Abstract
Purpose
We hypothesized that mutations in homologous recombination repair (HRR) genes beyond BRCA1 and BRCA2 improve outcomes for ovarian carcinoma (OC) patients treated with platinum therapy and would impact the relative benefit of adding prolonged bevacizumab.
Experimental design
We sequenced DNA from blood and/or neoplasm from 1,195 women enrolled in GOG-0218, a randomized phase III trial in advanced OC of bevacizumab added to carboplatin and paclitaxel. Defects in HRR were defined as damaging mutations in 16 genes. Proportional hazards models were used to estimate relative hazards for progression-free survival (PFS) and overall survival (OS).
Results
Of 1,195 women with OC, HRR mutations were identified in 307 (25.7%). Adjusted hazards for progression and death compared to those without mutations were lower for women with non-BRCA HRR mutations (HR 0.73, 95% CI 0.57 – 0.94, p=0.01 for PFS; HR 0.67, 95% CI 0.50 – 0.90, p=0.007 for OS) and BRCA1 mutations (hazard ratio (HR) 0.80, 95% CI 0.66 – 0.97, p=0.02 for PFS; HR 0.74, 95% CI 0.59 – 0.94, p=0.01 for OS) and were lowest for BRCA2 mutations (HR 0.52, 95% CI 0.40 – 0.67, p<0.0001 for PFS; HR 0.36, 95% CI 0.25 – 0.53, p<0.0001 for OS). A test of interaction showed no difference in the effect of bevacizumab on PFS between cases with and without mutations.
Conclusions
HRR mutations, including non-BRCA genes, significantly prolong PFS and OS in OC and should be stratified for in clinical trials. The benefit of adding bevacizumab was not significantly modified by mutation status.
Keywords: Ovarian cancer, BRCA, homologous recombination, bevacizumab, somatic mutation
INTRODUCTION
BRCA1 and BRCA2 are key components of the BRCA-Fanconi anemia DNA repair pathway that controls DNA repair via homologous recombination.(1–3) Germline and somatic mutations affecting homologous recombination repair (HRR) genes are relatively common, found in approximately one-third of ovarian carcinoma patients, with the majority of mutations occurring in BRCA1 and BRCA2.(4,5) Ovarian carcinoma patients with inherited mutations in BRCA1 and BRCA2 have longer five-year survival, likely due to improved sensitivity to platinum chemotherapy.(6–8) This may also be true for somatic and germline mutations in other HRR genes, though the number of cases with non-BRCA HRR mutations studied to date is small.(5) In addition, BRCA1 and BRCA2 mutated ovarian carcinomas may have a lower angiogenic profile(9) and more favorable immmunophenotype than those without mutations, which might also contribute to better outcomes.(10,11)
GOG-0218 was a randomized, phase III trial for patients with primary advanced ovarian, peritoneal, and fallopian tube carcinoma (OC), examining the addition of the anti-angiogenesis drug bevacizumab to standard chemotherapy with carboplatin and paclitaxel. Patients in this study who received extended bevacizumab had a significant prolongation in median progression-free survival (PFS) of 3.8 months.(12) In the parallel European ICON-7 trial, a non pre-specified subgroup analysis suggested that extended bevacizumab was of greatest benefit for patients with poor prognostic features such as stage IV disease, or unresectable or suboptimally resected tumors, demonstrating a benefit in overall survival (OS) in these “high-risk” patients.(13)
We hypothesized that OC patients with inherited mutations in HRR genes, including a select set of genes beyond BRCA1 and BRCA2, have longer survival and improved platinum sensitivity and may derive less benefit from extended bevacizumab. We tested this hypothesis using tissues from patients in GOG-218 who had provided consent for translational research.
MATERIALS AND METHODS
GOG-0218 clinical trial details have been previously described.(12) DNA from tumor and blood from patients on GOG-0218 were sequenced using BROCA, a targeted capture, massively parallel sequencing test developed at the University of Washington.(14) All available germline DNA samples extracted from blood were sequenced (N = 788). These patients have been previously described.(8) In order to improve detection of HRR deficiency in this group by detection of somatic mutations in HRR genes, we also sequenced DNA from neoplastic tissue from a subset of these patients with negative germline testing (N = 324). Finally, we sequenced DNA from neoplastic tissue from 407 additional cases that did not have available DNA from blood, detecting both germline and somatic mutations (bringing the total number of sequenced patients to 1195). Distinguishing between germline and somatic mutations could not be done with certainty in the group with only tumor sequencing. As the effect on outcome was predicted to be similar, germline and somatic mutations were combined for analyses. All patients provided written informed consent as approved by an institutional review board (IRB), in accordance with ethical guidelines as described in the U.S. Common Rule. As patients were not specifically consented for open access genomic data, complete sequencing data such as BAM files cannot be shared publicly.
