Abstract
Rationale
Genome-Wide Association Studies identified single nucleotide polymorphisms (SNPs) near the SORT1 locus strongly associated with decreased plasma low-density lipoprotein cholesterol (LDL-C) levels and protection from atherosclerotic cardiovascular disease and myocardial infarction. The minor allele of the causal SORT1 SNP locus creates a putative C/EBPα binding site in the SORT1 promoter, thereby increasing sortilin expression by 12-fold in liver, which is rich in this transcription factor. Our previous studies in mice have showed reductions in plasma LDL-C and its principal protein component, apolipoprotein B (apoB) with increased SORT1 expression, and in vitro studies suggested that sortilin promoted the presecretory lysosomal degradation of apoB associated with the LDL precursor, very-low density lipoprotein (VLDL).
Objective
To determine directly that SORT1 overexpression results in apoB degradation and to identify the mechanisms by which this reduces apoB and VLDL secretion by the liver, thereby contributing to understanding the clinical phenotype of lower LDL-C levels.
Methods and Results
Pulse-chase studies directly established that SORT1 overexpression results in apoB degradation. As noted above, previous work implicated a role for lysosomes in this degradation. Through in vitro and in vivo studies, we now demonstrate that the sortilin-mediated route of apoB to lysosomes is unconventional and intersects with autophagy. Increased expression of sortilin diverts more apoB away from secretion, with both proteins trafficking to the endosomal compartment in vesicles that fuse with autophagosomes to form amphisomes. The amphisomes then merge with lysosomes. Furthermore, we show that sortilin itself is a regulator of autophagy and that its activity is scaled to the level of apoB synthesis.
Conclusions
These results strongly suggest that an unconventional lysosomal targeting process dependent on autophagy degrades apoB that was diverted from the secretory pathway by sortilin, and provide a mechanism contributing to the reduced LDL-C found in individuals with SORT1 overexpression.
Subject Terms: Animal Models of Human Disease, Atherosclerosis, Genetic, Association Studies, Lipids and Cholesterol, Vascular Disease
Keywords: Apolipoprotein B, liver, amphisome, sortilin, autophagy, single nucleotide polymorphisms coronary disease, lipids and lipoprotein metabolism
INTRODUCTION
Genome Wide Association Studies (GWAS) have been used to identify novel genes involved in lipid metabolism and atherosclerosis1. These analyses have identified a single nucleotide polymorphism (SNP) in a locus on chromosome 1p13 harboring the gene SORT1, which encodes the protein sortilin-1 (hereafter referred to as sortilin), as strongly associated with lower low-density lipoprotein cholesterol (LDL-C) levels and a nearly 40% reduction in risk for myocardial infarction as compared to subjects lacking this polymorphism2. The SNP creates a binding site for the transcription factor C/EBPα, which is highly expressed in the liver3. The generation of this binding site is thought to underlie the significantly increased expression (over 12-fold) of hepatic SORT1 found in those either hetero- or homozygotic for the SNP2. Furthermore, increased expression of sortilin in mouse liver and in hepatic cells reduces very-low density lipoprotein (VLDL) production2, 4, 5, the precursor of LDL, which is expected to contribute to the lower LDL-C levels observed in the GWAS analysis.
Sortilin is a 95-kDa member of the Vps10p-domain receptor family6. This family of transmembrane receptors was first characterized in yeast as a sorting factor that directs proteins to the vacuole, the equivalent organelle to the lysosome in higher eukaryotes7. Sortilin is synthetized in the endoplasmic reticulum (ER) as a precursor protein harboring a pro-peptide that prevents the receptor from binding ligands prematurely. Sortilin is then converted to its mature form in the trans-Golgi network (TGN) by furin-mediated proteolytic cleavage8, at which point it can bind select ligands, one of which is apolipoprotein B100 (apoB100)2, 4, 9. Sortilin participates in trafficking cargo (i) to constitutive secretory vesicles, (ii) to the endosomal compartment, from which associated proteins can further travel to the plasma membrane, lysosomes, or other locations, and (iii) to secretory granules. A minor fraction of sortilin is also present at the plasma membrane where it acts both as a signaling and endocytosis receptor10.
ApoB100 is the principal protein component of VLDL as well as one of its products, LDL, and is among the strongest risk factors for the development of heart disease (e.g.,11). In contrast to many other secreted hepatic proteins whose secretion is regulated by synthesis, apoB100 secretion is primarily regulated by its degradation rate, which occurs both during VLDL assembly and maturation12, 13. We and others have discovered several apoB100 degradation pathways in hepatocytes, including proteasome-dependent ER-associated degradation (ERAD) and a post-ER presecretory process (PERPP) that in some examples utilizes macroautophagy (reviewed in14). Macroautophagy (hereafter referred to as autophagy) was initially characterized as a protein catabolic pathway to recycle amino acids during nutrient deprivation (reviewed in15); however, the contribution of autophagy to the regulation of lipid metabolism has increasingly gained importance. Besides the regulation of VLDL secretion through the degradation of apoB10014, different forms of selective autophagy regulate intracellular lipid breakdown directly through lipophagy or indirectly through degradation of other lipid droplet components16, 17, and control the rate of lipid synthesis through degradation of lipogenic enzymes18, 19.
We and others have demonstrated a major effect of sortilin on the secretion of apoB100-containing lipoproteins2, 4, 5, 9. Specifically, overexpression of SORT1 in mouse liver to mimic the increased hepatic expression found in minor vs. major allele homozygote individuals2 reduced apoB100 production in vivo. In cultured hepatic cells, sortilin overexpression similarly reduced apoB100 secretion by promoting its trafficking to lysosomes, where it was presumably degraded. The specific route through which apoB100 was delivered to the lysosome is unknown, though it has been assumed to be an example of the conventional direct pathway, in which vesicles bud from the trans-Golgi network (TGN), traffic to the endosomal compartment and fuse with lysosomes. Given the aforementioned role of autophagy in apoB100 degradation, we hypothesized an unconventional, indirect route. Through a series of in vitro and in vivo studies, we now demonstrate that sortilin can route apoB100 from the secretory pathway to the lysosome through an autophagic intermediate known as the amphisome, which results from the fusion of an endosome and an autophagosome. These findings provide novel mechanistic insights into the cellular basis for the clinical effects of SORT1 overexpression in the liver, and illuminate special features of sortilin-mediated degradation of apoB100 by the autophagic pathway.
METHODS
The authors declare that all supporting data are available within the article (and its online supplementary files). Detailed materials and methods are included in the online data supplement.
RESULTS
Increased sortilin expression stimulates autophagy-dependent apoB100 degradation in hepatic cells
Rat hepatoma McArdle RH-7777 cells are a well-characterized model of VLDL assembly and secretion20, 21. Therefore, we used McArdle RH-7777 cells stably expressing human apoB10022 (hereafter referred to as McA cells) to analyze the effects of sortilin overexpression on apoB100 metabolism in vitro.
To confirm that increased SORT1 expression reduces apoB100 secretion, as anticipated from our previous studies2, 4, we compared the secreted and intracellular levels of apoB100 in McA cells overexpressing sortilin (McA-sortilin) or GFP (McA-GFP, control) that were steady state radio-labeled for 4 h with35S-met/cys. Consistent with previous data, McA-sortilin cells showed a significant reduction in apoB100 secretion, intracellular apoB100, and total apoB100 (Figure 1A).
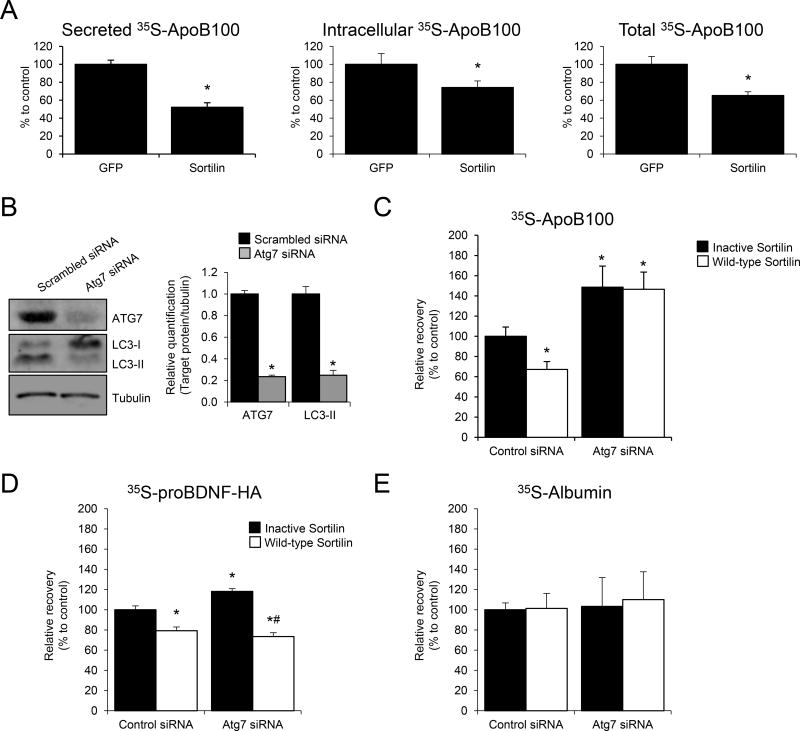
Figure 1. Sortilin overexpression increases apoB100 degradation in rat hepatic cells in an autophagy-dependent manner.
(A) McA rat hepatic cells stably expressing GFP or sortilin were cultured in normal growth medium. When cells reached sub-confluence (80%–90%), they were washed with PBS, and amino acid-starved for 1 h before being subjected to steady state metabolic labeling using a mixture of [35S]-methionine and cysteine. After 4 h, cells were harvested and apoB100 was immunoprecipitated, separated by SDS-PAGE, and bands quantified using a phosphorimager. The graphical data are displayed as the relative differences in secreted apoB100 (left panel), intracellular apoB100 (middle panel), and total (cell+medium), right panel apoB100 between the cells transfected with GFP (set to 100%) vs. sortilin. (B–E) McA hepatic cells stably expressing human apoB100 were transfected with Atg7 siRNA or with scrambled siRNA for 24 h. Then, cells were transfected with either a plasmid containing the full-length myc-tagged human sortilin (Wild-type Sortilin, pcDNA3.1-hSORT1-myc) or a dominant negative sortilin (“Inactive Sortilin”; pcDNA3.1-hPro-Sortilin) generated by mutation of the furin recognition site, which prevents sortilin binding to apoB100. (B) Western blot for ATG7, LC3-I and LC3-II levels 48 h after siRNA incubation. Tubulin was used as internal control. (C) Pulse-chase results showing the recoveries of apoB100 at the end versus beginning of the chase period, with “Inactive Sortilin, Control siRNA” set to 100%. (D) Relative recovery of proBDNF-HA from McA hepatic cells transfected with Atg7 siRNA or with scrambled siRNA for 24 h. Prior to the pulse-chase study, an expression plasmid for proBDNF-HA was co-transfected with an expression plasmid for either wild-type or inactive sortilin for 24 h. (E) Relative recovery of albumin. Numerical data represent the means ± SEM. *, p<0.05 versus Control siRNA; #, p<0.05 versus Atg7 siRNA using two-tailed Student’s t-test. The experiments were performed in triplicate.
