Abstract
Background Context
Chronic inflammation is an important component of intervertebral disc (IVD) degeneration, but there is limited knowledge about the identity and source of inflammatory cells involved with the degenerative processes. Macrophages can exhibit multiple phenotypes and are known inflammatory regulators in many tissues, but their phenotypes have not been characterized in IVD degeneration.
Purpose
To characterize accumulation and localization of macrophages in IVD degeneration.
Study Design/Setting
Exploratory study to characterize macrophage phenotypes in human cadaver IVDs and the effects of injury and degeneration using multiple immunohistochemistry methods.
Patient Sample
N.A.
Outcome Measures
% Positivity of immunohistochemical markers specific for CCR7, CD163 and CD206. Qualitative assessments of dual immunofluorescence and immunostaining localization.
Methods
Macrophages were identified in human cadaveric IVDs with immunohistochemistry using cell surface markers CCR7, CD163, and CD206, which are associated with pro-inflammatory M1, remodeling M2c, and anti-inflammatory M2a phenotypes, respectively. Variations in the accumulation and localization of macrophage markers with degenerative grade across subjects and within donors are described. Federal grant funded project with no relevant conflicts.
Results
Cells expressing all three macrophage markers were found in all degenerative IVDs, but not in the healthiest IVDs. Cells expressing CCR7 and CD163, but not CD206 significantly increased with degenerative grade. Many cells also coexpressed multiple macrophage markers. Across all degenerative grades, CCR7+ and CD163+ were significantly more present in unhealthy NP, AF, and EP regions exhibiting structural irregularities and defects. Positively stained cells in the NP and AF closely resembled resident IVD cells, suggesting that IVD cells can express macrophage cell surface markers. In the EP, there were increasing trends of positively stained cells with atypical morphology and distribution, suggesting a source for exogenous macrophage infiltration into the IVD.
Conclusions
Chronic inflammatory conditions of IVD degeneration appear to involve macrophages or macrophage-like cells, as expression of multiple macrophage markers increased with degeneration, especially around unhealthy regions with defects and the EP. Knowledge of macrophage phenotypes and their localization better elucidates the complex injury and repair processes in IVDs and may eventually lead to novel treatments.
Keywords: intervertebral disc degeneration, macrophages, macrophage phenotype, intervertebral disc injury, inflammation, vertebral endplates
INTRODUCTION
Intervertebral disc (IVD) degeneration is a major medical condition implicated in lower back pain, which is a leading cause of global disability.[1] Although painful IVD degeneration is a complex, multifactorial process, it is known to involve a chronic inflammatory state, as advancing degeneration is associated with elevated levels of pro-inflammatory cytokines, increased matrix degrading enzyme activity, sensitization of neurons, and neurovascular ingrowth into the IVD.[2–6] Overloading and injury to the IVD and surrounding spinal tissues can also induce pro-inflammatory, catabolic responses, tissue defects and altered biomechanics.[7, 8] The affected microenvironment is thought to attract inflammatory cells into the IVD, which in turn stimulates additional pro-inflammatory cytokine production by resident IVD cells.[6, 8–10]. The poor healing response of the IVD makes it particularly vulnerable to inflammatory challenges with little capacity to recover from injury.[11, 12] As a result, it is essential to better understand how inflammatory cells contribute to IVD degeneration, including how their accumulation, localization, and phenotypic characterization change with degeneration.
Inflammatory cells expressing the pan-phagocytic marker CD68 have been identified within degenerated and herniated IVDs, suggesting that phagocytic cells, such as macrophages, may play a critical role in IVD pathophysiology.[6, 13–16] In herniated IVDs, CD68+ cells were identified and described as exogenous macrophages that infiltrated the IVD.[6, 15, 16] Macrophage infiltration is also implicated in non-herniated IVD degeneration, although this topic is under-studied.[6, 13] CD68+ cells in IVD degeneration have also been characterized as transformed resident IVD cells with phagocytic potential [14, 17, 18] so their identification as exogenous macrophages is less clear. An important next step is to examine IVD degeneration with more precise macrophage markers not distinguished by CD68 in order to improve the characterization of inflammatory cells within the IVD and delineate their potential roles in IVD degeneration.
Macrophages exhibit a dynamic spectrum of phenotypes that possess widespread pro-inflammatory, remodeling, and anti-inflammatory functions that can critically contribute to acute and chronic disease. In response to acute injury, macrophages rapidly switch their behavior from a predominantly pro-inflammatory state (often called M1) in the early stages of healing (0–3 days) to a state that promotes resolution of inflammation and healing (often called M2) at later stages (4–18 days). This M1-to-M2 transition has been described in repair processes of a diverse array of tissues, including heart,[19] lung,[20] muscle,[21] skin,[22] and spinal cord.[23] It is unknown whether a similar progression of macrophage phenotypes occurs in the IVD. In addition to secreting TNFα and IL-1β, early M1 activation is critical for the initiation of angiogenesis.[24, 25] The role of M2 macrophages in tissue healing is not as well understood. M2 macrophages have been further subdivided into two different phenotypes, often referred to as M2a and M2c. In particular, M2c macrophages (stimulated in vitro by IL-10) have been described as the remodeling phenotype because they secrete high levels of MMP-7, -8, and -9.[26] While M2c macrophages, distinguished by the cell surface marker CD163, are beneficial for normal tissue repair processes,[27, 28] their presence has also been associated with pathological degenerative diseases including tendon disease,[29] spondyloarthropathy,[30] chronic venous ulcers,[31] macular degeneration,[32] and psoriasis.[33] Similarly, M2a macrophages (stimulated in vitro by IL-4) are often considered the anti-inflammatory, pro-healing phenotype, but have also been associated with fibrotic disease, including pulmonary fibrosis,[34, 35] renal fibrosis[36], glandular fibrosis[37], and fibrous encapsulation of biomaterials[38].
The present study evaluated the accumulation and localization of macrophages within the IVD with degeneration and identified different macrophage phenotypes in order to better understand their possible roles in IVD degeneration. Cell surface markers CCR7, CD163, and CD206 were selected to represent M1, M2c, and M2a macrophage phenotypes, respectively.[24, 25] Using multiple staining techniques with quantitative and qualitative analyses, histological sections from human cadaveric lumbar IVDs were evaluated for the presence of these macrophage markers. The main hypotheses were that multiple macrophage phenotypes exist within human IVDs, and that markers associated with pro-inflammatory M1 and remodeling M2c phenotypes, but not anti-inflammatory M2a increase with advancing IVD degeneration. Immunohistochemistry was used to locate these markers and evaluate cellular morphology to better identify how macrophages originate and accumulate in immune-privileged IVDs. Specifically, this study evaluated if cells immunopositive for macrophage markers are more prevalent in unhealthy regions with irregularities and defects than in healthy, unaffected regions and whether their presence in the NP, AF, or EP increases with degeneration.
MATERIALS AND METHODS
Human cadaveric IVD specimens
Twelve intact, non-herniated, lumbar IVDs were isolated from six human cadavers (age range: 8–93 years, 2 females & 6 males). Cadaveric tissue was used with permission following standard procedures from autopsy services. Samples were obtained as available excluding infectious conditions, bone cancer or other disorders that might impact ‘normal’ spinal aging and degeneration. There were no exclusions on sex or age although samples had more males than females and were skewed to older ages as is common for cadaveric specimens. Twelve IVDs were a sufficient number of samples to identify if large effects of degenerative grade were present using between and within subject comparisons and analyzing using multiple immunohistochemical analyses. A single lumbar spine from a 93-year old human cadaver was assessed for degeneration using histology, gross morphology and MRI in order to better assess differences in IVD degeneration within a single human, and to highlight degenerative changes independent of age. These IVDs were imaged by T2 magnetic resonance imaging in a clinical 1.5T magnet prior to fixation and additionally scored for degeneration using the Pfirrmann classification for MRI evaluation [39]. Lumbar spines were sectioned sagittally and assessed for morphological assessment using the Thompson scale [40]. Lumbar spines were then fixed, embedded, and stained for degenerative grade using the Rutges scale for histological observations [41]. Increased degenerative grade in the Rutges score corresponds to increasing grade in the Pfirmann MRI classification [39] and Thompson morphological grade [40] (Figure 1).
