Abstract
Objective
This meta-analysis compared diagnostic validity of electrocorticographic (ECoG) high-γ modulation (HGM) to electrical stimulation mapping (ESM) for pre-surgical language localization.
Methods
From a structured literature search, studies with electrode level data comparing ECoG HGM and ESM for language localization were included in the meta-analysis. Outcomes included global measures of diagnostic validity: area under the summary receiver operating characteristic (SROC) curve (AUC), and diagnostic odds ratio (DOR); as well as pooled estimates of sensitivity and specificity. Clinical and technical determinants of sensitivity/specificity were explored.
Results
Fifteen studies were included in qualitative synthesis, and 10 studies included in the meta-analysis (number of patients 1–17, mean age 10.3–53.6 years). Overt picture naming was the most commonly used task for language mapping with either method. ECoG HGM was analyzed at 50–400 Hz with different bandwidths in individual studies. For ESM, pulse duration, train duration, and maximum current varied greatly among studies. Sensitivity (0.23–0.99), specificity (0.48–0.96), and DOR (1.45–376.28) varied widely across studies. The pooled estimates are: sensitivity 0.61 (95% CI 0.44, 0.76), specificity 0.79 (95% CI 0.68, 0.88), and DOR 6.44 (95% CI 3.47, 11.94). AUC was 0.77. Results of bivariate meta-regression were limited by small samples for individual variables.
Conclusion
ECoG HGM is a specific but not sensitive method for language localization compared to gold-standard ESM. Given the pooled DOR of 6.44 and AUC of 0.77, ECoG HGM can fairly reliably ascertain electrodes overlying ESM cortical language sites.
Keywords: Functional brain mapping, Language localization, Epilepsy surgery
1. INTRODUCTION
To ensure safe and effective resective neurosurgery for epilepsy, tumors, and other brain lesions, it is often necessary to determine the functional localization of language cortex in individual patients. The conventional method of extra-operative electrical stimulation mapping (ESM) involves stimulation of implanted intracranial electrodes and observation for behavioral effects. ESM is associated with risks of after-discharges, seizures, and pain, which can all interfere with comprehensive mapping [1, 2]. There is also evidence for language thresholds to exceed after-discharge thresholds particularly in younger children [3]. Moreover, because it must be done sequentially for electrode pairs, ESM is time consuming, effectively limiting the number of sites that can be tested. The neurophysiological validity of stimulation-induced “all-or-none” interference with elementary language tasks to faithfully capture brain language representation is also questionable [4]. Hence, an alternative approach for functional localization has emerged, based on task-related modulation in electrocorticograph (ECoG) spectra [5]. This approach has usually focused on power modulations in the high-γ (typically >40 Hz) band, which have shown good correlation with neural firing rates and blood oxygen-level dependent response [6]. ECoG high-γ modulation (HGM) has been consistently observed during several language tasks with favorable spatial-temporal profile [7, 8]. However, clinical validation of ECoG HGM mapping against ESM is limited to small samples with variable results. This has frequently raised concerns whether ECoG HGM should be adopted in routine clinical practice, either as a supplement or replacement for ESM. Hence, this meta-analysis was performed to obtain pooled estimates of the diagnostic validity of ECoG HGM compared to ESM for pre-surgical language localization and to explore the sources of variability among the studies.
2. METHODS
2.1 Literature search
Electronic databases including PubMed, EMBASE (all resources), and Cochrane library (all registers) were systematically searched on December 16, 2016 for articles in English, with appropriate keywords related to functional mapping, high-frequency oscillations, and neurosurgery (Table e1). Studies comparing language localization with ECoG HGM and ESM were eligible for inclusion. For this study, we defined γ-band as ≥50 Hz [9, 10]. Studies which reported neither sensitivity/specificity, nor sufficient electrode level data to allow their calculation, were excluded. Studies where ESM did not interfere with language function, or where authors analyzed HGM in arbitrarily spatially restricted electrodes, were also excluded.
2.2 Data extraction
Following variables were extracted from the included studies: number of patients, mean age, native language, sample criteria, tasks used for ECoG HGM and ESM, frequency band for ECoG HGM analysis, ESM settings (pulse frequency, pulse duration, stimulus train duration, maximum stimulation current), and criterion for scoring ESM positive electrodes. Electrode level data (i.e. number of electrodes positive and negative for language by ECoG HGM and ESM respectively) were extracted and used for meta-analysis from studies which provided this detail; otherwise, reported sensitivity/specificity were extracted. Some of the studies reported electrode data for multiple subgroups based on implanted hemisphere, tasks used, or definition of language positive sites. Only one representative subgroup was included per study in the meta-analysis, since the subgroups were unlikely to be mutually independent. Studies which did not report electrode level data were reviewed but could not be included in the meta-analysis.
2.3 Outcomes
The primary outcome measure was the area under the summary receiver operating characteristic (SROC) curve (AUC), which represents a global measure of diagnostic validity from pooled data. Other outcomes included pooled estimates of diagnostic odds ratio (DOR), sensitivity, specificity, and metrics representing heterogeneity in the data. Determinants of sensitivity/specificity were also explored including mean age of patients, native language (English/others), minimum and maximum frequencies of the bandwidth used for ECoG HGM analysis, and pulse duration and maximum current strength used for ESM.
