Abstract
Background
Merkel cell carcinoma (MCC) incidence rates are rising and strongly age-associated, relevant for an aging population.
Objective
Determine MCC incidence in the United States and project incident cases through the year 2025.
Methods
Registry data were obtained from the SEER-18 database, containing 6,600 MCC cases. Age and sex-adjusted projections were generated utilizing US census data.
Results
Between 2000–2013, there was a 95% increase in the number of reported MCC cases, compared to 57% for melanoma and 15% for all ‘solid’ cancers. In 2013, the MCC incidence rate was 0.7 per 100,000 person-years in the US, corresponding to 2,488 cases. MCC incidence increased exponentially with age, from 0.1 to 1.0 to 9.8 (per 100,000 person-years) between age groups 40–44, 60–64, 85+ years, respectively. Due to aging of the “baby-boom” generation, US MCC incidence is predicted to climb to 2,835 cases in 2020 and 3,284 cases in 2025.
Limitations
Projections assume the age-adjusted incidence rate stabilizes and thus may be underestimates.
Conclusions
An aging population is driving brisk increases in the number of new MCC cases in the US. This growing impact combined with a rapidly evolving therapeutic landscape warrants expanded awareness of MCC diagnosis and management.
GRAPHICAL ABSTRACT
INTRODUCTION
Merkel cell carcinoma (MCC) is a neuroendocrine skin cancer with high metastatic potential, with one-third to one-half of patients developing recurrence or metastasis. In 2007, annual incidence of MCC in the US was estimated at 1500 cases per year.1 80% of MCCs are caused by a common virus (Merkel cell polyomavirus),2, 3 and the remaining 20% by extensive UV-mediated damage.4, 5,6–8 MCCs that are diagnosed at early stage have better outcome, and high dermatologist density has been associated with improved MCC-specific survival suggesting provider familiarity with MCC may positively impact patient outcomes.9 For patients with metastatic disease, immunotherapies have been recently demonstrated to be effective in MCC,10–12 and there is emerging evidence that these are most effective if given prior to any chemotherapy, highlighting the importance of proper up front systemic therapy.13 Therefore, updated incidence numbers can allow for better appreciation of the true impact of MCC and if increasing, proportionally increase its prominence in education for providers including those in primary care, dermatology, surgery and medical oncology, with hopes of improving patient outcomes.
From its first description by Toker in 1972,14 the observed incidence of MCC grew rapidly and this trend was sustained into the new millennium.15, 16 Increases were felt to initially represent an underappreciation/misdiagnosis of MCC cases that was improved in the 1990s with the widespread adoption of CK20 antibody immunohistochemistry. Over the past 10 years, the MCC incidence rates have been reported to continue to rise worldwide: in France,17 Sweden,18 Germany,19 Australia,20 China,21 and the United States.22 However, to our knowledge no estimates of total annual US incidence (number of cases) have been published within the last five years. Furthermore, a large population shift is anticipated, with most “baby boomers” passing the 65 year threshold, at which the risk of MCC markedly increases. Indeed, the percentage of Americans >65 years of age is expected to dramatically increase from 13% of the population in 2015 to 20% in 2025.23 Therefore, we used the SEER-18 registry, which captures approximately 28% of the US population,24 in order to estimate current MCC incidence, and cross reference these data with US census projections to forecast incidence in 10 years.
MATERIALS AND METHODS
SEER Database
De-identified national registry data from the Surveillance, Epidemiology, and End Results (SEER-18) database25, 26 was accessed using SEER*Stat 8.3.2 software in February 2017. Incidence data were collected from a SEER-18 “rate session”. The SEER-18 registry contains information from registries that are geographically represented across the US (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, Los Angeles, San Jose-Monterey, Rural Georgia, Alaska Native Tumor Registry, Greater California, Greater Georgia, Kentucky, Louisiana, and New Jersey). At the time of database access, data were available from 2000–2013. Rates were age and sex adjusted to the 2000 US Standard population (19 age groups – Census P25-1130). Data were selected for cases in the research database with known sex and age and tumors with SEER defined “malignant behavior”. Data were extracted for MCC (ICD-O-3 Hist/behavior code 8247/3), malignant melanoma (codes 8720/3–8761/3) and for the SEER defined site recode B ICD-O-3/WHO 2008 grouping “All Solid Tumors” (http://seer.cancer.gov/siterecode).
