Abstract
Objectives
To compare long-term outcomes of cancer patients who pursued fertility preservation (FP) to those who did not, and compare random-start (RS) and menstrual cycle-specific (CS) protocols for FP.
Design
Retrospective cohort.
Setting
Single urban academic institution.
Patient(s)
Oncology patients who contacted the FP patient navigator, 2005–2015.
Intervention(s)
None.
Main Outcome Measure(s)
Time to cancer treatment, disease-free survival, and reproductive outcomes in FP vs no-FP patients, and cycle outcomes for RS vs CS protocols. Data was analyzed by chi-square and logistic regression.
Result(s)
Of 497 patients that met inclusion criteria, 41% elected FP. The median number of days to cancer treatment was 33 and 19 days in the FP and no-FP groups, respectively (P<0.001). There was no difference in cancer recurrence or mortality. There were no differences in stimulation parameters, outcomes, or days to next cancer treatment in RS vs CS protocols. 21 patients returned to use cryopreserved specimens, resulting in 16 live births. 8/21 returning patients utilized a gestational carrier. 13 FP (6.4%) and 16 no-FP (5.5%) patients experienced a spontaneous pregnancy.
Conclusion(s)
FP is both safe and efficacious for eligible cancer patients. Only 10% of patients returned to use cryopreserved specimens, and almost half used a gestational carrier, suggesting the need for further research into reproductive decision-making in cancer survivors.
Keywords: cancer outcomes, fertility preservation, oncofertility, IVF, recurrence
INTRODUCTION
In the United States, more than 843,820 new female cancer cases were estimated to be diagnosed in 2016 (1). Fortunately, there has been significant improvement in cancer survival rates because of progress in diagnosing certain cancers at an earlier stage, as well as advancements in treatment. From 2002 to 2012, there was an 83% 5-year survival rate amongst women younger than 45 years diagnosed with cancer (2). Recent data from the National Cancer Institute indicate that nearly 250,000 cancer survivors are women of reproductive age, ages 20 to 39 years, with breast cancer being the most common in this age group (3, 4). As a result of the increase in the number of cancer survivors, greater attention has been focused on the delayed effects of cancer treatments on the future quality of life of the survivor, including fertility (5).
Because of the gonadotoxicity of chemotherapy and radiotherapy, 42% of female cancer survivors will develop treatment induced ovarian failure (6–8). Many cancer survivors are concerned that their reproductive potential will be compromised after cancer treatment (9–15). Doctors and other health care providers have become more aware and sensitive to the fertility needs of cancer patients as indicated by practice guidelines developed by the American Society of Reproductive Medicine (ASRM) and the American Society of Clinical Oncology (ASCO) (16–18). These guidelines state that all healthcare providers involved in the care of cancer patients need to be able to discuss the effects of cancer treatment on fertility and provide appropriate referrals to reproductive specialists when indicated (17, 18). Despite the recommendation that oncologists refer all patients who are undergoing gonadotoxic therapies, many oncologists do not refer their patients for fertility preservation (5, 19). Ovarian stimulation for infertility treatment can induce a hyper-estrogenic state, which is of particular concern in hormone sensitive cancers including breast cancer, endometrial cancer, and malignant melanoma. However, at this time there is very limited information about the longer-term effects of ovarian stimulation on cancer recurrence and mortality (20).
In 2005, Northwestern Memorial Hospital’s (NMH) Reproductive Endocrinology and Infertility (REI) division began providing fertility preservation (FP) via oocyte or embryo cryopreservation for women with a cancer diagnosis prior to undergoing cancer treatment (21). Since that time, the division has provided consultation to hundreds of women with a new cancer diagnosis, and a subgroup of these women did undergo ovarian stimulation to cryopreserve their oocytes and/or embryos. The initial appointment with reproductive endocrinology is facilitated by a patient navigator (PN) to ensure that these patients are seen quickly. A significant number of eligible cancer patients decline FP, citing reasons such as trauma from cancer diagnosis, emotional distress, financial constraints, partnered but unmarried, fear of exacerbating their disease or increasing the likelihood of a recurrence if they underwent ovarian stimulation. Another common concern is that pursing fertility preservation would cause a long delay to initiating cancer therapy (9, 22).
It has been proposed that a random-start (RS) protocol, which does not wait for menses to begin ovarian stimulation, as opposed to the traditional cycle-specific (CS) protocol, may decrease the number of days to next cancer treatment (23–25). While there is no reported difference between RS and CS protocols in ovarian stimulation outcome, the ability to initiate an ovarian stimulation cycle regardless of the menstrual cycle phase presents an opportunity to reduce the number of days until the next cancer treatment is administered (26–28). Studies have not documented the actual time to cancer treatment between CS and RS protocols, which could inform future implementation of an RS protocol for FP patients.