A subset of genes predicted to impact homologous recombination repair (HRR) when mutated was selected prior to analysis based on review of the available literature and expert opinion, including: ATM, ATR, BARD1, BLM, BRCA1, BRCA2, BRIP1, CHEK2, MRE11A, NBN, PALB2, RAD51C, RAD51D, RBBP8, SLX4, and XRCC2. Sequencing reads were aligned to the human reference genome (hg19). Variants were identified using GATK37 and Pindel after indel realignment and base quality calibration. Copy number variations (CNVs) were detected in germline samples as previously described.(14–16) Missense mutations were only included if proven to be damaging in functional assays or classified as likely pathogenic on ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/).(17)
Clinical information was collected as per the GOG-0218 protocol.(12) All pathology was centrally reviewed. PFS and OS were defined as the time between enrollment and progression or death, respectively. Proportional hazards models were used to provide estimates of the relative hazards for progression and death by genotype, adjusted by clinical characteristics. The effect of mutation status on the impact of bevacizumab on progression was assessed with a test of interaction. All P values are 2-sided.
RESULTS
Study Population
Of 1,873 women in GOG 218, 1,195 (63.8%) had DNA available for sequencing. Baseline characteristics of sequenced and not sequenced patients are described in Table 1. The distribution of race was significantly different between the groups, with more non-Hispanic Whites and fewer Asian/Pacific Islanders in the sequenced group (p<0.001, chi-square). There were more optimally debulked stage III patients and less stage IV patients in the sequenced group (p<0.001, chi-square). The distribution of histologic subtypes also differed, with fewer patients with clear cell carcinoma in the sequenced group (p=0.012, chi-square). The median PFS duration for those who were not sequenced was 10.5 months compared to 13.5 months for those sequenced (p<0.001) and the median overall survival for those not sequenced was 30.9 months compared to 46.2 months for those who were sequenced.
Table 1.
Clinical Characteristics of OC Patients, Sequenced Vs. Not Sequenced
| Characteristic | Sequenced | Not Sequenced | |
|---|---|---|---|
| N | 1195 | 678 | |
| Age | <40 | 40 (3.3%) | 23 (3.4%) |
| 40 – 49 | 176 (14.7%) | 90 (13.3%) | |
| 50 – 59 | 385 (32.2%) | 221 (32.6%) | |
| 60 – 69 | 374 (31.3%) | 236 (34.8%) | |
| 70 – 79 | 205 (17.2%) | 91 (13.4%) | |
| ≥80 | 15 (1.3%) | 17 (2.5%) | |
| Race/Ethnicity* | Non-Hispanic White | 1048 (87.7%) | 518 (76.4%) |
| Hispanic | 51 (4.3%) | 23 (3.4%) | |
| Non-Hispanic Black | 46 (3.8%) | 34 (5.0%) | |
| Asian/Pacific Islander | 31 (2.6%) | 86 (12.7%) | |
| Other or Unknown | 19 (1.6%) | 17 (2.5%) | |
| Disease Site | Ovary | 998 (83.5%) | 565 (83.3%) |
| Peritoneal | 171 (14.3%) | 103 (15.2%) | |
| Fallopian Tube | 26 (2.2%) | 10 (1.5%) | |
| Stage* | III/Optimal | 465 (38.9%) | 175 (25.8%) |
| III/Suboptimal | 453 (37.9%) | 299 (44.1%) | |
| IV | 277 (23.2%) | 204 (30.1%) | |
| Histology** | HG Serous | 971 (81.3%) | 526 (77.6%) |
| LG Serous | 46 (3.8%) | 25 (3.7%) | |
| Carcinoma, NS | 84 (6.2%) | 81 (14.2%) | |
| LG Endometrioid | 4 (0.3%) | 2 (0.3%) | |
| HG Endometrioid | 38 (3.2%) | 15 (2.2%) | |
| Clear Cell | 28 (2.3%) | 27 (4.0%) | |
| Mucinous | 7 (0.6%) | 12 (1.8%) | |
| Treatment | CT + P -> P | 408 (34.1%) | 217 (32.0%) |
| CT + B -> P | 386 (32.3%) | 239 (35.3%) | |
| CT + B -> B | 401 (33.6%) | 222 (32.7%) |
Abbreviations: HG (high-grade, grades 2 and 3), LG (low-grade, grade 1), NS (not specified), CT (chemotherapy), P (placebo), B (Bevacizumab).
