Abstract
Acute myeloid leukemia (AML) is a blood cancer that is poorly responsive to conventional cytotoxic chemotherapy and a diagnosis of AML is usually fatal. More effective and better-tolerated therapies for AML are desperately needed. Activating mutations in FMS-like tyrosine kinase 3 (FLT3) are one of the most frequently observed genetic defects in AML. FLT3 inhibitors have shown impressive anti-leukemic activity in clinical trials; however, sustained remissions using these inhibitors as monotherapy have not been achieved. Our previous studies have implicated impaired glutamine metabolism in response to FLT3 inhibitors as a dominant factor causing AML cell death. In this study, we have employed metabolic flux analysis to examine the effects of FLT3 inhibition on glutamine utilization in FLT3-mutated AML cells using stable isotope tracers. We found that the FLT3 inhibitor AC220 inhibited glutamine flux into the antioxidant factor glutathione profoundly due to defective glutamine import. We also found that the glutaminase inhibitor CB-839 similarly impaired glutathione production by effectively blocking flux of glutamine into glutamate. Moreover, the combination of AC220 with CB-839 synergized to deplete glutathione, induce mitochondrial reactive oxygen species, and cause loss of viability through apoptotic cell death. In vivo, glutaminase inhibition with CB-839 facilitated leukemic cell elimination by AC220 and improved survival significantly in a patient-derived xenograft AML mouse model. Therefore, targeting glutaminase in combination with FLT3 may represent an effective therapeutic strategy for improving treatment of FLT3-mutated AML.
Acute myeloid leukemia (AML) is a hematological cancer that is characterized by the aberrant growth of myeloid blast cells. AML is the most common adult leukemia and accounts for approximately 20% of childhood leukemias. With standard cytotoxic chemotherapy regimens, only 40% of AML patients <60 years of age will survive more than 5 years and outcomes are far worse in older patients who are unfit for intensive chemotherapy (median survival, <1 year) [1, 2]. Significant improvement of AML patient outcomes may require the development of targeted therapies that are both more effective and less toxic than current regimens. Activating mutations of FMS-like tyrosine kinase 3 (FLT3) are found in up to one-third of AML patients (30–35% of adult patients and ~15% of pediatric patients [3]) and confer a poor prognosis, especially with concomitant NPM1 and DNMT3A mutations [4]. Tyrosine kinase inhibitors targeting FLT3 have been shown to be effective in clinical trials for FLT3-mutated AML; unfortunately, the development of resistance inevitably leads to disease relapse [1, 3, 5]. Therefore, combination therapies incorporating other targeted drugs in addition to FLT3 inhibitors may be necessary to eradicate AML cells more effectively.
It has long been known that cancer cells are characterized by an increased rate of production of reactive oxygen species (ROS) relative to normal cells and an altered redox state [6]. Glutathione represents the major detoxification system for ROS in cells and targeting it as an anticancer strategy has been explored for several tumors, including AML [7]. AML cells have been found to have aberrant glutathione metabolism and are sensitive to therapies that further disrupt the glutathione pathway and it has been proposed that AML cells are “addicted” to glutathione [8]. Our previous work has shown that FLT3 inhibitors compromise glutamine metabolism and impair production of glutathione, leading to severe mitochondrial oxidative stress and apoptotic cell death [9]. In this study, we further investigated the impact of FLT3 inhibition on glutamine and glutathione metabolism and explored the therapeutic efficacy of combining a FLT3 inhibitor with a drug that further impairs metabolism of glutamine/glutathione.
Methods
Cell lines
The AML cell lines Molm13 and MV4-11, which have FLT internal tandem duplication mutations (FLT3-ITD+), EOL-1 (FLT3WT but FLT3-dependent [10]), and OCI-AML3 (FLT3WT) were obtained from D. Graham (Emory University, Atlanta, GA). All cell lines were NPMWT and DNMT3AWT except for OCI-AML3, which has an NPM1 mutation [11]. Cells were cultured in RPMI 1640 medium/10% FBS. Cell lines were authenticated by short tandem repeat examination and tested negative for mycoplasma using the e-Myco plus PCR Detection Kit (iNtRON).
Pharmacologic agents
AC220 (quizartinib) was purchased from Selleck Chemicals and, for in vivo experiments, was synthesized by the CU Medicinal Chemistry Core, as described previously [12]. CB-839 was purchased from MedChem Express and, for in vivo experiments, was obtained from Calithera Biosciences.