Figure 1. Comparison of histological grade with morphology and MRI findings.
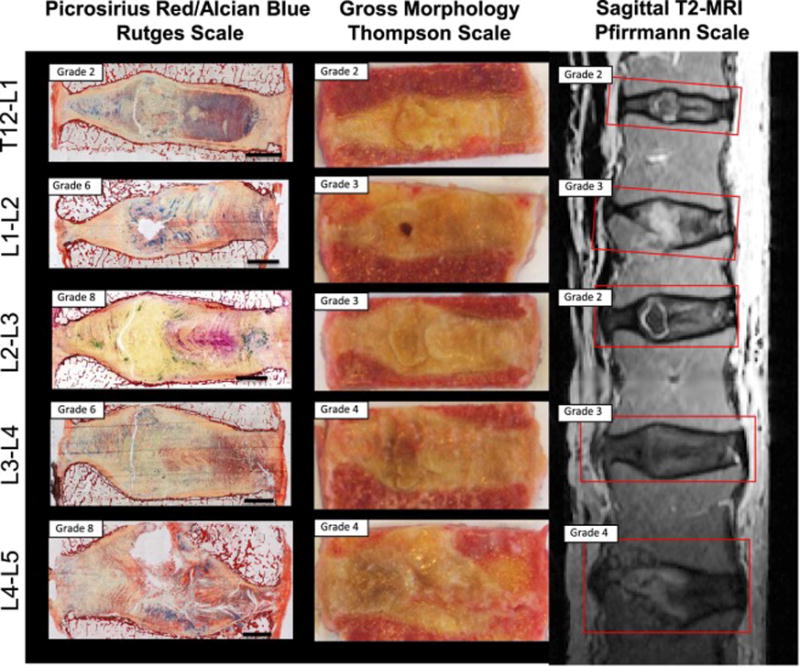
Five IVDs from a 93-year-old cadaveric donor were compared using the Rutges histological classification system, Thompson morphological scale, and Pfirrmann T2-weighted MRI grading scale. While scores are distinct, higher grades in all 3 scales indicated an increase in degeneration severity.
For histological processing in each sample, a central sagittal strip of tissue (~10 mm wide) with thin layers of superior and inferior vertebral endplates was fixed in zinc acetate, embedded in methyl-methacrylate (MMA) and sectioned for histology (5 μm thick), as previously described.[42, 43] Each IVD was assigned an averaged numerical grade of degeneration by three authors (KRN, BAW, JCI) who individually assessed the IVD based on a modified version[43] of the validated histological classification system of Rutges et al. [41]. The Rutges grading scheme takes several criteria into account, including the structure, organization, morphology, and staining intensity of NP, AF and endplate regions. Completely healthy IVDs are grade 0, while entirely degenerated IVDs are grade 10. Among the 12 IVDs examined, 3 were characterized as low degeneration (grades 0, 2, and 3), 4 were considered to have moderate degeneration (grades 4, 4, 6, and 6), and 5 were highly degenerated (grades 8, 8, 10, 10, and 10) (Table 1). Representative images of IVDs with low, moderate, and high degeneration are presented in Figure 2A.
Table 1.
Summary of human intervertebral disc specimens (12 IVDs from 6 cadavers).
| Degeneration | Degenerative Grade (0 to 10) | Age/Gender | Region or Vertebral Level |
|---|---|---|---|
| Low | 0 | 8/F | Lumbar |
| 2 | 93/M | T12/L1 | |
| 3 | 53/M | T12/L1 | |
|
| |||
| Moderate | 4 | 44/M | Lumbar |
| 4 | 81/F | Lumbar | |
| 6 | 93/M | L1/2 | |
| 6 | 93/M | L3/4 | |
|
| |||
| High | 8 | 93/M | L2/3 |
| 8 | 93/M | L4/5 | |
| 10 | 85/M | L2/3 | |
| 10 | 85/M | L3/4 | |
| 10 | 85/M | L4/5 | |
Figure 2. Macrophage markers were evaluated in human cadaveric IVDs of low, moderate, and high degenerative grades.
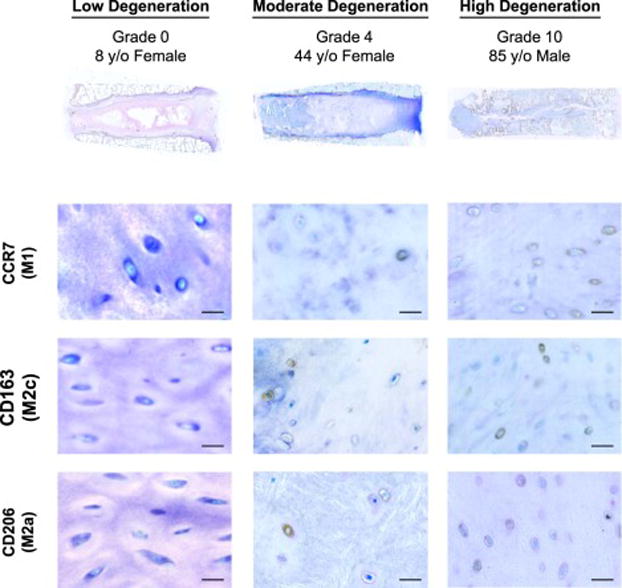
(A) Representative human IVDs with Rutges degeneration scale indicating low (grades 0–3), moderate (grades 4–6), and high (grades 8–10) degenerative grades. With (B) representative expression of macrophage phenotype markers CCR7 (M1), CD163 (M2c), and CD206 (M2a). CCR7+, CD163+, and CD206+ cells were identified in all moderate and high degeneration IVDs, while little or no positive staining was identified in low degeneration IVDs. Scale bar = 50 μm.
Immunohistochemistry
Prior to staining, tissue sections were deplasticized and rehydrated. Sections were then treated with proteinase K (S3020, Dako North America, Carpinteria, CA) followed by protein block (X0909, Dako North America). At room temperature, sections were incubated for one hour with either monoclonal mouse primary antibodies against human CCR7 (1:300 dilution, ab89064, Abcam, Cambridge, MA), monoclonal rabbit primary antibodies against human CD163 (1:100, ab126756, Abcam), polyclonal rabbit primary antibodies against human CD206 (1:100, ab64693, Abcam) or normal mouse serum (ab7486, Abcam) as a negative control. Then sections were incubated for 30 minutes at room temperature with anti-mouse or anti-rabbit IgG peroxidase secondary antibodies (ImmPRESS reagent, Vector Laboratories, Burlingame, CA). Afterwards the sections were treated with hydrogen peroxide block (ab94710, Abcam) for 10 minutes followed by diaminobenzidine-based peroxidase substrate for a minute (ImmPACT DAB Vector Laboratories). Finally, the sections were counterstained with toluidine blue before mounting. For each specimen, 15–20 composite (mosaic) images, each representing an area of ~75 mm2, were randomly acquired throughout the entire IVD at 20× magnification in bright field microscopy. If there were no cells present in the field of vision, the microscope position was moved to another randomly chosen area. The approximate location was then recorded. The total number of cells in each IVD was counted in consensus by 2 authors (KRN and BAW) using ImageJ version 1.45s (U.S. National Institutes of Health, Bethesda, MD). The percent positivity for each marker was calculated by dividing the number of positive cells by the total number of cells.