US Census data
For the years 2000–2013, US Census Population Data were accessed through a frequency session utilizing SEER*Stat 8.3.2 software (Populations- Total US 1969–2015 Katrina/Rita Adjustment). For the years 2015, 2020, and 2025 US population estimates were downloaded from the 2014 national population projections publicly available at census.gov.23
Statistical Analyses
Statistical analyses were performed in SEER*Stat software and standard errors/confidence intervals generated with the Tiwari et al 2006 modification for confidence intervals.27 Projected incidences were calculated using 2011–2013 incidence rates for each age and sex bracket (with multiple years allowing for reduced error in incidence rate) and total projected incidence was summed (Supplemental Table 1). Graphs were created in GraphPad Prism software.
RESULTS
Trends in MCC incidence rate and reported cases
A total of 6,600 cases of Merkel cell carcinoma (MCC) were reported to SEER between 2000 and 2013 (the most recent year for which data are were available at the time of extraction in February 2017). Age and sex adjusted incidence rates were calculated and normalized to the 2000 US standard population.
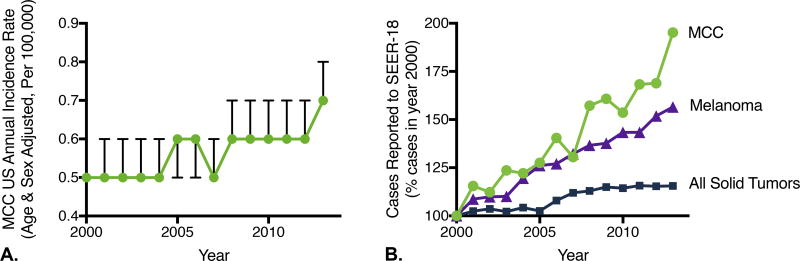
For all solid cancers, there was a significant decrease in the standardized incidence rate between 2000 (429 cases per 100,000, 95% CI 427.5–430.5) and 2013 (379.8 cases per 100,000, 95% CI 378.6–381.1). In contrast, for the most aggressive skin cancers (melanoma and Merkel cell carcinoma), incidence rates significantly increased. For MCC, the incidence rate rose from 0.5 cases per 100,000 in 2000 (95%CI 0.4–0.5) to 0.7 per 100,000 in 2013 (95% CI 0.7–0.8)(Figure 1A).
Figure 1. Changes in incidence of Merkel cell carcinoma (MCC) as compared to all solid tumors and melanoma, 2000–2013.
Data were extracted from the SEER-18 database, which captures 28% of the US population. A) US annual incidence rate of Merkel cell carcinoma The US annual incidence rate, age and sex adjusted to the 2000 US standard population (cases per 100,000 persons per year). Bars represent 95% confidence intervals. B) Cases reported to SEER with year 2000 as reference. The change in number of cases reported to SEER-18 (which reflects incidence rate and number of persons at risk in SEER catchment area) are shown, normalized to year 2000. The total number of solid tumors reported (blue squares) increased by 15% between 2000 and 2013, as compared 57 percent for melanoma (purple triangles), and 95% for MCC (green circles).
Next, we determined changes in the total number of cases reported annually to the SEER-18 database (28% of US population captured). The number of cases reflects the incidence rate, the population at risk, and the database capture efficiency. For all solid tumors, there was a modest 15.5% increase in total number of cases reported to SEER-18 (from 313,683 in 2000 to 362,397 in 2013). In contrast, for MCC a 95.2% increase was observed (from 334 cases captured by SEER in 2000 to 652 in 2013) (Figure 1B); this impressive increase exceeded even the 56.5% increase seen with melanoma (from 13,945 to 21,824 reported cases).
