Abstract
Rationale
Myocardial infarction (MI) is a major cause of adult mortality worldwide. The origin(s) of cardiac fibroblasts that constitute the post-infarct scar remain controversial, in particular the potential contribution of bone marrow lineages to activated fibroblasts within the scar.
Objective
To establish the origin(s) of infarct fibroblasts using lineage tracing and bone marrow transplants, and a robust marker for cardiac fibroblasts, the Collagen1a1-GFP reporter.
Methods and Results
Using genetic lineage tracing or bone marrow transplant, we found no evidence for Collagen-producing fibroblasts derived from hematopoietic or bone marrow lineages in hearts subjected to permanent left anterior descending (LAD) coronary artery ligation. In fact, fibroblasts within the infarcted area were largely of epicardial origin. Intriguingly, collagen-producing fibrocytes from hematopoietic lineages were observed attached to the epicardial surface of infarcted and sham-operated hearts in which a suture was placed around the LAD.
Conclusion
In this controversial field, our study demonstrated that the vast majority of infarct fibroblasts were of epicardial origin and not derived from bone marrow lineages, endothelial-to-mesenchymal transition (EndoMT) or blood. We also noted the presence of collagen-producing fibrocytes on the epicardial surface that resulted at least in part from the surgical procedure.
Subject Terms: Cardiovascular Disease, Fibrosis, Heart Failure, Myocardial Infarction, Remodeling
Keywords: Fibroblast, bone marrow, myocardial infarction, fibrosis, cardiac disease, bone marrow
INTRODUCTION
Myocardial infarction (MI) results in massive myocyte loss that severely compromises cardiac function. The adult myocardium lacks regenerative capacity, and lost myocardium is replaced by a fibrous scar. Although this scar provides vital chamber structural integrity, it often results in adverse myocardial stiffening and deleterious effects on cardiac function. Therefore, modulation of scar formation could have potential beneficial effects on post-MI remodeling and cardiac function, and understanding the sources of fibroblasts in the context of MI may assist future therapeutic approaches targeting the fibrotic process.
Cardiac fibroblasts are the main cell type responsible for extracellular matrix deposition in the heart1. During development, a majority of cardiac fibroblasts are derived from epicardium, although a subset enriched in the interventricular septum derives from endothelium/endocardium2, 3. Fibroblasts play a key role following infarction, as the outcome depends on the generation of a fibrous scar comprised largely of Collagen4. The origins of Collagen-producing fibroblasts following infarction are controversial. A subset of fibroblasts is produced by epithelial-to-mesenchymal transition (EMT) of adult epicardium following infarction5. Furthermore, adult endothelial-to-mesenchymal transition (EndoMT) has also been reported to contribute to scar formation6, although this has been recently challenged7, 8. Several studies have reported the presence9, 10 or absence7, 8, 11 of circulating fibroblast progenitors that make a significant contribution to the post-infarct fibroblast population.
Previously, we described cardiac fibroblast origins during development and in the context of a mouse model of pressure overload2. Fibroblasts were identified with a Collagen1a1-GFP reporter, that has superior specificity when compared to FSP1, αSMA, Vim or Thy1 stainings2, 8, 12. Here, using genetic lineage tracing and complementary bone marrow transplant experiments, we provide strong evidence that fibroblasts within the infarcted fibrotic area of the left ventricle do not arise from hematopoietic-lineages or infiltrating bone marrow derived cells, or from EndoMT, but rather are principally of epicardial origin. However, although never within the infarct scar itself, Collagen-producing blood-derived fibrocytes were observed at the epicardial surface of the infarcted area. Our results provide strong evidence that endogenous fibroblasts are the primary critical target for therapeutic targeting of post-infarct fibrosis.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Expanded methods are presented in the Online Data Supplement.