Image characterization
Image locations were noted as nucleus pulposus (NP), annulus fibrosus (AF), or endplate regions. The NP, AF, and EP were further identified as healthy and unhealthy regions based on criteria described by Rutges et al.[41] Healthy regions had well-organized extracellular matrix (ECM) and normal cell distribution patterns (i.e. homogenous structures, intact AF collagen lamellae, regular thickness of the IVD and clear boundaries between adjacent regions, and absence of tissue defects). Unhealthy regions were defined as areas with extensive damage and obvious defects in terms of ECM organization and cellular distribution patterns (i.e. granulation-like tissue, tears, cracks, ruptures, cell clustering, sclerosis, and irregular contours or decreased thickness of the EP) (Figure 3).
Figure 3. Healthy and unhealthy tissue regions were present within a moderately degenerated IVD (Rutges grade 4) from an 81-year-old female.
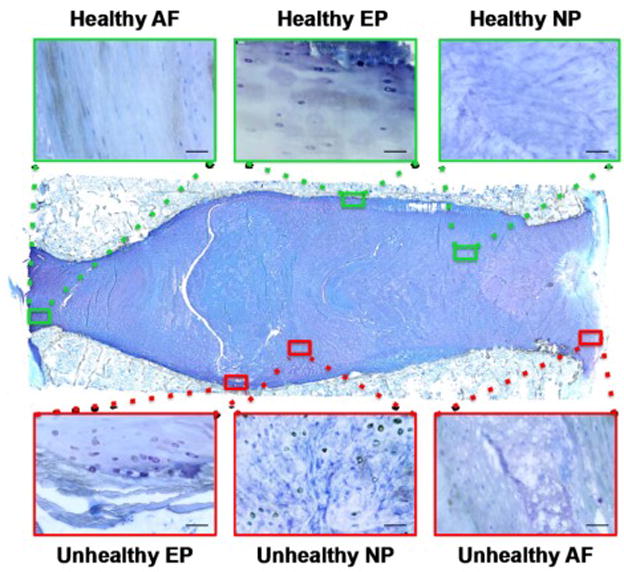
Characteristic healthy areas (green boxes) were identified in NP, AF, and EP regions by their organized collagenous structure, lack of structural discontinuities, and the appearance of a normal cell density and morphology. Characteristic unhealthy regions (red boxes) were identified by structural discontinuities, proximity to cracks and fissures, imperfections in the normal collagen orientation, accumulation of fibrous matrix, and irregularities in cell density, morphology or organization. Scale bar = 50 μm.
The criteria of distinguishing between healthy and unhealthy regions were discussed among co-authors to provide consensus among all authors prior to classification of the sections by the first author (KRN). Regions were also qualitatively compared with those of a grade 0 cadaveric IVD from an 8-year-old female, which served as a gold standard for characterizing healthy tissue structure, organization, and cell morphology. Embedding in MMA was selected since it maintains the best retention of original IVD structure by minimizing several processing artifacts that are more common with other histological techniques.[43, 44] Furthermore, a professional histotechnologist (DML) carefully sectioned and reviewed all samples prior to experimentation.
Dual-staining immunofluorescence
To assess for possible colocalization of markers, tissue sections were also stained for multiple macrophage markers using immunofluorescence. Following the same deplasticization, rehydration, proteinase K treatment and blocking steps as above, sections were incubated with one of three combined primary antibody mixtures for one hour at room temperature: (i) M1–M2a: monoclonal mouse antibodies against human CCR7 receptor (ab89064, Abcam) and polyclonal rabbit antibodies against human CD206 receptor (ab64693, Abcam), (ii) M1–M2c: monoclonal mouse antibodies against human CCR7 receptor (ab89064, Abcam) and monoclonal rabbit antibodies against CD163 receptor (ab126756, Abcam), or (iii) M2a–M2c: monoclonal mouse antibodies against CD206 receptor (ab8918, Abcam) and monoclonal rabbit antibodies against CD163 receptor (ab126756, Abcam). All primary antibodies were diluted 1:100. A combined mix of normal mouse (ab7486, Abcam) and rabbit serum (ab166640, Abcam) was used as a negative control. For 30 minutes at room temperature, sections were incubated with a secondary antibody mix consisting of Alexa Fluor® 594 donkey anti-mouse (1:700, 715-585-151, Jackson ImmunoResearch Laboratories, West Grove, PA) and Cy™5 donkey anti- rabbit antibodies (1:700, 711-175-152, Jackson ImmunoResearch Laboratories). DAPI nuclear counterstain was applied to each section (1:1000, 62248, Thermo Fisher Scientific, Waltham, MA) prior to mounting.
Within a few hours after mounting, ten images at 40× magnification were randomly taken throughout each IVD. Exposure times were fixed for each channel filter: 200 milliseconds for DAPI, 1 second for Texas Red 594, and 3 seconds for Cy5. Immunofluorescence intensities were qualitatively assessed to determine relative expression of markers to determine whether hybrid macrophage phenotypes existed.
Statistical analysis
Spearman’s non-parametric correlation was used to determine associations between percent positivity and degenerative grade were analyzed between all individual IVDs as independent samples, between average scores within each human subject, and between IVDs within a single human subject. Mann-Whitney non-parametric tests were conducted to compare mean percent positivity among healthy versus unhealthy regions in the NP, AF, and EP. Statistical analyses were performed using GraphPad Prism version 6.0f (GraphPad Software, San Diego, CA). P < 0.05 was considered statistically significant.
Funding
This study was funded by National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases grants R01AR0577397 and R01AR064157.
RESULTS
Expression of macrophage phenotype markers within the entire IVD
Cells expressing macrophage phenotype markers CCR7, CD163, and CD206 were identified in all IVDs with histomorphologic signs of degeneration (Figure 2). No CCR7+, CD163+, and CD206+ cells were found in IVDs with low degeneration, except in a grade 2 cadaveric IVD from a 93-year-old where < 8% positivity was determined for each of the three markers. The two IVDs that were absent of any macrophage markers were grades 0 and 3 cadaveric IVDs from an 8- and 53-year old, respectively.
Analysis of IVDs from all degenerative grades as independent samples found that the total CCR7 (correlation coefficient, R = 0.68, p = 0.027) and CD163 (R = 0.65, p = 0.024) both significantly increased with degeneration, while no significant correlations were determined for CD206 staining (Figure 4a). When controlling for age by comparing IVDs within the same 93-year-old cadaver, a similar increase in total CCR7 was also observed with degenerative grade (CCR7: R = 0.95, p = 0.017; CD163: R = 0.53, p = 0.35; CD206: R = 0.74, p = 0.13) (Figure 4b). Total percent positivity did not correlate with age for any of the three markers (CCR7: R = 0.49, p = 0.36; CD163: R = 0.38, p= 0.50; CD206: R = 0.20, p = 0.71) (Figure 4c).
Figure 4. Macrophage phenotype markers CCR7 and CD163 significantly correlated with degenerative grade but not age.