Association of Demographic Factors with MCC
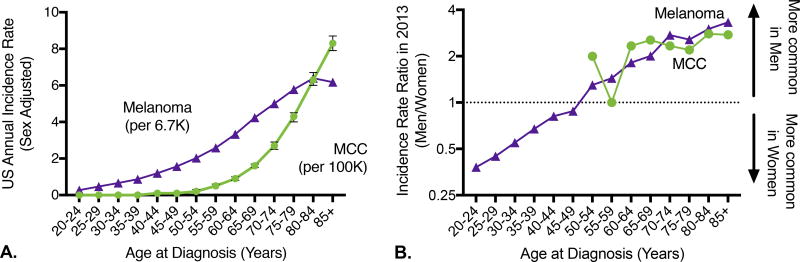
The incidence rate of MCC increases dramatically with age (Figure 2A; n = 6,600 MCC cases) and this effect is more pronounced than for melanoma (Figure 2A; n = 251,437 melanoma cases) or for solid tumors in general (Supplemental Figure 1). Specifically, the MCC incidence rate increases 10-fold between ages 40–44 (rate 0.1 cases/100,000/year, 95% CI 0–0.1) and 60–64 (rate 0.9/100,000/year, 95%CI 0.8–1) and 10-fold again between ages 60–64 and 85+ (rate 8.3 cases/100,000/year, 95% CI 7.9–8.7). This trend has been sustained, and data from 2011–2013 (the most recent years with data available, n=1778) are consistent: 0.1 cases/100,000 for ages 40–44, 1.0/100,000 for ages 60–64, and 9.8/100,000 for ages 85+. Unlike the rate of most cancers that decrease among the oldest (85+) individuals, the rate of MCC continues its significant rise. Consistent with this, in 2013 the median age at diagnosis for MCC was between 75–79 years for both men and women, as compared to 65–69 years for men with melanoma and 60–64 years for women with melanoma. 84% of persons with MCC were 65 years or older at diagnosis.
Figure 2. Merkel cell carcinoma disproportionately impacts individuals >=65 years of age.
A) Incidence rate by age. Incidence rate by age is shown for Merkel cell carcinoma (green circles, per 100,000 persons) and melanoma (purple triangles, per 6,667 persons). Unlike for melanoma, the incidence rate of MCC increases in individuals >= 85 years of age. N=6,600 cases of Merkel cell carcinoma and 251,437 cases of melanoma (all cases reported to SEER between 2000–2013 with associated age and sex information). 95% confidence intervals are shown. B) Relative incidence in men and women by age. Both MCC and melanoma have a strong male predominance in the oldest individuals. There are insufficient cases of MCC below age 50 to determine whether women in the ‘Gen-X’ and ‘Millenial’ generations will be at higher MCC risk relative to men, as they are for melanoma. Year 2013 only is shown due to rapid changes in melanoma risk for young women. Note that Y axis is on logarithmic scale.
Across all age groups in the US, the incidence rate of Merkel cell carcinoma is higher in men than in women, and this effect is most pronounced at the oldest age groups (Figure 2B). For melanoma, incidence rates are higher in men than women over the age of 50, and higher in women than men under age 50,28 suspected to be due in part to changing patterns of UV exposure including indoor tanning.29 MCC incidence below the age of 50 is too low to evaluate whether this trend towards increased risk in younger cohorts of women (“Gen-X” and “millennial” generations) will also hold true for MCC. Approximately 2/3 of cases of MCC are currently diagnosed in men and this was stable between 2000–2013.
Ultraviolet light is a well-established MCC risk factor.30 Consistent with this, observed MCC incidence rates were highest in non-Hispanic white individuals. In the most recent years for which data is available (2011–2013, n=1778) the age- and sex-adjusted incidence rate of MCC in non-Hispanic whites was 0.8 per 100,000 (95% CI 0.8–0.9) as compared to 0.3 per 100,000 (95% CI 0.3–0.4) in Hispanics and 0.1 per 100,000 (95% CI 0.1–0.2) in non-white, non-Hispanic individuals. The proportion of individuals presenting with MCC that were minority (defined as either Hispanic or non-white) increased significantly between 2000–2002 and 2011–2013 (from 7.5% to 9.7%, p = 0.045) and increases in MCC incidence rate were seen across all racial and ethnic groups.