To determine if autophagy is involved in the sortilin-mediated reduction in apoB100 recovery, we performed pulse-chase studies in autophagy-sufficient and deficient cells. McA cells were transiently transfected with siRNA to Atg7, which is essential for autophagosome formation and autophagy23, or a control siRNA. Twenty-four hours later, the cells were transfected with a plasmid expressing either myc-tagged human sortilin (wild-type) or sortilin in which the furin recognition site is mutated, precluding propeptide cleavage and subsequent ligand binding (inactive sortilin)8. The efficacy of the Atg7 siRNA was validated by observing lower ATG7 protein levels and a reduction in autophagosome maturation as indicated by a decrease in the phosphatidylethanolamine (PE)-conjugated form of the autophagosome membrane protein LC3 (LC3-II; Figure 1B).
As shown in Figure 1C, McA cells overexpressing wild-type sortilin showed decreased apoB100 recovery (i.e., greater levels of protein degradation) when compared to cells transfected with the plasmid encoding the inactive cleavage-deficient sortilin species. Notably, this effect was abrogated by Atg7 knockdown. We also noted that ATG7-deficient McA cells showed a higher relative recovery of apoB100 (i.e., less degradation), indicating that autophagy contributes to basal degradation of apoB100, as recently observed (Guo, Tuyama, et al., in preparation; the % attributable to autophagy was ~40% and to ERAD, ~50%). The increased level of apoB100 in ATG7-deficient cells was attributable to the accumulation of apoB100 intracellularly (Online Figure IA, B), consistent with increased sortilin expression diverting more apoB100 from the secretory pathway to a compartment from which it could no longer be secreted even when its degradation was inhibited. In additional pulse-chase studies (Online Figure II), by using combinations of ATG7 siRNA and the proteasome inhibitor MG132, we were able to determine that the overall contribution of autophagy to apoB100 degradation is similar to that of ERAD, (Online Figure IIA, B). To determine if inhibition of Atg7 in McA cells influenced SORT1 and Apob gene expression we measured mRNA levels in McA cells incubated with control or Atg7 siRNA by RT-PCR, but failed to observe any significant differences in their message levels (Online Figure IIIA, B).
Sortilin is known to bind a variety of ligands and promote their lysosomal degradation10, including pro-brain-derived neurotrophic factor (proBDNF)24. To determine if autophagy is a general degradation pathway for sortilin ligands, we co-transfected into ATG7-deficient or control McA cells plasmids expressing wild-type or the inactive sortilin species along with a plasmid encoding proBDNF, which contained a human influenza hemagglutinin (HA) tag (proBDNF-HA). We then performed pulse-chase experiments to measure proBDNF recovery. Similar to the effects observed on apoB100, proBDNF recovery was also reduced in cells expressing wild-type sortilin relative to cells expressing the inactive sortilin species. Importantly, this effect was maintained in ATG7-deficient cells, indicating that sortilin does not require the autophagy pathway to deliver proBDNF to the lysosome (Figure 1D). Notably, in both experiments there was no significant change in albumin relative recovery, suggesting that sortilin does not globally reduce protein secretion but exhibits ligand specificity (Figure 1E).
To independently investigate the requirement for autophagy in apoB100 degradation when sortilin is overexpressed, we also studied the effects of the autophagy inhibitor 3-methyladenine (3-MA)25 (Online Figure IV). First, we observed that in cells in which sortilin was not overexpressed, 3-MA increased the intracellular accumulation of apoB100, but not its secretion. In the sortilin overexpressing cells, 3-MA, as in the McA cells treated with ATG7 siRNA (Figure 1), the induction of apoB100 degradation was prevented. Besides confirming a role for autophagy of apoB100 in sortilin overexpressing cells, the data show that, in general, when autophagy is inhibited, the “rescued” apoB100 remains in the cell, apparently unavailable for secretion.
Previously published work demonstrated sortilin to be a bona fide cell surface LDL receptor whose overexpression and deficiency in cultured cells and mice is associated with increased and decreased LDL uptake and lysosomal degradation, respectively4. We also showed that the LDL receptor mediates the re-uptake and lysosomal degradation of newly secreted apoB100-lipoproteins26. To ensure that the apoB100 degradation we observed in this study was not attributable to sortilin-mediated re-uptake and subsequent delivery to lysosomes, we performed steady-state metabolic labeling experiments in the presence of either BSA or unlabeled LDL, which would compete with the newly-synthesized radio-labeled apoB100-lipoproteins for binding extracellular sortilin preventing the re-uptake of newly secreted apoB100 (Online Figure VA), but not of other proteins, such as albumin (Online Figure VB). As shown in Online Figure VC, sortilin overexpression led to decreased recovery of radiolabeled apoB100 with no change in the recovery of endogenously labeled albumin (Online Figure VD). These data demonstrate that the effect of sortilin on apoB100 degradation is independent of its role as a cell surface LDL receptor.
Effects of autophagy and sortilin over-expression on VLDL production in vivo
Our previous work suggested that increased sortilin expression reduced plasma LDL-C levels by promoting the presecretory lysosomal degradation of apoB1004. However, we did not establish whether sortilin chaperoned apoB100 directly to the lysosome or whether sortilin functioned through an indirect pathway for lysosomal delivery2, 4. Our studies in McA cells raised the possibility that sortilin does not directly promote apoB100 degradation, but in fact relies on an intact autophagy pathway. We therefore studied the effects of sortilin overexpression on apoB100 metabolism in mice in which hepatic autophagy was either active or inactive.
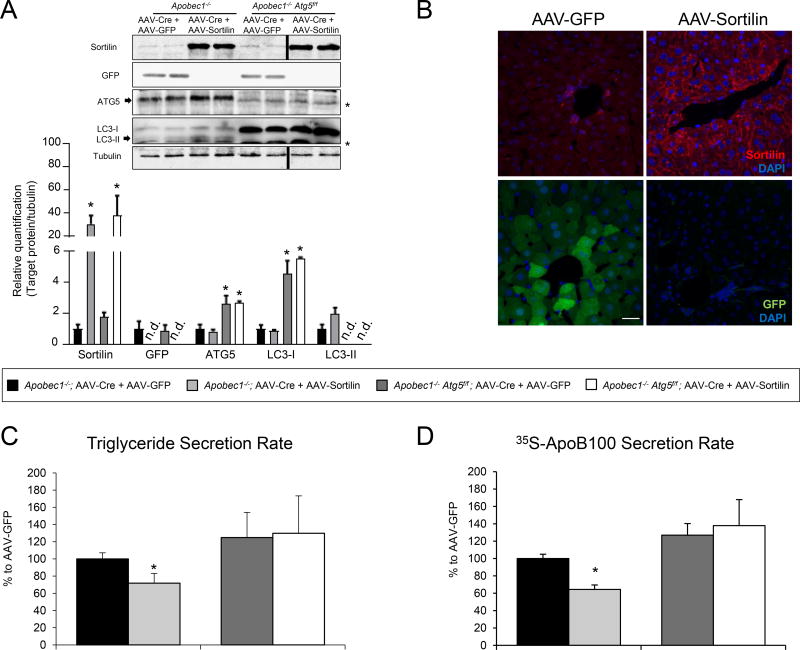
In contrast to human liver, which produces only apoB100, mouse liver produces apoB100 as well as apoB48, which is due to hepatic expression of the apob mRNA editing enzyme (Apobec1); in humans, Apobec1 is expressed only in the small intestine27, 28. To create a more “humanized” mouse model for our in vivo studies, we generated hepatic autophagy sufficient and deficient mice on an Apobec1−/−, apoB100-only background29. Thus, Apobec1−/− mice were crossed with mice with a floxed allele of the essential autophagy factor ATG5, Atg5f/f mice30. This led to the creation of an Apobec1−/− Atg5f/f mouse. By treating these mice with a combination of an AAV encoding Cre Recombinase (Cre) and either GFP (control) or sortilin-encoding AAV, we produced animals with acute hepatic sufficiency/deficiency of autophagy and normal/increased sortilin expression. We chose somatic gene deletion in contrast to constitutive deletion to avoid artefacts from the chronic sequelae of autophagy deficiency. Moreover, we chose to increase sortilin expression rather than developing a knock-out animal because of its clinical relevance in human subjects2. Immunoblot and histological analyses performed two weeks after AAV injection confirmed the efficacy of the GFP and SORT1 vectors at achieving homogeneous gene overexpression (Figure 2A, B). The efficacy of AAV.Cre in blocking Atg5 expression was also confirmed by western blot analysis (Figure 2A; (* denotes a non-specific band). Notably, ATG5 protein levels were dramatically reduced in Apobec1−/− Atg5f/f but not in Apobec1−/− mice. In addition, Apobec1−/− Atg5f/f mice showed an accumulation of the unlipidated form of LC3 (LC3-I). As a consequence, mice overexpressing hepatic sortilin had significantly reduced VLDL-triglyceride (TG) and apoB100 secretion rates when compared to mice overexpressing GFP. Notably, with autophagy deficiency, there was no increase over control where TG or apoB100 secretion was examined (Figure 2C, 2D), consistent with the results in vitro (Online Figure IA, IB; Online Figure IV); i.e., apoB100/VLDL diverted by sortilin overexpression does not appear to re-enter the secretory pathway to a significant extent when autophagy is inhibited. (Figure 2C, D).
Figure 2. Sortilin-mediated effects on VLDL production are abrogated in liver-specific autophagy deficient mice.