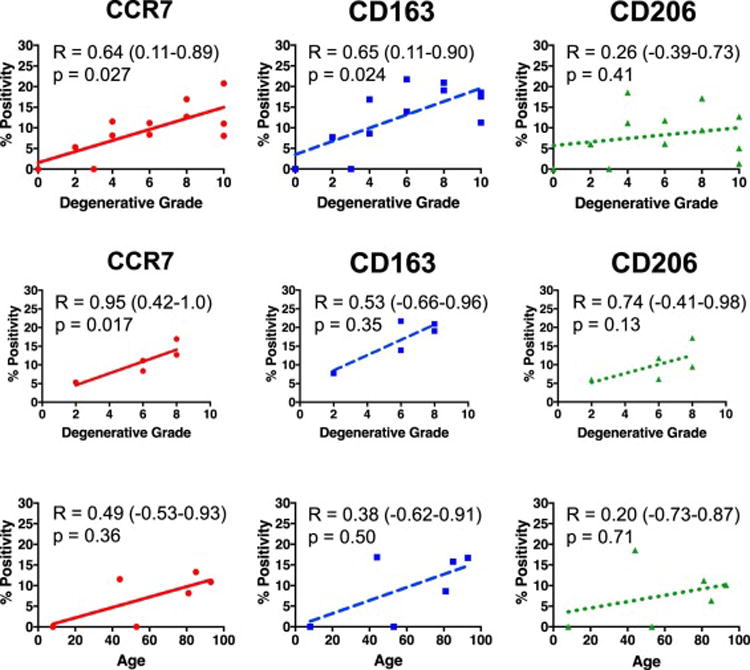
(A) Percent positivity for macrophage phenotypic markers CCR7 and CD163 but not CD206 were significantly associated with Rutges degenerative grade when averaged across all sections within an entire IVD (n=12). (B) IVDs with varying Rutges grade within a single 93-year old human also demonstrated strong correlations of CCR7 and CD163 with degenerative grade (n=5). (C) No macrophage phenotypic markers demonstrated any significant association with age when values for all IVDs from a single human were averaged (n=6). R = Spearman’s correlation coefficient (95% Confidence Interval).
Localization of macrophage phenotype markers
Different correlations with degeneration were observed for NP, AF, and EP regions. Within the NP, only CCR7 significantly correlated with degeneration (p < 0.001), while the AF had no significant associations of any marker (Figure 5). Furthermore, the EP had increasing trends of all three markers with degeneration (all p ≤ 0.05, Figure 5).
Figure 5. Percent positivity of macrophage phenotype markers significantly increased in NP and EP but not AF regions.
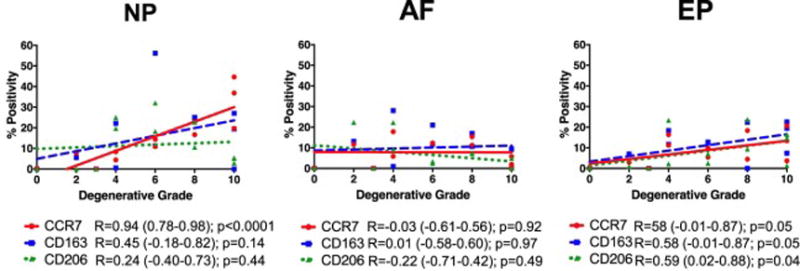
The percentage of cells immunopositive for CCR7, CD163, and CD206 were averaged within NP, AF, and EP regions for individual IVDs and correlated with Rutges degenerative grade. CCR7+, CD163+ and CD206+ cells all significantly increased with degenerative grade in endplate (EP) regions while only CCR7+ cells significantly increased in NP regions. No changes in percent positivity of any macrophage marker were observed with degenerative grade in AF regions. (N = 12). R = Spearman’s correlation coefficient (95% Confidence Interval).
In addition, CCR7+, CD163+, and CD206+ cells were present in healthy NP, AF, and EP regions (Figure 6), as well as in areas with histological ECM abnormalities suggestive of degenerative changes, including granulation-like tissue, tears, cracks, ruptures, cell clustering, sclerosis and other structural irregularities (Figure 7 and 8). Particularly in the NP and AF, positively stained cells shared morphological similarities to resident cells within the NP (round and oval with surrounding pericellular matrix) and AF (thin, slightly elongated and organized along concentric collagen lamellae),[41, 45, 46] as well as to those of the grade 0 cadaveric IVD (Figure 9). On the contrary, the EP boundaries between IVD and vertebral bone were frequently populated with disorganized immunopositive cells that exhibited morphology atypical of normal resident IVD cells, especially compared with endplate cells of the grade 0 IVD (Figure 9). Cells near the endplate are normally thin, elongated, and aligned along collagen fibers that run parallel and horizontal to the vertebral bone.[45, 46] In contrast, many positively stained cells in the endplate region were large, with irregularly rounded shapes, and organized in patterns that were less aligned with collagen orientation.
Figure 6. Macrophage phenotype markers were observed in healthy areas in the NP, AF, and EP regions of IVDs with moderate and high degeneration.
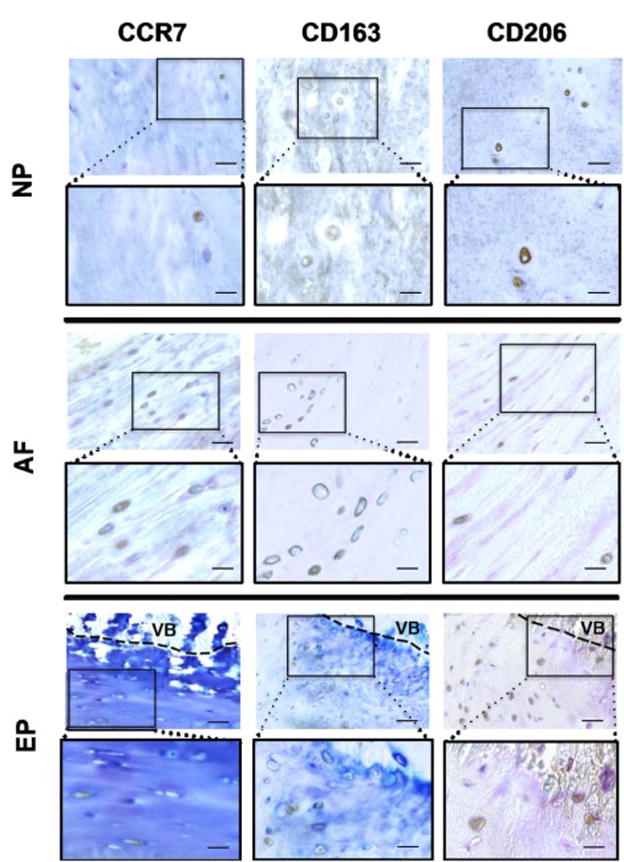
Healthy areas were identified as containing well organized extracellular matrix and normal cell distribution patterns, including homogenous structures, intact AF collagen lamellae, absence of cell clustering, and clearly defined boundaries between NP, AF and EP. Scale bar (in unboxed images) = 50 μm, (in boxed images) = 25 μm. VB = vertebral bone.
Figure 7. Macrophage phenotype markers were observed in unhealthy areas in the NP, AF, and EP regions of IVDs with moderate and high degeneration.
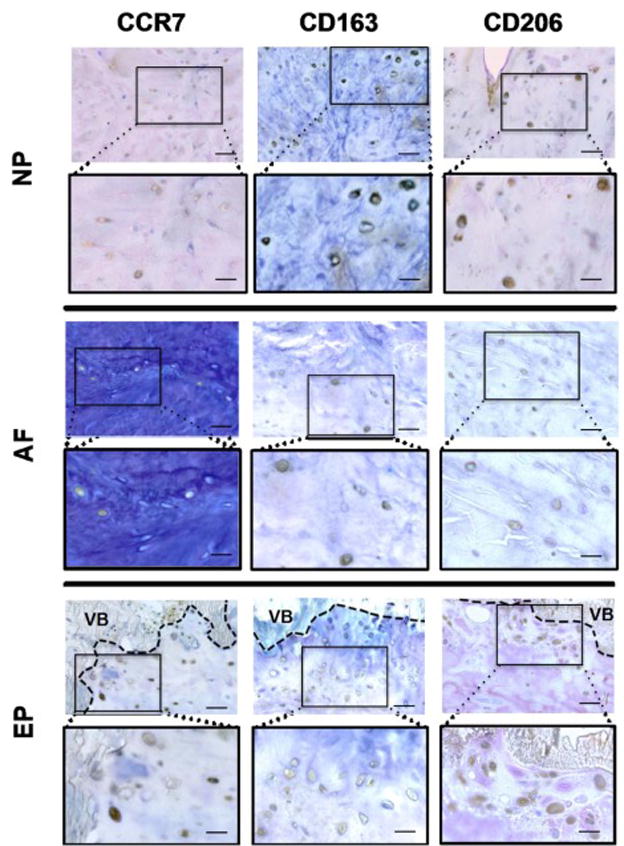
Unhealthy areas were identified as irregularities and obvious defects in extracellular matrix organization and cellular distribution patterns including proximity to tears, cracks and ruptures, granulation-like tissue, irregularities in collagen fiber orientation, cell clustering and cells with irregular size, shape or organization pattern. Scale bar (in unboxed images) = 50 μm, (in boxed images) = 25 μm. VB = vertebral bone.
