days after the ED visit to ascertain whether the infant remained well (and therefore bacterial meningitis was excluded clinically).
Statistical analysis
We report the rates of bacteremia and concurrent bacterial infections by age, with 95% confidence intervals using the exact binomial method. Statistical analyses were performed using SAS software version 9.4 (Cary, NC).
Results
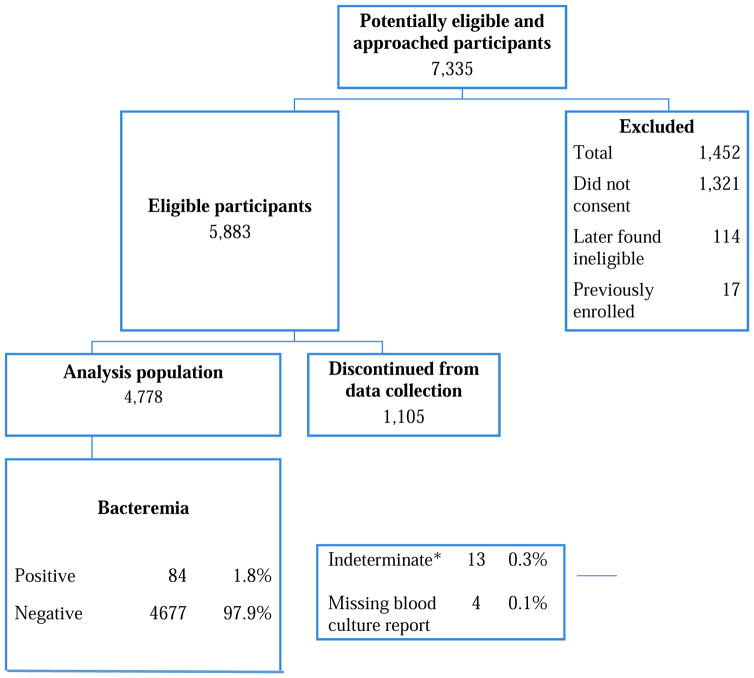
Of 7,335 infants approached, 4,778 (65.1%) infants had blood cultures performed; 84 cultures were positive (1.8%; 95% CI 1.4–2.2%) (Figure 1). Of enrolled patients who met inclusion criteria for the current analysis (4,761), 1,515 (32%) were ≤ 28 days old and 2, 074 (44%) were girls. The race/ethnicity distribution was white (57%), African American (24%), Asian (3%), other (9%) and unknown (7%); 30% reported Hispanic ethnicity. A total of 4,771 had information about meningitis: 3,559 had a CSF culture obtained in the ED and an additional 1,212 had absence of meningitis confirmed through follow-up (7 patients had missing CSF information).
Figure 1.
Patient enrollment
*Indeterminate included cultures with multiple organisms where one could have been a pathogen, or positive gram stain but no bacterial growth
The spectrum and frequency of bacterial species causing bacteremia, as well as bacterial co-infections are demonstrated in Table 1. Eleven infants (13.1%; 95% CI 7.5–21.9%) had associated bacterial meningitis and 36 (42.9%; 95% CI 32.8–53.5%) had concurrent urinary tract infections. Of the 33 infants whose blood cultures grew Escherichia coli, 30 (91%) had concurrent urinary tract infections. Urine cultures were missing for 104 patients. Group B streptococcus accounted for 24% of the bacteremia and 54% of the concurrent bacterial meningitis, of which 5/11 were in infants ≤ 28 days old. Staphylococcal aureus accounted for 13% of the bacteremia. An additional 13 infants were diagnosed with bacterial meningitis and had negative blood cultures. The cerebrospinal fluid cultures of these infants grew Escherichia coli (n=3), Enterococcus faecalis (n=3), Group B Streptococcus (n=3), Klebsiella oxytoca (n=1), Listeria monocytogenes (n=2), and Staphylococcal aureus (n=1). In 3 infants the CSF grew contaminant organisms and in one case the culture report was missing.
Table 1.