The aim of the current study was to quantify the delay to treatment in patients that elect FP, and determine if there is an association between ovarian stimulation for FP and cancer recurrence and mortality. Furthermore, for patients who underwent ovarian stimulation for FP, we examined whether RS vs CS stimulation starts impacted IVF cycle outcomes and time to cancer treatment. Finally, we explored pregnancy rates and outcomes following cancer treatment.
MATERIALS AND METHODS
Study Population
This was an IRB approved study. Subjects were identified from a FP patient log of women who had been diagnosed with cancer and contacted the FP PN at Northwestern Memorial Hospital from January 2005 through January 2016, regardless of whether they ultimately elected to undergo ovarian stimulation. The initial FP patient list included 1054 subjects. Subjects were excluded from the initial list if they presented for non-cancer related FP or for reasons other than FP, were older than 45 years at the time of PN consultation, initially met with the FP PN with a diagnosis of cancer recurrence, or had chemotherapy treatment before PN consultation. We chose to exclude patients with recurrent cancer at presentation because our main end point was cancer recurrence. We excluded both recurrent cancer diagnosis as well as recent chemotherapy from ovarian stimulation outcomes because the history of chemotherapy could directly affect ovarian stimulation outcomes and therefore could be a confounder. We also excluded patients in which PN consultation to next cancer treatment was >100days from the time to next treatment analysis because we felt that the decision to pursue or to not pursue fertility preservation would not have impacted their cancer care. These patients, however, were included in the other analyses, including cancer recurrence, mortality, and stimulation outcomes.
For each patient, cancer diagnosis (breast cancer, hematological cancers, gynecological cancers, and other cancers), treatment history, dates of initial contact with the FP PN, subsequent cancer treatment dates (surgery, chemotherapy, tamoxifen, and/or bone marrow or stem cell transplant), cancer relapse (defined as recurrence of same primary cancer type), and mortality data were collected. Patient mortalities were identified using medical records as well as obituaries. Pregnancy outcomes were also recorded. Cancer recurrence and mortality data were collected from oncology and pathology notes. Pregnancy information, both spontaneous and as a result of using cryopreserved gametes, was also collected.
Patients that underwent a fertility preservation cycle were further stratified by whether they underwent a cycle specific (CS) or random start (RS) protocol. Controlled ovarian hyperstimulation (COH) outcome data were examined, as well as embryo disposition preferences, future pregnancy data and last encounter with a Northwestern provider.
Controlled Ovarian Hyperstimulation
Our protocol has been documented in previous studies (21, 29). Briefly, COH was started using recombinant FSH with or without urinary menotropins with dosage based on age and ovarian reserve measurements. Over time, our practice has evolved to include more RS protocols, and thus patients who desire to begin stimulation immediately can do so. For a CS protocol, gonadotropins were initiated on the third day of menses, whereas for a RS protocol, gonadotropins were initiated at any point in the menstrual cycle. Response to medication was evaluated with regular ultrasounds and E2 measurements, and dosage adjusted accordingly. For a CS protocol, once the leading follicle grew to at least 12mm in diameter or E2 reached 300 pg/mL, the patient began a daily injection of GnRH antagonist to prevent ovulation. For RS, antagonist was started once the new lead follicle grew to at least 12mm. Per our institutional IVF protocol for all patients, when at least three follicles measured 16mm in diameter, final follicular maturation was triggered by an injection of human chorionic gonadotropin and oocyte retrieval was performed 36 hours later. Slow cooling was used at our clinic for oocyte cryopreservation until 2008, when vitrification became standard protocol. Of note, we do not prescribe letrozole or other aromatase inhibitors or selective estrogen receptor modulators, during stimulation for patients with hormone-sensitive cancers like breast cancer.
Statistical Methods
Data were analyzed by chi-square test. Subsequently, linear and logistic regression analyses were performed to adjust for potentially confounding variables. Statistical analyses were performed with SPSS IBM Statistics 24.0 for Windows (SPSS, Chicago, IL). All P values were two-sided, and a P value of <0.05 was considered statistically significant.
RESULTS
Demographics
497 cancer patients were included in the analysis in which 204 (41.0%) proceeded with ovarian stimulation for FP prior to initiating their next cancer treatment (Table 1). Median age at time of contact with the PN was 31 years (range: 15–42 years) for FP patients and 33 years (range: 15–45 years) for patients that declined FP (P = 0.008) (Table 1).
Table 1.