Figure 8. Macrophage phenotype markers were strongly stained among cell clusters.
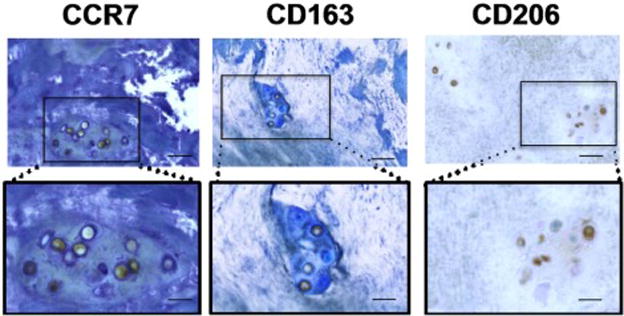
Cell clustering in degenerated IVDs were identified by their presence in a distinct area of granulation-like tissues. Cells within granulation tissue clusters stained strongly for CCR7, CD163, and CD206 macrophage phenotype markers. Immunopositive cells within these clusters had cell shape and size that resembled resident NP cells, but at greatly increased cell density. Scale bar (in unboxed images) = 50 μm, (in boxed images) = 25 μm.
Figure 9. Immunopositive cells had cell morphologies that were similar to resident AF and NP cells but distinct from resident EP cells.
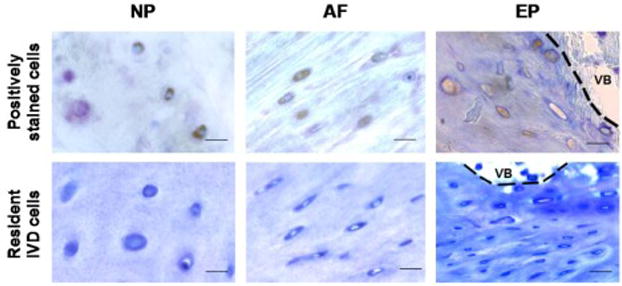
Positively stained cells in the NP and AF generally had similar morphology to normal resident IVD cells. In contrast, degenerated IVDs had immunopositive cells near the EP boundary that often had cell morphology with larger size, different shape, lack of smooth edges, and irregular organization as compared to normal resident IVD cells. Normal resident cells are represented by sections from the grade 0 cadaveric IVD from the 8-year-old female. Scale bar = 25 μm. VB = vertebral bone.
Compared with healthy NP, AF, and EP, total mean CCR7 and CD163 positivity was determined to be significantly higher in unhealthy tissue (both p < 0.01), while no differences were found regarding CD206 (Figure 10).
Figure 10. CCR7+ and CD163+ cells were significantly increased in unhealthy NP, AF, and EP regions as compared to healthy regions.
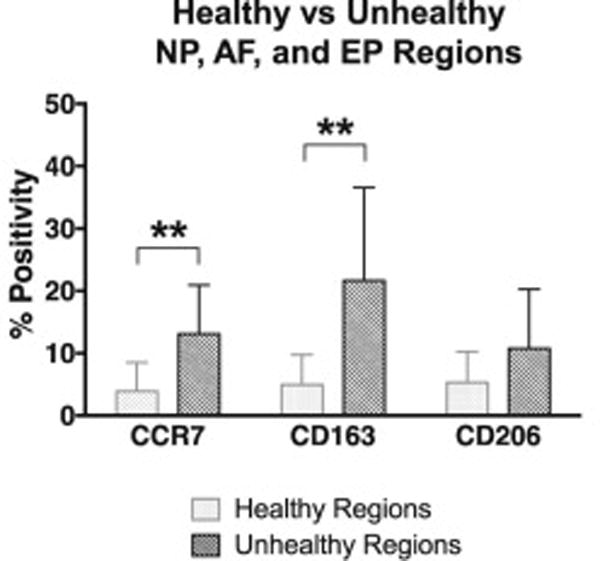
Unpaired Mann-Whitney non-parametric test comparing CCR7, CD163, and CD206 positivity in healthy versus unhealthy regions in NP, AF, and EP. N = 12. Bar graphs with error bars represent mean positivity with standard deviation. Asterisks (**) = p < 0.01.
Dual-staining immunofluorescence
Based on qualitative observations of relative immunofluorescence intensities, some cells exhibited cells with a distinct macrophage phenotype with higher expression of CCR7 (Figure 11A, B), CD163 (Figure 11D, E) or CD206 (Figure 11G, H), while other cells exhibited mixed macrophage phenotypes with colocalization of markers (CCR7-CD206, CCR7-CD163, or CD206-CD163) (Figure 11C, F, I).
Figure 11. IVD cells stained positively for single and multiple macrophage phenotype markers using dual-staining immunofluorescence.
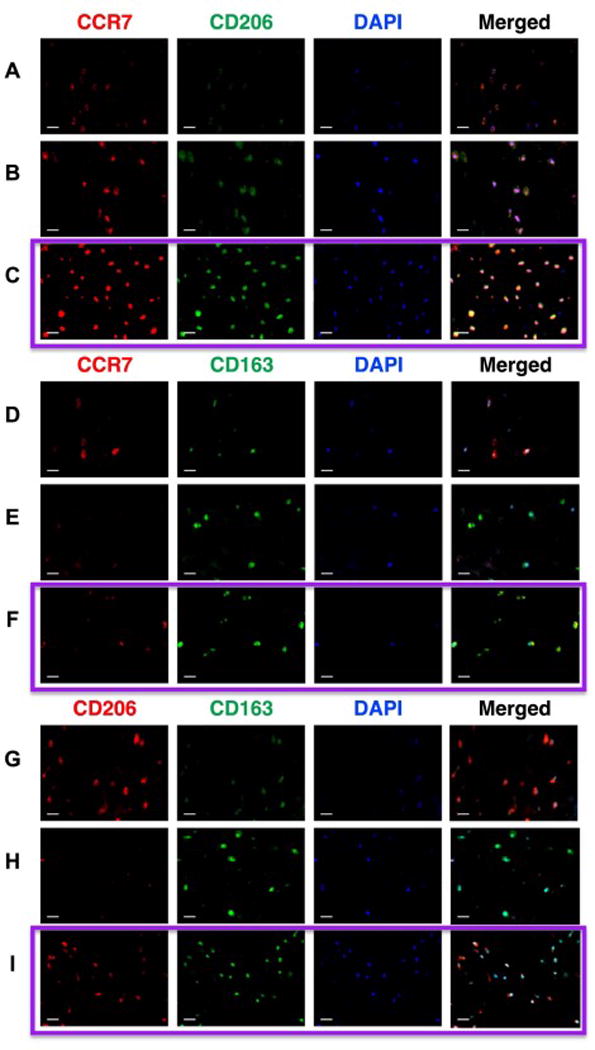
Dual-staining immunofluorescence imaging was used to characterize the presence of single and dual stained cells. Representative dual-stained images from multiple IVDs of (A–C) CCR7-M1/CD206-M2a, (D–F) CCR7-M1/CD163-M2c, and (G–I) CD206-M2a/CD163-M2c with DAPI nuclear staining. (C,F,I) Images within the purple boxes demonstrate co-localized cells that positively stained for multiple macrophage phenotype markers (C: CCR7-CD206, F: CCR7-CD163, I: CD206-CD163), suggestive of mixed macrophage phenotypes. (A,B,D,E,G,H) Cells were also identified that predominantly expressed single macrophage phenotype markers, confirming chromogenic findings. Scale bar = 25 μm.