Cell viability assays
Cells were seeded at 0.5–1.0 × 105/mL in triplicate wells of 48-well tissue culture plates. Where indicated, the cells were treated with drug for a period of 48–72 hours. After treatment, a sample of cells from each well was stained with propidium iodide (PI; 10 µg/mL) and viable cells (PI−) were counted with a flow cytometer (Guava easyCyte 8HT). Alternatively, cells were stained using 7-aminoactinomycin D/anti-Annexin V (Nexin reagent, Millipore EMD) to detect apoptotic cells.
ROS and glutathione measurements
Cells (1 × 105) were treated with drug as indicated for 20–24 hours, washed in PBS, resuspended in 150 µL of 10 µmol/L MitoPY1 (Tocris Bioscience) in serum-free medium and incubated at 37°C for 60–90 min. Cells were washed in cold fluorescence-activated cell sorting (FACS) buffer, resuspended in FACS buffer, and immediately analyzed by flow cytometry. To measure glutathione levels, the GSH-Glo Assay (Promega) was utilized according to the manufacturer’s instructions.
Metabolic tracing and extracellular flux experiments
Cells were seeded at 3 × 105/mL (replicates of three) and treated with vehicle (dimethylsulfoxide) or drug (AC220 or CB-839) for 8 hours, followed by incubation in regular RPMI 1640 (for unlabeled samples) or glutamine-free RPMI 1640 supplemented with 13C5,15N2-labeled L-glutamine (Cambridge Isotope Laboratories) for up to 12 hours in the presence of vehicle or drug. Flash-frozen cell pellets (~5 × 105 cells) or supernatants (50 µL) were extracted and subjected to analysis by ultra-high-performance liquid chromatography and mass spectrometry as described previously [13]. Metabolite assignments, isotopologue distributions, and correction for expected natural abundances of 13C and 15N isotopes were performed using MAVEN (Princeton University, Princeton, NJ) and validated manually. Extracellular flux analysis of oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) was performed using the Seahorse XF24 Analyzer as described previously [12] except that the assay medium contained 2 g/L glucose and 2 mmol/L glutamine.
Mouse experiments
NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice were obtained from The Jackson Laboratory and bred in-house. The patient sample for xenograft (obtained from D. Pollyea, University of Colorado, Aurora, CO) came from a 54-year-old female with AML expressing FLT3-ITD and NPM1 mutations. The leukemia was engrafted into female NSG mice as described previously [9]. Treatment was started when peripheral blast count was between 19% and 37% (mean 28% for all groups), which occurred at 4 weeks after transplantation for all mice. AC220, prepared as described previously [12], was delivered once daily orally at 5 mg/kg, CB-839 (in 25% HP-β − ΧΔ/10 mmol/L citrate, pH 2.0) twice daily orally at 200 mg/kg, and the combination at the same doses in the same manner. For monitoring leukemic burden in mice, blood was stained with anti-human CD45-FITC and HLA-ABC-PE-Cy7 and analyzed by flow cytometry as described previously [9]. Mice were sacrificed when leukemic burden in peripheral blood was ≥80% or upon any signs of distress. Complete blood cell counts and serum chemistry analysis were performed as described previously [12]. The institutional animal care and use committee at the University of Colorado approved all mouse experiments under protocol #41414(05)1E.
Statistics
All data are expressed as the mean ± SEM. Comparisons between two values were performed by Mann–Whitney U test unless otherwise indicated. Significance was defined at *p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.001 (Figs. 1–3). Combination indices (CI values) were calculated using the median effect principle and the combination index–isobologram theorem (CompuSyn).
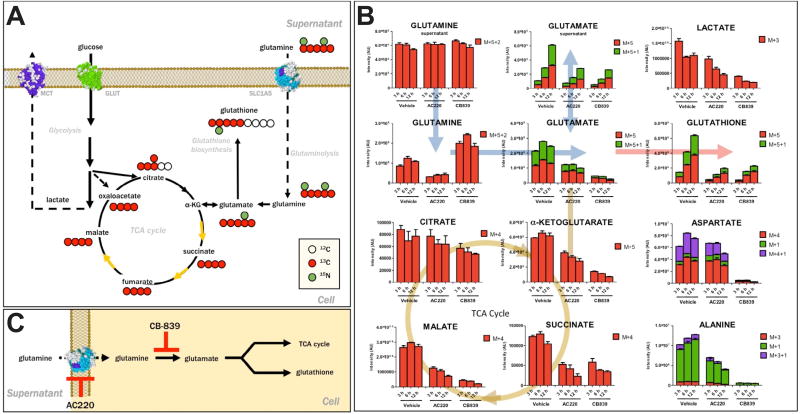
Figure 1.