RESULTS
Collagen1a1-GFP comprehensively labels fibroblasts of the infarcted area
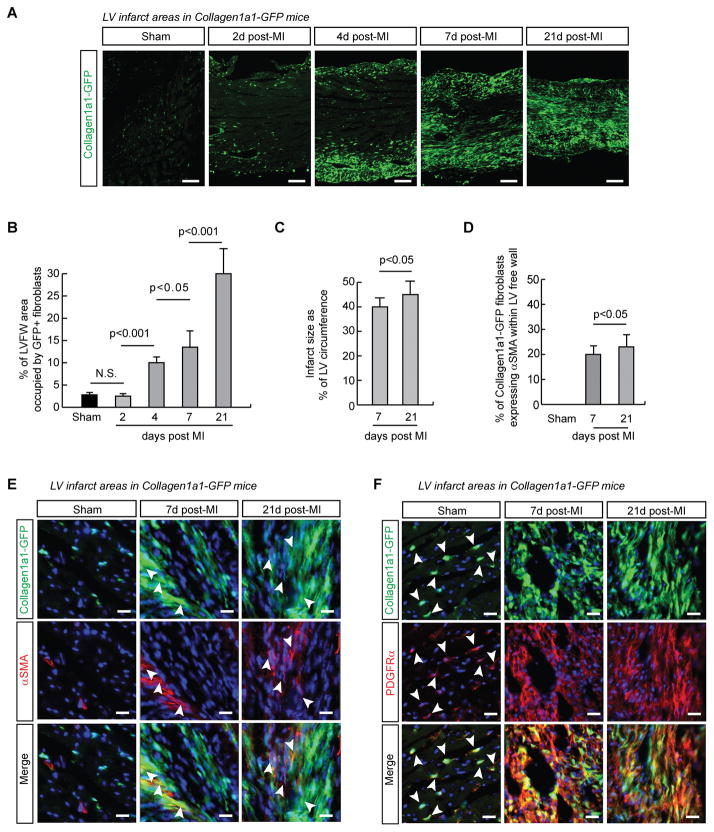
We previously reported that cell types within the myocardium, including endothelial cells, resident immune cells, vascular smooth muscle cells and pericytes do not express appreciable levels of Collagen1a1-GFP relative to fibroblasts2. The infarcted area of hearts from Collagen1a1-GFP+/− mice was imaged at distinct stages following myocardial infarction induced by permanent left anterior descending (LAD) coronary artery ligation, by 21 days post-surgery, fibroblasts had completely invested the infarcted area (Figure 1A). Quantification of the Collagen1a1-GFP+ area of the left ventricular free wall showed that, compared to 2.8±0.4 % of the myocardium in Sham operated controls, fibroblasts occupied 2.5±0.3% (2 days), 10±2% (4 days), 13.5±4% (7 days) and 30±6% (21 days) in infarcts (Figure 1B). Infarcts represented 40±3 and 45±5 % of the LV circumference at 7 days and 21 days, respectively (Figure 1C and Online Figure I A). Echocardiography revealed that LV function was significantly reduced in MI groups (Online Figure II and Supplemental Video I). Trichrome staining with fluorescence imaging of adjacent sections clearly showed colocalization of Collagen and Collagen1a1-GFP signal in infarcts (Online Figure I B). Markers including alpha Smooth Muscle Actin (αSMA) Thy1, and PDGFRα have been commonly utilized to identify activated fibroblasts within the infarcted area 7, 8, 10. We found that a subset of Collagen1a1-GFP fibroblasts in the scar co-expressed αSMA. This population was significantly higher at 21 days (23%) compared to 7 days (19%) post-infarct (Figure 1D, 1E). Thy-1 was highly expressed in immune cells and endothelium, and was expressed in only a subset of fibroblasts (Online Figure III). However, virtually all Collagen1a1-GFP fibroblasts expressed the mesenchymal marker PDGFRα (Figure 1F).
Figure 1. Collagen1a1-GFP comprehensively labels infarct fibroblasts.
A. Collagen1a1-GFP expression marks activated fibroblasts successively invading the infarcted area. The epicardial surface is in the lower part of the images. B. Quantification of the percentage of the left ventricular free wall (LVFW) area occupied by Collagen1a1-GFP+ fibroblasts in Sham operated (n =4) and infarcted hearts at 2d (n=3), 4d (n=3), 7d (n=4) and 21d (n=4) post infarction. ANOVA with Bonferroni’s post-hoc test. C. Quantification of infarct size as the percentage of left ventricle (LV) circumference from trichrome stained sections (n=5 hearts per time point). Un-paired, 2-tailed Student’s t-test. D. Quantification of αSMA+ fibroblasts in Sham LV, and infarcts 7d and 21d after surgery (n=3 hearts per time point). Un-paired, 2-tailed Student’s t-test. E. Immunofluorescence images showing a subset of myofibroblasts expresses Collagen1a1-GFP and αSMA (arrowheads). F. Fibroblasts co-express Collagen1a1-GFP and PDGFRα in Sham operated (arrowheads) and infarcted hearts. DAPI, blue. Scale bars are 20μm, except in A 100μm.