Age and sex matched Apobec1−/− and Apobec1−/− Atg5f/f mice received a single intraperitoneal injection containing a combination of two different adeno-associated vectors (AAV) under control of the liver-specific thyroxine-binding globulin promoter. All the mice received Cre recombinase-AAV (AAV-Cre), combined with either GFP or sortilin-containing AAV two weeks before the experiments. (A) Immunoblot analysis of GFP, sortilin, ATG5, LC3 and tubulin in liver homogenates from the indicated mice. * denote non-specific bands. (B) Immunodetection of sortilin (red) and GFP (green) in Apobec1−/− mice injected with AAV-GFP or AAV-Sortilin. DAPI (blue) was used to stain the nuclei. White bar; 25 µm. (C–D) Secretion rates of triglyceride and apoB100 were determined in vivo as described in the Methods in the indicated mice after infection with AAV-GFP (black bars) or AAV-Sortilin (open bars). (C) VLDL-triglyceride secretion rate, and (D) ApoB100 secretion rate. The total amount of plasma proteins radiolabeled with [35S]-methionine and cysteine were precipitated using trichloroacetic acid, and these data were used to normalize the triglyceride and apoB100 secretion rates. Numerical data represent the mean ± SEM of 5 to 6 animals/group. *, p<0.05; Student’s t-test.
Sortilin associates with autophagic vesicles
Previous work showed that the interaction between sortilin and apoB100 was stabilized in the presence of bafilomycin31, a vacuolar HC-ATPase [V-ATPase] inhibitor that alkalinizes the lysosome, thereby blocking substrate degradation32. This finding, together with the results shown above that autophagy is required for the in vitro and in vivo effects of sortilin on apoB100/VLDL secretion, prompted us to consider that the interaction between sortilin and apoB100 is also autophagy-dependent. To test this hypothesis, McA cells stably expressing sortilin (McA-sortilin) were treated with DMSO (control), bafilomycin, or 3-MA and after a 3 hour incubation intracellular proteins were crosslinked and sortilin was immunoprecipitated to capture the interaction between sortilin and apoB100. Western blot analyses of total cell homogenates showed that apoB100 and sortilin levels were unaffected by the treatments (Figure 3A); however, in cells treated with bafilomycin there was increased recovery of apoB100 after sortilin immunoprecipitation, consistent with previous observations31. In contrast, treatment with 3-MA, which prevents autophagosome formation25, reduced sortilin interaction with apoB100. Neither apoB100 nor sortilin was detected in IgG control samples (Figure 3B).
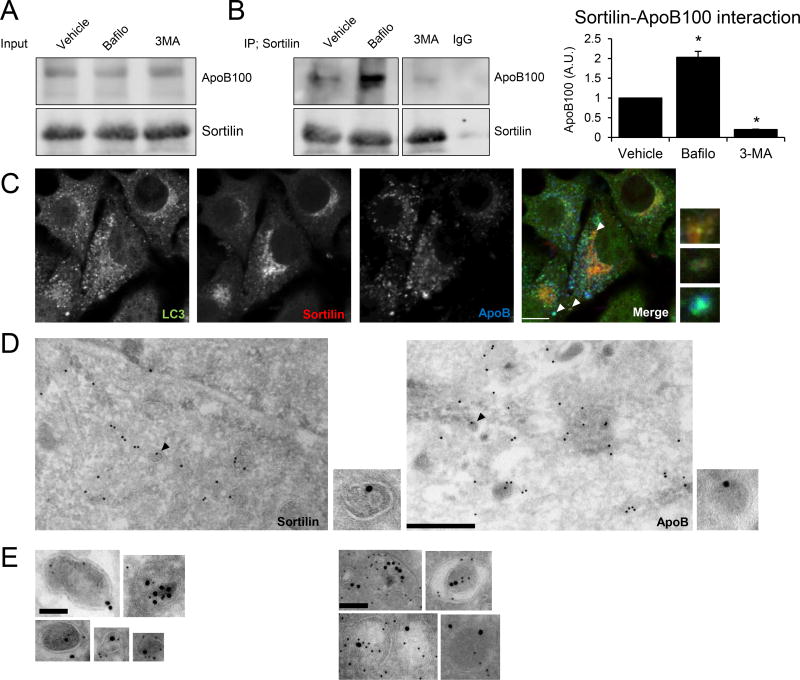
Figure 3. Sortilin and apoB100 are present in autophagic vacuoles in transformed and primary hepatic cells.
(A, B) McA cells overexpressing sortilin (McA-sortilin) were incubated with bafilomycin (100 nM) (Bafilo), 3-methyladenine (10 mM) (3-MA), or vehicle (water) for 3 h. After treatment, cells were washed and intracellular proteins cross-linked. Cellular sortilin was immunoprecipitated and the presence of sortilin or apoB100 was detected by western blotting (see methods for details). (A) ApoB100 and sortilin in the total cell homogenates represent the input for the co-immunoprecipitation analyses. (B) ApoB100 and sortilin detected after immunoprecipitation. Rabbit IgG was used as a control. The bands shown are cropped from the same western blot. The relative amount of apoB100 was quantified from 3 different experiments. (C) McA-sortilin cells were treated with bafilomycin, fixed and permeabilized to detect the presence of LC3 (green), sortilin (red), and apoB (blue) using confocal microscopy. Individual images are represented in greyscale. Insets show magnified areas with co-localization, indicated by the white arrowheads. White bar, 10 µM. (D) McA-sortilin cells exposed to bafilomycin were subjected to immuno-electron microscopy for sortilin (left) and apoB (right), as shown by immunogold labeling. Insets show magnified areas where sortilin and apoB co-localize within double membrane (i.e., autophagic) vacuoles. These structures are indicated with black arrowheads. Black bar; 500 nm. (E) Rabbit-anti sortilin antibody was detected using protein A conjugated to a 5 nm gold particle, while chicken-anti apoB antibody was detected using a secondary antibody conjugated to a 16 nm gold particle in McA-sortilin cells treated with bafilomycin. (F) Sortilin and apoB100 were detected in hepatocytes isolated from Apobec1−/− mice treated with the AAV-Sortilin expression vector. Once isolated, cells were exposed to vinblastine (50 µM) for 3 h. Black bar; 100 nm. Numerical data represent the means ± SEM. *, p<0.05 versus vehicle-treated cells by two-tailed Student’s t-test.
Next, we used confocal microscopy to determine the relative localization of sortilin, apoB (predominately apoB100, but also some apoB4833), and LC3 (an integral component of autophagosomes) in McA-sortilin-expressing cells pretreated with bafilomycin. As shown in Figure 3C, we found that the three proteins co-localized. We next used ultrastructural analysis of immunogold-staining to confirm the presence of sortilin and apoB in autophagic vacuoles, which are double membrane vesicles representing either autophagosomes or amphisomes. McA-sortilin cells were again treated with bafilomycin for 3 h and antibodies were used to detect sortilin or apoB. In this case, both proteins resided in the lumen of double-membrane vesicles of variable sizes and shapes (Figure 3D). We also simultaneously immunodetected sortilin and apoB using two gold-labeled secondary antibodies with different particle sizes (18 nm anti-chicken (apoB), 5 nm anti-rabbit (sortilin)). As shown in Figure 3E, double stained images additionally revealed both apoB and sortilin in the same double membrane structures (Figure 3E).
Although McA cells are enriched in apoB100 as a consequence of stable expression of the human protein, some apoB48 is still made due to persistent Apobec1 expression. To confirm that the data generated in the McA cells reflect the subcellular localization of apoB100 and not apoB48, and to further validate our data in a more humanized model, we generated primary hepatocytes from the livers of Apobec1−/− mice overexpressing sortilin through AAV-mediated transduction. We then treated these cells for 3 hours with vinblastine to prevent autophagosome-lysosome fusion and to inhibit autophagy34. Using the co-immunodetection protocol described above, we again observed sortilin and apoB100 co-localization inside autophagic vacuoles (Figure 3F).
Sortilin colocalizes with endosome and amphisome, but not phagophore, markers
Autophagy begins with the formation of a double membrane (phagophore) that sequesters cytosolic material as it seals into a double membrane (autophagosome). Autophagosomes then fuse then with lysosomes, which provide the hydrolases required for cargo degradation. However, a fraction of cellular autophagosomes can instead fuse first with endosomes or endosome-derived vesicles to generate amphisomes, which then fuse with lysosomes to complete cargo degradation35. The finding that 3-MA treatment decreases the association between sortilin and apoB100 (Figure 3B) suggests that sortilin and apoB100 interaction is dependent on the formation of autophagosomes. Hence, we aimed to determine more precisely at which step sortilin is incorporated into an autophagic vesicle. To this end, we performed co-localization studies with different organelle markers and, in agreement with previous reports4, indirect immunofluorescence microscopy showed sortilin co-localization with the TGN marker giantin and the early endosome marker EEA1. Importantly, sortilin also co-localized with the late endosome/lysosome marker LAMP1, as well as with the recycling endosome marker Rab11 (Figure 4A, C), which functions in amphisome formation35.
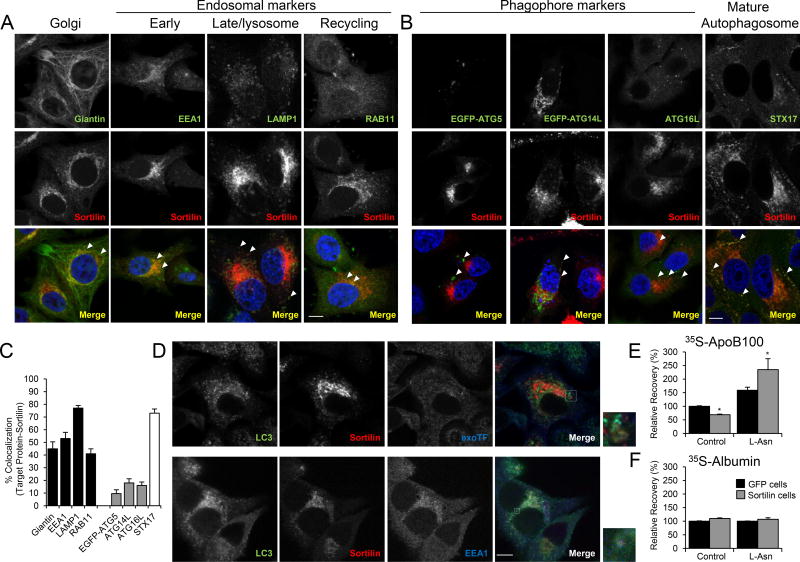
Figure 4. Sortilin co-localizes with endosome and autophagosome/amphisome markers, but not with phagophore markers.