2.4 Statistical analysis
The meta-analysis of ESM and ECoG HGM comparisons presented unique challenges, since each study contributed multiple patients, each having multiple electrodes for eventual analysis. These electrodes cannot be regarded as independent observations since they are nested by patients in each study, necessitating a multilevel approach. Further, the sensitivity and specificity of each study is correlated and requires a bivariate model for their joint distribution. Due to these considerations, sensitivity, specificity, and DOR for individual studies was first calculated, along with 95% confidence interval (CI), from electrode data. Equality of sensitivities and specificities across studies were tested using χ2 test to explore heterogeneity in the data. Then, pooled estimates of sensitivity, specificity, and DOR were obtained with bivariate random effects meta-analysis using the restricted maximum likelihood method. AUC was estimated from a hierarchical SROC curve obtained by modeling its slope in the logit space as the geometric mean of slopes of 2 regression lines, logit(sensitivity) on logit(1 – specificity) and vice versa [11]. This ensures the symmetry of the SROC curve with respect to sensitivity and specificity and also accounts for potential differences in the precision of the estimates from included studies. Pooled DOR was obtained using DerSimonian and Laird (DSL) estimator, along with Higgin’s I2 statistic which represents the proportion of observed variance from the “true” heterogeneity in effect size [12]. The DSL method incorporates study-specific heterogeneities using inverse variance approach to adjust weight assigned to each study. A bi-variate meta-regression was performed to explore determinants of the joint distribution of sensitivity and false positive rate (FPR = 1 – specificity) using the linear mixed model described by Reitsma et. al. [13]. Odds ratios (OR) with 95% CI were obtained for sensitivity and FPR for all covariates using inverse logit transformation on the fitted models. This is essentially an extension of random effects approach and assumes the (logit transformed) sensitivities and specificities of the analyzed studies to be approximately normally distributed with the variability resulting from unmeasured differences in the study population or test performance. This framework also incorporates possible correlation between sensitivity and specificity, sampling error, and provision for including covariates. All analyses were performed using the “MADA” library in R [14].
3. RESULTS
Fifteen studies were included, having 1 to 17 patients, with mean age varying from 10.3 to 53.6 years (Table 1) [15–29]. Six of the studies included native speakers of languages other than English. Overt picture naming was the most common task used both for ECoG HGM as well as ESM; however, a multitude of tasks/task-combinations were used for language mapping (Tables 1, 3). The frequency band for ECoG power modulation varied from 50 to 400 Hz with different bandwidths. The pulse frequency used for ESM was identical across the studies at 50 Hz, but the pulse duration (200–500 us), train duration (2–10 s), and maximum current (5–15 mA) varied greatly. Five studies did not provide electrode level data, allowing only 10 studies to be included in the meta-analysis (Fig. 1) [15–17, 19–22, 25].
TABLE 1.
Studies included in qualitative and quantitative (*) synthesis for comparison of electrical stimulation mapping (ESM) and electrocorticographic (ECoG) high-γ modulation for extra-operative language localization
| Study | n | Age (years, mean ± SD) |
Native language |
Sample criteria | ECoG task(s) | Frequency band (Hz) for ECoG HGM |
ECoG signal processing |
ESM task(s) | ESM pulse duration, train duration, maximum current |
ESM+ definition |
Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arya, 2015*^ | 7 | 10.3 ± 4.1 | English | Excluded those unable to converse | Conversational Speech | 70–116 | Custom block design based on SIGFRIED (500 ms windows) | Picture naming | 500 μs, 5s, 10 mA | Naming and/or oral motor deficits | 0.89 | 0.63 |
| Arya, 2017*^ | 17 | 11.3 ± 4.4 | English | Included patients able to name pictures | Overt picture naming | 70–116 | Custom block design based on SIGFRIED (500 ms windows) | Picture naming | 500 μs, 5 s, 10–15 mA | Naming and/or oral motor deficits | 0.47 | 0.81 |
| Babajani- Feremi, 2016* | 9 | 23 ± 8 | English | LH language dominance; No known frontal lobe pathology that could affect language representation | Overt object naming | 50–119 | TPC to identify clusters of bins spanning ≥20 Hz and 150 ms within [0 3]s time window | Sentence reading; comprehension of spoken sentences; sentence repetition; confrontation naming; modified token test | 500 μs, 3–5 s, 5 mA | Speech arrest or disruption, not accompanied by positive motor signs | 1.00 | 0.83 |
| Bauer, 2013^ | 8 | 36.1 ± 7.8 | Dutch | Age >12 years; no language/other major cognitive impairment; LH language on IAT | Speaking (retrospective 90 s epochs) | 65–95 | TPC | Object/picture naming | NA, 4–7s, 10 mA | Speech arrest/disruption | 0.22 | 0.82 |
| Genetti, 2015* | 12 | 23.1 ± 13 | French German, English^ | Semantic congruency decision | 70–160 | TPC using 80 ms sliding Hamming windows with 87.5% overlap ratio | Automatic speech (counting or naming) | 300 μs, 2–5 s, 10 mA | 0.50 | 0.92 | ||
| Kojima, 2012*^ | 13 | 16.3 ± 8.2 | LH language by IAT; Excluded “massive” MCD (peri-Sylvian, peri-Rolandic, hemispheric) | Response to an auditory question | 50–120 | TPC | Response to auditory question(s), picture naming, counting, reciting alphabet | 300 μs, 5–10 s, 9 mA | Naming difficulty OR mouth/throat sensorimotor symptoms | 0.91 | 0.61 | |