Bacteremia pathogens and concurrent bacterial infections by age
| Bacteremia1 | Concurrent UTI2 | Concurrent bacterial meningitis2 | ||||
|---|---|---|---|---|---|---|
| ≤ 28 days | > 28 days | ≤ 28 days | > 28 days | ≤ 28 days | > 28 days | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| N | 47 (56.0) | 37 (44.0) | 18 (38.3) | 18 (48.6) | 9 (19.1) | 2 (5.4) |
| Bacteremia organisms | ||||||
| Escherichia coli | 16 (19.0) | 17 (20.2) | 14 (87.5) | 16 (94.1) | 1 (6.3) | 0 (0) |
| Group B streptococcus | 15 (17.9) | 5 (6.0) | 0 (0) | 0 (0) | 5 (33.3) | 1 (20.0) |
| Staphyloccus aureus | 5 (6.0) | 6 (7.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Enterobacter cloacae | 2 (2.4) | 3 (3.6) | 1 (50.0) | 2 (66.7) | 1 (50.0) | 0 (0) |
| Klebsiella pneumoniae | 2 (2.4) | 0 (0) | 1 (50.0) | 0 (0) | 1 (50.0) | 0 (0) |
| Enterococcus species | 1 (1.2) | 1 (1.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Neisseria meningitidis | 0 (0) | 2 (2.4) | 0 (0) | 0 (0) | 0 (0) | 1 (50.0) |
| Moraxella species | 0 (0) | 2 (2.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Listeria monocytogenes | 1 (1.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Citrobacter freundii | 1 (1.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Salmonella species | 1 (1.2) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 0 (0) |
| Flavobacterium | 1 (1.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Lactose fermenting negative bacilli | 1 (1.2) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 0 (0) |
| Streptococcus pneumoniae | 1 (1.2) | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 0 (0) |
| Pseudomonas | 0 (0) | 1 (1.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
The percentages in this column are based on the 84 total patients with bacteremia
The percentages in this column are based on the total number of patients with the corresponding bacteremia organism in the matching age group.
The prevalence of bacteremia by week of age is demonstrated in Table 2. The highest frequency was among infants 8–14 days old. Among infants ≤ 28 days-old there was week-to-week variation in bacteremia prevalence; prevalence overall was 3.1% (95% CI 2.3–4.1%) vs 1.1% (95% CI 0.8–1.6%) among infants 29–60 days-old. Among the infants 29–60 days there was little variation in prevalence of bacteremia by week of age. The frequency of contaminated samples (n=182) by week of age ranged from 1.9–6.7%. Of the 24 cases of bacterial meningitis, 19 occurred in infants 28 days-old and younger (1.3%, 95% CI 0.8–2.0%) and 5 were in infants 29 days-old and older (0.2%, 95% CI 0.1–0.4%). There were no cases of bacterial meningitis in infants older than 42 days (6 weeks).
Table 2.
Bacteremia by week of age
| Age (days) | Proportion, 95% CI |
|---|---|
| 0–7 | 4/156 (2.6%, 1.0% – 6.4%) |
| 8–14 | 19/356 (5.3%, 3.4% – 8.2%) |
| 15–21 | 15/449 (3.3%, 2.0% – 5.4%) |
| 22–28 | 9/554 (1.6%, 0.9% – 3.1%) |
| 29–35 | 6/654 (0.9%, 0.4% – 2.0%) |
| 36–42 | 11/774 (1.4%, 0.8% – 2.5%) |
| 43–49 | 5/778 (0.6%, 0.3% – 1.5%) |
| 50–56 | 9/729 (1.2%, 0.7% – 2.3%) |
| 57–60 | 6/311 (1.9%, 0.9% – 4.1%) |
Limitations
The study has some limitations related to infant enrollment and laboratory testing. The study population was a convenience sample based on the availability of research or clinical staff to approach families and complete enrollment procedures. We do not know how many eligible subjects were not approached. The intent was to enroll infants at risk of infection because of young age, but who otherwise were not critically-ill appearing. Because there were no specific criteria or definition for critically-ill, there was likely some variation in how it was defined. The consent rate of 60%–70% may also have allowed for a study sample with potentially biases reported results. However, the rates of bacteremia in the enrolled population reflect those reported in the recent literature, suggesting that our sample is similar and generalizable.1,3–5 While all enrolled infants had blood cultures obtained, not all had CSF samples collected, potentially resulting in missed bacterial meningitis. However, all young infants managed without performing lumbar punctures had telephone follow up, and we included in the analysis only those infants in whom we could confirm that bacterial meningitis had not occurred (by either laboratory diagnosis or clinical follow-up). Despite the large sample, few young infants had bacterial meningitis, limiting analysis by week of age. Finally, urine culture results were missing for 2%.