(A) McA cells overexpressing sortilin (McA-sortilin) were fixed and immunostained for the Golgi marker giantin, the early endosome antigen 1 (EEA1), the late endosome and lysosomal marker lysosome associated protein 1 (LAMP1), and the recycling endosome marker Ras-related protein 11 (Rab11) (green). Sortilin immunostaining is represented in red, and nuclei were stained with DAPI (blue). (B) McA-sortilin cells were transiently transfected with plasmids containing the phagophore markers ATG5 or ATG14L tagged with EGFP (green). The phagophore marker ATG16L was detected by immunostaining (green). Syntaxin 17 (STX17) was used as a mature autophagosome marker (green). Sortilin immunostaining is represented in red, and nuclei are in blue. (C) Co-localization percentage between the target protein marker and sortilin was calculated by computerized image analysis (ImageJ) using the plugin JACoP. (D) Top panels; McA-sortilin cells were incubated with Alexa 568-labeled transferrin (exoTF) (25µg/ml) for 10 min, fixed, and permeabilized. Endogenous levels of LC3 (green), sortilin (red), and exoTF (blue) were co-localized using confocal microscopy. Bottom panels: co-localization between LC3 (green), sortilin (red), and EEA1 (blue) was detected using confocal microscopy. Insets show magnified areas with co-localization of the indicated proteins, denoted by the white arrowheads. Individual images are represented in greyscale. White bars, 10 µM. (E, F) McA cells stably expressing GFP (McA-GFP) or sortilin (McA-sortilin) were cultured in normal growth medium. Three hours before performing the pulse-chase analysis, cells were treated with L-asparagine (L-Asn, 30 mM) for 3 h to inhibit the fusion of amphisomes with the lysosomes. (E) Relative recovery for apoB100 or (F) albumin was determined by pulse-chase analysis. Numerical data represent the means ± SEM. *, p<0.05; two-tailed Student’s t-test. The experiments were performed in triplicate.
To further characterize the timing of sortilin colocalization with autophagy-related structures and to determine whether sortilin is incorporated into autophagosomes versus pre-autophagosomes (phagophores), we transiently transfected McA-sortilin cells with plasmids encoding EGFP-tagged ATG5 or ATG14L, proteins present at the limiting membrane (phagophore) during autophagosome formation and that dissociate during autophagosome maturation36. Confocal microscopy revealed that the degree of co-localization between sortilin and tagged ATG5 or ATG14L was significantly lower than that between sortilin and TGN or endosome markers. Similarly, sortilin co-localized at a greatly reduced level with the endogenous phagophore marker ATG16L (Figure 4B, C). We next determined the degree of co-localization between sortilin and syntaxin 17 (STX17), a protein only present in fully formed, mature autophagosomes37. In this case, a high degree of co-localization was evident (Figure 4B, C). These data suggest that sortilin becomes incorporated into the autophagosome after it is fully formed, presumably via fusion of an endosome in which it resides with an autophagosome, thereby generating an amphisome.
To confirm that sortilin is present in amphisomes, which have both autophagosome and endosome markers38, we incubated McA-sortilin cells with exogenous Alexa 568-labeled transferrin (a marker of the endosomal compartment) for 10 min and performed co-localization experiments. Confocal microscopy revealed that the autophagosome marker LC3 co-localized with exogenous transferrin and with sortilin (Figure 4D). These results were confirmed and extended by co-localizing the endosome marker EEA1 with sortilin and LC3, supporting our conclusion that sortilin is present in the amphisome.
To further test the involvement of an amphisomal intermediate in sortilin-mediated apoB100 degradation, we treated sortilin overexpressing McA cells and appropriate GFP-overexpressing control cells with L-asparagine (L-Asn), a specific inhibitor of amphisome-lysosome fusion38. We hypothesized that if sortilin facilitated apoB100 trafficking to the lysosome through an amphisome intermediate, disrupting this fusion event would decrease sortilin-mediated apoB100 degradation. Indeed, we observed that L-Asn increased the relative recovery of apoB100 in both sets of cells, but a greater effect was observed in sortilin overexpressing cells (Figure 4E). In contrast, L-Asn had no effect on albumin recovery in either cell line (Figure 4F).
Sortilin and apoB100 are enriched in autophagic vacuoles and co-localize with amphisome markers in mouse liver
To demonstrate sortilin-dependent autophagic degradation of apoB100 in vivo, we injected Apobec1−/− mice with AAV-Sortilin or AAV-GFP (control) and quantitated the amount of apoB100 in hepatic autophagic vacuoles. We hypothesized that increased SORT1 expression would consequently increase the autophagic vacuole localization of apoB100. Two weeks after AAV injection, mice were fasted overnight and 3 hours prior to sacrifice they were injected with vinblastine to increase the recovery of autophagic vacuoles34. Fraction purity was confirmed by western blot analysis of organelle-specific markers in each subcellular fraction (Figure 5A, Online Figure VIA), as previously described34. As expected, LC3-II was enriched in the autophagosomal/lysosomal fractions. The conjugated protein was also more abundant in autophagic vacuoles since degradation had not yet occurred in this compartment, as indicated by the low level of mature cathepsin D in this fraction.
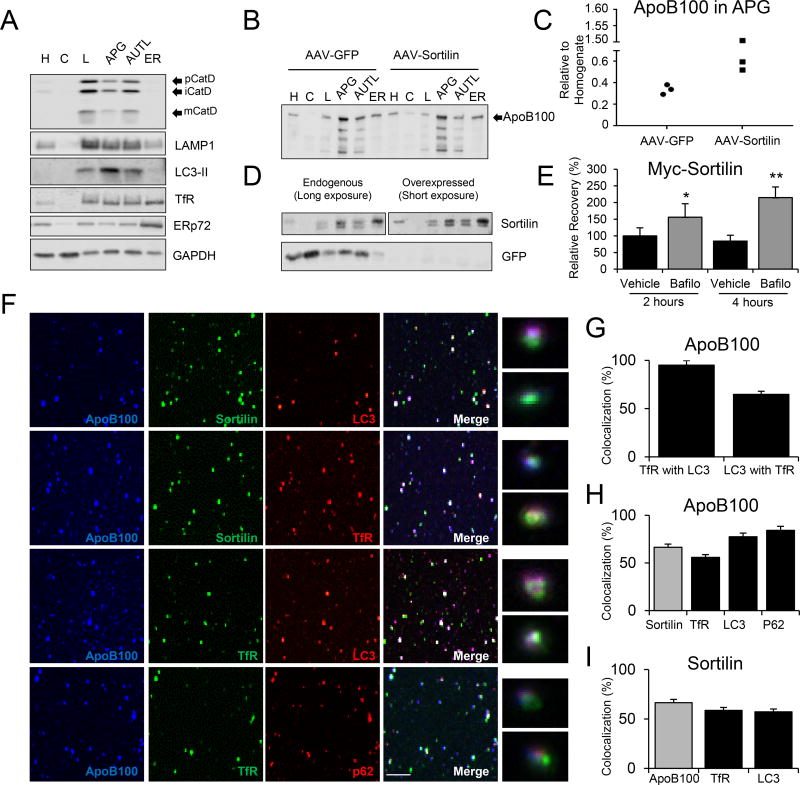
Figure 5. Sortilin and apoB100 are present in amphisomes in vivo.
Apobec1−/− mice (n=3/group) were injected with a liver-specific AAV containing sortilin (AAV-Sortilin) or GFP (AAV-GFP) prior to isolation of hepatic autophagic vacuoles (see methods for details). (A) Enrichment of the different fractions assessed by western blotting using the lysosomal marker cathepsin D (mature (mCatD), immature (iCatD), and pro-Cathepsin D (pCatD)), endosomal/lysosomal marker LAMP1, autophagosome marker LC3, endosomal marker transferrin receptor (TfR), endoplasmic reticulum (ER) marker ERp72, and cytosolic marker GAPDH. (B) Relative distributions of apoB100 in subcellular fractions. Intact apoB100 is indicated with an arrow. (C) ApoB100 content in autophagic vacuole fraction (APG) in comparison to homogenate (H) normalized to total protein content. (D) Top panels: Relative distribution of sortilin between AAV-GFP (endogenous) and AAV-Sortilin (overexpressed) injected mice. Due to large differences in expression, representative western blots show two different exposure times. Bottom panel: GFP relative distribution. C: cytosol, L: lysosome, AUTL: autolysosome. (E) McA cells transiently transfected using a myc-tagged sortilin plasmid and treated with bafilomycin (100 nM) or vehicle (DMSO) for 3 h. Cells were subjected to a pulse-chase analysis at two different chase points, 2 and 4 h (see methods for details). Myc-sortilin was immunoprecipitated using an anti-Myc antibody, separated by SDS-PAGE and quantified by phosphorimaging. Experiments were performed in duplicate. (F) The presence of amphisomes in the APG fraction and of sortilin and apoB100 in these amphisomes was confirmed by spotting vesicles on a cover slip and staining with indicated antibodies. Single channels, merged images, and inserts showing triple co-localization at higher magnification are shown. (G–I) Quantification of co-localization of TfR and LC3 to determine amphisome abundance (G), and of apoB100 (H) and sortilin (I) with indicated markers. Numerical data represents the average ± SEM. Statistical analysis performed using two-tailed t-test. *, p<0.05 versus 2 h vehicle control. #, p<0.05 versus 2 h Bafilo treatment.
Next, the presence and distribution of apoB100, sortilin, and GFP in the different subcellular fractions were analyzed by western blotting (Figure 5B–D). ApoB100 was present in its native full-length form in the total homogenate (H) and ER fractions, and was significantly depleted from the cytosolic (C) fraction. In contrast, the autophagic (APG; containing autophagosomes and amphisomes38) and autolysosomal (AUTL) fractions showed an enrichment in full-length and fragmented forms of apoB100 (Figure 5B). If hepatic sortilin overexpression promotes apoB100 delivery to autophagic vacuoles, we reasoned that with sortilin overexpression, apoB100 would be enriched in the APG fraction relative to the total liver homogenate (H). Despite not reaching statistical significance (p=0.167, two-tailed Student’s t-test), all of the mice (n=3/group) injected with AAV-Sortilin showed increased apoB100 in the APG fraction in comparison to their littermate controls, which were injected with AAV-GFP (Figure 5C).