| Korostenskaja, 2014* | 1 | 13 | Picture naming, story listening | 60–170 | Custom block design based on SIGFRIED (500 ms windows) | Picture naming | 200 μs, 5 s, 10 mA | 0.25 | 0.91 | |||
| Miller, 2011*^ | 7 | 27.3 ± 9 | Verb generation | 76–200 | Custom method based on BCI2000 using 80 ms windows with 40 ms overlap | Verb generation | NA, 3 s, 10 mA | 0.74 | 0.48 | |||
| Mooij, 2016 | 9 | 30.6 ± 10.9 | Dutch | LH or bilateral language by IAT/fMRI | Story and music listening | 65–95 | Custom method with γ-band time- domain signal reconstruction and discriminant analysis | Picture naming | 200 μs, 4–7 s, 10 mA | 0.35 | 0.93 | |
| Ogawa, 2017^ | 7 | 43.6 ± 12.6 | Japanese | Brain tumors in language dominant hemisphere | Picture Naming | 60–170 | Custom method using a variance metric (R2) for task-related increase in γ-power | Sentence repetition, picture naming, word reading | 500 μs, NA, 15 mA | 0.90 | 0.90 | |
| Ruescher, 2013*^ | 3 | 43.3 ± 4.9 | German | Conversational speech | 60–400 | TPC (500 ms window) | Battery including reading, counting, object naming, command execution, token test, sentence repetition | 250 μs, 10 s, 15 mA | Oral motor interference | 0.22 | 0.97 | |
| Sinai, 2005*^ | 13 | 33.8 ± 11.2 | LH language dominance, IQ> 80, no language impairment | Picture Naming | 80–100 | TPC with mixed effects model (100 ms epochs with 50% overlap) | Picture naming, sentence comprehension (modified token test), paragraph reading, spontaneous speech | 300 μs, 1–5 s, 15 mA | Naming and/or oral motor deficits | 0.43 | 0.84 | |
| Tamura, 2016 | 4 | 53.6 ± 17.3 | Japanese | Tumor(s) involving dominant frontal/temporal lobes | Story listening | 80–160 | TPC | Spontaneous speech, word repetition, picture naming | 300 μs, NA, 15 mA | Speech arrest/aphasia | 0.94 | 0.95 |
| Towle, 2008 | 12 | 19.8 ± 8.9 | Drug-resistant epilepsy | Word repetition, new word identification, conversation | 70–100 | TPC | 300 μs, 2–10 s, 10 mA | 0.63 | 0.57 | |||
| Wang, 2016*^ | 7 | 36.4 ± 20 | English | Picture naming, word repetititon | 70–110 | Trial-averaged or single trial amplitude change | NA, NA, 12 mA | 0.70 | 0.84 |
(ECoG electro-corticographic, ESM electrical stimulation mapping, IQ intelligence quotient, fMRI functional magnetic resonance imaging, IAT intra-carotid amobarbital test [Wada test], LH left hemisphere, MCD malformations of cortical development, n number of patients, NA not available, RH right hemisphere, SD standard deviation, SIGFRIED signal modeling for real-time identification and event detection, TPC Trial-averaged Power Comparison (between task and baseline),
these studies include other subgroups as well and only one representative subgroup is included in this table, for other subgroups see table 3).
TABLE 3.
Subgroups other than those included in meta-analysis for studies that analyzed outcomes in multiple different groups based on criteria for defining electrodes overlying language sites on ESM, tasks used for language mapping, and brain lobes.
| Study | Criteria for subgroups | Subgroups | Sensitivity | Specificity |
|---|---|---|---|---|
| Arya, 2015 | Definition of ESM+ | Only naming deficits | 0.83 | 0.63 |
| Only oral motor | 1.00 | 0.69 | ||
| Either | 0.89 | 0.63 | ||
| Arya, 2017 | Hemisphere, definition of ESM+, task (overt or covert) used for ECoG HGM | LH, overt, ESM+ either naming/oral motor | 0.47 | 0.81 |
| LH, overt, ESM+ only naming | 0.40 | 0.76 | ||
| LH, covert, ESM+ either naming/oral motor | 0.14 | 0.91 | ||
| LH, covert, ESM+ only naming | 0.05 | 0.87 | ||
| RH, overt, ESM+ only oral motor | 0.44 | 0.81 | ||
| RH, overt, ESM+ only oral motor | 0.20 | 0.96 | ||
| Bauer, 2013 | Task used for ECoG HGM | Speaking (retrospective 90 s epochs) | 0.22 | 0.82 |
| Listening (retrospective 90 s epochs) | 0.17 | 0.83 | ||
| Verb generation (overt = 6, covert = 1) | 0.16 | 0.88 | ||
| Picture naming | 0.21 | 0.9 | ||
| Kojima, 2012 | Brain lobe | Left temporal lobe | 0.91 | 0.61 |
| Left frontal lobe | 0.83 | 0.64 | ||
| Miller, 2011 | Task used for ESM | Noun reading | 0.89 | 0.66 |
| Verb generation | 0.74 | 0.48 | ||
| Ogawa, 2017 | Task used for ECoG HGM | Picture naming | 0.90 | 0.90 |
| Word reading (Kanji, Hiragana) | 0.89 | 0.89 | ||
| Ruescher, 2013 | Definition of ESM+ | Only oral motor | 0.22 | 0.97 |
| Only speech difficulties | 0.00 | 0.94 | ||
| Sinai, 2005 | Definition of ESM+ | Only naming | 0.38 | 0.78 |
| Oral motor | 0.46 | 0.81 | ||
| Either | 0.43 | 0.84 | ||
| Wang, 2016 | Task used for ECoG HGM | Picture naming | 0.63 | 0.81 |
| Word repetition | 0.76 | 0.86 | ||
| Overall | 0.70 | 0.84 |
(ECoG electro-corticography; ESM electrical stimulation mapping; HGM high-gamma modulation; LH left hemisphere; RH right hemisphere)
Figure 1.
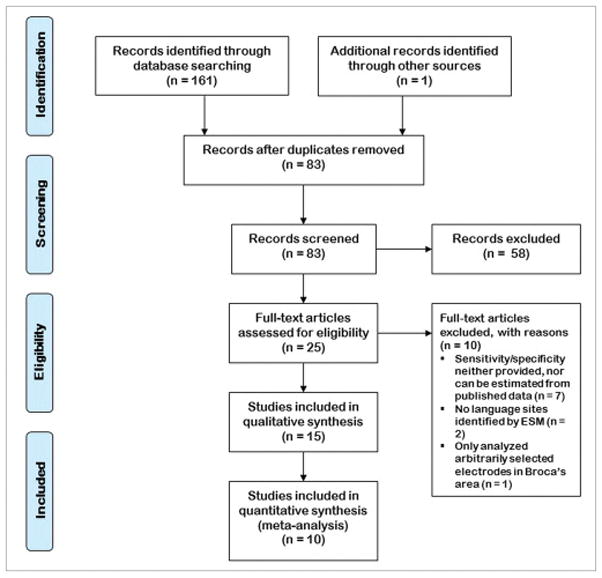
The PRISMA flow diagram
3.1 Diagnostic accuracy meta-analysis
Sensitivity (0.23–0.99), specificity (0.48–0.96), and DOR (1.45–376.28) varied widely across individual studies (Fig. 2) [17, 22, 25]. For studies that provided electrode level data, this was also substantiated by the test for equality of sensitivity and specificity which showed significant heterogeneity (p<0.0001 for both sensitivity and specificity), and the large confidence intervals around these data points (Fig. 3). The pooled estimates were: sensitivity 0.61 (95% CI 0.44, 0.76) and specificity 0.79 (95% CI 0.68, 0.88). The pooled DSL estimate for DOR was found to be 6.44 (95% CI 3.47, 11.94) with low heterogeneity (I2 = 23.1%) [30]. The AUC was estimated to be 0.77. The pooled estimates along with confidence and prediction ellipsoids for the joint distribution and SROC curve are shown in Figure 4.