P-value <0.001, chi-square,
P-value 0.012, chi-square.
Sequencing results
In total, 307/1,195 (25.7%) sequenced patients had damaging mutations in one of the selected genes affecting HRR. There were 148 (12.4%) with mutations in BRCA1, 78 (6.5%) with mutations in BRCA2, and 81 (6.8%) with mutations in other HRR genes. Ten patients (0.8%) had more than one mutation. Of these 10, three were in the BRCA1 mutation group, with additional mutations in RBBP8 (one) and NBN (two), and four were in the BRCA2 group (one with CHEK2, one with RBBP8, one with both ATM and CHEK2, and one with BRIP1 and CHEK2). Of the 81 patients in the other HRR group, there were 84 mutations in the following genes: 12 in ATM, five in ATR, three in BARD1, five in BLM, 19 in BRIP1, two in CHEK2, two in MRE11A, three in NBN, six in PALB2, seven in RAD51C, seven in RAD51D, eight in RBBP8, two in SLX4, and three in XRCC2. Details of the patients with non-BRCA HRR mutations are provided in Supplemental Table 1.
In the subset of 324 patients with negative germline testing who had subsequent sequencing of neoplastic DNA, there were 32 (9.9%) somatic mutations in HRR genes (17 (5.2%)) in BRCA1, seven (2.2%) in BRCA2, and eight (2.5%) in other HRR genes). In a small exploratory analysis, these 32 “definitely somatic” mutation carriers had significantly lower hazards of both progression and death when compared to 174 patients with “definitely germline” mutations (Supplemental Figure 1). Mutation rates by the source of sequenced DNA are described in more detail in Supplemental Table 2.
Clinical Characteristics by Mutation Status
The proportion of patients in each mutation group by clinical characteristics is shown in Table 2. Mutation status did not differ by disease stage or primary site. Mutations in HRR genes were found in all histologic subtypes. The overall mutation rate in the high-grade serous carcinomas was 27.0% (262/971), which was not significantly different from the rate in unspecified carcinoma (22/101, 21.8%, p=0.29), endometrioid (10/42, 23.8%, p=0.73), clear cell (6/28, 21.4%, p=0.67), or mucinous carcinoma (2/7, 28.6%, p=1.0). The low-grade serous carcinomas had a lower HRR mutation rate of 10.9% (5/46, 95% confidence interval 4.8–23.1%, p=0.02, Fisher’s exact) when compared to high-grade serous carcinomas.
Table 2.
Clinical Characteristics and Mutation Category in OC Patients
| Characteristic | BRCA1 | BRCA2 | Other HRR | WT | |
|---|---|---|---|---|---|
| N | 148 | 78 | 81 | 888 | |
| Disease Site | Ovary | 131 (88.5%) | 65 (83.3%) | 68 (85.2%) | 734 (82.7%) |
| Peritoneal | 16 (10.8%) | 12 (15.4%) | 12 (14.8%) | 131 (14.8%) | |
| Fallopian Tube | 1 (0.7%) | 1 (1.3%) | 1 (1.2%) | 23 (2.6%) | |
| Stage | III/Optimal | 59 (39.9%) | 26 (33.3%) | 37 (45.7%) | 343 (38.6%) |
| III/Suboptimal | 58 (39.2%) | 29 (37.2%) | 25 (30.9%) | 341 (38.4%) | |
| IV | 31 (20.9%) | 23 (29.5%) | 19 (23.5%) | 204 (23.0%) | |
| Histology | HG Serous | 127 (85.8%) | 70 (89.7%) | 65 (80.2%) | 709 (79.8%) |
| LG Serous* | 4 (2.7%) | 0 | 1 (1.2%) | 41 (4.6%) | |
| Carcinoma, NS | 12 (8.1%) | 6 (7.7%) | 4 (12.6%) | 79 (8.9%) | |
| Endometrioid | 3 (2.0%) | 1 (1.3%) | 6 (7.4%) | 32 (3.6%) | |
| Clear Cell | 2 (1.4%) | 1 (1.3%) | 3 (3.7%) | 22 (2.5%) | |
| Mucinous | 0 | 0 | 2 (2.5%) | 5 (0.6%) | |
| Treatment | CT + P -> P | 53 (35.8%) | 28 (35.9%) | 27 (33.3%) | 300 (33.8%) |
| CT + B -> P | 34 (23.0%) | 19 (24.4%) | 26 (32.1%) | 307 (34.6%) | |
| CT + B -> B | 61 (41.2%) | 31 (38.3%) | 28 (34.6%) | 281 (31.6%) |
Abbreviations: HG (high-grade, grades 2 and 3), LG (low-grade, grade 1), NS (not specified), CT (chemotherapy), P (placebo), B (Bevacizumab).