Sortilin was mostly present in the ER and the APG fractions, but was depleted in the lysosome (L) and AUTL fractions. Like apoB100 (Figure 5B), sortilin also underwent fragmentation, but only a single proteolytic band was apparent (Figure 5D), which was present in the three autophagy-related fractions (L, APG, AUTL), but not in ER and total homogenate fractions. For both apoB100 and sortilin, the fragments are an expected consequence of cathepsin D proteolysis. It is important to note that the relative distribution of sortilin in the livers of AAV-GFP injected mice, in which sortilin is expressed at wild type levels, is essentially identical to that of sortilin in AAV-Sortilin-injected mice (Figure 5D, Online Figure VIB). Analysis of sortilin’s intracellular distribution was alternatively assessed in McA wild-type cells and McA-sortilin cells exposed to vehicle or bafilomycin using confocal microscopy (Online Figure VII). In the control, sortilin localized near the nucleus, whereas bafilomycin treatment promoted a dispersed distribution of sortilin in both cell types (Online Figure VII). GFP was only present in mice injected with AAV-GFP and showed the same distribution as other cytosolic proteins (i.e., GAPDH, as shown in Figure 5A), but was also present in the autophagic compartments as a result of bulk cytosolic sequestration (Figure 5D, Online Figure VIC).
Because the sortilin sub-cellular distribution follows the same pattern as apoB100 (i.e., more abundant in the APG than in the AUTL fraction; Figure 5B, D), we hypothesized that sortilin is also degraded in the lysosome. To test this hypothesis, McA cells were transfected with a plasmid encoding Myc-tagged full-length sortilin, the cells were treated with bafilomycin for 3 hours to inhibit lysosomal degradation, and a pulse-chase analysis was performed. We found that bafilomycin treatment increased sortilin levels in a time-dependent manner, suggesting that sortilin is indeed degraded by the lysosome (Figure 5E). These results are consistent with previous data39–41 and are also consistent with the presence of sortilin in autophagic vacuoles that ultimately fuse with the lysosome.
To further establish amphisomes as an intermediate in the pathway of sortilin-mediated apoB100 degradation, we asked whether endosomal cargo resided in the different isolated compartments. As shown in Figure 5A, we found that transferrin receptor (TfR) was detected in lysosomes—as expected from the delivery of endocytic cargo to lysosomes—but was also present in the APG and AUTL fractions. This observation suggests either possible endosomal contamination of the fractions or the presence of hybrid autophagosome/endosome components (i.e., amphisomes). To distinguish between these possibilities, we spotted vesicles from the APG fraction on a glass coverslip and immunostained them with different antibodies. As shown in Figures 5F–I, we confirmed that about half of the LC3-positive compartments were also positive for the endosomal marker, transferrin receptor, and that all of the transferrin receptor-positive compartments had LC3 label. Therefore, the double-labeled compartments represent bona fide autophagosomes, but not endosomes. Although four color labeling was not possible due to antibody availability, triple labeling confirmed that apoB100 and sortilin co-localized in ~75% of these examples, and that they both also co-localized with LC3 and transferrin receptor. Overall, these data confirm the presence of both sortilin and apoB100 in amphisomes.
Increased sortilin expression enhances autophagic flux
Sortilin is a critical mediator of lysosomal trafficking and degradation, which suggests that the receptor may also play a more global role in autophagy beyond its role in apoB metabolism. To test this hypothesis, we used primary mouse hepatocytes transduced with an adenovirus encoding the LacZ reporter gene (AV-LacZ, control) or a full-length human sortilin construct (AV-Sortilin). After 24 h, cells were incubated in the presence of the lysosomal protease inhibitors leupeptin and E64D, or vehicle (water) for 4 h, and intracellular levels of the autophagosome marker LC3 were determined by immunofluorescence microscopy.
As shown in Figure 6A, while no significant differences in LC3 fluorescence levels were observed between untreated AV-LacZ and AV-Sortilin hepatocytes, upon treatment with the protease inhibitors both sets of cells showed an increase in LC3, but the relative increase was significantly greater in the sortilin-overexpressing cells. We next collected total cell lysates to determine changes in LC3-II, which is the form of LC3 associated with autophagic vacuoles that consequently undergo lysosomal degradation42. However, we failed to note significant differences in steady-state levels of LC3-II between cells infected with AV-LacZ or AV-Sortilin (Figure 6B); in contrast, treatment with the protease inhibitors increased LC3-II levels in both cell types with a significantly more pronounced effect in sortilin-overexpressing cells (Figure 6B). Overall, these results suggest that sortilin overexpression enhances autophagic flux in both transformed and primary hepatic cells.
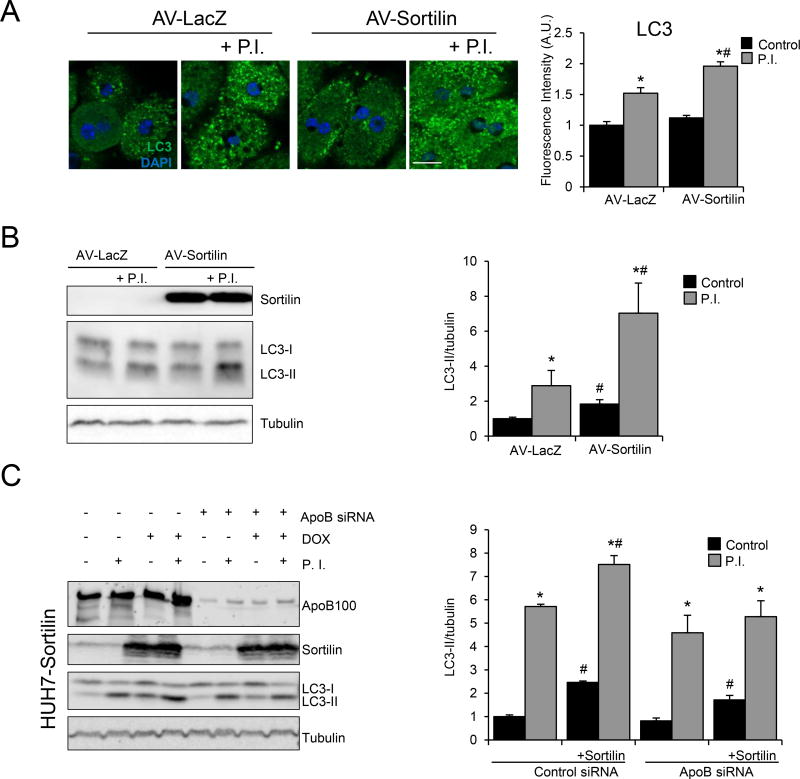
Figure 6. Sortilin overexpression in primary hepatocytes increases autophagic flux.
(A) Mouse primary hepatocytes were isolated and plated to 80–90% confluence and subsequently infected with an adenovirus (AV) containing β-galactosidase (LacZ) or sortilin for 24 h. After incubation, cells were maintained in the presence or absence of the lysosomal protease inhibitors (P.I.) E46D (30 µM) and leupeptin (100 µM) for 4 h before being fixed, permeabilized, and stained for LC3 (green). Nuclei were stained with DAPI (blue). White bar; 10µm. Graph bars show LC3 fluorescence levels from the different treatments measured using ImageJ. (B) Cells subjected to the same experimental conditions were harvested and total protein homogenates were separated by SDS-PAGE. Representative western blot images are shown for sortilin, LC3, and tubulin (loading control). Graph bars show the quantification of LC3-II levels/tubulin. (C) Doxycycline (DOX)-inducible HUH7 hepatocytes overexpressing sortilin (HUH7-sortilin) were transfected with siRNA targeting human apoB100 or with a scrambled siRNA (control) for 24 h before changing the medium to DOX-containing medium or control media. Twenty-four hours later, cells were incubated with protease inhibitors (P.I.) for 4 h before being harvested for protein analysis. Representative western blot images are shown for apoB100, sortilin, LC3, and tubulin (loading control). Graph bars show the quantification of LC3-II levels/tubulin. Numerical data represent the means ± SEM. *, p<0.05 control treatment versus P.I. treatment. #, p<0.05 control cells versus sortilin-overexpressing cells; two-tailed Student’s t-test.
We also measured p62 protein levels, which usually correlate inversely with autophagic activity43, in the total liver homogenate of mice injected with AAV-GFP or AAV-Sortilin (n=6/group) two weeks prior to analysis. As anticipated, p62 levels in total liver homogenate were reduced in mice injected with AAV-Sortilin, confirming that sortilin overexpression promotes autophagic activity in the liver (Online Figure VIIIA).
ApoB100 expression influences sortilin-dependent autophagic flux
To determine if the stimulatory effect of sortilin on autophagy was related to its ability to increase apoB100 delivery for autophagy, we studied HUH7 human hepatic cell lines engineered to overexpress sortilin under the control of an inducible promoter4. These cells harbor the full-length human sortilin gene under the control of a doxycycline (DOX)-inducible promoter. To assess the differential effect of sortilin on autophagic flux based on apoB100 abundance, we used siRNA to reduce expression of apoB100 in these cells.
First, the HUH7 sortilin expressing cells were treated with scrambled siRNA for 24 h and then exposed to DOX for 48 h to induce sortilin overexpression. After incubation with protease inhibitors, the cells were harvested and LC3-II levels were measured to determine autophagic flux. Similar to our findings in mouse primary hepatocytes (Figure 6A, B), we observed a significant increase in LC3-II flux (as reflected by LC3-II degradation) in cells where sortilin expression was induced (Figure 6C). This sortilin-induced increase in LC3-II flux was absent when cells were treated with apoB100 siRNA (Figure 6C). Expression of sortilin in HUH7 cells also increased steady-state levels of LC3-II (Figure 6C), but this increase was independent of the level of apoB100. The increase in LC3-II flux was attributed neither to DOX (Online Figure VIIIB) nor to sortilin overexpression increasing LC3 transcription (Online Figure VIIIC). Instead, the effect of sortilin overexpression on autophagy is consistent with effects observed upon expression of other autophagy receptors, such as optineurin or TRIM3044, 45. These results further establish the role of sortilin in the regulation of autophagy and demonstrate that recognition between an autophagy receptor and its cargo can induce this pathway.