Discussion
In this large prospective cohort of 4,778 previously-healthy term febrile infants ≤ 60 days old evaluated in United States EDs, the overall prevalence of bacteremia was 1.8%, and Escherichia coli and Group B streptococcus were the most common pathogens identified. The prevalence was higher among infants in the first four weeks of life than in the older infants. Among the infants 29–60 days old, the frequency was lower overall and we observed no significant variation by week of age among that group. The bacteremia rate in our whole cohort is similar to that in other large cohorts of young febrile infants. 1,4,5 There are differences, however, in study design, setting, and population: 1) the data reported in the present study were prospectively gathered, allowing for real-time evaluation and data queries; 2) all infants were enrolled in the ED and had blood cultures obtained; and 3) the study included multiple sites, allowing for broad geographic representation and ample sample sizes at each week of age.
Escherichia coli was the most common etiologic agent of bacteremia in our study population. This is similar to the epidemiology in the current reported literature. 1,3,4 Escherichia coli urinary tract infections were the most frequent identified site infections among infants with bacteremia. The rates of bacteremia that we report, and the spectrum of pathogens likely result in part from study inclusion criteria, which involved only previously healthy term infants, high rates of peripartum maternal group B Streptococcus screening and treatment, and high population rates of immunization for Streptococcus pneumoniae. The exclusion of critically-ill infants (by study protocol) also contributed to the lower rates of bacteremia than in other reported literature. Of note, the prevalence of contaminated blood cultures in young febrile infants collected in the ED setting was high, and similar to that reported by others.2,4 There appeared to be no association between week of age and blood culture contamination rates.
The epidemiology of bacteremia and bacterial meningitis are important contributing factors in determining the best approach to the diagnostic evaluation and disposition of young febrile infants. Bacteremia prevalence was highest in infants ≤ 28 days. Although lower in infants 29–60 days old, the bacteremia rates did not vary week-by-week in the second month of life. As expected, bacterial meningitis prevalence was also higher in infants ≤ 28 days old, and there were no cases of bacterial meningitis after the 6th week of life.
Conclusion
In this large, prospective cohort study of febrile infants 60 days-old and younger the overall prevalence of bacteremia was 1.8 %. We found Escherichia coli to be the most frequent etiology of bacteremia, followed by Group B streptococcus, Staphylococcus aureus, and Enterobacter cloacae, together accounting for 81% of pathogenic positive blood cultures. In infants 28 days and younger the prevalence of bacteremia and bacterial meningitis was higher than that observed in infants 29 days and older and a low threshold for complete laboratory evaluation and hospitalization for the youngest group is warranted.
Acknowledgments
Funding source: The research reported in this publication was supported in part by grant H34MCO8509 from Health Resources and Services Administration, Emergency Services for Children and by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R01HD062477. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This project is also supported in part by the Health Resources and Services Administration (HRSA), Maternal and Child Health Bureau (MCHB), Emergency Medical Services for Children (EMSC) Network Development Demonstration Program under cooperative agreements U03MC00008 for $3,000,000, and is partially supported by MCHB cooperative agreements: U03MC00001, U03MC00003, U03MC00006, U03MC00007, U03MC22684, and U03MC22685. This information or content and conclusions are those of the author and should not be construed as the official position or policy of, nor should any endorsements be inferred by HRSA, HHS or the U.S. Government.