Estimates and Forecasts of Number of Merkel Cell Carcinoma Incident Cases in the US
Data from the SEER-derived incidence rates were combined with US census population data to estimate the total US MCC incidence (cases per year) from 2000–2013 and project incidence for 2015, 2020 and 2025. For these analyses, for the years 2000–2013 we utilized the incidence rate for each individual age and sex bracket observed for that particular year. For the years 2015 and later, we used the incidence rate observed for each individual age and sex bracket in 2011–2013 (the most recent years for which data was available; Supplemental Table 1). In order to be conservative (erring towards underestimate), the adjusted incidence rate was not increased but instead held rate stable; thus, projections reflect only anticipated changes in population demographics.
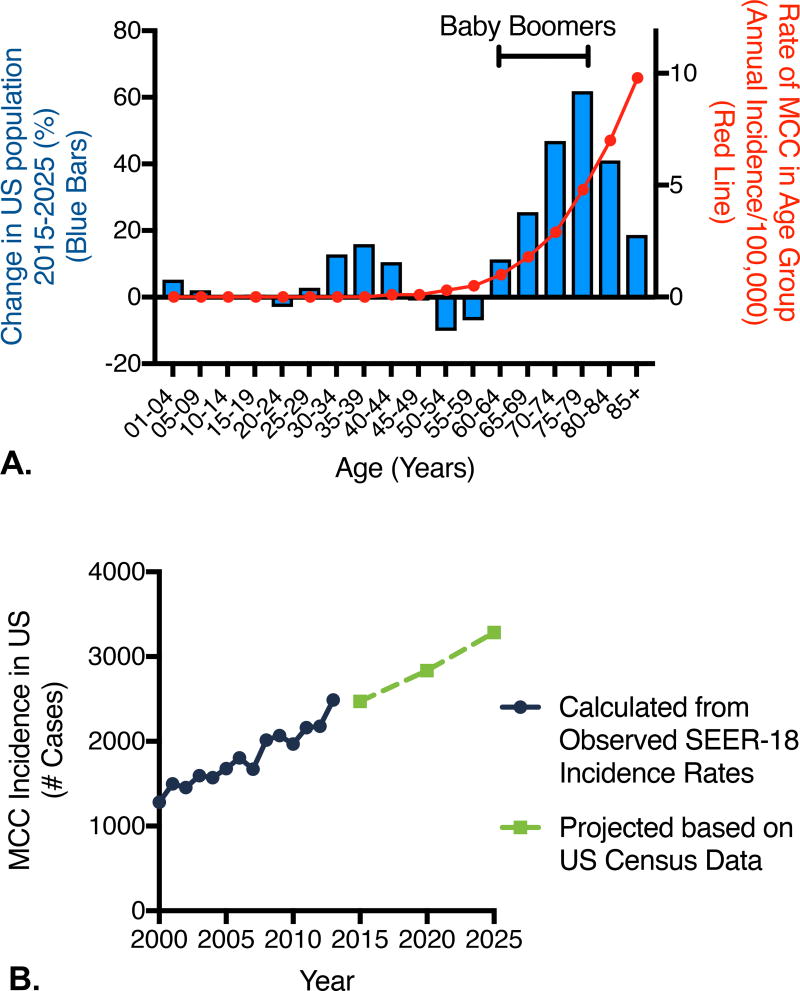
Based on US census reports, due to the aging of the “baby boom” generation there is anticipated to be a large and disproportionate increase in the population aged 65 and older between 2015 and 2025 (Figure 3A).31 These individuals will increase from 13% of the US population to 20% of the total population. This means that there will be a large increase in the individuals who are at higher risk for MCC.
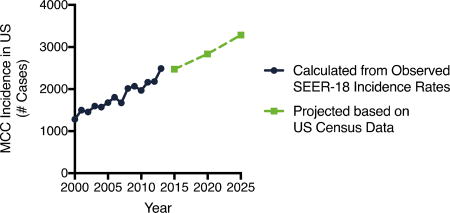
Figure 3. Observed and projected MCC incidence.