The cytosolic tail of sortilin facilitates both apoB100 degradation and enhanced autophagic flux
To determine if the sortilin-dependent increase in autophagic flux is related to its role as a mediator of TGN and endocytic trafficking, we studied two sortilin mutants that compromise Golgi-to-endosome transport in HUH7 cells46. We previously showed that these sortilin mutated forms have no effect on apoB100 secretion4. The LAYA mutant lacks the tyrosine and di-leucine sorting motifs located on the cytoplasmic tail46. The STOP mutant lacks the entire cytoplasmic tail due to the generation of a premature stop codon (Figure 7A). HUH7 sortilin expressing cells under the control of the DOX-inducible promoter were used as controls (Figure 7B).
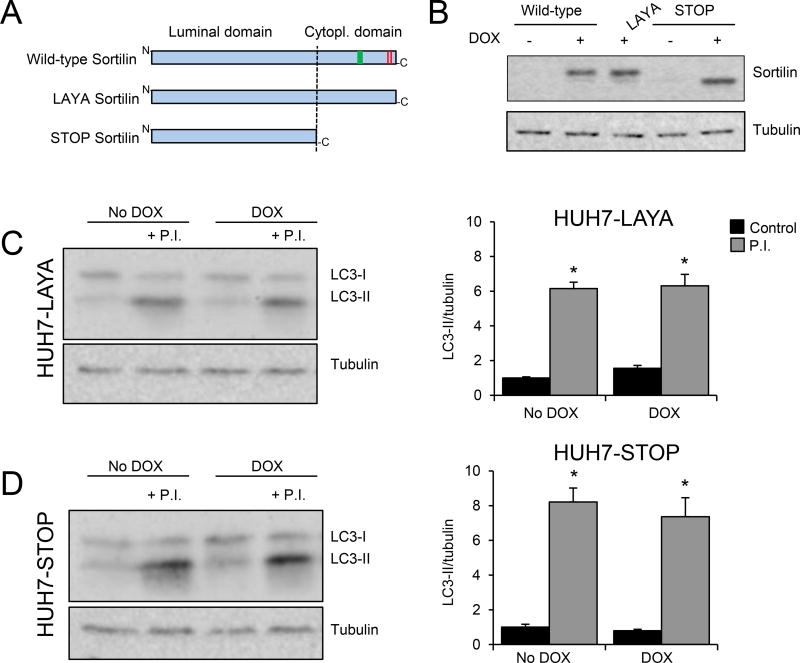
Figure 7. Sortilin targeting motifs are required to induce autophagy in HUH7 cells.
Autophagic flux was determined in doxycycline (DOX)-inducible HUH7 cells stably overexpressing the LAYA-sortilin mutant (lacking two sorting motifs present in the cytoplasmic tail: dileucine, red; tyrosine, green)) or the STOP-sortilin mutant (lacking the entire cytoplasmic tail). (A) Schematic representation of the constructs used. (B) Western blotting analysis showing the expression of sortilin after 48 h treatment with 700 ng/ml of DOX from cells expressing a wild-type, LAYA, or STOP sortilin construct. (C, D) HUH7 cells overexpressing the sortilin LAYA mutant (HUH7-LAYA) or the sortilin STOP mutant (HUH7-STOP) were incubated with regular medium (no DOX) or DOX-supplemented medium (700 ng/ml) for 48 h. Four hours before harvesting, cells were maintained with growth medium in the presence or absence of a combination of the lysosomal protease inhibitors (P.I.) E46D (30 µM) and leupeptin (100 µM). Total protein homogenates were separated on an acrylamide gel to quantify the levels of LC3-II and tubulin (as a control). Representative western blot images are shown for LC3 and tubulin (left panels), as are the corresponding LC3-II/tubulin quantifications (right panels). Experiments were performed in triplicate. Numerical data represent the means ± SEM. *, p<0.05 vehicle versus P.I. treatment. Two-tailed Student’s t-test.
In contrast to the positive effects of sortilin overexpression on autophagy, as described above, expression of the Sortilin STOP or LAYA proteins blocked sortilin-induced autophagic flux (Figure 7C–F). From these results, along with previous findings4, we conclude that these sorting elements are required for sortilin to direct apoB100 for autophagic degradation as well as to increase the degradative capacity of the autophagy pathway.
DISCUSSION
Nine years ago a single nucleotide polymorphism near the SORT1 locus was discovered by GWAS to be strongly associated with reduced LDL-C, protection from atherosclerotic cardiovascular disease (ASCVD), and, in minor allele homozygotes, a 12-fold increase in hepatic SORT1 expression2. Indeed, our previous studies in mice and hepatic cells established that increased sortilin expression reduces apoB-lipoprotein production, which can be reversed in vitro with a lysosomal inhibitor and in vivo by the use of sortilin mutants compromised for lysosomal trafficking4. These findings helped explain the GWAS data and are consistent with sortilin being a member of the Vps10p sorting family, which bind protein cargo in the Golgi for vesicular transport to the endosomal compartment. From here, these cargo molecules are distributed to multiple locations, including the lysosome47. Nonetheless, significant gaps remained in our understanding of sortilin’s role in apoB100 and VLDL metabolism.
Two open questions that we have answered in this report are the prior lack of direct evidence showing that increased SORT1 expression promotes apoB100 degradation, and, assuming that it does, the cellular itinerary taken by apoB100 during the degradation process. We now show that: 1) increased SORT1 expression in hepatic cells decreases recovery of apoB100 by diverting it from the secretory pathway and promoting its autophagic degradation, and 2) increased expression of sortilin traffics the diverted apoB100 to autophagic vacuoles through amphisome intermediates, which represent the fusion of endosomes with autophagosomes. Furthermore, our data demonstrate that sortilin is a global regulator of autophagic capacity, a phenomenon that is dependent both on the presence of an embedded region required for intracellular trafficking and the level of apoB100 expression. Taken together, these findings provide a novel mechanistic basis for the lower LDL-C in individuals homozygotic for the SORT1 allele associated with increased hepatic expression of sortilin.
Because sortilin begins to interact with cargo in the TGN, our results position degradation of apoB100 after SORT1 overexpression as another example of post-ER presecretory proteolysis (PERPP), a phenomenon we initially reported in 200148. One example of PERPP is N-3 fatty acid-induced autophagic degradation of apoB100 that is damaged by lipid peroxidation21. It was not obvious that overexpression of SORT1 would be another example for two reasons, one being the differences in the metabolic perturbations. The other is that the conventional understanding of lysosomal targeting by either a mannose 6-phosphate receptor (M6PR) dependent or independent (e.g., sortilin) mechanism in what has been termed the direct pathway, is that sorting receptors bind and concentrate client proteins in areas of the TGN from which vesicles bud and traffic to the endosomal compartment. This is followed by endosomal maturation and eventual fusion with lysosomes (reviewed in49). Thus, there is no requirement for entry into the autophagic machinery during this itinerary. That sortilin and apoB100 co-localize in amphisomes, structures resulting from the fusion of early or late endosomes and autophagosomes38, means that unlike classical transport to lysosomes, apoB100 targeting is unconventional and indirect.
Our finding that sortilin affects autophagic capacity in a manner dependent on the level of apoB100 expression is consistent with previous studies on Aβ oligomers, which are linked to the pathogenesis of Alzheimer disease. As recently shown, these oligomers, like apoB100, are sortilin ligands and can trigger autophagic flux in cultured neurons; however, pre-incubation with SORT1 siRNA blocked these effects50. Interestingly, both Aβ oligomers and apoB100 are aggregation-prone21, 51, implying that sortilin may be especially adept at handling insoluble ligands. Our results are also reminiscent of the increase in autophagy observed upon expression of other autophagy receptors, such as optineurin or TRIM30, further supporting the model that recognition between an autophagy receptor and its ligand-cargo is capable of inducing this pathway44, 45.
Based on our data, adjusting the level of autophagy to the level of apoB100 expression may represent one strategy to meet the challenges that this protein presents to hepatic cells. ApoB100 is a 4536 amino acid protein with extensive hydrophobic patches and multiple intramolecular disulfide bonds. Its folding and assembly into nascent pre-VLDL particles requires processing in the ER, with still more maturation of pre-VLDL occurring in the Golgi to achieve a secretion-competent state (reviewed in12, 13). In order to destroy apoB100 and thus regulate its levels, autophagy/PERPP can be viewed as a quality or quantity control pathway that serves as a late-stage checkpoint to protect hepatic cells from the accumulation of apoB100 and VLDL particles that are otherwise too large to be handled by the ER-Associated Degradation pathway52, which requires retro-translocation from the ER and degradation by the proteasome. Given the inefficiency in ultimately forming mature VLDL particles, the level of apoB100 expression will have an impact on the abundance of the potentially toxic intermediates, and increasing autophagy can be viewed as a protective, homeostatic response.
Although we have only investigated the role of sortilin in apoB100 degradation, which involves diverting apoB100 from the secretory pathway, we recognize that sortilin can also facilitate apoB100/VLDL secretion, as revealed in mice deficient in whole body or hepatic sortilin (e.g.,4, 7, 9). Because sortilin initially serves to target cargo from the TGN to the endosomal compartment47, the subsequent and divergent steps (ultimately to either the lysosome for degradation or the plasma membrane for secretion) are expected to require different mechanisms. Indeed, it has recently become more generally appreciated that specialized vesicles can traffic cargo proteins that, like apoB100, have either secretory or degradation fates (reviewed in53). Notably, a fraction of such vesicles, dubbed perhaps paradoxically as secretory autophagosomes, fuse with early or late endosomes to form amphisomes so that the ferried cargo is eventually degraded by the lysosome. Further studies, beyond the scope of present report will be required to determine the relationship between this process (“secretory autophagy”) and the degradation mediated by increased sortilin expression. At a minimum, even if the two processes are unique, the involvement of an amphisome intermediate appears to be common and essential. Further studies will also be needed to determine the mechanistic relationships, if any, in the aforementioned phenotypic similarity (decreased apoB and VLDL secretion) in SORT1 overexpressing versus deficient mice. Because no individual with SORT1 deficiency has been described, we have exclusively focused in this report on the clinically relevant setting, i.e., SORT1 overexpression, and have summarized our present understanding of the cellular relationships between this overexpression and pre-secretory apoB100 metabolism in Online Figure IX. It is also important to place the present findings in the context of other data. Our laboratory and the groups of Janet and Charles Sparks have reported that insulin, a physiological regulator of VLDL production in mammals, promotes the degradation of apoB100 by autophagy54, 55. In subsequent studies, the Sparks have shown that insulin increases the interaction of apoB100 with sortilin prior to degradation31, 56. Based on our data, we suggest that increased sortilin-apoB100 interaction provides a plausible mechanism for insulin-induced apoB100 autophagy. That sortilin is an important mediator of the effects of insulin on apoB100/VLDL metabolism is also supported by work from the Rader and Tall laboratories in which hepatic sortilin expression was suppressed and VLDL production was increased in insulin-resistant (ob/ob) mice. Notably, these events were reversed when sortilin expression was restored5.