DISCUSSION
Healthy IVDs are considered immune-privileged and painful IVD degeneration is associated with chronic inflammation, yet surprisingly little is known about macrophage phenotypes within the IVD. This study tested the hypothesis that multiple macrophage phenotypes exist within human IVDs and increase with degeneration. This study is the first to report the presence of CCR7+, CD163+, and CD206+ cells in human IVDs, and we determined that macrophages and/or macrophage-like cells of varying phenotypes are present in human IVDs. The most important findings were that CCR7+ and CD163+ cells increased with degeneration grade and were significantly more present in IVD regions with structural irregularities and defects, suggesting increased M1 and M2c macrophage phenotypes. In addition, many immunopositive cells within the NP and AF regions had similar morphology and localization as healthy IVD cells, supporting the notion that resident IVD cells can exhibit macrophage-like properties. On the contrary, immunopositive cells in the EP significantly increased with degeneration grade for all macrophage markers and had irregular shape and morphology suggesting exogenous macrophage infiltration into the IVD EP.
The strong associations of CCR7 and CD163, but not CD206 in unhealthy regions and with advancing degeneration are consistent with the concept of IVD degeneration as a frustrated healing process involving an imbalance of macrophage phenotypes resulting in chronic catabolism. Specifically, the M1 phenotype (CCR7+) is known to secrete high levels of pro-inflammatory cytokines, such as TNFα and IL-1β, the M2c phenotype (CD163+) has recently been found to produce elevated levels of MMP needed for ECM remodeling, and the anti-inflammatory M2a phenotype (CD206+) is generally associated with the final stages of tissue repair, wound healing, ECM deposition, and fibrosis. [24, 25] As a result, the increase in M1-like and M2c-like cells but not M2a is consistent with the concept that cells in degenerated IVDs remain in pro-inflammatory and remodeling states without ever transitioning to final states of wound healing. It is doubtful that macrophages were originally native to the IVD because no positively stained cells were found within the healthiest and youngest IVD (the grade 0 cadaveric IVD from an 8-year-old female), confirming the theory that IVDs normally begin as immune-privileged organs that are avascular and isolated from the host immune system prior to degenerative changes.[47] Interestingly we did not identify any significant associations with age, which does not seem to be an accurate proxy for IVD degeneration. This point was highlighted by the strong significant increase in CCR7+ cells with degeneration grade across spinal levels of the same 93-year-old cadaver that had no variation with age. Instead, cells positive for macrophage phenotypic markers were found to accumulate in degenerated IVDs and unhealthy regions involving defect areas within IVDs, suggesting that macrophages play a role in IVD injury and healing. IVD degeneration has been considered a cycle of frustrated healing, in which altered stresses on the IVD affect the ability of cells to repair disrupted ECM, leading to repeated tissue injury [48]. The large avascular structure of the IVD further limits its innate healing capacity. [11] Furthermore, pro-inflammatory and catabolic factors have all been consistently associated with IVD degeneration, [3, 49–51]. Therefore this study highlights the potential role of macrophages and multiple phenotypic states involved in the IVD degeneration. While the present immunolocalization study provides additional context to help explain degenerative processes, future studies are necessary to identify specific roles of these immunopositive cells in situ and to determine how they contribute to IVD injury, healing, and degeneration.
Dual-staining immunofluorescence also showed frequent colocalization of macrophage phenotype markers throughout the IVD, indicating that the population of positive cells was quite heterogeneous, consisting of mixed, and perhaps transitory, macrophage phenotypes. Phenotype plasticity is one of the major hallmarks of macrophages and thus measurements of multiple markers are useful.[24, 25, 52, 53] The mixed profile of phenotypes in IVDs could reflect the gradual, extracellular changes and complex tissue-derived signals that occur throughout IVD degeneration, as it is now known that the microenvironment of macrophages is crucial for determining their phenotypes.[54] Specific functions of different macrophage phenotypes are known in other organ systems. For instance, the concept of relative imbalance in proportions of M1-like and M2-like macrophages has been implicated in obesity,[55] kidney disease,[36] atherosclerosis,[53] chronic venous ulcers,[31] and spinal cord injury,[23]. It is generally hypothesized that an impaired M1-to-M2 transition leads to failed repair and chronic inflammatory states. It is possible that strategies that promote transition to an anti-inflammatory M2a phenotype could improve the poor innate healing of IVDs. However, multiple inflammatory cells play varying roles and express multiple phenotypic markers in disease states, and this is expected in the IVD. Therefore, identification and localization of additional inflammatory cells in human IVDs of varying degenerative grades will further clarify the processes of IVD injury, repair and degeneration.
The grade 0 cadaveric IVD from the 8-year-old female has potential limitations as a control since IVDs in the first decade have morphological differences with older IVDs. Nevertheless, the grade 3 IVD from the 53-year old male also had 0% positivity for the macrophage markers, suggesting our findings are unlikely to be affected by morphological differences in adolescent IVDs. The resemblance of positively stained cells in the NP and AF to native IVD cells supports the hypothesis that a subset of resident IVD cells can eventually take on macrophage-like phenotypes. Based on cytomorphology of CD68+ cells found within the IVD, Nerlich et al. similarly suggested that altered resident IVD cells are capable of undergoing phagocytic conversion.[14] Moreover, human and bovine NP cells can behave as competent phagocytes in vitro, highlighting their potential role in maintaining IVD homeostasis through removal of apoptotic cells.[17, 18] However, it is also known macrophage phenotypic changes are associated with alterations in their function, [56] so it is also possible that immunopositive cells in different local tissue microenvironments are serving distinct roles. It remains to be seen if the presence of macrophages during IVD injury and degeneration can trigger the differentiation of IVD cells towards macrophage-like phenotypes or whether these populations are true macrophages.
The increasing trends with degeneration of CCR7+, CD163+ and CD206+ cells in the EP and the irregular morphology and organization of these cells compared to that of normal endplate cells provide support for the hypothesis that exogenous macrophage infiltration through the endplates occurs, particularly since cell migration from endplate to NP regions is well-described in IVDs.[57] Peng et al. previously proposed that IVD degeneration and discogenic back pain is a result of tissue injury followed by the infiltration of macrophages that promote the ingrowth of blood vessels and nerves and fibrosis.[13] The importance of macrophage presence in the EP is highlighted by our findings that all 3 macrophage markers significantly increased with degenerative grade in the EP, while only CCR7+ significantly increasing in the NP, and no macrophage markers increased in the AF region. The trends in % positivity with degenerative grade for macrophage markers for the whole IVD therefore did not always match trends of individual regions. Specifically, the positive correlation between CCR7+ in the whole IVD was similar to positive correlations found in both NP and EP regions, highlighting the presence of M1 (pro-inflammatory) phenotypes in both NP and EP. The positive correlation between CD163+ in the whole IVD was driven by % positivity of EP cells with a moderate, non-significant trend in the NP. CD206+ was not significantly correlated with degenerative grades in the whole IVD, despite a moderate and significant correlation in the EP, suggesting that increased presence of CD206+ in the EP was not reinforced by trends in other regions so that M2a (anti-inflammatory) phenotypes may only exist in the EP.
Positively stained cells with rounded NP cell-like morphology were consistently identified in every cell cluster in this study. Cell clustering is a characteristic histological feature commonly found in degenerated IVDs [45, 58, 59]. The presence of all three macrophage markers localized within each cell cluster suggests multiple inflammatory phenotypes by NP cells in an attempt to heal focal areas of the IVD. Yet, cells within clusters are known to have diverse responses, such as expressing senescence markers, MMPs[45] and stress proteins (i.e. heat shock proteins, Hsp 27 and Hsp 72),[59]. These diverse responses to environmental factors associated with IVD injury and degeneration may indicate the involvement of inflammatory and native IVD cells.