Demographic Information for patients who contacted the patient navigator
| Patient Characteristics (n=497) |
Initiated Fertility Preservation (n=204a) |
Did Not Undergo Fertility Preservation (n=293) |
P value |
|---|---|---|---|
|
| |||
| Age | 0.008 | ||
| Median (Range) |
31 (15–42) |
33 (15–45) |
|
|
| |||
| Gravidity | 0.025 | ||
| Median | 0 | 0 | |
| Mean (Range) |
0.41 (0–4) |
0.75 (0–7) |
|
|
| |||
| Parity | 0.032 | ||
| Median | 0 | 0 | |
| Mean (Range) |
0.23 (0–3) |
0.42 (0–4) |
|
|
| |||
| Breast Cancer | 114 | 148 | NS |
|
| |||
| Hematologic Cancers (lymphoma, leukemia) | 37 | 58 | |
|
| |||
| Gynecologic Cancers (cervical, ovarian, uterine, endometrial) | 24 | 41 | |
|
| |||
| Other Cancers | 29 | 46 | |
|
| |||
| Median follow-up time (Days between PN meeting and latest research update) | 1377 | 1763 | NS |
n=9 that started an ovarian stimulation cycle but did not complete oocyte retrieval. These patients were included in Table 1.
The most common diagnosis for all women who contacted the PN was breast cancer (262 patients, 52.7%), followed by hematologic cancer (95 patients, 19.1%), and gynecologic cancer (65 patients, 13.1%). The remaining 75 patients (15.1%) presented with a variety of other types of cancer, including colon, brain, thyroid, and lung cancer. Overall, there was no significant difference in whether a patient underwent FP based on their cancer diagnosis (Table 1). The median number of days to cancer treatment after consulting the PN was 33 (range: 16–95) in the FP group and 19 (range 0–98) in the group that declined FP (P < 0.001) (Supplemental Table 1). Specifically with GYN cancers, although not statistically significant, the longer delay between PN and treatment was because these patients were undergoing GYN surgeries, and so surgeries were further delayed after oocyte retrieval to allow for the ovaries to decrease to a more normal size. Of note, we also ran the analysis including patients who required more than 100d for their next cancer treatment after meeting with the PN and this did not alter significance.
We further broke down the patients with breast cancer into those who had surgery as first treatment and those who had neoadjuvant chemotherapy prior to surgery (Table 2). In our practice, patients who have surgery as their initial treatment undergo ovarian stimulation after surgery and prior to the initiation of chemotherapy. We found no differences in the days between surgery to chemotherapy for patients undergoing initial surgery between patients that did FP and those who did not. We also found no difference in the days to initiating chemotherapy in the patients undergoing neo-adjuvant chemotherapy. Therefore, in patients with breast cancer who elected FP there did not appear to be a delay to initiating cancer treatments.
Table 2.
Days until next cancer treatment for breast cancer patients undergoing surgery vs neoadjuvant therapy
| Breast Cancer Patient Treatment Course | Initiated Fertility Preservation a | Did Not Undergo Fertility Preservation |
|---|---|---|
|
| ||
| Surgery initially (days between surgery and start of chemotherapy or radiation) | n=59 | n=78 |
| Median (Range) |
47 (19–801) |
40 (9–1871) |
|
| ||
| Neoadjuvant chemotherapy initially (days between PN and start of chemotherapy) | n=21 | n=25 |
| Median (Range) |
32 (18–70) |
16 (1–92) |
PN, patient navigator.
n=61 underwent surgery only; n=17 did not have exact next treatment dates available in our records; n=1 >100d until next treatment. These patients were not included in Table 2.
n=3 started an ovarian stimulation cycle but did not complete oocyte retrieval. These patients were included in the “Initiated Fertility Preservation” group.
Of the 293 cancer patients (59.0%) that did not undergo FP, the primary reason cited was insufficient time to complete an ovarian stimulation cycle prior to initiation of cancer treatment (86 patients, 29.4%). Of the remaining patients, 14 (4.8%) were deemed medically ineligible for ovarian stimulation, 20 (6.8%) were prescribed cancer treatment that was not gonadotoxic or posed no threat to fertility, 20 (6.8%) were not interested in future childbearing, 7 (2.4%) found FP cost-prohibitive, 3 (1.0%) were overwhelmed from their recent cancer diagnosis, and 2 (0.7%) already had embryos frozen prior to contacting the PN. There was no reason documented for declining FP in 100 patients (33.4%).
Nine of the 204 patients who elected FP started ovarian stimulation, but had their cycle cancelled prior to egg retrieval. Eight of these patients were cancelled due to poor response to ovarian stimulation, and one was cancelled due to non-compliance with medication. Median age for the cancelled stimulation group is 31 years (range: 25–40 years). 6 of the patients had breast cancer, 1 endometrial cancer, 1 lymphoma, and 1 osteosarcoma.