Other HRR (other homologous recombination repair) refers to those with mutations in non-BRCA genes including ATM, ATR, BARD1, BLM, BRIP1, CHEK2, MRE11A, NBN, PALB2, RAD51C, RAD51D, RBBP8, SLX4, and XRCC2.
Low-grade serous carcinoma had a significantly lower total mutation rate when compared to high-grade serous carcinoma, p=0.02, Fisher’s exact.
Effect of Mutation Status on Survival
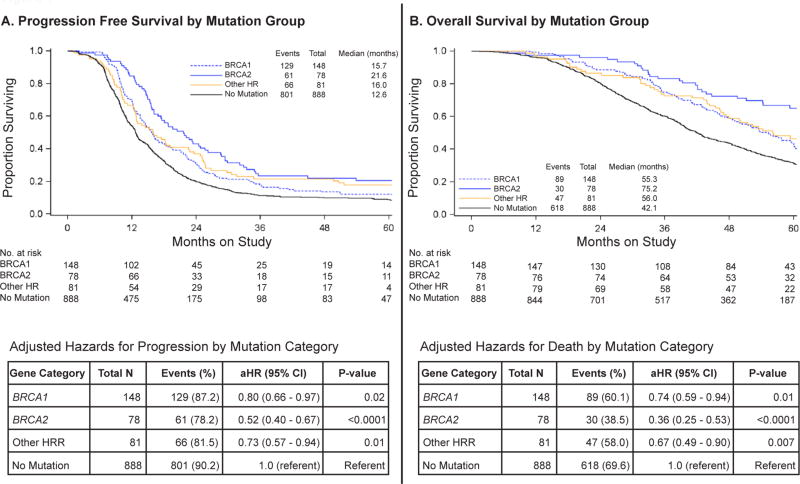
Damaging mutations in BRCA1, BRCA2, or other non-BRCA HRR genes were all associated with longer PFS and OS relative to cases without mutations (Figure 1). After adjusting for study treatment, stage of disease, size of residual disease, and initial performance status, adjusted hazard ratios for both progression and death were significantly lower in cases with HRR mutations when compared to those with no mutations (Figure 1). This effect was strongest for BRCA2 mutations and was similar for BRCA1 and non-BRCA HRR mutations.
Figure 1. Progression-free and overall survival in OC patients by mutation category.
A. – B. Kaplan-Meier curves for progression-free and overall survival by mutation category, with adjusted hazard ratios for progression or death. Hazard ratios were adjusted for study treatment, stage of disease, size of residual disease, and initial performance status. The no mutation group was the referent group. Events were progression or death in 60 months. Abbreviations: aHR (adjusted hazard ratio).