Participating centers and investigators in alphabetical order by center:
Ann & Robert H. Lurie Children’s Hospital (Elizabeth C. Powell, MD, MPH)
Bellevue Hospital Center (Deborah A. Levine, MD; Michael G. Tunik, MD)
Boston Children’s Hospital (Lise E. Nigrovic, MD, MPH)
Children’s Hospital of Colorado (Genie Roosevelt, MD)
Children’s Hospital of Michigan (Prashant Mahajan, MD, MPH, MBA)
Children’s Hospital of Philadelphia (Elizabeth R. Alpern, MD, MSCE)
Children’s Hospital of Pittsburgh (Melissa Vitale, MD)
Children’s Hospital of Wisconsin (Lorin Browne, DO; Mary Saunders, MD)
Children’s National Medical Center (Shireen M. Atabaki, MD, MPH)
Cincinnati Children’s Hospital Medical Center (Richard M. Ruddy, MD)
Hasbro Children’s Hospital (James G. Linakis, MD, PhD)
Helen DeVos Children’s Hospital (John D. Hoyle, Jr., MD)
Hurley Medical Center (Dominic Borgialli, DO, MPH)
Jacobi Medical Center (Stephen Blumberg, MD; Ellen F. Crain, MD, PhD)
Johns Hopkins Children’s Center (Jennifer Anders, MD)
Nationwide Children’s Hospital (Bema Bonsu, MD; Daniel M. Cohen, MD)
Nemours/Alfred I. DuPont Hospital for Children (Jonathan E. Bennett, MD)
New York Presbyterian-Morgan Stanley Children’s Hospital (Peter S. Dayan, MD, MSc)
Primary Children’s Medical Center (Richard Greenberg, MD)
St. Louis Children’s Hospital (David M. Jaffe, MD; Jared Muenzer, MD);
Texas Children’s Hospital (Andrea T. Cruz, MD, MPH, Charles Macias, MD)
University of California Davis Medical Center (Nathan Kuppermann, MD, MPH; Leah Tzimenatos, MD)
University of Maryland (Rajender Gattu, MD)
University of Michigan (Alexander J. Rogers, MD)
University of Rochester (Anne Brayer, MD)
Women and Children’s Hospital of Buffalo (Kathleen Lillis, MD).
The authors thank the research coordinators in PECARN and the project staff at the Data Coordinating Center at the University of Utah.
Footnotes
Meetings: Work to be presented in part at the Pediatric Academic Societies Annual Meeting, San Francisco, CA, May 8, 2017.
Financial disclosure: Octavio Ramilo, MD, Division of Pediatric Infectious Diseases and Center for Vaccines and Immunity, Nationwide Children’s Hospital and The Ohio State University, reports personal fees from HuMabs, Abbvie, Janssen, Medimmune and Regeneron, and grants from Janssen. All these fees and grants are not related to the current work. The other authors have no financial relationships relevant to this article to disclose.
Conflicts of interest: The authors have no conflicts of interest relevant to this article to disclose.
Contributors’ Statement:
Elizabeth C. Powell, MD, MPH: conceived and designed the study, supervised patient enrollment and data abstraction, contributed to data analysis, drafted the initial manuscript and approved the final manuscript.
Prashant V. Mahajan, MD, MPH, MBA: conceived and designed the study, obtained funding, supervised patient enrollment and data abstraction, contributed to data analysis, revised and approved the final manuscript.
Genie Roosevelt, MD, MPH: supervised patient enrollment and data abstraction, contributed to study design, revised and approved the final manuscript.
John D. Hoyle, Jr, MD: supervised patient enrollment and data abstraction, contributed to study design, revised and approved the final manuscript.
Rajender Gattu, MD: supervised patient enrollment and data abstraction, contributed to study design, revised and approved the final manuscript.
Andrea T. Cruz, MD, MPH: supervised patient enrollment and data abstraction, contributed to study design, revised and approved the final manuscript.