A) Explanation for ongoing brisk rise in MCC incidence. Projected change in US population based on US census projections (bars) with MCC incidence rate per 100,000 from 2011–2013 (red line) (most recent years of available data) overlaid. The baby boom generation in 2025 is indicated by the bracket (ages 61–79 in 2025) and account for much of the anticipated rise in MCC incidence. B) Observed incidence and projected annual incidence for MCC from 2000–2025, based on SEER-18 data and US census projections. Estimated number of cases in 2015 in the US is 2,472 cases and in 2025 3,284 new cases per year.
In 2013, the total US incidence of MCC (comparing age and sex bracketed observed incidence rates to US census report of population at risk) was calculated as 2488 cases (Figure 3B). Given the rise in the aging population, and assuming incidence rates for any given age group remain stable, the total incidence of MCC in 2020 is projected to be 2,835 cases. Given the further increases in populations at higher risk of MCC, the projected annual incidence of MCC in the US increases to 3,284 cases in 2025 (Figure 3B).
To determine the approximate accuracy of our approach, we retrospectively performed similar forecasts (projecting 2008 using 2003 data, and 2013 using 2008 data). When we performed such calculations, the observed numbers of incident cases were 9–13% greater than our projections, indicating that our methods were underestimating true incidence. This was due to increases in the age and sex adjusted incidence rate (assumed to be stable for the projections). If one were to instead allow for a 10% increase in incidence rate, the projected annual incidence of MCC would increase to approximately 3,500 cases per year in 2025.
The methods of Bashir and Esteve were next utilized to determine the proportion of increase in incident cases due to increased population size versus the proportion due to the aging of the population.32 From 2015 to 2025, we forecast a total increase in incident MCC cases of 812 cases per year (from 2,472 cases per year in the US in 2015 to 3,284 incident cases per year in the US in 2025). Of this increase, only 200 cases are explained by growth in population. The remaining 612 cases are instead due to the aging of the population, largely the aging of the baby boomers.
Ideally, incidence forecasts would effectively control for race and ethnicity. However, due to the relative rarity of MCC in non-white populations, forecasts accounting for each racial and ethnic group could not be performed with adequate precision. We did perform forecasts in the largest subset of patients with MCC (non-Hispanic whites) using race- and ethnicity- specific (as well as age- and sex-specific) incidence rates and population forecasts. By these methods, the number of incident cases in non-Hispanic white individuals in the US is predicted to be 3,077 cases in 2025. Assuming this represents approximately 90% of total cases of MCC (based on current data from 2011–2013, as above), this brings the total estimate of MCC incident cases in the US to 3,419 cases in 2025, which is roughly concordant with our projected annual incidence in 2025 of 3,284 cases as derived above.
DISCUSSION
Merkel cell carcinoma is an aggressive skin cancer that is associated with Merkel cell polyomavirus and sun exposure. The incidence of MCC has risen over the past several decades. Here we report ongoing increases in incidence, with the number of incident cases rising by >95% since the year 2000, which is well above the increase in incident cases of all solid tumors (15%) and even above that of the rapidly increasing melanoma (57%). We further project incident cases over the next 5 and 10 years, utilizing population projections from the US census. We estimate current annual incidence at 2,500 cases per year in the US, rising to approximately 3,250 cases in the year 2025 based on the established relationship of age and MCC risk.