Cancer Outcomes
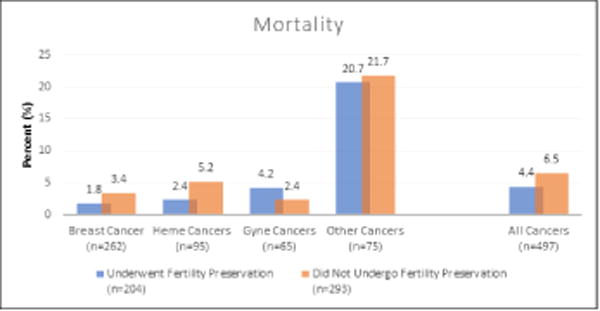
Median follow up time is reported in Table 1. Of the patients that underwent FP, 16 (7.8%) experienced a recurrence in their cancer compared to 18 (6.1%) patients who declined FP (NS). Mortality was reported in 9 (4.4%) patients that underwent FP and 19 (6.5%) patients that declined FP (NS). There was no statistically significant difference in mortality amongst breast cancer patients who did and did not undergo FP. Recurrence and mortality rates by cancer diagnosis are displayed in Figure 1.
Figure 1.
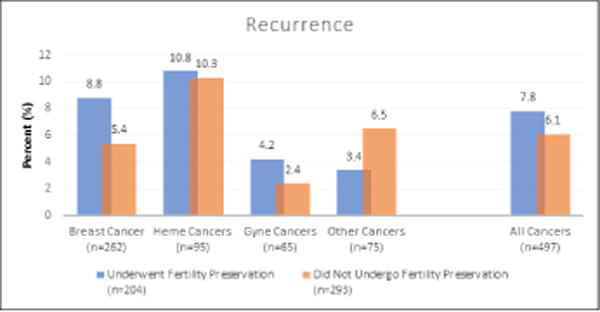
Cancer recurrence and mortality in patients who contacted the patient navigator
[Note from authors: place an “A” next to “Figure1A”]
A: There was no statistically significant difference in cancer recurrence between the patients who did or did not pursue fertility preservation.
[Note from authors: place “B” next to “Figure1B”]
B: There were no statistically significant differences in mortality found in patients who did and did not choose to undergo fertility preservation.
CS versus RS Protocol for Ovarian Stimulation
Of the 204 patients who elected FP, there were 195 patients analyzed with complete stimulation information; as noted previously, 9 patients initiated but did not complete a stimulation cycle. 173 underwent a CS protocol and 22 underwent an RS protocol. There were no statistically significant differences between the two stimulation protocols for demographics or cycle-related outcomes (Table 3). We then analyzed only patients who were not taking oral contraceptive pills prior to ovarian stimulation (n=52) and found no statistically significant differences (Supplemental Table 2).
Table 3.
COH cycle characteristics of patients who underwent a cycle specific vs. random start protocol and completed an oocyte retrieval
| Stimulation Measures (n=195a) |
Cycle Specific (n=173) |
Random Start (n=22) |
P value |
|---|---|---|---|
|
| |||
| Age | |||
| Median (Range) |
31 (15–42) |
30 (19–39) |
NS |
|
| |||
| BMI | |||
| Median (Range) |
22.96 (17.69–61.27) |
24.59 (16.20–34.40) |
NS |
|
| |||
| AMH (ng/ml) | |||
| Median (Range) |
1.70 (0–10.70) |
4.33 (0–10.39) |
NS |
|
| |||
| Peak Estradiol (pg/ml) | |||
| Median (Range) |
1445 (109–3518) |
1336 (55–4341) |
NS |
|
| |||
| Number of days of stimulation | |||
| Median (Range) |
10 (6–16) |
10 (7–15) |
NS |
|
| |||
| Total FSH Dosage | |||
| Median (Range) |
2100 (300–7500) |
1525 (750–5400) |
NS |
|
| |||
| Total HMG Dosage | |||
| Median (Range) |
825 (225–2850) |
750 (600–1800) |
NS |
|
| |||
| Total number of oocytes Retrieved | |||
| Median (Range) |
11 (0–55) |
13 (1–30) |
NS |
|
| |||
| Number of mature oocytes | |||
| Median (Range) |
8 (0–40) |
10 (1–21) |
NS |
|
| |||
| Number of oocytes cryopreserved | |||
| Median (Range) |
9 (0–37) |
12 (0–25) |
NS |
|
| |||
| Number of embryos cryopreserved | |||
| Median (Range) |
6 (0–16) |
6 (0–16) |
NS |
|
| |||
| Number of days to next cancer treatment (after PN meeting)b | n=132 | n=18 | |
| Median (Range) |
33 (16–95) |
36 (16–85) |
NS |
AMH, anti-Müllerian hormone; BMI, body mass index; COH, controlled ovarian hyperstimulation; E2, estradiol; HMG, human menotropin gonadotropin; PN, patient navigator.
n=9 started but did not complete full stimulation cycle. These patients were not included in Table 3.
n=29 did not have next treatment date available in our records; n=16 >100d until next treatment, thus not included in this analysis.