FLT3 inhibitor AC220 impairs glutamine flux comparable to the glutaminase inhibitor CB-839 in AML cells. (A) 13C 15N-Glutamine tracing allows determining the fate of labeled carbon and nitrogen atoms derived from glutamine into various downstream metabolites. (B) Glutamine flux analysis of Molm13 AML cells treated with vehicle (dimethylsulfoxide), AC220 (2 nmol/L), or CB-839 (500 nmol/L). Cells were pretreated for 8 hours followed by 13C,15N-glutamine labeling for 3, 6, and 12 hours, as indicated, followed by ultra-high-performance liquid chromatography and mass spectrometry analysis. Levels of 13C- and 15N-containing isotopologues (based on signal intensity) are shown. (C) Diagram depicting mechanism of inhibition of glutamine metabolism by AC220 and CB-839.
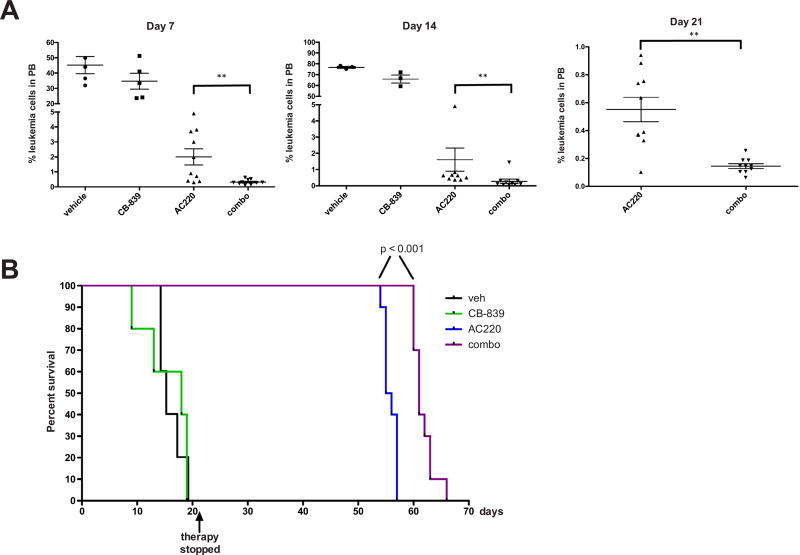
Figure 3.
CB-839 cooperates with AC220 in eliminating FLT3-mutated AML cells in vivo and improves survival. NSG mice were engrafted with primary FLT3-ITD+ leukemia and groups of mice were treated with vehicle (n = 5), CB-839 (n = 5; 200 mg/kg twice daily), AC220 (n = 10; 5 mg/kg once daily), or CB-839 and AC220 in combination (n = 10) for 21 days. (A) Leukemic burden was monitored weekly by peripheral blood (PB) draws and quantitation of leukemic cells (human CD45+, HLA-ABC+ cells) by flow cytometry on the indicated days is shown. (B) Kaplan–Meier curves showing survival of mice receiving the indicated therapy. Statistical significance was determined using the log–rank (Mantel–Cox) test.
Results and discussion
Given our previous results showing that FLT3 inhibition severely diminishes glutamine and glutamine-derived metabolites such as glutathione, we wanted to gain a further understanding of how FLT3 inhibitors influence glutamine metabolism in AML cells. To this end, we used metabolic flux analysis using 13C,15N-labeled glutamine [14] (Fig. 1A) in Molm13 AML(FLT3-ITD+) cells. These cells were treated with vehicle, the FLT3 inhibitor AC220 (quizartinib), or the known inhibitor of glutamine metabolism CB-839, which inhibits the enzyme glutaminase. As shown in Fig. 1B, AC220-treated cells displayed lower levels of intracellular labeled glutamine, most consistent with impaired transport into the cell. Unsurprisingly, flux of glutamine into glutamate and downstream metabolites, α-ketoglutarate (feeds into TCA cycle), glutathione (important for antioxidant metabolism), and alanine were also impaired. In contrast, CB-839 caused an accumulation of labeled glutamine coupled with depletion of labeled glutamate, consistent with blocked glutaminase activity that converts glutamine to glutamate and impaired flux into downstream metabolites, including glutathione. Although cysteine levels are usually rate limiting for glutathione synthesis, cysteine levels were not significantly changed (Supplementary Table E1, online only, available at www.exphem.org), so glutamate depletion appears sufficient to prevent glutathione production. Overall levels of glutamate and glutathione (as measured in unlabeled cells) were also found to be decreased in both AC220- and CB-839-treated cells (Supplementary Figure E1A and Supplementary Table E1, online only, available at www.exphem.org). Therefore, although AC220 and CB-839 impair glutamine metabolism through distinct mechanisms (Fig. 1C), both drugs similarly inhibit glutathione production. Moreover, in cells treated with both AC220 and CB-839, there is a combinatorial effect of these drugs in blocking glutamine flux into glutathione (Supplementary Figure E1B, online only, available at www.exphem.org). Although the drug combination modestly decreased the OCR (Supplementary Figure E1C, online only, available at www.exphem.org), indicating reduced mitochondrial respiration, the ECAR was increased (Supplementary Figure E1D, online only, available at www.exphem.org), indicating increased use of glycolysis, which is likely to maintain energy production.