Interaction Between Mutation Status and the Effect of Bevacizumab on Progression
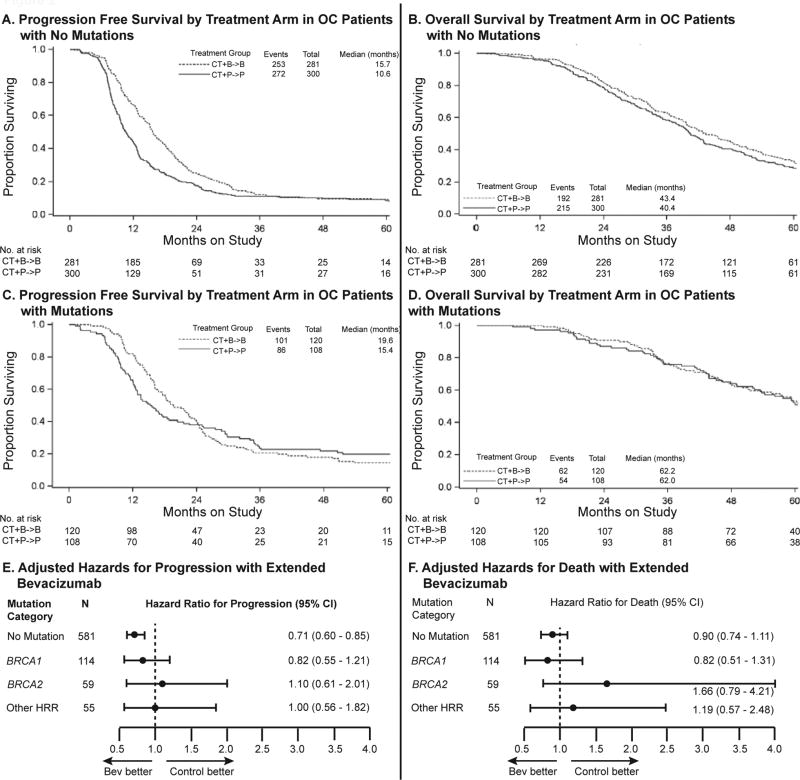
For this analysis, only patients from arm 1 (carboplatin/paclitaxel with placebo) and arm 3 (carboplatin/paclitaxel with bevacizumab and bevacizumab maintenance) were included (N = 809). In patients with no mutations (N = 581), extended bevacizumab significantly prolonged PFS (15.7 months vs. 10.6 months, HR 0.71, 95% CI 0.60 – 0.85, p=0.0001), Figure 2A. In those with mutations (N = 228), extended bevacizumab conferred a median PFS of 19.6 months vs. 15.4 months, (HR 0.95, 95% CI 0.71 – 1.26, Figure 2C). Using a test of interaction, mutation status did not significantly modify the effect of extended bevacizumab on progression (0.95/0.71) = 1.33, 95% CI 0.95 – 1.85, p = 0.10.
Figure 2. Effect of extended bevacizumab on survival in OC patients with and without mutations.
A. – D. Kaplan-Meier curves for progression-free and overall survival in OC patients with and without mutations in genes affecting HRR. E. – F. Adjusted hazard ratios for progression or death by individual mutation categories. Using a test of interaction, mutation status did not significantly modify the effect of extended bevacizumab on progression (0.95/0.71) = 1.33, 95% CI 0.95 – 1.85, p = 0.10.
DISCUSSION
GOG-0218 was a large randomized, placebo-controlled phase III clinical trial with standardized treatment, central pathology review, and follow up, providing a unique opportunity to assess the impact of HRR deficiency on clinical outcomes. In this trial, patients with OC with defective HRR, defined by damaging somatic or germline mutations in one of 16 genes, had significantly prolonged PFS and OS when compared to those without mutations (Figure 1). This is the first study in OC large enough to separately assess the impact on outcomes of non-BRCA HRR mutations, which are less common than mutations in BRCA1 and BRCA2, though our smaller single institution study showed a trend towards improved overall survival with mutations in a subset of non-BRCA genes.(5) We carefully selected a list of 14 non-BRCA genes a priori that we thought would be most likely to impact outcomes based on in vitro data. OCs with mutations in other HRR pathway genes such as FANCM, FANCA, and FANCI were not classified as HRR deficient. Interestingly, the non-BRCA HRR mutations were associated with PFS and OS curves that were almost identical to BRCA1 mutations, supporting our a priori selection of HRR genes as meaningful.
As expected, BRCA1 and BRCA2 mutations were associated with longer survival, with the most profound effect seen for BRCA2 mutations with a median survival advantage of 33 months compared to those without mutations. These patients were treated years before poly (ADP-ribose) polymerase (PARP) inhibitors became available, and these outcome differences would likely be even greater with current treatments. Previous studies have also demonstrated a better outcome for BRCA2 compared to BRCA1 mutations (5–8), but ours is the first large study to include somatic mutations and to assess outcomes relative to mutation status for patients treated on a phase III clinical trial with standardized treatment and follow-up. Given the meaningful impact of HRR deficiency on both PFS and OS, the presence of HRR mutations, including BRCA1 and BRCA2, should be carefully considered in the design and analyses of OC clinical trials.
Mutations in HRR genes were found in all histologic subtypes of OC, and rates were only significantly lower in low-grade serous carcinomas, 5/46 (10.9%) vs. 262/971 (27%) in high-grade serous carcinomas, P = 0.02, (Table 2). All tumors in GOG-0218 underwent centralized review by gynecologic pathologists, minimizing pathologic misclassification. These results are similar to our recent observation that germline HRR mutations are distributed across many histological sub-types without a clear predilection for high-grade serous carcinomas.(8) Therefore, HRR status cannot be assumed by histologic type. Some clinical trials targeting HRR deficiency, such as those using PARP inhibitors, are restricted to patients with high-grade serous carcinomas,(18) or to those with either high-grade serous or high-grade endometrioid histology.(19) Our data do not support restricting access to these clinical trials, or to germline genetic testing or tumor sequencing for HRR defects based on histologic subtype alone.