Alexander J. Rogers, MD: supervised patient enrollment and data abstraction, contributed to study design, revised and approved the final manuscript.
Shireen M. Atabaki, MD: supervised patient enrollment and data abstraction, contributed to study design, revised and approved the final manuscript.
David M. Jaffe, MD: supervised patient enrollment and data abstraction, contributed to study design, revised and approved the final manuscript.
T. Charles Casper, PhD: had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Casper revised and approved the final manuscript.
Octavio Ramilo, MD: conceived and designed the study, obtained funding, revised and approved the final manuscript.
Nathan Kuppermann, MD, MPH: conceived and designed the study, obtained funding, supervised patient enrollment and data abstraction, contributed to data analysis, revised and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Biondi E, Evans R, Mischler M, et al. Epidemiology of Bacteremia in Febrile Infants in the United States. Pediatrics. 2013;132:990–996. doi: 10.1542/peds.2013-1759. [DOI] [PubMed] [Google Scholar]
- 2.Herz A, Greenhow T, Alcantara J, Hansen J, Baxter R, Black S, Shinefield H. Changing epidemiology of outpatient bacteremia in 3-to-36-month-old children after the introduction of the heptavalent-conjugated pneumococcal vaccine. Pediatr Infect Dis J. 2006;25:293–300. doi: 10.1097/01.inf.0000207485.39112.bf. [DOI] [PubMed] [Google Scholar]
- 3.Greenhow T, Hung YY, Herz AM, et al. The changing epidemiology of serious bacterial infections in young infants. Pediatr Infect Dis J. 2014;33:595–599. doi: 10.1097/INF.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 4.Greenhow T, Hung Y, Herz A. Changing epidemiology of bacteremia in Infants Aged 1 week to 3 months. Pediatrics. 2012;129:e590–e596. doi: 10.1542/peds.2011-1546. [DOI] [PubMed] [Google Scholar]
- 5.Greenhow T, Hung Y, Pantell RH. Management and outcomes of previously healthy, full term, febrile infants ages 7 to 90 days. Pediatrics. 2016;138:e20160270. doi: 10.1542/peds.2016-0270. [DOI] [PubMed] [Google Scholar]
- 6.Aronson PL, Thurm C, Alpern ER, et al. Variation in care of the febrile young infant <90 days in US pediatric emergency departments. Pediatrics. 2014;134:667–677. doi: 10.1542/peds.2014-1382. [DOI] [PubMed] [Google Scholar]
- 7.Nigrovic L, Mahajan PV, Blumberg S, Browne L, Linakis JG, Ruddy RM, Bennett JE, Rogers AJ, Tzimenatos L, Powell EC, Alpern ER, Casper C, Ramilo O, Kuppermann N. The Performance of the Yale Observation Scale Score and Unstructured Clinician Suspicion to Identify Febrile Infants 60 Days of Age or Younger with Serious Bacterial Infections. Pediatrics. 2017 doi: 10.1542/peds.2017-0695. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhansali P, Wiedermann BL, Pastor W, McMillan J, Shah N. Management of Hospitalized Febrile Neonates Without CSF Analysis: A Study of US Pediatric Hospitals. Hosp Pediatr. 2015;5(10):528–533. doi: 10.1542/hpeds.2014-0175. [DOI] [PubMed] [Google Scholar]
- 9.Mahajan P, Kuppermann N, Mejias A, et al. Association of RNA biosignatures with bacterial infections in febrile infants aged 60 days or younger. JAMA. 2016;316(8):846–857. doi: 10.1001/jama.2016.9207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahajan P, Kuppermann N, Suarez N, et al. Febrile Infant Working Group for the Pediatric Emergency Care Applied Research Network. RNA transcriptional biosignature analysis for identifying febrile infants with serious bacterial infections in the emergency department: a feasibility study. Pediatr Emerg Care. 2015;31(1):1–5. doi: 10.1097/PEC.0000000000000324. [DOI] [PMC free article] [PubMed] [Google Scholar]