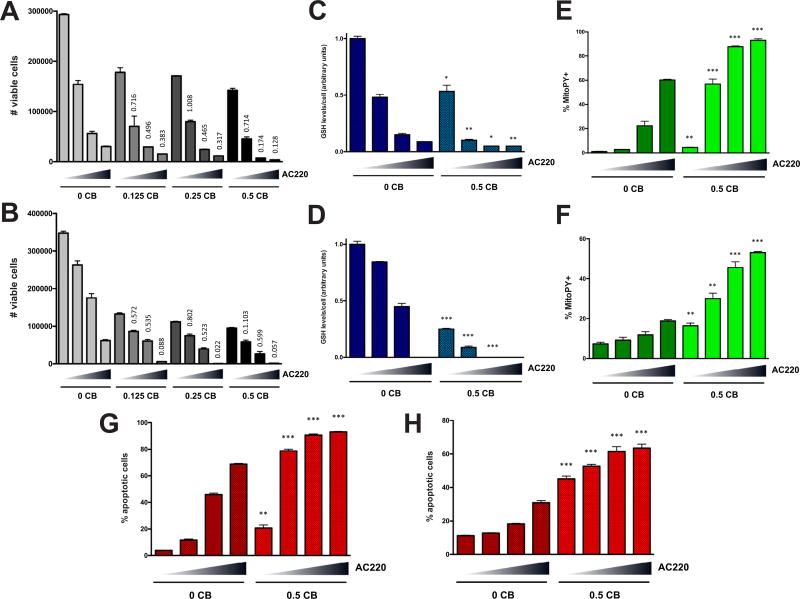
Our previous results indicate that glutathione is critical for reducing mitochondrial ROS and maintaining survival of AML cells [9]. Therefore, we tested whether AC220 and CB-839 have a combinatorial effect on AML cell viability. Indeed, AC220 showed synergistic cell killing in combination with CB-839 in Molm13 and EOL-1 cells (Figs. 2A and 2B; CI values <1 indicate synergy). Although AC220 was sufficient to cause decreases in glutathione in these cell lines, the decreases were more severe in combination with CB-839 (Figs. 2C and 2D). Consistent with effects on glutathione, AC220 caused dramatic accumulation of mitochondrial ROS (as measured by staining with MitoPY1) that was exacerbated by CB-839 (Figs. 2E and 2F) and this mirrored the amount of apoptosis that was induced (Figs. 2G and 2H). Similar effects of AC220/CB-839 on cell viability, glutathione levels, and mitochondrial ROS were observed in another FLT-3 mutated cell line, MV4-11, at high doses (Supplementary Figures E2A–E2C, online only, available at www.exphem.org), whereas no major synergistic effects on cell viability were seen in the FLT3WT cell line OCI-AML3, which is largely unresponsive to FLT3 inhibition (Supplementary Figure E2D, online only, available at www.exphem.org).
Figure 2.
AC220 and CB-839 have combinatorial effects on cell viability, glutathione, mitochondrial ROS, and apoptosis in AML cells. Molm13 (A) or EOL-1 (B) cells were treated with CB-839 (µmol/L) and AC220 alone or in combination as indicated (triangle: 0, 1, 2, and 3 nmol/L AC220 for Molm13; 0, 0.25. 0.5, and 1 nmol/L for EOL-1) for 48 hours and viable cells (based on PI exclusion) were counted by flow cytometry. Molm13 (C) or EOL-1 (D) cells were treated with CB-839 and AC220 as in (A) and (B) for 20 hours, and glutathione (GSH) levels were measured. Molm13 (E) or EOL-1 (F) cells were treated as in (A) and (B) for 20 hours, and mitochondrial ROS levels were measured using the fluorogenic dye MitoPY1 (for mitochondrial peroxides). Molm13 (G) or EOL-1 (H) cells were treated as in (A) and (B) for 48 hours, and apoptosis was measured by Annexin V staining and flow cytometry. For (A) and (B), CI values are shown above columns for each drug combination (CI values >1 indicate synergism). For (C–H), asterisks indicate statistical significance (unpaired t test, two-tailed) for comparisons of vehicle- and CB-839-treated cells under the same AC220 treatment conditions.