Embryo Disposition Decisions and Pregnancy Outcomes Following Cancer Treatment
We found that 86% of patients who underwent fertility preservation have had contact with a Northwestern provider within the past year. Twenty-one of 204 FP patients (10.3%) returned to use cryopreserved specimens—19 patients had cryopreserved embryos and 2 patients had cryopreserved oocytes (Supplemental Table 3). One patient who returned to use cryopreserved oocytes did not have any viable embryos develop to transfer, while the other successfully conceived and eventually gave birth. Of the patients who returned to use cryopreserved oocytes or embryos, 12/21 (57.1%) had a live birth, including 3 sets of twins. One patient had two live births. A gestational carrier was used in 8 out of the 21 returning patients (38.1%). Thirteen of the 204 FP patients (6.4%) experienced a live birth from a spontaneous pregnancy (two of these patients had two live births), including one twin pregnancy, whereas 16/293 (5.5%) patients who did not elect FP had a live birth, including two sets of twins. Of this group, one patient had two spontaneous pregnancies result in live birth. Median time from FP to return to use cryopreserved specimens was 797 days (range 201–2210). Patients who returned to use oocytes/embryos were more likely to be married at the time of cryopreservation (76.2% vs 23.8%, P = 0.015). There were no differences in age, AMH, or cancer type between patients who returned and those who did not. For oocyte/embryo disposition elected at the time of cryopreservation, 33.8% of patients chose to donate to research, 34.3% to continue cryopreservation, 24.5% to donate to another couple or family member, and 7.4% to discard.
DISCUSSION
This is one of the largest studies to date assessing long-term outcomes in patients who pursued FP and those who did not. Compared to patients that did not elect FP, cancer patients who had ovarian stimulation for FP had an approximately 2 week delay to initiation of cancer treatment, which is unlikely to be clinically significant. Other papers that examined this topic either only included patients with a single type of cancer (i.e. breast or lymphoma) or did not have a comparison group (30, 31). Importantly, we found that there was no increase in cancer recurrence or mortality rates in patients who elected FP. Additionally, for patients who pursued FP, there were similar IVF cycle outcomes between CS and RS protocols, and the RS protocol did not significantly shorten time to next cancer treatment. Finally, the small number of patients that did return to use cryopreserved embryos had satisfactory live birth rates.
There have been multiple other studies that have shown equivalent outcomes between CS and RS protocols in terms of number of oocytes and/or embryos cryopreserved (26, 32–34). Two of these studies reported a higher number of oocytes retrieved in RS protocols, however no difference in mature oocytes (32, 34). In contrast with our results, two of these studies also reported increased days of stimulation and increased total gonadotropin administered with a RS protocol (26, 27, 32). while one study reported similar values between the two groups, as was found in the present study. Number of days to next cancer treatment was not reported in any of the aforementioned studies; however, a decreased amount of time from initial presentation for fertility preservation and oocyte retrieval was noted for RS protocols (26, 32–34). It is also interesting to note the large number of our patients who were on hormonal birth control prior to cycle start. This is likely because most of our patients were young, reproductive aged women who were using this for contraception.
Similar to our FP outcome data, in a retrospective review of 176 cancer patients who underwent oocyte cryopreservation over a 9-year period, Druckenmiller et al (2016) reported that 6% of patients returned to use their oocytes, with a 44% live birth rate, consistent with their center’s rates for non-cancer patients. They also reported that 3 out of the 10 women who returned required gestational carriers (35). Other studies have reported a higher return rate. Cardozo et al reported similar IVF outcomes between FP patients and age-matched controls, but in that study there was a larger proportion of patients who returned to use cryopreserved embryos (21/63, 33%) (36). Our study found that 76.2% of FP patients who returned were married, which could explain the difference in findings. In a retrospective review of 54 women who underwent embryo cryopreservation over 17 years, Dolmans et al reported 17% returned to use embryos, and a 44% live birth rate (37). Another review of 38 FP patients reported a 26% rate of return, and 40% of the patients who returned used a gestational carrier (38).
These rates of return, as well as live birth rates, are valuable in counseling patients during their initial FP consultation. To date, 25/204 (12.3%) of the FP patients have experienced a live birth (12 from cryopreserved specimens and 13 from spontaneous pregnancies) compared to 16/293 (5.5%) of patients who did not pursue FP. It could be argued that the live birth rate from cryopreserved specimens (12/204, 5.9%) is similar to spontaneous live birth rates in both the FP group (13/204, 6.4%) and the no-FP group (5.5%); however, it is difficult to compare these percentages without knowing the denominator of patients who actually attempted pregnancy. Additionally, given that the reproductive window is often shortened after chemotherapy, as time from cancer treatment increases, we hypothesize that there will be less spontaneous live births in both groups, and more live births from cryopreserved specimens in the FP group. Prospective studies could better address this comparison.