No evidence for fibroblasts of hematopoietic origin within infarct tissue
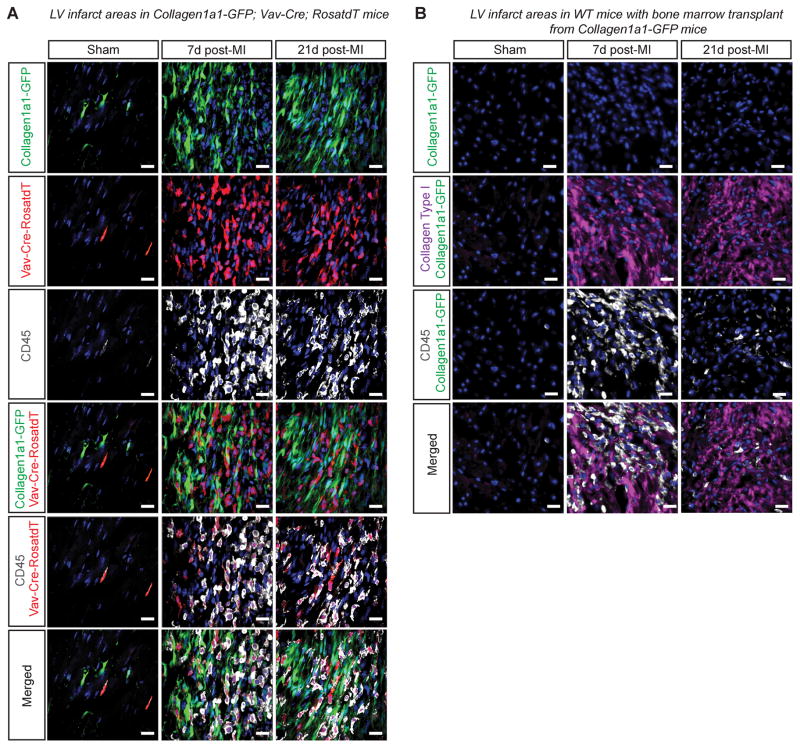
To investigate a contribution of hematopoietic lineages to fibroblasts of the infarct, we generated Vav-Cre+/−;RosatdT/+; Collagen1a1-GFP+/− mice and subjected them to left anterior descending (LAD) coronary artery ligation. As previous studies demonstrated a maximal contribution of bone marrow derived cells to infarct fibroblasts at day 7 post-infarct, we harvested and examined hearts at day 7 and day 21 post-MI9, 10. We were unable to detect any Collagen1a1-GFP+/− fibroblasts that were labeled by Vav-Cre+/−;RosatdT/+ within infarcted myocardium 7 or 21 days post-infarct. In fact, all Vav-Cre labelled cells expressed the leukocyte marker CD45 (Figure 2A).
Figure 2. Bone marrow derived cells do not give rise to infarct fibroblasts.
A. 7 and 21 days following infarction, Vav-Cre labelled cells in infarcted hearts were CD45+ hematopoietic cells and not Collagen1a1-GFP+ fibroblasts. Scale bars are 20μm. B. No Collagen1a1-GFP+ bone marrow derived cells were found in the infarct area 7 and 21 days following infarction in wild-type recipients of bone marrow from Collagen1a1-GFP mice. Collagen type I and CD45 staining indicate infarct area of left ventricle. DAPI, blue. Scale bars are 20μm.
Previous studies reporting the presence of bone-marrow derived fibroblasts in infarcts have relied on bone marrow transplantation rather than a genetic lineage tracing approach9, 10. As bone marrow transplants include both hematopoietic and bone marrow stromal cells which might contribute to infarct scar fibroblasts, we also performed bone marrow-transplant experiments. (Online Figure IV A–C). To investigate potential mobilization of cells expressing Collagen1a1-GFP from the bone marrow to the heart post-infarct, two months following recovery from transplant, mice that had received bone marrow from Collagen1a1-GFP+/− mice were subjected to permanent LAD coronary artery ligation. Histological analyses of cardiac tissue from recipient mice post-infarct demonstrated that no Collagen1a1-GFP labeled fibroblasts were detected within infarcted myocardium of chimaeric mice at either 7 or 21 days post-infarct (Figure 2B). Thus, infarct fibroblasts were not recruited from bone marrow.