Figure 2.
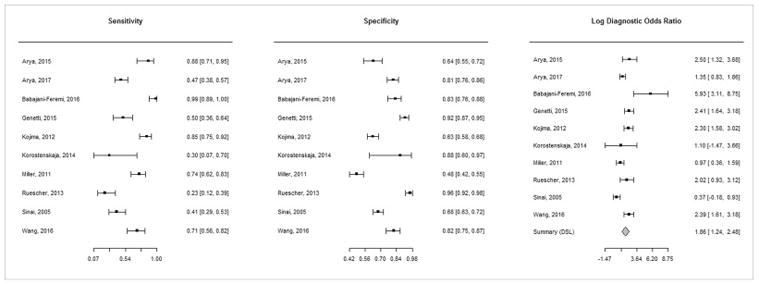
Forest plots of sensitivity, specificity, and diagnostic odds ratio (DOR) showing study specific estimates with 95% confidence intervals for these metrics.
The estimates for DOR are on a logarithmic scale. Since the sensitivity/specificity pairs from all studies are correlated, separate pooled estimates are not calculated for them. Instead, a pooled estimate for DOR is obtained.
Figure 3.
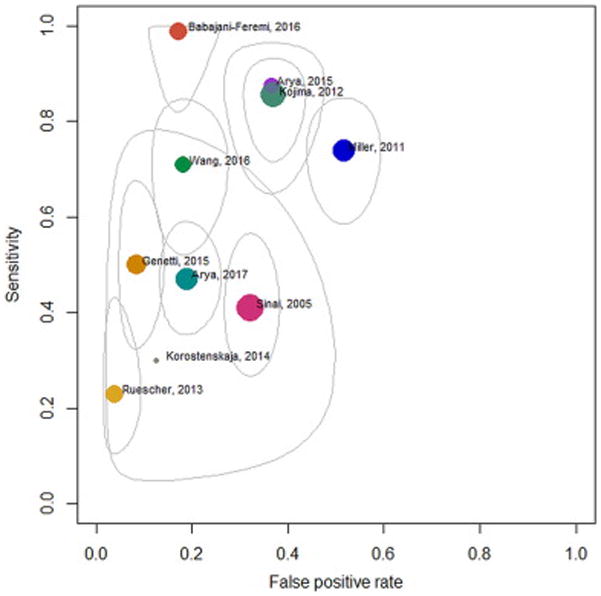
Confidence ellipsoids for the uncertainty in the pair of sensitivity and false positive rate
Confidence regions for the study specific paired estimates of sensitivity and false positive rate (colored bubble), plotted in the receiver operating characteristic (ROC) space. The size of the bubble is proportional to the total number or electrodes in the study.
Figure 4.
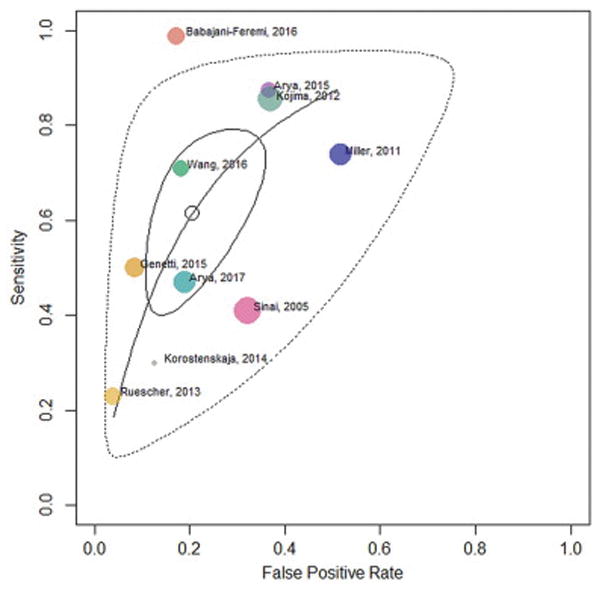
Summary Receiver Operating Characteristic (SROC) curve
SROC curve is shown with pooled estimate (open circle), 95% confidence region (solid closed curve), and 95% prediction region (dotted closed curve). This curve is obtained by modeling its slope in the logit space as the geometric mean of the slopes of the two regression lines, logit (sensitivity) on logit (false positive rate) and vice versa (Rutter-Gatsonis Hierarchical SROC). Study specific estimates (colored bubbles) are also shown. The size of the bubble is proportional to the total number or electrodes in the study.
3.2 Meta-regression
A bivariate meta-regression for the joint distribution of sensitivity and FPR found maximum current used for ESM (OR 39.31, 95% CI 4.02, 384.25, p = 0.001) to significantly determine sensitivity. Also, studies including speakers of languages other than English had significantly higher specificity (lower FPR, OR 0.06, 95% CI 0.01, 0.28, p = 0.001) compared to studies of English speakers (Table 2).
TABLE 2.