The role of anti-angiogenesis targeted therapy in ovarian carcinomas with and without HRR deficiency has not been previously defined. In the subset of patients with HRR mutations in GOG-0218, extended bevacizumab did not confer a statistically significant prolongation in PFS (Figure 2C). However, there was insufficient evidence that bevacizumab had a different effect on PFS or OS in HRR-mutated versus non-mutated cases, using a test of interaction (Figure 2). The power of this analysis was limited by not having translational samples from all participants in GOG-0218, therefore the number of mutation carriers was relatively small. Sequenced patients were less likely to be stage IV and had better PFS and OS, which also may have reduced our ability to detect a difference. Within the similar ICON7 trial, a subset of 359 cases subjected to expression profiling, proliferative and mesenchymal subtypes may have derived greater benefit from bevacizumab therapy, however tests of interaction were not done to formally test that hypothesis. The mutation status of the ICON7 cancers was not determined.(20) Further studies are needed to identify ovarian carcinomas best treated with bevacizumab, but our data do not currently support BRCA mutation status as a determinant.
This study has several limitations. While germline sequencing data is available on all patients (by sequencing DNA from blood or tumor), around 40% of patients did not have tumor sequenced. This likely underestimated the true mutation frequency by missing some somatic mutations. In addition, as germline and somatic HRR mutations are rarely present in the same patient, utilizing a “germline negative” group (N = 324/1195, 27.1%) for additional tumor sequencing likely overestimated the somatic mutation rate by excluding those with germline mutations who were unlikely to have somatic mutations (Supplemental Table 2). Exome sequencing data from The Cancer Genome Atlas (TCGA) demonstrated somatic or germline mutations in DNA-repair genes in around 40% of patients with high-grade serous histology ovarian carcinomas,(4) compared to 27% in patients with high-grade serous carcinomas in this trial. This difference is likely due to a combination of 1) our pre-selected shorter HRR gene list chosen to maximize likelihood of therapeutic impact, 2) our incomplete tumor sequencing data in 40% of cases, and 3) our more stringent definition of damaging mutations compared to TCGA. Finally, mutations in non-BRCA “other” HRR genes were too few to allow a survival analysis by individual gene, necessitating combining these patients into one group for analysis.
In conclusion, HRR deficiency is a strong predictor of both PFS and OS in ovarian carcinoma, including mutations in 14 non-BRCA HRR genes (ATM, ATR, BARD1, BLM, BRIP1, CHEK2, MRE11A, NBN, PALB2, RAD51C, RAD51D, RBBP8, SLX4, and XRCC2). Histologic subtype does not provide sufficient information to predict either inherited or somatic HRR mutations. There is insufficient evidence that HRR mutations should be a criterion, either positive or negative, in the decision to use bevacizumab maintenance therapy for advanced ovarian carcinoma.
Supplementary Material
Translational Significance.
In the setting of a large clinical trial in primary ovarian cancer patients, we have demonstrated that damaging mutations in a selected set of genes affecting homologous recombination repair beyond BRCA1 and BRCA2 are associated with improved progression-free and overall survival, controlling for known prognostic features, with an outcome similar to that seen for BRCA1 mutations. Cases with BRCA2 mutations had even better outcomes. Given the magnitude of these effects, consideration should be given to assessing homologous recombination repair in the design and analyses of ovarian carcinoma clinical trials. We found mutations in homologous recombination repair genes including BRCA1 and BRCA2 in all histologic subtypes, with only low-grade serous having a significantly lower mutation frequency. Our data do not support restricting access to clinical trials, or to germline genetic testing, on the sole basis of histology. We did not see a differential effect of the impact of bevacizumab on progression-free survival by mutation status. Therefore, mutation status should not be used to decide who is a candidate for bevacizumab.
Acknowledgments
Conflict of Interest Statements: Dr. Backes has received research grants from Eisai Incorporated, ImmunoGen, and Clovis. Dr. Burger has received honoraria for leading web based tumor board discussions for Roche. Dr. Tewari reports participation in advisory boards for Genentech and was a member of Roche’s speaker’s bureau for 2 years. Dr. Mannel is on an advisory board for Genentech.