Fibroblasts of hematopoietic origin (fibrocytes) locate to the epicardial surface following infarct
Intriguingly, in wild type mice that had received a bone marrow transplant from Collagen1a1-GFP+ mice, although we did not observe any Collagen1a1-GFP+ cells within the infarct scar, we did observe a small population of Collagen1a1-GFP+ cells at the surface of infarcted hearts that co-expressed CD45 (Figure 3A). Collagen1a1-GFP+/CD45+ cells were also observed at the surface of infarcted hearts of Collagen1a1-GFP+/− transgenic mice, indicating that the presence of these cells was not due to the bone marrow transplant procedure (Figure 3A). Further investigation revealed that these cells were not observed in sham-operated mice that were generated by opening of the chest cavity and pericardium, but were observed in sham-operated mice that were generated by opening of the chest cavity and pericardium followed by placement of an inert suture around the LAD (sham-plus-suture) (Figure 3B). This suggested that, in infarcted mice, recruitment of Collagen1a1-GFP+/CD45+ cells to the surface of the heart was triggered by coronary ligation surgery and/or the presence of a suture. Indeed, fibrocytes were found proximal to the suture in both sham-operated and infarcted hearts (Online Figure V). Examination of hearts from Vav-Cre+/−;RosatdT/+; Collagen1a1-GFP+/− mice revealed that the Collagen1a1-GFP+/CD45+ cells observed at the surface of infarcted hearts were Vav-Cre lineage traced and expressed the fibrocyte marker Cxcr4+, suggesting that these cells were fibrocytes, cells of hematopoietic origin expressing Cxcr4 and Collagen13 (Figure 3C). The potential functional significance or relevance of these fibrocytes remains to be addressed.
Figure 3. Fibroblasts of hematopoeitic origin (fibrocytes) locate to the epicardial surface following infarct or sham operation with suture.
A. In wild-type recipients of Collagen1a1-GFP bone marrow, Collagen1a1-GFP+ cells were spherical CD45+ fibrocytes found outside the infarcts 7d post-infarction. Collagen1a1-GFP+;CD45+ cells were also found outside infarcts of Collagen1a1-GFP mice 7d post-infarction. Similar results were obtained 21d post-infarction. B. Collagen1a1-GFP+;CD45+ cells were not found on the surface of hearts from Collagen1a1-GFP sham-operated mice 7d post-sham operation, when the sham was performed by opening the chest and pericardium of anesthetized mice without needle punch or suture around the LAD. In contrast, when the sham operation was performed in a more invasive manner, also including a needle punch and suture around the LAD, Collagen1a1-GFP+;CD45+ cells were found on the surface of hearts 7d post-operation. Similar results were obtained 21d post-sham or post-sham-with-suture surgery. Scale bars represent 50μm. C. Collagen1a1-GFP+;CD45+ cells on the surface of hearts from infarcted Collagen1a1-GFP+/−;Vav-Cre+/−;RosatdT/+ mice co-expressed Cxcr4 and were labelled by Vav-Cre. The dashed lines represent the epicardium. DAPI, blue. Scale bars are 150μm and 50μm (insets).