Merkel cell carcinoma particularly affects the elderly; this relationship to age is much more pronounced than for melanoma or solid tumors in general. This relationship is observed despite the fact that infection with Merkel cell polyomavirus often occurs before adulthood.33–36 Given the critical role that the immune system plays in MCC surveillance as evidenced both by the observation of worse outcomes in immunosuppressed populations37 and better outcomes in patients with brisk immune responses,38, 39 as well as the excellent responses to immunotherapy amongst patients with MCC,10, 13 it is plausible that the predilection of MCC for older individuals may represent diminished immunity in these populations. Indeed, immunosenescence is a well characterized phenomenon with diminished B and T cell function as well as response to vaccination in older individuals.40
Our study had several limitations. Although large, including more than 6,000 patients from a database encompassing more than one-quarter the US population, there may be some geographic differences in incidence not reflected in the available data. Projections are limited to the US. Future studies could consider doing similar projections in other US (eg. National Cancer Data Base or National Program for Cancer Registries) or European/worldwide databases. For the projections of MCC incidence, we held the rate of MCC incidence for any given age steady despite the observed increases in adjusted-rates over the past decade, and thus the projected incidence of 3,250 cases may be an underestimate of true incidence. Our projections cannot take into account skin tone or changes in sun exposure pattern that may occur across the next ten years, although changes in these factors are unlikely to have substantial effect in the short term. In addition, we lack immunosuppression data which can affect risk, although patients with immunosuppression currently represent <10% of those diagnosed with MCC.30 Finally, our data report on incidence only, not prevalence or mortality.
In conclusion, the incidence of Merkel cell carcinoma is increasing and will very likely continue to rise as the baby boom population enters the higher-risk age groups for MCC. We estimate this will exceed 2,800 MCC cases per year in 2020 and 3,250 cases per year in 2025 in the US. Because of its high propensity for spread, the need for adjuvant radiation in many cases,41 and the clear role for early immunotherapy in the metastatic setting, both early detection and optimal management will be critical for improved outcomes. These ongoing increases in MCC incidence strongly advocate for increased specialty-appropriate MCC-specific education to the broad set of providers that care for MCC patients.
Supplementary Material
Acknowledgments
Funding/Support: NIH T32-CA009515, NIH K24-CA139052, NIH R01CA176841, Prostate Cancer Foundation Award #15CHAS04, UW MCC Patient Gift Fund, and the Bloom endowment at UW.
Conflict of Interest: Dr. Nghiem has received consulting fees from EMD Serono, Pfizer, and research grant support from Bristol-Meyers Squibb.
ABBREVIATIONS AND ACRONYMS
- MCC
Merkel cell carcinoma
- MCPyV
Merkel cell polyomaviurs
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Prior Presentation: This work has been submitted for AAD Annual Meeting February 2018
IRB: De-identified national registry data was utilized/exempt.
References
- 1.Lemos B, Nghiem P. Merkel cell carcinoma: more deaths but still no pathway to blame. J Invest Dermatol. 2007;127:2100–3. doi: 10.1038/sj.jid.5700925. [DOI] [PubMed] [Google Scholar]
- 2.Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Houben R, Shuda M, Weinkam R, Schrama D, Feng H, Chang Y, et al. Merkel cell polyomavirus-infected Merkel cell carcinoma cells require expression of viral T antigens. J Virol. 2010;84:7064–72. doi: 10.1128/JVI.02400-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harms PW, Vats P, Verhaegen ME, Robinson DR, Wu YM, Dhanasekaran SM, et al. The Distinctive Mutational Spectra of Polyomavirus-Negative Merkel Cell Carcinoma. Cancer Res. 2015;75:3720–7. doi: 10.1158/0008-5472.CAN-15-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goh G, Walradt T, Markarov V, Blom A, Riaz N, Doumani R, et al. Mutational landscape of MCPyV-positive and MCPyV-negative Merkel cell carcinomas with implications for immunotherapy. Oncotarget. 