Financial Support: This work was supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469), the Gynecologic Oncology Group Statistical and Data Center (CA 37517), the Gynecologic Oncology Group Tissue Bank (U10 CA27469, U24 CA114793, and U10 CA180868), NRG Oncology Grant (1 U10 CA180822), R01CA157744 (M. King), RO1CA175716 (T. Walsh and M. King), and by the Ovarian Cancer Research Foundation Alliance (E. Swisher and B. Norquist), The Breast Cancer Research Foundation (M. King), the Department of Defense Ovarian Cancer Research Program OC120312 (E. Swisher), the V Foundation Translational Research Award (E. Swisher), The Women’s Reproductive Health Research Career Development Award 5K12HD001264-13 (B. Norquist), The Nancy Kelley Jensen Faculty Fellowship for Gynecologic Oncology Research (B. Norquist), and the Wendy Feuer Research Fund for the Prevention and Treatment of Ovarian Cancer (E. Swisher).
Role of Funding Sources: The funders/sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
The following Gynecologic Oncology Group member institutions participated in the primary treatment studies: Cancer Trials Support Unit, University of Oklahoma Health Sciences Center, University of California Medical Center at Irvine-Orange Campus, Fred Hutchinson Cancer Research Center, Ohio State University Comprehensive Cancer Center, Gynecologic Oncology Network/Brody School of Medicine, Mayo Clinic, Metro-Minnesota CCOP, Abramson Cancer Center of the University of Pennsylvania, University of North Carolina at Chapel Hill, Abington Memorial Hospital, University of Alabama at Birmingham, Rush University Medical Center, Walter Reed National Military Medical Center, Washington University School of Medicine, University of Kentucky, University of Iowa Hospitals and Clinics, Roswell Park Cancer Institute, Duke University Medical Center, University of Colorado Cancer Center – Anschutz Cancer Pavilion, University of Chicago, Cleveland Clinic Foundation, Women’s Cancer Center of Nevada, University of Hawaii, Mount Sinai School of Medicine, Northwestern University, Fox Chase Cancer Center, Yale University, Women and Infants Hospital, University of California at Los Angeles Health System, Memorial Sloan Kettering Cancer Center, Indiana University Hospital/Melvin and Bren Simon Cancer Center, University of Minnesota Medical Center-Fairview, University of Mississippi Medical Center, University of Pittsburgh Cancer Institute, University of New Mexico, Case Western Reserve University, The Hospital of Central Connecticut, University of Texas-Galveston, Cancer Research for the Ozarks NCORP, Cooper Hospital University Medical Center, University of Virginia, Moffitt Cancer Center and Research Institute, State University of New York Downstate Medical Center, University of Wisconsin Hospital and Clinics, Carle Cancer Center, Northern Indiana Cancer Research Consortium, Penn State Milton S. Hershey Medical Center, Wake Forest University Health Sciences, Stony Brook University Medical Center, University of Massachusetts Memorial Health Care, Fletcher Allen Health Care, Michigan Cancer Research Consortium Community Clinical Oncology Program, MD Anderson Cancer Center, Gynecologic Oncology of West Michigan PLLC, Central Illinois CCP, Delaware Christiana Care CCOP, Virginia Commonwealth University, University of Cincinnati, Tufts-New England Medical Center, Scot and White Memorial Hospital, Tacoma General Hospital, Northern New Jersey CCOP, Virginia Mason CCOP, New York University Medical Center, Aurora Women’s Pavilion of Aurora West Allis Medical Center, Wisconsin NCI Community Oncology Research Program, Evanston CCOP-North Shore University Health System, Cancer Research Consortium of West Michigan NCORP, Missouri Valley Cancer Consortium CCOP, William Beaumont Hospital, Colorado Cancer Research Program NCORP, Saint Louis-Cape Girardeau CCOP, University of Texas Southwestern Medical Center, University of Illinois, Kalamazoo CCOP, Upstate Carolina CCOP, Heartland Cancer Research CCOP and Wichita CCOP.