There is little in the published literature quantifying the delay to cancer treatment, if any, in patients who elect FP. Baynosa et al performed a retrospective review of 82 breast cancer patients, and found no significant difference in time to chemotherapy between patients who elected FP and those who did not (30). This difference from our study results may be due to the fact that medical treatment for breast cancer is often delayed by surgery, regardless of whether a patient chooses FP, and our study included all cancer diagnoses, not only breast cancer. Additionally, in institutions that do not have a formalized FP program or dedicated PN for FP patients, delay to treatment may be longer.
The main strength of this study is that it is one of the largest studies with follow-up to date; however, the main limitation is that it is a retrospective review, with the possibility that outcome data could be missing for patients who have transferred elsewhere for their oncology, fertility, or pregnancy care. There is also an element of selection bias when comparing patients who pursue FP with those who do not, since healthier patients or patients with a better prognosis may be more willing to delay cancer treatment for FP procedures. Additionally, our patient population may be slightly different than other centers, given that financial barriers are lessened by a low package price for oocyte cryopreservation and generous payment plans. This is reflected in the fact that only 2.6% of the patients who declined FP cited cost as the reason. Finally, because cryopreserved gametes are stored at a long-term storage facility, the number of patients returning to use their gametes may also be under-reported; however, given that 86% of patients are still active patients within our hospital system, we feel that most pregnancies would have been captured. In addition, longer follow up is needed to more definitely answer questions about the longer-term impact of undergoing FP. However, with a median follow-up of about 4 years, there was not an increase in either cancer recurrence or mortality in patients who elected to pursue FP, which is reassuring. Future studies should also focus on hormone-sensitive cancers, and whether concomitant treatment with medications like letrozole is necessary, given that our protocol does not use letrozole and we did not see an increase in adverse cancer outcomes in breast cancer patients who pursued FP to date.
Taken together, the results of our study suggest that FP is both safe and efficacious for eligible cancer patients, and there does not appear to be a significant advantage to either the CS or RS protocol. An unexpected finding from our study was that very few FP patients returned to use cryopreserved oocytes or embryos. Given the resource-intense nature of cryopreservation for FP, more research is needed to understand why the utilization rate is so low, and better predict which patients will return for cryopreserved specimens. Additionally, a large proportion of returning FP patients utilized a gestational carrier, suggesting that providers should counsel women about this possibility at the time of cryopreservation, and ensure appropriate infectious disease testing is performed.
To date, the vast majority of data on FP patient outcomes is from retrospective reviews, similar to this study. While a randomized trial would not be feasible or ethical in this population, a nationwide registry in which FP patients could be followed prospectively would likely provide the most definitive answers about long term outcomes, both oncologic and reproductive, in this patient population.
Supplementary Material
Capsule.
Fertility preservation is both safe and efficacious for eligible cancer patients, regardless of stimulation protocol used.
Acknowledgments
The authors wish to thank all of the faculty, nurses, and staff at Northwestern Fertility and Reproductive Medicine for the excellent care they provide patients. We also wish to thank Mr. Christopher Novak for reviewing this paper.
Supported Northwestern Memorial Foundation Evergreen Grant (to MEP) and P50 HD076188 (MEP, PI: T. Woodruff).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Partially presented at the American Society for Reproductive Medicine 2016 meeting in Salt Lake City, UT, Society for Reproductive Investigation 2016 meeting, Montreal, QC, Canada, and Society for Reproductive Investigation 2017 meeting, Orlando, FL.