Bi-variate meta-regression
| Sensitivity | FPR | ||||
|---|---|---|---|---|---|
| Co-variate | Included Studies [References] | OR (95% CI) | p-value | OR (95% CI) | p-value |
| Mean age (years) | 15 [15–29] | 4.17 (0.75, 23.03) | 0.194 | 0.51 (0.11, 2.49) | 0.315 |
| Native language (Non English vs. English) | 10 [15–19, 23–25, 27, 29] | 0.55 (0.05, 5.59) | 0.083 | 0.06 (0.01, 0.28) | 0.001* |
| ECoG bandwidth lower bound (Hz) | 15 [15–29] | 18.96 (0.13, 2783.28) | 0.317 | 0.04 (0.00, 3.43) | 0.398 |
| ECoG bandwidth upper bound (Hz) | 15 [15–29] | 4.75 (1.20, 18.73) | 0.078 | 0.73 (0.22, 2.35) | 0.054 |
| ESM maximum current (mA) | 15 [15–29] | 39.31 (4.02, 384.25) | 0.001* | 0.45 (0.03, 6.82) | 0.616 |
| ESM pulse duration (ms) | 12 [15–17, 19–21, 23–28] | 0.13 (0.01, 2.50) | 0.087 | 0.07 (0.01, 0.75) | 0.325 |
(Bivariate random effects models were fitted to the pairs of transformed sensitivities (Sensitivityt) and false positive rates (FPRt): (Sensitivityt, FPRt) = ax + b, where: a = slope, b = intercept, and x = the covariate.
The transformation used was: x ⟼ ta (x): = a log x – (2 – a) log (1 – x)
Note that for α = 1, the logit transformation results. Hence, the odds ratios [OR] were obtained by inverse logit transformation, and represent the proportional gain in sensitivity or FPR per unit change in the covariate, for continuous variables. CI confidence interval, ECoG electrocorticographic, ESM electrical stimulation mapping, *p ≤ 0.05)
4. DISCUSSION
This meta-analysis showed that ECoG HGM is a specific (0.79, 95% CI 0.68, 0.88) but not sensitive (0.61, 95% CI 0.44, 0.76) modality for language localization compared to ESM as the current clinical gold-standard. Note that CI around the pooled estimate included 0.5 for sensitivity but not specificity. Pooled DOR of 6.44 (95% CI 3.47, 11.93) indicates that electrode sites with HGM, compared to those without, have a 6.44 times greater odds of being classified as ESM-defined language cortex. Given this pooled DOR and an AUC of 0.77 from SROC curve, ECoG HGM was a good binary classifier of electrodes overlying language cortex (Fig. 4). Thus, with good specificity and DOR, ECoG HGM can be relied upon to ascertain electrodes which are likely to be ESM− for language, but probably not otherwise.
The data showed significant heterogeneity as evident from large confidence ellipsoids around study-specific estimates (Fig. 3), and tests for equality of sensitivity and specificity across studies. However, the meta-regression performed to explore sources of this heterogeneity was not informative, in our opinion (Table 2). The maximum current used for ESM was found to significantly determine sensitivity, and the specificity of studies including non-English speaking patients was significantly higher than that of studies including native English speakers. However, it is possible that these findings represent aberrations due to data architecture, and lack physiological basis. A majority of the studies (n = 9) used 10–15 mA as the maximum current for ESM. However, one study which reported 100% sensitivity, performed ESM at only up to 5 mA (Fig. 5) [17]. Similarly, the effect of native language was driven by 2 studies only. Hence, the observed statistical significance in the regression analysis could have been due to the small number of data points and the effects of these outliers.
Figure 5.
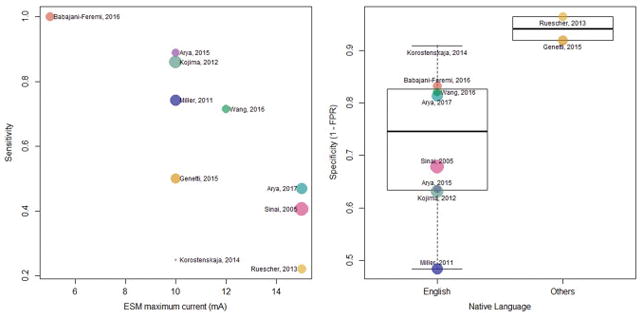
Determinants of pairs of sensitivity and false positive rate on bi-variate meta-regression
Bi-variate meta-regression showed maximum frequency used for electrocorticographic (ECoG) high-γ modulation (HGM), and maximum current used for electrical stimulation mapping (ESM) to be significant determinants of sensitivity (upper panel). Studies including native speakers of languages other than English showed significantly lower sensitivity and higher specificity (lower panel).
Additional potential sources of variability in the sensitivity/specificity of ECoG HGM compared to ESM, include the methods for performing, analyzing, and interpreting these techniques. Regarding ESM, although the pulse frequency was consistent across the studies, there was variability in the pulse duration, train duration, and maximum current used. These variables affect the ability to perform sufficient trials of a given language task during stimulations, and to observe for interference with task performance. For trial-based tasks like picture naming, the train duration should be sufficient to allow the patient to see and name at least 2–4 pictures during stimulation, to permit observation for consistent interference. Also, it is known that the current strength required to cause interference with language tasks, is a function of age, after-discharge thresholds, and prior stimulation [31, 32]. Especially in children, a higher current strength, often above the after-discharge threshold, is required for interference with language task(s) [1, 3, 33]. In fact, the optimal current strength for ESM is not well-established, and varies across centers. Some evidence suggests that ESM may relatively poorly localize language cortex in young children compared to older individuals [33]. However, lack of separate pediatric electrode-level data in studies including both children and adults, precludes a subgroup analysis for HGM and ESM diagnostic comparison (Table 3).
Further, there was no uniformity in the language tasks used for ESM (Tables 1, 3). Studies on ESM have shown that stimulation of different regions of the cortex can produce task-specific interference. For example, ESM in peri-sylvian cortex is known to interfere with orofacial motor sequencing, phoneme identification, and automatic speech tasks [34, 35]. Whereas, repetition tasks, visual naming, and auditory naming sites have been identified in mid-to-posterior superior temporal gyrus, posterior inferior frontal gyrus, and adjacent regions [35, 36]. Limited evidence suggests further variability in verb generation sites including frontal lobe (anterior to visual naming sites), and temporo-parietal cortex [37].