References
- 1.Howlett NG, Taniguchi T, Olson S, Cox B, Waisfisz Q, De Die-Smulders C, et al. Biallelic inactivation of BRCA2 in Fanconi anemia. Science. 2002;297(5581):606–9. doi: 10.1126/science.1073834. [DOI] [PubMed] [Google Scholar]
- 2.Moynahan ME, Chiu JW, Koller BH, Jasin M. Brca1 controls homology-directed DNA repair. Mol Cell. 1999;4(4):511–8. doi: 10.1016/s1097-2765(00)80202-6. [DOI] [PubMed] [Google Scholar]
- 3.Moynahan ME, Pierce AJ, Jasin M. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol Cell. 2001;7(2):263–72. doi: 10.1016/s1097-2765(01)00174-5. [DOI] [PubMed] [Google Scholar]
- 4.Kanchi KL, Johnson KJ, Lu C, McLellan MD, Leiserson MD, Wendl MC, et al. Integrated analysis of germline and somatic variants in ovarian cancer. Nat Comm. 2014;5:3156. doi: 10.1038/ncomms4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pennington KP, Walsh T, Harrell MI, Lee MK, Pennil CC, Rendi MH, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20(3):764–75. doi: 10.1158/1078-0432.CCR-13-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alsop K, Fereday S, Meldrum C, deFazio A, Emmanuel C, George J, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30(21):2654–63. doi: 10.1200/JCO.2011.39.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolton KL, Chenevix-Trench G, Goh C, Sadetzki S, Ramus SJ, Karlan BY, et al. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA. 2012;307(4):382–90. doi: 10.1001/jama.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norquist BM, Harrell MI, Brady MF, Walsh T, Lee MK, Gulsuner S, et al. Inherited Mutations in Women With Ovarian Carcinoma. JAMA Oncol. 2016;2(4):482–90. doi: 10.1001/jamaoncol.2015.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vogel TJ, Dellorusso C, Welcsh P, Shah CA, Goff BA, Garcia RL, et al. Angiogenic alterations associated with circulating neoplastic DNA in ovarian carcinoma. Transl Oncol. 2012;5(4):247–51. doi: 10.1593/tlo.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strickland KC, Howitt BE, Shukla SA, Rodig S, Ritterhouse LL, Liu JF, et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget. 2016;7(12):13587–98. doi: 10.18632/oncotarget.7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birkbak NJ, Kochupurakkal B, Izarzugaza JM, Eklund AC, Li Y, Liu J, et al. Tumor mutation burden forecasts outcome in ovarian cancer with BRCA1 or BRCA2 mutations. PLoS One. 2013;8(11):e80023. doi: 10.1371/journal.pone.0080023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365(26):2473–83. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 13.Oza AM, Cook AD, Pfisterer J, Embleton A, Ledermann JA, Pujade-Lauraine E, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015;16(8):928–36. doi: 10.1016/S1470-2045(15)00086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walsh T, Lee MK, Casadei S, Thornton AM, Stray SM, Pennil C, et al. Detection of inherited mutations for breast and ovarian cancer using genomic capture and massively parallel sequencing. Proc Natl Acad Sci USA. 2010;107(28):12629–33. doi: 10.1073/pnas.1007983107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abel HJ, Duncavage EJ, Becker N, Armstrong JR, Magrini VJ, Pfeifer JD. SLOPE: a quick and accurate method for locating non-SNP structural variation from targeted next-generation sequence data. Bioinformatics. 2010;26(21):2684–8. doi: 10.1093/bioinformatics/btq528. [DOI] [PubMed] [Google Scholar]
- 16.Nord AS, Lee M, King MC, Walsh T. Accurate and exact CNV identification from targeted high-throughput sequence data. BMC Genomics. 2011;12:184. doi: 10.1186/1471-2164-12-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen PL, Chen CF, Chen Y, Xiao J, Sharp ZD, Lee WH. The BRC repeats in BRCA2 are critical for RAD51 binding and resistance to methyl methanesulfonate treatment. Proc Natl Acad Sci U S A. 1998;95(9):5287–92. doi: 10.1073/pnas.95.9.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. The New England journal of medicine. 2016;375(22):2154–64. doi: 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]
- 19.Swisher EM, Lin KK, Oza AM, Scott CL, Giordano H, Sun J, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017;18(1):75–87. doi: 10.1016/S1470-2045(16)30559-9. [DOI] [PubMed] [Google Scholar]
- 20.Kommoss S, Winterhoff B, Oberg AL, Konecny GE, Wang C, Riska SM, et al. Bevacizumab May Differentially Improve Ovarian Cancer Outcome in Patients with Proliferative and Mesenchymal Molecular Subtypes. Clin Cancer Res. 2017;23(14):3794–801. doi: 10.1158/1078-0432.CCR-16-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.