Another context to place the present findings in is apoB100 degradation in general. As noted in Results, sortilin overexpression explains almost 30% of the autophagic degradation of apoB100, consistent with the degree of reduction of apoB100 in pre-clinical models (4 and the present results). Though this may appear modest, in individuals homozygotic for the SORT1 allele associated with hepatic overexpression, there is a 40% reduction in CVD risk. Similar to loss-of-function PCSK9 mutations57, this likely represents the clinical benefits of a lifelong mild reduction in LDL-C.
In summary, we have shown that increased hepatic SORT1 expression, to mimic individuals homozygotic for the minor allele, diverts apoB100 from the secretory pathway to a degradation process that requires autophagy. This indirect route is distinct from the more conventional, direct trafficking route from the TGN to the lysosome. The complexity of this pathway likely evolved to enable sortilin to serve a dual role in lipoprotein metabolism, namely to promote apoB100 degradation as well as VLDL secretion, with the impact on each aspect appropriately scaled to metabolic factors. Future studies will undoubtedly uncover other novel mechanistic features of the apoB100 degradation pathway, some of which may be therapeutically applicable. Nevertheless, the present results significantly extend the mechanistic basis for the decreased LDL-C levels in humans with increased hepatic SORT1 expression.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Genome-wide association studies (GWAS) identified a genetic variant present in the SORT1 gene promoter associated with lower LDL cholesterol (LDL-C), less cardiovascular disease, and increased expression of the encoded protein sortilin 1, or sortilin, in the liver.
Subsequent studies in mice showed that hepatic sortilin overexpression reduced the production of apolipoprotein B100 (apoB) and VLDL (precursor of LDL), by diversion from the secretory pathway.
In studies in vitro, sortilin bound apoB and its increased expression resulted in lysosomal degradation of apoB and VLDL (apoB/VLDL).
What New Information Does This Article Contribute?
We show that when sortilin is overexpressed in hepatic cells, it induces an unconventional form of lysosomal targeting of apoB/VLDL, in which sortilin and apoB/VLDL traffic to the endosomal compartment in vesicles that fuse with autophagosomes to form amphisomes, which merge with lysosomes.
Sortilin is also a novel regulator of autophagy; when overexpressed, sortilin increases the degradative capacity, or flux, of this pathway.
Studies of the effects of sortilin overexpression on apoB/VLDL production in vivo were consistent with the in vitro studies. Overall, our studies suggest that the cell biological fate of apoB/VLDL in humans with sortilin overexpression and diversion of apoB/VLDL from the secretory pathway is degradation through a pre-lysosomal, autophagy-dependent intermediate process.
A polymorphism in the SORT1 gene is associated by GWAS with lower LDL-C and decreased CVD risk. Given these desirable associations, the underlying mechanisms are of considerable interest. There is increased mRNA and protein (sortilin) encoded by SORT1 in the livers of individuals with the polymorphism. Consistent with this finding, when sortilin was overexpressed in hepatic cells or mice, the secretion of apoB/VLDL was decreased. Thus, we explored the cell biological fate of apoB/VLDL diverted from secretion by sortilin. Sortilin sorts cargo from the trans-Golgi network to lysosomes (and the plasma membrane), and we hypothesized that the effects of sortilin overexpression on apoB/VLDL secretion represented conventional targeting to lysosomal degradation. Instead, we surprisingly found that the route is indirect and intersects with autophagy. Vesicles that contain sortilin and apoB/VLDL leave the secretory pathway and fuse with autophagic vacuoles, forming unusual structures called amphisomes. These, in turn, fuse with lysosomes. Although sortilin amplifies this autophagic degradation, blocking this unique pathway does not increase apoB/VLDL secretion, demonstrating that regulation of secretion resides with the activity of sortilin. The results provide mechanistic insight into the apoB100 degradation that results from sortilin-induced diversion of nascent VLDL from the secretion pathway. This degradation is an unusual example of the utilization of amphisomes, a rarely sighted autophagic intermediate, in the degradation process of apoB and nascent VLDL.
Acknowledgments
We thank Fengxia Liang, Chris Petzold, and Kristen Dancel-Manning, of the Microscopy Laboratory at New York University School of Medicine for their expertise in electron microscopy. We also would like to recognize our recently deceased (Dec. 3, 2017) colleague, Dr. Janet D. Sparks, whose many notable findings were influential in guiding our thinking.
SOURCES OF FUNDING
These studies were supported by NIH grants HL58541 and 127930 (EAF), AG031782 and DK098408 (AMC), DK79307 (JLB), HL059407 (DJR), a Scientist Development Grant from the American Heart Association 16SDG27550012 (JA), and a postdoctoral fellowship from the American Diabetes Association Grant 1-15-MI-03 (JMM).
Nonstandard Abbreviations and Acronyms
- ApoB100
apolipoprotein B100
- LC3
microtubule-associated protein light chain 1A/1B-light-chain 3
- LDL
low density lipoprotein
- VLDL
very-low density lipoprotein
- TGN
Trans-Golgi network
- McA cells
Rat hepatoma McArdle RH-7777 cells
- Apobec1
apoB mRNA editing enzyme 1
- SNP
Single nucleotide polymorphism
- C/EBPα
CCAAT/enhancer-binding protein alpha
Footnotes
DISCLOSURES
None.
References
- 1.Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, Pirruccello JP, Ripatti S, Chasman DI, Willer CJ, Johansen CT, Fouchier SW, Isaacs A, Peloso GM, Barbalic M, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–13. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Musunuru K, Strong A, Frank-Kamenetsky M, Lee NE, Ahfeldt T, Sachs KV, Li X, Li H, Kuperwasser N, Ruda VM, Pirruccello JP, Muchmore B, Prokunina-Olsson L, Hall JL, Schadt EE, Morales CR, Lund-Katz S, Phillips MC, Wong J, Cantley W, Racie T, Ejebe KG, Orho-Melander M, Melander O, Koteliansky V, Fitzgerald K, Krauss RM, Cowan CA, Kathiresan S, Rader DJ. From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature. 2010;466:714–9. doi: 10.1038/nature09266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calkhoven CF, Gringhuis SI, Ab G. The chicken CCAAT/enhancer binding protein alpha gene. Cloning, characterisation and tissue distribution. Gene. 1997;196:219–29. doi: 10.1016/s0378-1119(97)00230-8. [DOI] [PubMed] [Google Scholar]
- 4.Strong A, Ding Q, Edmondson AC, Millar JS, Sachs KV, Li X, Kumaravel A, Wang MY, Ai D, Guo L, Alexander ET, Nguyen D, Lund-Katz S, Phillips MC, Morales CR, Tall AR, Kathiresan S, Fisher EA, Musunuru K, Rader DJ. Hepatic sortilin regulates both apolipoprotein B secretion and LDL catabolism. The Journal of clinical investigation. 2012;122:2807–16. doi: 10.1172/JCI63563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ai D, Baez JM, Jiang H, Conlon DM, Hernandez-Ono A, Frank-Kamenetsky M, Milstein S, Fitzgerald K, Murphy AJ, Woo CW, Strong A, Ginsberg HN, Tabas I, Rader DJ, Tall AR. Activation of ER stress and mTORC1 suppresses hepatic sortilin-1 levels in obese mice. The Journal of clinical investigation. 2012;122:1677–87. doi: 10.1172/JCI61248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen CM, Nielsen MS, Nykjaer A, Jacobsen L, Tommerup N, Rasmussen HH, Roigaard H, Gliemann J, Madsen P, Moestrup SK. Molecular identification of a novel candidate sorting receptor purified from human brain by receptor-associated protein affinity chromatography. The Journal of biological chemistry. 1997;272:3599–605. doi: 10.1074/jbc.272.6.3599. [DOI] [PubMed] [Google Scholar]
- 7.Strong A, Patel K, Rader DJ. Sortilin and lipoprotein metabolism: making sense out of complexity. Current opinion in lipidology. 2014;25:350–7. doi: 10.1097/MOL.0000000000000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munck Petersen C, Nielsen MS, Jacobsen C, Tauris J, Jacobsen L, Gliemann J, Moestrup SK, Madsen P. Propeptide cleavage conditions sortilin/neurotensin receptor-3 for ligand binding. The EMBO journal. 1999;18:595–604. doi: 10.1093/emboj/18.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kjolby M, Andersen OM, Breiderhoff T, Fjorback AW, Pedersen KM, Madsen P, Jansen P, Heeren J, Willnow TE, Nykjaer A. Sort1, encoded by the cardiovascular risk locus 1p13.3, is a regulator of hepatic lipoprotein export. Cell metabolism. 2010;12:213–23. doi: 10.1016/j.cmet.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Carlo AS, Nykjaer A, Willnow TE. Sorting receptor sortilin-a culprit in cardiovascular and neurological diseases. Journal of molecular medicine. 2014;92:905–11. doi: 10.1007/s00109-014-1152-3. [DOI] [PubMed] [Google Scholar]
- 11.Lamarche B, Tchernof A, Mauriege P, Cantin B, Dagenais GR, Lupien PJ, Despres JP. Fasting insulin and apolipoprotein B levels and low-density lipoprotein particle size as risk factors for ischemic heart disease. Jama. 1998;279:1955–61. doi: 10.1001/jama.279.24.1955. [DOI] [PubMed] [Google Scholar]