2016;7:3403–15. doi: 10.18632/oncotarget.6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harms PW, Collie AM, Hovelson DH, Cani AK, Verhaegen ME, Patel RM, et al. Next generation sequencing of Cytokeratin 20-negative Merkel cell carcinoma reveals ultraviolet-signature mutations and recurrent TP53 and RB1 inactivation. Mod Pathol. 2016;29:240–8. doi: 10.1038/modpathol.2015.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong SQ, Waldeck K, Vergara IA, Schroder J, Madore J, Wilmott JS, et al. UV-Associated Mutations Underlie the Etiology of MCV-Negative Merkel Cell Carcinomas. Cancer Res. 2015;75:5228–34. doi: 10.1158/0008-5472.CAN-15-1877. [DOI] [PubMed] [Google Scholar]
- 8.Paulson KG, Lemos BD, Feng B, Jaimes N, Penas PF, Bi X, et al. Array-CGH reveals recurrent genomic changes in Merkel cell carcinoma including amplification of L-Myc. J Invest Dermatol. 2009;129:1547–55. doi: 10.1038/jid.2008.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Criscito MC, Martires KJ, Stein JA. A population-based cohort study on the association of dermatologist density and Merkel cell carcinoma survival. J Am Acad Dermatol. 2017;76:570–2. doi: 10.1016/j.jaad.2016.10.043. [DOI] [PubMed] [Google Scholar]
- 10.Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N Engl J Med. 2016;374:2542–52. doi: 10.1056/NEJMoa1603702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Angelo SP, Russell J, Hassel JC, Lebbe C, Chmielowski B, Rabinowits G, et al. First-line (1L) avelumab treatment in patients (pts) with metastatic Merkel cell carcinoma (mMCC): Preliminary data from an ongoing study. Journal of Clinical Oncology. 2017;35:9530. [Google Scholar]
- 12.Topalian SL, Bhatia S, Hollebecque A, Awada A, De Boer JP, Kudchadkar RR, et al. CT074 - Non-comparative, open-label multiple cohort phase 1/2 study to evaluate nivolumab (NIVO) in patients with virus-associated tumors (CheckMate 358): Efficacy and safety in Merkel cell carcinoma (MCC); AACR Annual Meeting; 2017. [Google Scholar]
- 13.Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D'Angelo SP, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17:1374–85. doi: 10.1016/S1470-2045(16)30364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toker C. Trabecular carcinoma of the skin. Arch Dermatol. 1972;105:107–10. [PubMed] [Google Scholar]
- 15.Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49:832–41. doi: 10.1016/s0190-9622(03)02108-x. [DOI] [PubMed] [Google Scholar]
- 16.Hodgson NC. Merkel cell carcinoma: changing incidence trends. J Surg Oncol. 2005;89:1–4. doi: 10.1002/jso.20167. [DOI] [PubMed] [Google Scholar]
- 17.Fondain M, Du Thanh A, Bessaoud F, Dereure O, Tretarre B, Guillot B. Epidemiological trends in Merkel cell carcinoma in southern France: a registry-based study. Br J Dermatol. 2016 doi: 10.1111/bjd.14950. [DOI] [PubMed] [Google Scholar]
- 18.Zaar O, Gillstedt M, Lindelof B, Wennberg-Larko AM, Paoli J. Merkel cell carcinoma incidence is increasing in Sweden. J Eur Acad Dermatol Venereol. 2016;30:1708–13. doi: 10.1111/jdv.13698. [DOI] [PubMed] [Google Scholar]
- 19.Eisemann N, Jansen L, Castro FA, Chen T, Eberle A, Nennecke A, et al. Survival with nonmelanoma skin cancer in Germany. Br J Dermatol. 2016;174:778–85. doi: 10.1111/bjd.14352. [DOI] [PubMed] [Google Scholar]
- 20.Youlden DR, Soyer HP, Youl PH, Fritschi L, Baade PD. Incidence and survival for Merkel cell carcinoma in Queensland, Australia, 1993–2010. JAMA Dermatol. 2014;150:864–72. doi: 10.1001/jamadermatol.2014.124. [DOI] [PubMed] [Google Scholar]
- 21.Song PI, Liang H, Wei WQ, Jiang YQ, Smith JS, Qiao YL. The clinical profile of Merkel cell carcinoma in mainland China. Int J Dermatol. 2012;51:1054–9. doi: 10.1111/j.1365-4632.2011.05251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitzgerald TL, Dennis S, Kachare SD, Vohra NA, Wong JH, Zervos EE. Dramatic Increase in the Incidence and Mortality from Merkel Cell Carcinoma in the United States. Am Surg. 2015;81:802–6. doi: 10.1177/000313481508100819. [DOI] [PubMed] [Google Scholar]