This study used human cadaveric IVDs that provided insights relevant to chronic human IVD degeneration. The relatively small sample size of human IVDs does not provide sufficient statistical power to conclude that there are no aging effects, yet does not change our conclusion that CCR7+ and CD163+ significantly correlated with degenerative grade. The importance of degenerative grade, independent of aging, was reinforced by the significant associations of CCR7+ and CD163+ with degenerative grade in a single human with varying degenerative grade across spinal levels. The samples were also skewed for high ages as is common with cadaveric samples. We believe this study motivates future investigations with greater numbers of IVDs and other species in order to determine potential aging effects and to explore mechanisms for these observations. For example, a definitive test of macrophage migration would require lineage-tracing studies that would be impossible to do on post-mortem human tissue. Moreover, methods were limited to immunohistochemical techniques for surface markers to identify macrophage phenotypes, which do not directly provide information about cellular functions. The immunostaining methods on methacrylate embedded samples have been developed over many years to produce relatively little background [42, 43]. Negative controls used for all antibodies were ‘clean’. We consulted with our master histologist (DML) who was blinded to the sample identification to characterize cells as positive or negative in the few instances that lacked clarity.
CONCLUSIONS
This is the first study to identify and localize multiple macrophage phenotypic markers in human IVDs. The increase in CCR7+ and CD163+ cells, without significant correlations in CD206+ cells in degenerated IVDs and around unhealthy tissue regions indicated that macrophages and macrophage-like cells play an important role in IVD degeneration and suggests an imbalance of macrophage phenotypes involved in a complex, incomplete healing process. The significant increase in all 3 macrophage phenotypic markers in the EP, suggests important roles for macrophages in this region. The localization and morphology of CCR7+, CD163+ and CD206+ cells within human IVDs further highlights a potential interplay between native IVD cells and infiltrating macrophages. Overall, these results indicate that the inflammatory component of IVD degeneration is a complex process involving interactions of multiple cell types at multiple stages of healing. Understanding macrophage accumulation, localization and phenotypes within the IVD can improve knowledge about IVD pathophysiology and may help identify future therapeutic targets.
Acknowledgments
This study was funded by National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases grants R01AR0577397 and R01AR064157.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buchbinder R, Blyth FM, March LM, Brooks P, Woolf AD, Hoy DG. Placing the global burden of low back pain in context. Best practice & research Clinical rheumatology. 2013;27(5):575–89. doi: 10.1016/j.berh.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Cornejo MC, Cho SK, Giannarelli C, Iatridis JC, Purmessur D. Soluble factors from the notochordal-rich intervertebral disc inhibit endothelial cell invasion and vessel formation in the presence and absence of pro-inflammatory cytokines. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2015;23(3):487–96. doi: 10.1016/j.joca.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le Maitre CL, Hoyland JA, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis research & therapy. 2007;9(4):R77. doi: 10.1186/ar2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Purmessur D, Freemont AJ, Hoyland JA. Expression and regulation of neurotrophins in the nondegenerate and degenerate human intervertebral disc. Arthritis research & therapy. 2008;10(4):R99. doi: 10.1186/ar2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nature reviews Rheumatology. 2014;10(1):44–56. doi: 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shamji MF, Setton LA, Jarvis W, et al. Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis and rheumatism. 2010;62(7):1974–82. doi: 10.1002/art.27444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacLean JJ, Lee CR, Grad S, Ito K, Alini M, Iatridis JC. Effects of immobilization and dynamic compression on intervertebral disc cell gene expression in vivo. Spine. 2003;28(10):973–81. doi: 10.1097/01.BRS.0000061985.15849.A9. [DOI] [PubMed] [Google Scholar]
- 8.Walter BA, Likhitpanichkul M, Illien-Junger S, Roughley PJ, Hecht AC, Iatridis JC. TNFalpha transport induced by dynamic loading alters biomechanics of intact intervertebral discs. PloS one. 2015;10(3):e0118358. doi: 10.1371/journal.pone.0118358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takada T, Nishida K, Maeno K, et al. Intervertebral disc and macrophage interaction induces mechanical hyperalgesia and cytokine production in a herniated disc model in rats. Arthritis and rheumatism. 2012;64(8):2601–10. doi: 10.1002/art.34456. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Tian Y, Phillips KL, et al. Tumor necrosis factor alpha- and interleukin-1beta-dependent induction of CCL3 expression by nucleus pulposus cells promotes macrophage migration through CCR1. Arthritis and rheumatism. 2013;65(3):832–42. doi: 10.1002/art.37819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kandel R, Roberts S, Urban JP. Tissue engineering and the intervertebral disc: the challenges. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2008;17(Suppl 4):480–91. doi: 10.1007/s00586-008-0746-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Purmessur D, Walter BA, Roughley PJ, Laudier DM, Hecht AC, Iatridis J. A role for TNFalpha in intervertebral disc degeneration: a non-recoverable catabolic shift. Biochemical and biophysical research communications. 2013;433(1):151–6. doi: 10.1016/j.bbrc.2013.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng B, Hao J, Hou S, et al. Possible pathogenesis of painful intervertebral disc degeneration. Spine. 2006;31(5):560–6. doi: 10.1097/01.brs.0000201324.45537.46. [DOI] [PubMed] [Google Scholar]
- 14.Nerlich AG, Weiler C, Zipperer J, Narozny M, Boos N. Immunolocalization of phagocytic cells in normal and degenerated intervertebral discs. Spine. 2002;27(22):2484–90. doi: 10.1097/00007632-200211150-00012. [DOI] [PubMed] [Google Scholar]
- 15.Kawaguchi S, Yamashita T, Yokogushi K, Murakami T, Ohwada O, Sato N. Immunophenotypic analysis of the inflammatory infiltrates in herniated intervertebral discs. Spine. 2001;26(11):1209–14. doi: 10.1097/00007632-200106010-00008. [DOI] [PubMed] [Google Scholar]
- 16.Koike Y, Uzuki M, Kokubun S, Sawai T. Angiogenesis and inflammatory cell infiltration in lumbar disc herniation. Spine. 2003;28(17):1928–33. doi: 10.1097/01.BRS.0000083324.65405.AE. [DOI] [PubMed] [Google Scholar]
- 17.Jones P, Gardner L, Menage J, Williams GT, Roberts S. Intervertebral disc cells as competent phagocytes in vitro: implications for cell death in disc degeneration. Arthritis research & therapy. 2008;10(4):R86. doi: 10.1186/ar2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen YF, Zhang YZ, Zhang WL, et al. Insights into the hallmarks of human nucleus pulposus cells with particular reference to cell viability, phagocytic potential and long process formation. International journal of medical sciences. 2013;10(13):1805–16. doi: 10.7150/ijms.6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Troidl C, Mollmann H, Nef H, et al. Classically and alternatively activated macrophages contribute to tissue remodelling after myocardial infarction. Journal of cellular and molecular medicine. 2009;13(9B):3485–96. doi: 10.1111/j.1582-4934.2009.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnston LK, Rims CR, Gill SE, McGuire JK, Manicone AM. Pulmonary macrophage subpopulations in the induction and resolution of acute lung injury. American journal of respiratory cell and molecular biology. 2012;47(4):417–26. doi: 10.1165/rcmb.2012-0090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnold L, Henry A, Poron F, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. The Journal of experimental medicine. 2007;204(5):1057–69. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirza RE, Fang MM, Ennis WJ, Koh TJ. Blocking interleukin-1beta induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes. 2013;62(7):2579–87. doi: 10.2337/db12-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(43):13435–44. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spiller KL, Anfang RR, Spiller KJ, et al. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 2014;35(15):4477–88. doi: 10.1016/j.biomaterials.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spiller KL, Nassiri S, Witherel CE, et al. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials. 2015;37:194–207. doi: 10.1016/j.biomaterials.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lurier EB, Dalton D, Dampier W, et al. Transcriptome analysis of IL-10-stimulated (M2c) macrophages by next-generation sequencing. Immunobiology. 2017;222(7):847–56. doi: 10.1016/j.imbio.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans BJ, Haskard DO, Sempowksi G, Landis RC. Evolution of the Macrophage CD163 Phenotype and Cytokine Profiles in a Human Model of Resolving Inflammation. International journal of inflammation. 2013;2013:780502. doi: 10.1155/2013/780502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Philippidis P, Mason JC, Evans BJ, et al. Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: antiinflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circulation research. 2004;94(1):119–26. doi: 10.1161/01.RES.0000109414.78907.F9. [DOI] [PubMed] [Google Scholar]