Fibroblasts of epicardial origin, rather than from Endothelial-to-mesenchymal transition (EndoMT), contribute to infarct formation
EndoMT is considered a potential source of fibroblasts in fibrosis, and has been suggested to contribute to post-infarct fibroblast accumulation6. To investigate this possibility, we generated Tie2-Cre+/−;RosatdT/+;Collagen1a1-GFP+/− mice that were subjected LAD coronary artery ligation. As previously reported, in Sham-operated hearts, we observed a population of Tie2-Cre lineage traced fibroblasts (Figure 4A)2, 3. Quantitative analyses demonstrated that this population represented 3.8% (n=3) of fibroblasts in the left ventricular free-wall of Sham-operated mice. Importantly, similar proportions of Tie2-Cre+/−;RosatdT+/− Collagen1a1-GFP expressing fibroblasts was observed 7 days (3.7%, n=3) and 21 days (4.3%, n=3) post-infarction (Online Table I). Collagen1a1-GFP intensity within fibroblasts was not correlated with lineage origin (Figure 4A), suggesting that the cellular context rather than developmental origin determines the level of Collagen production. Furthermore, the presence of αSMA+ Tie2-Cre+/−;RosatdT+/− fibroblasts suggested that this sub-population was able to acquire a myofibroblast phenotype (Online Figure VI A). Greater than 92% of fibroblasts of the left ventricular free wall derive from the epicardium during development2. To investigate whether fibroblasts within the infarct area are also of epicardial origin, we generated Wt1-Cre+/−;RosatdT/+;Collagen1a1-GFP+/− mice, and subjected them to permanent LAD coronary artery ligation. Histological analyses of hearts from these mice demonstrated that Collagen1a1-GFP+/− fibroblasts within the infarct area in the left ventricular free wall were almost exclusively Wt1-Cre lineage-traced, representing over 96% of Collagen1a1-GFP+ fibroblasts in the left ventricular free wall and remote myocardium of Sham-operated or 7 days or 21 days infarcted left ventricle (Figure 4B; Online Table I).
Figure 4. The great majority of fibroblasts within infarcted areas display an epicardial signature.
A. Tie2-Cre labelled fibroblasts were relatively scarce at 7d or 21d post-infarction, representing a similar fraction of fibroblasts to that observed in sham-operated hearts. B. Wt1-Cre labeled fibroblasts represent the vast majority of infarct fibroblasts at 7d or 21d post-infarction. C. Quantification of Tie2-Cre and Wt1-Cre lineage traced fibroblasts in Sham-operated and infarcted hearts. Data are presented as absolute counts and percentages. D. A negative binomial regression analysis was run to statistically assess the main effect of the lineage (Tie2-Cre, WT1-Cre), intervention (sham, 7 days post MI, 21 day post MI) and sampling area (scar vs remote) on numbers of the lineage traced fibroblasts. Only the Cre-lineage, but not intervention or area of sampling had a significant effect on number of labelled fibroblasts. DAPI, blue. Scale bars represent 20μm.
A negative binomial regression was run to statistically assess the main effect of the Cre-lineage (WT1-Cre, Tie2-Cre), intervention (Sham, 7 days post MI, 21 days post MI) and sampling area (infarct vs remote) on numbers of the lineage traced fibroblasts (Figure 4C, 4D). Only the Cre-lineage, but not intervention or area of sampling had a significant effect on number of labelled fibroblasts (Figure 4D). Hence, although absolute numbers of fibroblasts increased by several fold following MI (Online Table I), the lack of significant variation in the relative numbers of fibroblasts derived from endothelial and epicardial lineages suggested that EndoMT was not occurring to an appreciable extent in the infarcted or remote myocardium, and was therefore not significantly contributing to extracellular matrix deposition and scar formation. Rather, our observations suggest that the vast majority of infarct fibroblasts, including αSMA+ myofibroblasts (Online Figure VI B), arose from epicardial lineages.
DISCUSSION
In this study, using the Collagen1a1-GFP reporter, a robust cardiac fibroblast marker2, 12, we specifically addressed the contribution of bone marrow and blood/endothelial lineages to the infarct scar in the permanent LAD-occlusion model. By lineage tracing and bone marrow transplant experiments, we could find no evidence for contribution of hematopoietic or Endo-MT-derived fibroblasts to the infarct scar. Collagen-producing fibrocytes of hematopoietic origin were found on the epicardial surface of the infarct area, but were not observed to an appreciable extent within infarcts.
Imaging fibroblasts in infarcted heart has proven challenging, notably because of the promiscuity of commonly used markers such as αSMA or Thy-114. The Collagen1a1-GFP reporter faithfully labels cardiac fibroblasts2, 12, and has enabled us to visualize fibroblast invasion of the infarcted area. We found that in the context of infarction, the mesenchymal marker PDGFRα also labels all fibroblasts, as we and others have reported previously in healthy and hypertrophic heart 2, 12. Hence, these markers are suitable for analyzing the lineage origins of fibroblasts constituting the infarct scar, a question that has remained controversial. Perhaps not surprisingly, we observed that 19% and 23% of fibroblasts expressed αSMA 7 days and 21 days post-infarct, respectively, which is markedly more than what we previously observed in the context of pressure-overload induced cardiac hypertrophy2. The presence of more numerous αSMA-expressing cells following infarction as compared with hypertrophy has previously been observed and could be due to increased mechanical stress on fibroblasts in infarcts15.