- 12.Brodsky JL, Fisher EA. The many intersecting pathways underlying apolipoprotein B secretion and degradation. Trends in endocrinology and metabolism: TEM. 2008;19:254–9. doi: 10.1016/j.tem.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ginsberg HN, Fisher EA. The ever-expanding role of degradation in the regulation of apolipoprotein B metabolism. Journal of lipid research. 2009;50(Suppl):S162–6. doi: 10.1194/jlr.R800090-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher EA. The degradation of apolipoprotein B100: multiple opportunities to regulate VLDL triglyceride production by different proteolytic pathways. Biochimica et biophysica acta. 2012;1821:778–81. doi: 10.1016/j.bbalip.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng Y, He D, Yao Z, Klionsky DJ. The machinery of macroautophagy. Cell research. 2014;24:24–41. doi: 10.1038/cr.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaushik S, Cuervo AM. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nature cell biology. 2015;17:759–70. doi: 10.1038/ncb3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–5. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider JL, Suh Y, Cuervo AM. Deficient chaperone-mediated autophagy in liver leads to metabolic dysregulation. Cell metabolism. 2014;20:417–32. doi: 10.1016/j.cmet.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shpilka T, Welter E, Borovsky N, Amar N, Shimron F, Peleg Y, Elazar Z. Fatty acid synthase is preferentially degraded by autophagy upon nitrogen starvation in yeast. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:1434–9. doi: 10.1073/pnas.1409476112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meex SJ, Andreo U, Sparks JD, Fisher EA. Huh-7 or HepG2 cells: which is the better model for studying human apolipoprotein-B100 assembly and secretion? Journal of lipid research. 2011;52:152–8. doi: 10.1194/jlr.D008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan M, Maitin V, Parathath S, Andreo U, Lin SX, St Germain C, Yao Z, Maxfield FR, Williams KJ, Fisher EA. Presecretory oxidation, aggregation, and autophagic destruction of apoprotein-B: a pathway for late-stage quality control. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5862–7. doi: 10.1073/pnas.0707460104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao ZM, Blackhart BD, Linton MF, Taylor SM, Young SG, McCarthy BJ. Expression of carboxyl-terminally truncated forms of human apolipoprotein B in rat hepatoma cells. Evidence that the length of apolipoprotein B has a major effect on the buoyant density of the secreted lipoproteins. The Journal of biological chemistry. 1991;266:3300–8. [PubMed] [Google Scholar]
- 23.Mizushima N, Noda T, Yoshimori T, Tanaka Y, Ishii T, George MD, Klionsky DJ, Ohsumi M, Ohsumi Y. A protein conjugation system essential for autophagy. Nature. 1998;395:395–8. doi: 10.1038/26506. [DOI] [PubMed] [Google Scholar]
- 24.Evans SF, Irmady K, Ostrow K, Kim T, Nykjaer A, Saftig P, Blobel C, Hempstead BL. Neuronal brain-derived neurotrophic factor is synthesized in excess, with levels regulated by sortilin-mediated trafficking and lysosomal degradation. The Journal of biological chemistry. 2011;286:29556–67. doi: 10.1074/jbc.M111.219675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seglen PO, Gordon PB. 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proceedings of the National Academy of Sciences of the United States of America. 1982;79:1889–92. doi: 10.1073/pnas.79.6.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams KJ, Brocia RW, Fisher EA. The unstirred water layer as a site of control of apolipoprotein B secretion. The Journal of biological chemistry. 1990;265:16741–4. [PubMed] [Google Scholar]
- 27.Chen SH, Habib G, Yang CY, Gu ZW, Lee BR, Weng SA, Silberman SR, Cai SJ, Deslypere JP, Rosseneu M, et al. Apolipoprotein B-48 is the product of a messenger RNA with an organ-specific in-frame stop codon. Science. 1987;238:363–6. doi: 10.1126/science.3659919. [DOI] [PubMed] [Google Scholar]
- 28.Powell LM, Wallis SC, Pease RJ, Edwards YH, Knott TJ, Scott J. A novel form of tissue-specific RNA processing produces apolipoprotein-B48 in intestine. Cell. 1987;50:831–40. doi: 10.1016/0092-8674(87)90510-1. [DOI] [PubMed] [Google Scholar]
- 29.Hirano K, Young SG, Farese RV, Jr, Ng J, Sande E, Warburton C, Powell-Braxton LM, Davidson NO. Targeted disruption of the mouse apobec-1 gene abolishes apolipoprotein B mRNA editing and eliminates apolipoprotein B48. The Journal of biological chemistry. 1996;271:9887–90. doi: 10.1074/jbc.271.17.9887. [DOI] [PubMed] [Google Scholar]
- 30.Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–6. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 31.Chamberlain JM, O'Dell C, Sparks CE, Sparks JD. Insulin suppression of apolipoprotein B in McArdle RH7777 cells involves increased sortilin 1 interaction and lysosomal targeting. Biochemical and biophysical research communications. 2013;430:66–71. doi: 10.1016/j.bbrc.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M, Agostinis P, Aguirre-Ghiso JA, Ahn HJ, Ait-Mohamed O, Ait-Si-Ali S, Akematsu T, Akira S, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elzinga BM, Baller JF, Mensenkamp AR, Yao Z, Agellon LB, Kuipers F, Verkade HJ. Inhibition of apolipoprotein B secretion by taurocholate is controlled by the N-terminal end of the protein in rat hepatoma McArdle-RH7777 cells. Biochimica et biophysica acta. 2003;1635:93–103. doi: 10.1016/j.bbalip.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Marzella L, Ahlberg J, Glaumann H. Isolation of autophagic vacuoles from rat liver: morphological and biochemical characterization. The Journal of cell biology. 1982;93:144–54. doi: 10.1083/jcb.93.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szatmari Z, Sass M. The autophagic roles of Rab small GTPases and their upstream regulators: a review. Autophagy. 2014;10:1154–66. doi: 10.4161/auto.29395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mizushima N, Yamamoto A, Hatano M, Kobayashi Y, Kabeya Y, Suzuki K, Tokuhisa T, Ohsumi Y, Yoshimori T. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. The Journal of cell biology. 2001;152:657–68. doi: 10.1083/jcb.152.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Itakura E, Kishi-Itakura C, Mizushima N. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell. 2012;151:1256–69. doi: 10.1016/j.cell.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Berg TO, Fengsrud M, Stromhaug PE, Berg T, Seglen PO. Isolation and characterization of rat liver amphisomes. Evidence for fusion of autophagosomes with both early and late endosomes. The Journal of biological chemistry. 1998;273:21883–92. doi: 10.1074/jbc.273.34.21883. [DOI] [PubMed] [Google Scholar]
- 39.Canuel M, Lefrancois S, Zeng J, Morales CR. AP-1 and retromer play opposite roles in the trafficking of sortilin between the Golgi apparatus and the lysosomes. Biochemical and biophysical research communications. 2008;366:724–30. doi: 10.1016/j.bbrc.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 40.Dumaresq-Doiron K, Jules F, Lefrancois S. Sortilin turnover is mediated by ubiquitination. Biochemical and biophysical research communications. 2013;433:90–5. doi: 10.1016/j.bbrc.2013.02.059. [DOI] [PubMed] [Google Scholar]
- 41.McCormick PJ, Dumaresq-Doiron K, Pluviose AS, Pichette V, Tosato G, Lefrancois S. Palmitoylation controls recycling in lysosomal sorting and trafficking. Traffic. 2008;9:1984–97. doi: 10.1111/j.1600-0854.2008.00814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. The EMBO journal. 2000;19:5720–8. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD, Adeli K, Adhihetty PJ, Adler SG, Agam G, Agarwal R, Aghi MK, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12:1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ying H, Turturro S, Nguyen T, Shen X, Zelkha R, Johnson EC, Morrison JC, Yue BY. Induction of autophagy in rats upon overexpression of wild-type and mutant optineurin gene. BMC cell biology. 2015;16:14. doi: 10.1186/s12860-015-0060-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomar D, Singh R, Singh AK, Pandya CD, Singh R. TRIM13 regulates ER stress induced autophagy and clonogenic ability of the cells. Biochimica et biophysica acta. 2012;1823:316–26. doi: 10.1016/j.bbamcr.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 46.Nielsen MS, Madsen P, Christensen EI, Nykjaer A, Gliemann J, Kasper D, Pohlmann R, Petersen CM. The sortilin cytoplasmic tail conveys Golgi-endosome transport and binds the VHS domain of the GGA2 sorting protein. The EMBO journal. 2001;20:2180–90. doi: 10.1093/emboj/20.9.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hermey G. The Vps10p-domain receptor family. Cell Mol Life Sci. 2009;66:2677–89. doi: 10.1007/s00018-009-0043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fisher EA, Pan M, Chen X, Wu X, Wang H, Jamil H, Sparks JD, Williams KJ. The triple threat to nascent apolipoprotein B. Evidence for multiple, distinct degradative pathways. The Journal of biological chemistry. 2001;276:27855–63. doi: 10.1074/jbc.M008885200. [DOI] [PubMed] [Google Scholar]
- 49.Braulke T, Bonifacino JS. Sorting of lysosomal proteins. Biochimica et biophysica acta. 2009;1793:605–14. doi: 10.1016/j.bbamcr.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 50.Takamura A, Sato Y, Watabe D, Okamoto Y, Nakata T, Kawarabayashi T, Oddo S, Laferla FM, Shoji M, Matsubara E. Sortilin is required for toxic action of Abeta oligomers (AbetaOs): extracellular AbetaOs trigger apoptosis, and intraneuronal AbetaOs impair degradation pathways. Life Sci. 2012;91:1177–86. doi: 10.1016/j.lfs.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 51.Finder VH, Glockshuber R. Amyloid-beta aggregation. Neuro-degenerative diseases. 2007;4:13–27. doi: 10.1159/000100355. [DOI] [PubMed] [Google Scholar]
- 52.Fisher EA, Williams KJ. Autophagy of an oxidized, aggregated protein beyond the ER: a pathway for remarkably late-stage quality control. Autophagy. 2008;4:721–3. doi: 10.4161/auto.6346. [DOI] [PubMed] [Google Scholar]
- 53.Ponpuak M, Mandell MA, Kimura T, Chauhan S, Cleyrat C, Deretic V. Secretory autophagy. Current opinion in cell biology. 2015;35:106–16. doi: 10.1016/j.ceb.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andreo U, Guo L, Chirieac DV, Tuyama AC, Montenont E, Brodsky JL, Fisher EA. Insulin-stimulated degradation of apolipoprotein B100: roles of class II phosphatidylinositol-3-kinase and autophagy. PloS one. 2013;8:e57590. doi: 10.1371/journal.pone.0057590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sparks JD, O'Dell C, Chamberlain JM, Sparks CE. Insulin-dependent apolipoprotein B degradation is mediated by autophagy and involves class I and class III phosphatidylinositide 3-kinases. Biochemical and biophysical research communications. 2013;435:616–20. doi: 10.1016/j.bbrc.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sparks CE, Sparks RP, Sparks JD. The enigmatic role of sortilin in lipoprotein metabolism. Current opinion in lipidology. 2015;26:598–600. doi: 10.1097/MOL.0000000000000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cohen JC, Boerwinkle E, Mosley TH, Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–72. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.