The scoring of electrodes as ESM+ for language was also variable across studies. It is important to note that while ESM is often performed between electrode pairs, HGM analysis is based on referential ECoG recordings which yield estimates of activation at individual electrodes. In clinical practice, it is common to score an electrode as ESM+ only if stimulation consistently interferes with language task in two or more tested pairs including that electrode. For electrodes tested only in a single pair, it is customary, at least in our centers, to score them same as the pair. While many studies have conformed to this clinical practice [16, 18, 26], others have used the more liberal “next-neighbor” approach of scoring all electrodes surrounding the one with language interference as being positive by ESM [17, 19]. A similar approach has been used in a study demonstrating the clinical utility of ESM [38].
Methodological variability was even greater for language mapping with ECoG HGM, which was performed in most centers purely for research purposes. There was almost no uniformity in the equipment and computational methods used for signal acquisition, pre-processing, and analysis. Although a majority of studies averaged the power change over a number of trials of a given language task [17–20, 23, 26, 28], others used block designs based on signal modeling for real-time identification and event detection (SIGFRIED) algorithm of BCI2000 [15, 21, 24], or custom methods specific to their labs [22, 29]. Channels with excessive artifact or epileptiform activity also confound power estimation for ECoG HGM signal analysis, particularly with trial-averaging. However, many of the studies have specified removing noisy channels before re-referencing to the common average [15–18, 24, 27]. The frequency bandwidth used for analysis of task-related power modulations, and the language tasks themselves, varied widely across studies (Tables 1, 3). The criteria for scoring an electrode as positive for language-related HGM were also inconsistent. Some studies used pre-specified statistical cut-offs, whereas others arbitrarily chose electrodes based on location or those with highest power differential compared to baseline [15, 16, 23, 26, 27]. These factors along with the variability in electrode coverage in individual patients, and potential reorganization of brain networks due to chronic epilepsy, made evidence synthesis and interpretation of pooled data very challenging.
Like any other evidence synthesis, we could only attempt to reconcile disparate studies, but could not improve upon the heterogeneous source data. Several eligible studies did not provide electrode level data, thus precluding them from meta-analysis. Many of those who did provide such data, analyzed it in multiple different subgroups based on anatomic location of analyzed electrodes, tasks used for ESM/ECoG HGM, or criteria for scoring ESM+ electrodes, while we could include only one subgroup per study (Table 3). Further, individual patients in each study contributed multiple electrodes towards the meta-analysis, whereas we had to regard the patients as independent observations. There is a lack of mathematical methods to model such a multilevel nested analysis for diagnostic accuracy comparisons [11]. Hence, our pooled estimates should only be regarded as suggestive of general trends.
Compared to ESM, ECoG HGM has a number of potential advantages for clinical practice. Because it is based on passive recordings, ECoG HGM carries no risk of pain, after-discharges, or stimulated seizures, and it can be used to rapidly, and less tediously, survey all electrodes simultaneously at the bedside. However, the results of our meta-analysis suggest that ECoG HGM is a specific, but not sensitive, classifier of ESM language sites. If one assumes that ESM provides the ground truth for cortical language representation and accurately predicts the outcome of cortical resection, our results would argue that ECoG HGM cannot totally replace ESM. Rather, ECoG HGM can be used to predict ESM− sites with good specificity and thus prioritize other sites for ESM before they are resected, but it cannot rule out function at a site or declare it safe for resection without also testing it with ESM. This conclusion is somewhat counterintuitive given that ECoG HGM being an activation modality, potentially shows all sites participating in a task, while ESM, which supposedly creates a transient lesion, should detect the subset of those sites which are critical for the task performance.
Although the studies reviewed here all used ESM as the gold-standard, there are a number of reasons to question this assumption. There is limited data about the geometry of current spread in brain tissue after stimulation of subdural electrodes [39], and important knowledge gaps remain about the physiological and physical determinants of language response inhibition thresholds during ESM [3]. Also, remote after-discharges noted during and after ESM suggest that electrical stimulation of a localized area of cortex can have distant neurophysiologic effects mediated by preferentially connected pathways [31, 40]. These factors may be partly responsible for the imperfect correlation between ESM findings and long-term post-operative language outcomes [4, 41, 42].
5. CONCLUSION
Our meta-analysis underscored the heterogeneity in performing, analyzing, and interpreting ECoG HGM language mapping in available studies. If ECoG HGM is to provide a potentially safer and more patient-friendly modality for language mapping in future, then there is a need for uniform methods for signal acquisition, processing, analysis, and interpretation. Most importantly, the results of ECoG HGM should be validated against long-term post-operative language outcomes, preferably in larger, more homogeneous patient populations. Since ECoG HGM simultaneously generates information about all implanted electrodes compared to ESM which is usually limited to a subset of electrodes, larger sample studies will be needed to generate more reliable estimates of predictive values that can guide clinical practice.
Supplementary Material
Table e1. Structured literature search strategy
HIGHLIGHTS.
ECoG high-γ modulation is a specific method for pre-surgical language localization
It can reliably ascertain language sites localized by electrical stimulation mapping
It can be a potentially safer technique for language mapping in selected patients
Acknowledgments
RA wishes to acknowledge Dr. Susan T. Herman of Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, for support through the American Epilepsy Society’s EpiPORT program; Dr. Phillip Doebler of Technical University, Dortmund, Germany, for help with the MADA package in R; and Dr. Yujing Wang of Johns Hopkins University for sharing raw data from her publication.
Footnotes
Contributions: Study concept, design, literature search, data extraction: NEC, RA; data analysis: RA, PSH; first draft: RA; critical review: NEC, PSH. All authors approve the final version.