Previous studies have reported a significant contribution of bone marrow derived fibroblasts to infarcts in mice. Notably, van Amerongen et al.10 found a strong Luciferase signal associated with infarcts of mice that had received bone marrow transplants from a Collagen1a2-Luciferase reporter line10. However, the in vivo imaging utilized did not allow for determination of whether these Collagen-producing bone marrow-derived cells were present within the infarct itself or surrounding the heart. In the event of the latter, this observation would be consistent with our own observation that Collagen1a1-GFP+ fibrocytes accumulated at the surface of the heart post-surgery. Our Vav-Cre lineage tracing studies demonstrated that these fibrocytes were of hematopoietic origin, and thus confirmed that fibrocytes observed post bone marrow transplant were derived from bone marrow hematopoietic cells and not bone marrow mesenchymal cells. However, we also observed these cells in sham-operated animals in which a suture had been placed around the LAD, suggesting that their presence at the surface of infarcted hearts was at least partially a response to the presence of the unligated suture and/or the surgical procedure directed at the epithelial/epicardial surface of the heart. Indeed, fibrocytes have been shown to be recruited in various cases of wound healing involving damage to epithelium16.
Another potential source of fibroblasts following infarction is EndoMT6. We did not observe an increase in the relative number of Tie2-Cre lineage traced fibroblasts at baseline, in Sham, or in infarcted hearts, arguing against a meaningful contribution from endothelium/endocardium to fibroblasts within the infarct.
Our data showed that greater than 96% of fibroblasts present in the infarct scar of mice subjected to permanent ligation of the LAD were of epicardial origin. It should be noted that these observations are limited to the model used in this study. Previous studies looking at ischemic reperfusion injury or non-ischemic heart failure have reported direct fibrocyte contribution to myocardial fibrosis17, 18. A previous study noted that, post-infarction, EMT of adult epicardial cells contributed to fibroblasts in regions directly underlying the epicardium5, suggesting that it is likely that most fibroblasts within the scar derive from resident fibroblasts in the vicinity of the infarct, including the border zone and sub-epicardial and sub-endocardial layers. Using Thy-1 as a fibroblast marker, another study reported an epicardial origin for the vast majority of fibroblasts of the post-infarct scar7. However, Thy-1 labels many cell types including immune cells19, lymphatic endothelium20 and pericytes21. Furthermore we previously found that approximately a third of Collagen1a1-GFP fibroblasts did not express Thy-12, that was recently also reported by another group22. A more recent study reported that infarct myofibroblasts were derived from Tcf21+ resident fibroblasts and not from monocytes/macrophages8. Hence, by combining the robust Collagen1a1-GFP fibroblast marker with epicardial, endothelial and pan-hematopoietic genetic lineage tracing and bone marrow transplants, our study clearly demonstrates that infarct fibroblasts derive from the epicardial lineage and not from bone marrow or hematopoietic lineages, nor from EndoMT. However, we did observe Cxcr4+;CD45+;Collagen1a1-GFP+ fibrocytes at the surface of hearts in which a suture was positioned around the LAD which is likely to resolve some seemingly contradictory data in the field.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Cardiac fibroblasts produce the extracellular matrix, including Collagen type I, required for scar formation following myocardial infarction.
Multiple sources of fibroblasts have been reported, including epicardium, endothelium, and bone marrow derived lineages.
Whether or not bone marrow derived lineages make a major contribution to scar formation post myocardial infarction is unclear.
What New Information Does This Article Contribute?
Using genetic lineage tracing and bone marrow transplantation, we showed that collagen-producing fibroblasts in the heart post-myocardial infarction were not derived from hematopoietic or bone-marrow derived lineages.
Fibrocytes, collagen-producing cells of hematopoietic lineage, were at the surface of the heart, near the suture placed on the coronary artery to produce myocardial infarction.
Collagen-producing fibroblasts in the infarct area were not derived from adult endothelium and were essentially of epicardial origin.