- 29.Dakin SG, Martinez FO, Yapp C, et al. Inflammation activation and resolution in human tendon disease. Science translational medicine. 2015;7(311):311ra173. doi: 10.1126/scitranslmed.aac4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baeten D, Demetter P, Cuvelier CA, et al. Macrophages expressing the scavenger receptor CD163: a link between immune alterations of the gut and synovial inflammation in spondyloarthropathy. The Journal of pathology. 2002;196(3):343–50. doi: 10.1002/path.1044. [DOI] [PubMed] [Google Scholar]
- 31.Sindrilaru A, Peters T, Wieschalka S, et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. The Journal of clinical investigation. 2011;121(3):985–97. doi: 10.1172/JCI44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao X, Shen D, Patel MM, et al. Macrophage polarization in the maculae of age-related macular degeneration: a pilot study. Pathology international. 2011;61(9):528–35. doi: 10.1111/j.1440-1827.2011.02695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fuentes-Duculan J, Suarez-Farinas M, Zaba LC, et al. A subpopulation of CD163-positive macrophages is classically activated in psoriasis. The Journal of investigative dermatology. 2010;130(10):2412–22. doi: 10.1038/jid.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murthy S, Larson-Casey JL, Ryan AJ, He C, Kobzik L, Carter AB. Alternative activation of macrophages and pulmonary fibrosis are modulated by scavenger receptor, macrophage receptor with collagenous structure. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2015;29(8):3527–36. doi: 10.1096/fj.15-271304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murray LA, Chen Q, Kramer MS, et al. TGF-beta driven lung fibrosis is macrophage dependent and blocked by Serum amyloid P. The international journal of biochemistry & cell biology. 2011;43(1):154–62. doi: 10.1016/j.biocel.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 36.Anders HJ, Ryu M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney international. 2011;80(9):915–25. doi: 10.1038/ki.2011.217. [DOI] [PubMed] [Google Scholar]
- 37.Furukawa S, Moriyama M, Tanaka A, et al. Preferential M2 macrophages contribute to fibrosis in IgG4-related dacryoadenitis and sialoadenitis, so-called Mikulicz’s disease. Clinical immunology (Orlando, Fla) 2015;156(1):9–18. doi: 10.1016/j.clim.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 38.Kao WJ, McNally AK, Hiltner A, Anderson JM. Role for interleukin-4 in foreign-body giant cell formation on a poly(etherurethane urea) in vivo. Journal of biomedical materials research. 1995;29(10):1267–75. doi: 10.1002/jbm.820291014. [DOI] [PubMed] [Google Scholar]
- 39.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26(17):1873–8. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 40.Thompson JP, Pearce RH, Schechter MT, Adams ME, Tsang IK, Bishop PB. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine. 1990;15(5):411–5. doi: 10.1097/00007632-199005000-00012. [DOI] [PubMed] [Google Scholar]
- 41.Rutges JP, Duit RA, Kummer JA, et al. A validated new histological classification for intervertebral disc degeneration. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2013;21(12):2039–47. doi: 10.1016/j.joca.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 42.Laudier D, Schaffler MB, Flatow EL, Wang VM. Novel procedure for high-fidelity tendon histology. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2007;25(3):390–5. doi: 10.1002/jor.20304. [DOI] [PubMed] [Google Scholar]
- 43.Walter BA, Torre OM, Laudier D, Naidich TP, Hecht AC, Iatridis JC. Form and function of the intervertebral disc in health and disease: a morphological and stain comparison study. Journal of anatomy. 2014 doi: 10.1111/joa.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erben RG. Embedding of bone samples in methylmethacrylate: an improved method suitable for bone histomorphometry, histochemistry, and immunohistochemistry. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 1997;45(2):307–13. doi: 10.1177/002215549704500215. [DOI] [PubMed] [Google Scholar]
- 45.Roberts S, Evans H, Trivedi J, Menage J. Histology and pathology of the human intervertebral disc. The Journal of bone and joint surgery American volume. 2006;88(Suppl 2):10–4. doi: 10.2106/JBJS.F.00019. [DOI] [PubMed] [Google Scholar]
- 46.Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis research & therapy. 2003;5(3):120–30. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun Z, Zhang M, Zhao XH, et al. Immune cascades in human intervertebral disc: the pros and cons. International journal of clinical and experimental pathology. 2013;6(6):1009–14. [PMC free article] [PubMed] [Google Scholar]
- 48.Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31(18):2151–61. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 49.Weiler C, Nerlich AG, Bachmeier BE, Boos N. Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: a study in surgical specimen and autopsy controls. Spine. 2005;30(1):44–53. doi: 10.1097/01.brs.0000149186.63457.20. discussion 4. [DOI] [PubMed] [Google Scholar]
- 50.Crean JK, Roberts S, Jaffray DC, Eisenstein SM, Duance VC. Matrix metalloproteinases in the human intervertebral disc: role in disc degeneration and scoliosis. Spine. 1997;22(24):2877–84. doi: 10.1097/00007632-199712150-00010. [DOI] [PubMed] [Google Scholar]
- 51.Weiler C, Nerlich AG, Zipperer J, Bachmeier BE, Boos N. 2002 SSE Award Competition in Basic Science: expression of major matrix metalloproteinases is associated with intervertebral disc degradation and resorption. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2002;11(4):308–20. doi: 10.1007/s00586-002-0472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nature reviews Immunology. 2008;8(12):958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khallou-Laschet J, Varthaman A, Fornasa G, et al. Macrophage plasticity in experimental atherosclerosis. PloS one. 2010;5(1):e8852. doi: 10.1371/journal.pone.0008852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lavin Y, Winter D, Blecher-Gonen R, et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159(6):1312–26. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. The Journal of clinical investigation. 2007;117(1):175–84. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McWhorter FY, Davis CT, Liu WF. Physical and mechanical regulation of macrophage phenotype and function. Cellular and molecular life sciences : CMLS. 2015;72(7):1303–16. doi: 10.1007/s00018-014-1796-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim KW, Lim TH, Kim JG, Jeong ST, Masuda K, An HS. The origin of chondrocytes in the nucleus pulposus and histologic findings associated with the transition of a notochordal nucleus pulposus to a fibrocartilaginous nucleus pulposus in intact rabbit intervertebral discs. Spine. 2003;28(10):982–90. doi: 10.1097/01.BRS.0000061986.03886.4F. [DOI] [PubMed] [Google Scholar]
- 58.Johnson WE, Eisenstein SM, Roberts S. Cell cluster formation in degenerate lumbar intervertebral discs is associated with increased disc cell proliferation. Connective tissue research. 2001;42(3):197–207. doi: 10.3109/03008200109005650. [DOI] [PubMed] [Google Scholar]
- 59.Sharp CA, Roberts S, Evans H, Brown SJ. Disc cell clusters in pathological human intervertebral discs are associated with increased stress protein immunostaining. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2009;18(11):1587–94. doi: 10.1007/s00586-009-1053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