Disclosure(s): Research reported in this paper was supported by NINDS of the National Institutes of Health (NIH) under award number R01NS091139 (PI: NEC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. There are no other conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jayakar P, Lesser RP. Extraoperative functional mapping. In: Engel JJ, Pedley TA, editors. Epilepsy: A comprehensive textbook. 2. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. pp. 1851–1858. [Google Scholar]
- 2.Aungaroon G, Zea Vera A, Horn PS, Byars AW, Greiner HM, Tenney JR, Arthur TM, Crone NE, Holland KD, Mangano FT, Arya R. After-discharges and seizures during pediatric extra-operative electrical cortical stimulation functional brain mapping: Incidence, thresholds, and determinants. Clin Neurophysiol. 2017;128:2078–2086. doi: 10.1016/j.clinph.2017.06.259. [DOI] [PubMed] [Google Scholar]
- 3.Zea Vera A, Aungaroon G, Horn PS, Byars AW, Greiner HM, Tenney JR, Arthur TM, Crone NE, Holland KD, Mangano FT, Arya R. Language and motor function thresholds during pediatric extra-operative electrical cortical stimulation brain mapping. Clin Neurophysiol. 2017;128:2087–2093. doi: 10.1016/j.clinph.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Hamberger MJ. Cortical language mapping in epilepsy: a critical review. Neuropsychol Rev. 2007;17:477–89. doi: 10.1007/s11065-007-9046-6. [DOI] [PubMed] [Google Scholar]
- 5.Crone NE, Sinai A, Korzeniewska A. High-frequency gamma oscillations and human brain mapping with electrocorticography. Prog Brain Res. 2006;159:275–95. doi: 10.1016/S0079-6123(06)59019-3. [DOI] [PubMed] [Google Scholar]
- 6.Lachaux JP, Fonlupt P, Kahane P, Minotti L, Hoffmann D, Bertrand O, Baciu M. Relationship between task-related gamma oscillations and BOLD signal: new insights from combined fMRI and intracranial EEG. Hum Brain Mapp. 2007;28:1368–75. doi: 10.1002/hbm.20352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crone NE, Boatman D, Gordon B, Hao L. Induced electrocorticographic gamma activity during auditory perception. Brazier Award-winning article, 2001. Clin Neurophysiol. 2001;112:565–82. doi: 10.1016/s1388-2457(00)00545-9. [DOI] [PubMed] [Google Scholar]
- 8.Crone NE, Hao L, Hart J, Jr, Boatman D, Lesser RP, Irizarry R, Gordon B. Electrocorticographic gamma activity during word production in spoken and sign language. Neurology. 2001;57:2045–53. doi: 10.1212/wnl.57.11.2045. [DOI] [PubMed] [Google Scholar]
- 9.Crone NE, Korzeniewska A, Franaszczuk PJ. Cortical gamma responses: searching high and low. Int J Psychophysiol. 2011;79:9–15. doi: 10.1016/j.ijpsycho.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engel J, Jr, Bragin A, Staba R, Mody I. High-frequency oscillations: what is normal and what is not? Epilepsia. 2009;50:598–604. doi: 10.1111/j.1528-1167.2008.01917.x. [DOI] [PubMed] [Google Scholar]
- 11.Schwarzer G, Carpenter JR, Rücker G. Meta-Analysis with R. 1. New York, NY: Springer International Publishing; 2015. [Google Scholar]
- 12.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 13.Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58:982–90. doi: 10.1016/j.jclinepi.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 14.Doebler P, Holling H. [Accessed: December 24, 2016];Meta-Analysis of Diagnostic Accuracy with mada. URL: https://cran.r-project.org/web/packages/mada/vignettes/mada.pdf.
- 15.Arya R, Wilson JA, Vannest J, Byars AW, Greiner HM, Buroker J, Fujiwara H, Mangano FT, Holland KD, Horn PS, Crone NE, Rose DF. Electrocorticographic language mapping in children by high-gamma synchronization during spontaneous conversation: comparison with conventional electrical cortical stimulation. Epilepsy Res. 2015;110:78–87. doi: 10.1016/j.eplepsyres.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Arya R, Wilson JA, Fujiwara H, Rozhkov L, Leach JL, Byars AW, Greiner HM, Vannest J, Buroker J, Milsap G, Ervin B, Minai A, Horn PS, Holland KD, Mangano FT, Crone NE, Rose DF. Presurgical language localization with visual naming associated ECoG high- gamma modulation in pediatric drug-resistant epilepsy. Epilepsia. 2017;58:663–673. doi: 10.1111/epi.13708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Babajani-Feremi A, Narayana S, Rezaie R, Choudhri AF, Fulton SP, Boop FA, Wheless JW, Papanicolaou AC. Language mapping using high gamma electrocorticography, fMRI, and TMS versus electrocortical stimulation. Clin Neurophysiol. 2016;127:1822–36. doi: 10.1016/j.clinph.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 18.Bauer PR, Vansteensel MJ, Bleichner MG, Hermes D, Ferrier CH, Aarnoutse EJ, Ramsey NF. Mismatch between electrocortical stimulation and electrocorticography frequency mapping of language. Brain Stimul. 2013;6:524–31. doi: 10.1016/j.brs.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Genetti M, Tyrand R, Grouiller F, Lascano AM, Vulliemoz S, Spinelli L, Seeck M, Schaller K, Michel CM. Comparison of high gamma electrocorticography and fMRI with electrocortical stimulation for localization of somatosensory and language cortex. Clin Neurophysiol. 2015;126:121–30. doi: 10.1016/j.clinph.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Kojima K, Brown EC, Rothermel R, Carlson A, Matsuzaki N, Shah A, Atkinson M, Mittal S, Fuerst D, Sood S, Asano E. Multimodality language mapping in patients with left-hemispheric language dominance on Wada test. Clin Neurophysiol. 2012;123:1917–24. doi: 10.1016/j.clinph.2012.