References
- 1.American Cancer Society. Cancer Facts & Figures 2016. Atlanta: American Cancer Society; 2016. [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, et al. SEER cancer statistics review, 1975–2010. National Cancer Institute; 2013. [Google Scholar]
- 3.Rodriguez-Wallberg KA, Oktay K. Fertility preservation in women with breast cancer. Clin Obstet Gynecol. 2010;53:753. doi: 10.1097/GRF.0b013e3181f96e00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ries LAG, Harkins D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, et al. SEER cancer statistics review, 1975–2003. 2006 [Google Scholar]
- 5.Letourneau JM, Ebbel EE, Katz PP, Katz A, Ai WZ, Chien AJ, et al. Pretreatment fertility counseling and fertility preservation improve quality of life in reproductive age women with cancer. Cancer. 2012;118:1710–7. doi: 10.1002/cncr.26459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eskander RN, Randall LM, Berman ML, Tewari KS, Disaia PJ, Bristow RE. Fertility preserving options in patients with gynecologic malignancies. Am J Obstet Gynecol. 2011;205:103–10. doi: 10.1016/j.ajog.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsen EC, Müller J, Schmiegelow K, Rechnitzer C, Andersen AN. Reduced ovarian function in long-term survivors of radiation-and chemotherapy-treated childhood cancer. J Clin Endocrinol Metab. 2003;88:5307–14. doi: 10.1210/jc.2003-030352. [DOI] [PubMed] [Google Scholar]
- 8.Gardino SL, Jeruss JS, Woodruff TK. Using decision trees to enhance interdisciplinary team work: the case of oncofertility. J Assist Reprod Genet. 2010;27:227–31. doi: 10.1007/s10815-010-9413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Partridge AH, Gelber S, Peppercorn J, Sampson E, Knudsen K, Laufer M, et al. Web-Based Survey of Fertility Issues in Young Women With Breast Cancer. J Clin Oncol. 2004;22:4174–83. doi: 10.1200/JCO.2004.01.159. [DOI] [PubMed] [Google Scholar]
- 10.Partridge AH, Pagani O, Abulkhair O, Aebi S, Amant F, Azim HA, Jr, et al. First international consensus guidelines for breast cancer in young women (BCY1) Breast. 2014;23:209–20. doi: 10.1016/j.breast.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Loren AW, Mangu PB, Beck LN, Brennan L, Magdalinski AJ, Partridge AH, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2500–10. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stein DM, Victorson DE, Choy JT, Waimey KE, Pearman TP, Smith K, et al. Fertility preservation preferences and perspectives among adult male survivors of pediatric cancer and their parents. J Adolesc Young Adult Oncol. 2014;3:75–82. doi: 10.1089/jayao.2014.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crawshaw MA, Glaser AW, Hale JP, Sloper P. Male and female experiences of having fertility matters raised alongside a cancer diagnosis during the teenage and young adult years. European Journal of Cancer Care. 2009;18:381–90. doi: 10.1111/j.1365-2354.2008.01003.x. [DOI] [PubMed] [Google Scholar]
- 14.Yee S, Abrol K, McDonald M, Tonelli M, Liu KE. Addressing oncofertility needs: views of female cancer patients in fertility preservation. Journal of psychosocial oncology. 2012;30:331–46. doi: 10.1080/07347332.2012.664257. [DOI] [PubMed] [Google Scholar]
- 15.Achille MA, Rosberger Z, Robitaille R, Lebel S, Gouin J-P, Bultz BD, et al. Facilitators and obstacles to sperm banking in young men receiving gonadotoxic chemotherapy for cancer: the perspective of survivors and health care professionals. Hum Reprod. 2006;21:3206–16. doi: 10.1093/humrep/del307. [DOI] [PubMed] [Google Scholar]
- 16.Wallace WHB, Anderson RA, Irvine DS. Fertility preservation for young patients with cancer: who is at risk and what can be offered? Lancet Oncol. 2005;6:209–18. doi: 10.1016/S1470-2045(05)70092-9. [DOI] [PubMed] [Google Scholar]
- 17.Society for Assisted Reproductive Technology, American Society for Reproductive Medicine. Assisted reproductive technology in the United States: 2000 results generated from the American Society for Reproductive Medicine/Society for Assisted Reproductive Technology Registry. Fertil Steril. 2004;81:1207–20. doi: 10.1016/j.fertnstert.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, et al. American Society of Clinical Oncology Recommendations on Fertility Preservation in Cancer Patients. J Clin Oncol. 2006;24:2917–31. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 19.Bann CM, Treiman K, Squiers L, Tzeng J, Nutt S, Arvey S, et al. Cancer Survivors’ Use of Fertility Preservation. J Womens Health. 2015;24:1030–7. doi: 10.1089/jwh.2014.5160. [DOI] [PubMed] [Google Scholar]