Following myocardial infarction, dead myocardium is replaced by scar tissue that ensures structural integrity of the left ventricular chamber. Fibroblasts play an essential role in scar formation, primarily by producing extracellular matrix. Studies using bone marrow transplantation or genetic lineage tracing have produced conflicting conclusions as to contribution of bone marrow derived fibroblasts to scar formation after myocardial infarction. By using both of these approached, in combination with a murine Collagen-GFP reporter line in which collagen-producing fibroblasts are faithfully labelled, we observed that collagen-producing cells of bone marrow origin located to the surface of hearts of sham-operated and infarcted mice, but were not observed within the healthy myocardium or in the infarct area. We found no evidence for adult endothelial contribution to fibroblasts, and observed that the vast majority of the fibroblasts in the infarcted area were of epicardial origin. Understanding how fibroblasts are recruited for scar formation might have implications for treatment of patients with myocardial infarction and development of therapies to modulate the fibrotic process.
Acknowledgments
We thank D. Brenner for providing the Collagen1a1-GFP reporter mice.
SOURCES OF FUNDING
TMM was supported by AHA postdoctoral fellowship 11POST7310066. PC was supported by the Marie Curie International Outgoing Fellowship within the 7th European Community Framework Program under grant agreement No 623739 - The Cardiac Code. MC received funding from the Spanish Ministry of Education, Culture and Sports, Predoctoral Fellowship program (FPU, AP2008-00508) and Travel Grant Program (TTFPU11-AP2008-00508). JB is supported by the European Commission’s Marie Sklodowska-Curie Individual Fellowship (Titin Signals, 656636). SME is funded by grants from the National Heart, Lung, and Blood Institute, and the Leducq Foundation. MP and TMM acknowledge the generosity of the Leducq Foundation (SHAPEHEART).
Nonstandard Abbreviations and Acronyms
- EMT
epithelial-to-mesenchymal transition
- endoMT
endothelial-to-mesenchymal transition
- FSP1
fibroblast-specific protein 1
- GFP
green fluorescent protein
- LAD
left anterior descending artery
- MI
Myocardial Infarction
- PDGFRα
platelet-derived growth factor receptor alpha
- αSMA
alpha smooth muscle actin
- Thy1
thymus cell antigen 1
Footnotes
DISCLOSURES
None to declare.
References
- 1.Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovascular research. 2005;65:40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 2.Moore-Morris T, Guimaraes-Camboa N, Banerjee I, Zambon AC, Kisseleva T, Velayoudon A, Stallcup WB, Gu Y, Dalton ND, Cedenilla M, Gomez-Amaro R, Zhou B, Brenner DA, Peterson KL, Chen J, Evans SM. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. The Journal of clinical investigation. 2014;124:2921–2934. doi: 10.1172/JCI74783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali SR, Ranjbarvaziri S, Talkhabi M, Zhao P, Subat A, Hojjat A, Kamran P, Muller AM, Volz KS, Tang Z, Red-Horse K, Ardehali R. Developmental heterogeneity of cardiac fibroblasts does not predict pathological proliferation and activation. Circulation research. 2014 doi: 10.1161/CIRCRESAHA.115.303794. [DOI] [PubMed] [Google Scholar]
- 4.Sun Y, Weber KT. Infarct scar: A dynamic tissue. Cardiovascular research. 2000;46:250–256. doi: 10.1016/s0008-6363(00)00032-8. [DOI] [PubMed] [Google Scholar]
- 5.Zhou B, Honor LB, He H, Ma Q, Oh JH, Butterfield C, Lin RZ, Melero-Martin JM, Dolmatova E, Duffy HS, Gise A, Zhou P, Hu YW, Wang G, Zhang B, Wang L, Hall JL, Moses MA, McGowan FX, Pu WT. Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. The Journal of clinical investigation. 2011;121:1894–1904. doi: 10.1172/JCI45529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aisagbonhi O, Rai M, Ryzhov S, Atria N, Feoktistov I, Hatzopoulos AK. Experimental myocardial infarction triggers canonical wnt signaling and endothelial-to-mesenchymal transition. Disease models & mechanisms. 2011;4:469–483. doi: 10.1242/dmm.006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruiz-Villalba A, Simon AM, Pogontke C, Castillo MI, Abizanda G, Pelacho B, Sanchez-Dominguez R, Segovia JC, Prosper F, Perez-Pomares JM. Interacting resident epicardium-derived fibroblasts and recruited bone marrow cells form myocardial infarction scar. Journal of the American College of Cardiology. 2015;65:2057–2066. doi: 10.1016/j.jacc.2015.03.520. [DOI] [PubMed] [Google Scholar]