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korostenskaja M, Chen PC, Salinas CM, Westerveld M, Brunner P, Schalk G, Cook JC, Baumgartner J, Lee KH. Real-time functional mapping: potential tool for improving language outcome in pediatric epilepsy surgery. J Neurosurg Pediatr. 2014;14:287–95. doi: 10.3171/2014.6.PEDS13477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller KJ, Abel TJ, Hebb AO, Ojemann JG. Rapid online language mapping with electrocorticography. J Neurosurg Pediatr. 2011;7:482–90. doi: 10.3171/2011.2.PEDS1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mooij AH, Huiskamp GJ, Gosselaar PH, Ferrier CH. Electrocorticographic language mapping with a listening task consisting of alternating speech and music phrases. Clin Neurophysiol. 2016;127:1113–9. doi: 10.1016/j.clinph.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa H, Kamada K, Kapeller C, Prueckl R, Takeuchi F, Hiroshima S, Anei R, Guger C. Clinical Impact and Implication of Real-Time Oscillation Analysis for Language Mapping. World Neurosurg. 2017;97:123–131. doi: 10.1016/j.wneu.2016.09.071. [DOI] [PubMed] [Google Scholar]
- 25.Ruescher J, Iljina O, Altenmuller DM, Aertsen A, Schulze-Bonhage A, Ball T. Somatotopic mapping of natural upper- and lower-extremity movements and speech production with high gamma electrocorticography. Neuroimage. 2013;81:164–77. doi: 10.1016/j.neuroimage.2013.04.102. [DOI] [PubMed] [Google Scholar]
- 26.Sinai A, Bowers CW, Crainiceanu CM, Boatman D, Gordon B, Lesser RP, Lenz FA, Crone NE. Electrocorticographic high gamma activity versus electrical cortical stimulation mapping of naming. Brain. 2005;128:1556–70. doi: 10.1093/brain/awh491. [DOI] [PubMed] [Google Scholar]
- 27.Tamura Y, Ogawa H, Kapeller C, Prueckl R, Takeuchi F, Anei R, Ritaccio A, Guger C, Kamada K. Passive language mapping combining real-time oscillation analysis with cortico-cortical evoked potentials for awake craniotomy. J Neurosurg. 2016;125:1580–1588. doi: 10.3171/2015.4.JNS15193. [DOI] [PubMed] [Google Scholar]
- 28.Towle VL, Yoon HA, Castelle M, Edgar JC, Biassou NM, Frim DM, Spire JP, Kohrman MH. ECoG gamma activity during a language task: differentiating expressive and receptive speech areas. Brain. 2008;131:2013–27. doi: 10.1093/brain/awn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Fifer MS, Flinker A, Korzeniewska A, Cervenka MC, Anderson WS, Boatman-Reich DF, Crone NE. Spatial-temporal functional mapping of language at the bedside with electrocorticography. Neurology. 2016;86:1181–9. doi: 10.1212/WNL.0000000000002525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lesser RP, Luders H, Klem G, Dinner DS, Morris HH, Hahn JF, Wyllie E. Extraoperative cortical functional localization in patients with epilepsy. J Clin Neurophysiol. 1987;4:27–53. doi: 10.1097/00004691-198701000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Roux FE, Durand JB, Djidjeli I, Moyse E, Giussani C. Variability of intraoperative electrostimulation parameters in conscious individuals: language cortex. J Neurosurg. 2017;126:1641–1652. doi: 10.3171/2016.4.JNS152434. [DOI] [PubMed] [Google Scholar]
- 33.Schevon CA, Carlson C, Zaroff CM, Weiner HJ, Doyle WK, Miles D, Lajoie J, Kuzniecky R, Pacia S, Vazquez B, Luciano D, Najjar S, Devinsky O. Pediatric language mapping: sensitivity of neurostimulation and Wada testing in epilepsy surgery. Epilepsia. 2007;48:539–45. doi: 10.1111/j.1528-1167.2006.00962.x. [DOI] [PubMed] [Google Scholar]
- 34.Ojemann G, Mateer C. Human language cortex: localization of memory, syntax, and sequential motor-phoneme identification systems. Science. 1979;205:1401–3. doi: 10.1126/science.472757. [DOI] [PubMed] [Google Scholar]
- 35.Ojemann GA. Electrical-Stimulation and the Neurobiology of Language. Behavioral and Brain Sciences. 1983;6:221–226. [Google Scholar]
- 36.Hamberger MJ, Goodman RR, Perrine K, Tamny T. Anatomic dissociation of auditory and visual naming in the lateral temporal cortex. Neurology. 2001;56:56–61. doi: 10.1212/wnl.56.1.56. [DOI] [PubMed] [Google Scholar]
- 37.Ojemann JG, Ojemann GA, Lettich E. Cortical stimulation mapping of language cortex by using a verb generation task: effects of learning and comparison to mapping based on object naming. J Neurosurg. 2002;97:33–8. doi: 10.3171/jns.2002.97.1.0033. [DOI] [PubMed] [Google Scholar]
- 38.Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J Neurosurg. 1989;71:316–26. doi: 10.3171/jns.1989.71.3.0316. [DOI] [PubMed] [Google Scholar]
- 39.Koessler L, Colnat-Coulbois S, Cecchin T, Hofmanis J, Dmochowski JP, Norcia AM, Maillard LG. In-vivo measurements of human brain tissue conductivity using focal electrical current injection through intracerebral multicontact electrodes. Hum Brain Mapp. 2017;38:974–986. doi: 10.1002/hbm.23431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishitobi M, Nakasato N, Suzuki K, Nagamatsu K, Shamoto H, Yoshimoto T. Remote discharges in the posterior language area during basal temporal stimulation. Neuroreport. 2000;11:2997–3000. doi: 10.1097/00001756-200009110-00034. [DOI] [PubMed] [Google Scholar]
- 41.Krauss GL, Fisher R, Plate C, Hart J, Uematsu S, Gordon B, Lesser RP. Cognitive effects of resecting basal temporal language areas. Epilepsia. 1996;37:476–83. doi: 10.1111/j.1528-1157.1996.tb00594.x. [DOI] [PubMed] [Google Scholar]
- 42.Cervenka MC, Corines J, Boatman-Reich DF, Eloyan A, Sheng X, Franaszczuk PJ, Crone NE. Electrocorticographic functional mapping identifies human cortex critical for auditory and visual naming. Neuroimage. 2013;69:267–76. doi: 10.1016/j.neuroimage.2012.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table e1. Structured literature search strategy