- 20.Matthews ML, Hurst BS, Marshburn PB, Usadi RS, Papadakis MA, Sarantou T. Cancer, fertility preservation, and future pregnancy: a comprehensive review. Obstet Gynecol Int. 2012;2012:953937. doi: 10.1155/2012/953937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klock SC, Zhang JX, Kazer RR. Fertility preservation for female cancer patients: early clinical experience. Fertil Steril. 2010;94:149–55. doi: 10.1016/j.fertnstert.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 22.Shnorhavorian M, Harlan LC, Smith AW, Keegan TH, Lynch CF, Prasad PK, et al. Fertility preservation knowledge, counseling, and actions among adolescent and young adult patients with cancer: A population-based study. Cancer. 2015;121:3499–506. doi: 10.1002/cncr.29328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sönmezer M, Türkçüoğlu I, Coşkun U, Oktay K. Random-start controlled ovarian hyperstimulation for emergency fertility preservation in letrozole cycles. Fertil Steril. 2011;95:2125.e9–e11. doi: 10.1016/j.fertnstert.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi C, Matsubayashi H, Mizuta S, Yamaguchi K, Nishiyama R, Takaya Y, et al. Comparison between random-start and conventional-start stimulation according to anti-Mullerian hormone (AMH) levels in patients without cancer. Fertil Steril. 2016;106:e193. [Google Scholar]
- 25.Robertson DM, Gilchrist RB, Ledger WL, Baerwald A. Random start or emergency IVF/in vitro maturation: a new rapid approach to fertility preservation. Womens Health. 2016;12:339–49. doi: 10.2217/whe-2015-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cakmak H, Katz A, Cedars MI, Rosen MP. Effective method for emergency fertility preservation: random-start controlled ovarian stimulation. Fertil Steril. 2013;100:1673–80. doi: 10.1016/j.fertnstert.2013.07.1992. [DOI] [PubMed] [Google Scholar]
- 27.Cakmak H, Rosen MP. Ovarian stimulation in cancer patients. Fertil Steril. 2013;99:1476–84. doi: 10.1016/j.fertnstert.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 28.Kim JH, Kim SK, Lee HJ, Lee JR, Jee BC, Suh CS, et al. Efficacy of Random-start Controlled Ovarian Stimulation in Cancer Patients. J Korean Med Sci. 2015;30:290–5. doi: 10.3346/jkms.2015.30.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pavone ME, Innes J, Hirshfeld-Cytron JE, Kazer R, Zhang J. Comparing thaw survival, implantation and live birth rates from cryopreserved zygotes, embryos and blastocysts. J Hum Reprod Sci. 2011;4:23–8. doi: 10.4103/0974-1208.82356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baynosa J, Westphal LM, Madrigrano A, Wapnir I. Timing of breast cancer treatments with oocyte retrieval and embryo cryopreservation. Journal of the American College of Surgeons. 2009;209:603–7. doi: 10.1016/j.jamcollsurg.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Allen PB, Pavone ME, Smith KN, Kazer R, Rademaker AW, Lawson AK, et al. The Impact of Fertility Preservation on Treatment Delay and Progression-Free Survival in Women with Lymphoma: A Single-centre Experience. Br J Haematol. 2016 doi: 10.1111/bjh.14466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Wolff M, Capp E, Jauckus J, Strowitzki T, Germeyer A, group. Fs Timing of ovarian stimulation in patients prior to gonadotoxic therapy: an analysis of 684 stimulations. Eur J Obstet Gynecol Reprod Biol. 2016;199:146–9. doi: 10.1016/j.ejogrb.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 33.Cakmak H, Tran ND, Zamah AM, Cedars MI, Rosen MP. A novel “delayed start” protocol with gonadotropin-releasing hormone antagonist improves outcomes in poor responders. Fertil Steril. 2014;101:1308–14. doi: 10.1016/j.fertnstert.2014.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simi G, Obino MER, Casarosa E, Litta P, Artini PG, Cela V. Different stimulation protocols for oocyte cryropreservation in oncological patients: a retrospective analysis of single university centre. Gynecol Endocrinol. 2015;31:966–70. doi: 10.3109/09513590.2015.1080237. [DOI] [PubMed] [Google Scholar]
- 35.Druckenmiller S, Goldman KN, Labella PA, Fino ME, Bazzocchi A, Noyes N. Successful Oocyte Cryopreservation in Reproductive-Aged Cancer Survivors. Obstet Gynecol. 2016;127:474–80. doi: 10.1097/AOG.0000000000001248. [DOI] [PubMed] [Google Scholar]
- 36.Cardozo ER, Thomson AP, Karmon AE, Dickinson KA, Wright DL, Sabatini ME. Ovarian stimulation and in-vitro fertilization outcomes of cancer patients undergoing fertility preservation compared to age matched controls: a 17-year experience. J Assist Reprod Genet. 2015;32:587–96. doi: 10.1007/s10815-015-0428-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donnez J, Dolmans M-M, Pellicer A, Diaz-Garcia C, Ernst E, Macklon KT, et al. Fertility preservation for age-related fertility decline. Lancet. 2015;385:506–7. doi: 10.1016/S0140-6736(15)60198-2. [DOI] [PubMed] [Google Scholar]
- 38.Robertson AD, Missmer SA, Ginsburg ES. Embryo yield after in vitro fertilization in women undergoing embryo banking for fertility preservation before chemotherapy. Fertil Steril. 2011;95:588–91. doi: 10.1016/j.fertnstert.2010.04.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