- 8.Kanisicak O, Khalil H, Ivey MJ, Karch J, Maliken BD, Correll RN, Brody MJ, SCJL, Aronow BJ, Tallquist MD, Molkentin JD. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nature communications. 2016;7:12260. doi: 10.1038/ncomms12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mollmann H, Nef HM, Kostin S, von Kalle C, Pilz I, Weber M, Schaper J, Hamm CW, Elsasser A. Bone marrow-derived cells contribute to infarct remodelling. Cardiovascular research. 2006;71:661–671. doi: 10.1016/j.cardiores.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 10.van Amerongen MJ, Bou-Gharios G, Popa E, van Ark J, Petersen AH, van Dam GM, van Luyn MJ, Harmsen MC. Bone marrow-derived myofibroblasts contribute functionally to scar formation after myocardial infarction. The Journal of pathology. 2008;214:377–386. doi: 10.1002/path.2281. [DOI] [PubMed] [Google Scholar]
- 11.Yano T, Miura T, Ikeda Y, Matsuda E, Saito K, Miki T, Kobayashi H, Nishino Y, Ohtani S, Shimamoto K. Intracardiac fibroblasts, but not bone marrow derived cells, are the origin of myofibroblasts in myocardial infarct repair. Cardiovascular pathology: the official journal of the Society for Cardiovascular Pathology. 2005;14:241–246. doi: 10.1016/j.carpath.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Acharya A, Baek ST, Huang G, Eskiocak B, Goetsch S, Sung CY, Banfi S, Sauer MF, Olsen GS, Duffield JS, Olson EN, Tallquist MD. The bhlh transcription factor tcf21 is required for lineage-specific emt of cardiac fibroblast progenitors. Development (Cambridge, England) 2012;139:2139–2149. doi: 10.1242/dev.079970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Molecular medicine (Cambridge, Mass) 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 14.Moore-Morris T, Guimaraes-Camboa N, Yutzey KE, Puceat M, Evans SM. Cardiac fibroblasts: From development to heart failure. Journal of molecular medicine (Berlin, Germany) 2015;93:823–830. doi: 10.1007/s00109-015-1314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braitsch CM, Kanisicak O, van Berlo JH, Molkentin JD, Yutzey KE. Differential expression of embryonic epicardial progenitor markers and localization of cardiac fibrosis in adult ischemic injury and hypertensive heart disease. Journal of molecular and cellular cardiology. 2013;65:108–119. doi: 10.1016/j.yjmcc.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blakaj A, Bucala R. Fibrocytes in health and disease. Fibrogenesis & tissue repair. 2012;5:S6. doi: 10.1186/1755-1536-5-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, Crawford JR, Pilling D, Gomer RH, Trial J, Frangogiannis NG, Entman ML. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:18284–18289. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu PY, Mariani J, Finch S, McMullen JR, Sadoshima J, Marshall T, Kaye DM. Bone marrow-derived cells contribute to fibrosis in the chronically failing heart. The American journal of pathology. 2010;176:1735–1742. doi: 10.2353/ajpath.2010.090574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raff MC. Surface antigenic markers for distinguishing t and b lymphocytes in mice. Transplantation reviews. 1971;6:52–80. doi: 10.1111/j.1600-065x.1971.tb00459.x. [DOI] [PubMed] [Google Scholar]
- 20.Jurisic G, Iolyeva M, Proulx ST, Halin C, Detmar M. Thymus cell antigen 1 (thy1, cd90) is expressed by lymphatic vessels and mediates cell adhesion to lymphatic endothelium. Experimental cell research. 2010;316:2982–2992. doi: 10.1016/j.yexcr.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell stem cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’Antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA, Tallquist MD. Revisiting cardiac cellular composition. Circulation research. 2016;118:400–409. doi: 10.1161/CIRCRESAHA.115.307778. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.