- 23.Colby SL, Ortman JM. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. US Census Bureau. 2015:P25–1143. [Google Scholar]
- 24.Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RG, Barzi A, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017 doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 25.Sihto H, Bohling T, Kavola H, Koljonen V, Salmi M, Jalkanen S, et al. Tumor infiltrating immune cells and outcome of Merkel cell carcinoma: a population-based study. Clin Cancer Res. 2012;18:2872–81. doi: 10.1158/1078-0432.CCR-11-3020. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg PD, Cheever MA, Fefer A. Eradication of disseminated murine leukemia by chemoimmunotherapy with cyclophosphamide and adoptively transferred immune syngeneic Lyt-1+2-lymphocytes. The Journal of experimental medicine. 1981;154:952–63. doi: 10.1084/jem.154.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15:547–69. doi: 10.1177/0962280206070621. [DOI] [PubMed] [Google Scholar]
- 28.Guy GP, Jr, Thomas CC, Thompson T, Watson M, Massetti GM, Richardson LC, et al. Vital signs: melanoma incidence and mortality trends and projections - United States, 1982–2030. MMWR Morb Mortal Wkly Rep. 2015;64:591–6. [PMC free article] [PubMed] [Google Scholar]
- 29.Wehner MR, Chren MM, Nameth D, Choudhry A, Gaskins M, Nead KT, et al. International prevalence of indoor tanning: a systematic review and meta-analysis. JAMA Dermatol. 2014;150:390–400. doi: 10.1001/jamadermatol.2013.6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heath M, Jaimes N, Lemos B, Mostaghimi A, Wang LC, Penas PF, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375–81. doi: 10.1016/j.jaad.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.U.S. Census Population Projections. 2014 http://www.census.gov/population/projections/data/national/2014.html.
- 32.Bashir S, Esteve J. Analysing the difference due to risk and demographic factors for incidence or mortality. Int J Epidemiol. 2000;29:878–84. doi: 10.1093/ije/29.5.878. [DOI] [PubMed] [Google Scholar]
- 33.Carter JJ, Paulson KG, Wipf GC, Miranda D, Madeleine MM, Johnson LG, et al. Association of Merkel cell polyomavirus-specific antibodies with Merkel cell carcinoma. J Natl Cancer Inst. 2009;101:1510–22. doi: 10.1093/jnci/djp332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kean JM, Rao S, Wang M, Garcea RL. Seroepidemiology of human polyomaviruses. PLoS Pathog. 2009;5:e1000363. doi: 10.1371/journal.ppat.1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tolstov YL, Pastrana DV, Feng H, Becker JC, Jenkins FJ, Moschos S, et al. Human Merkel cell polyomavirus infection II. MCV is a common human infection that can be detected by conformational capsid epitope immunoassays. Int J Cancer. 2009;125:1250–6. doi: 10.1002/ijc.24509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen T, Hedman L, Mattila PS, Jartti T, Ruuskanen O, Soderlund-Venermo M, et al. Serological evidence of Merkel cell polyomavirus primary infections in childhood. J Clin Virol. 2011;50:125–9. doi: 10.1016/j.jcv.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 37.Paulson KG, Iyer JG, Blom A, Warton EM, Sokil M, Yelistratova L, et al. Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage. J Invest Dermatol. 2013;133:642–6. doi: 10.1038/jid.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paulson KG, Iyer JG, Tegeder AR, Thibodeau R, Schelter J, Koba S, et al. Transcriptome-wide studies of merkel cell carcinoma and validation of intratumoral CD8+ lymphocyte invasion as an independent predictor of survival. J Clin Oncol. 2011;29:1539–46. doi: 10.1200/JCO.2010.30.6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paulson KG, Iyer JG, Simonson WT, Blom A, Thibodeau RM, Schmidt M, et al. CD8+ lymphocyte intratumoral infiltration as a stage-independent predictor of Merkel cell carcinoma survival: a population-based study. Am J Clin Pathol. 2014;142:452–8. doi: 10.1309/AJCPIKDZM39CRPNC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goronzy JJ, Weyand CM. Understanding immunosenescence to improve responses to vaccines. Nat Immunol. 2013;14:428–36. doi: 10.1038/ni.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.NCCN. Merkel Cell Carcinoma: Version 1.2018. NCCNorg; 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.