We next wanted to test the therapeutic efficacy of AC220 combined with CB-839 in vivo. For this purpose, we used a patient-derived xenograft model of FLT3-mutated AML. Primary leukemic cells from a patient with FLT3-ITD+ AML were engrafted into NSG mice and, after mean leukemic burden in the peripheral blood reached ~30% (4 weeks after transplantation; Supplementary Figure E3A, online only, available at www.exphem.org), therapy was initiated using vehicle, CB-839, AC220, or CB-839 and AC220 in combination. Although CB-839 was ineffective by itself, leukemia cell numbers in the peripheral blood were reduced in the AC220 group and even further reduced in the combination group after only 4 days of therapy (Supplementary Figure E3A, online only, available at www.exphem.org). Although leukemia cell numbers continued to decline in the AC220 group on days 7, 14, and 21 (after which therapy was stopped), they declined even more significantly in the combination group (Fig. 3A). Supplementary Figure E3B (online only, available at www.exphem.org) shows that, after cessation of therapy, leukemic burden remained lower in the combination group compared with the AC220 group throughout the course of the experiment, demonstrating that a deeper remission was achieved. Moreover, the combination therapy extended survival of mice by ~1 week compared with AC220 therapy alone (Fig. 3B). It should be noted that mice receiving combination therapy exhibited no evident changes in appearance, or behavior and there were no significant changes in weight after 2 weeks of therapy (Supplementary Figure E3C, online only, available at www.exphem.org). Although complete blood cell counts showed a small but significant reduction in red blood cell numbers, no other hematopoietic changes were observed (Supplementary Figure E3D, online only, available at www.exphem.org). Moreover, markers of liver and kidney toxicity were not elevated (Supplementary Figure E3E, online only, available at www.exphem.org). Together, these data demonstrate the potential of using CB-839 as an adjuvant to FLT3 inhibitor therapy for the improved treatment of FLT3-mutated AML with minimal toxicity.
Several recent studies have shown that targeting glutamine uptake or metabolism can have antileukemic activity in AML [15–18]. Indeed, our own previous work suggests that inhibition of glutamine and redox metabolism by FLT3 inhibitors is causative in AML cell death [9]. Most studies, however, have not focused on the role of glutamine in generating glutathione or its importance for the maintenance of the redox state. Here, we demonstrate that inhibition of glutaminase using CB-839 decreases glutathione levels and has a combinatorial effect when used together with AC220, leading to severe glutathione depletion. Moreover, we show that CB-839 sensitizes FLT3-mutated AML cells to AC220-induced mitochondrial ROS accretion and subsequent apoptosis. In vivo, whereas CB-839 was insufficient to have a major antileukemic effect by itself, it clearly cooperated with AC220 in eliminating leukemic cells in a mouse model of AML and improved survival significantly. Therefore, glutaminase inhibition in combination with FLT3 inhibition may represent a very effective and less toxic therapeutic strategy for the treatment of FLT3-mutated AML compared with conventional cytotoxic chemotherapy.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Cancer Institute of the National Institutes of Health (K22-CA133182) and the Cancer League of Colorado to MAG and from the Leukemia & Lymphoma Society (6160-12) and the V Foundation (T2016-012) to JD.
Footnotes
Supplementary data related to this article can be found online at https://doi.org/10.1016/j.exphem.2017.09.007.
References
- 1.Bose P, Vachhani P, Cortes JE. Treatment of relapsed/refractory acute myeloid leukemia. Curr Treat Options Oncol. 2017;18:17. doi: 10.1007/s11864-017-0456-2. [DOI] [PubMed] [Google Scholar]
- 2.De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6:e441. doi: 10.1038/bcj.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Small D. FLT3 mutations: biology and treatment. Hematology Am Soc Hematol Educ Program. 2006:178–184. doi: 10.1182/asheducation-2006.1.178. [DOI] [PubMed] [Google Scholar]
- 4.Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374:2209–2221. doi: 10.1056/NEJMoa1516192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pratz KW, Luger SM. Will FLT3 inhibitors fulfill their promise in acute meyloid leukemia? Curr Opin Hematol. 2014;21:72–78. doi: 10.1097/MOH.0000000000000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glasauer A, Chandel NS. Targeting antioxidants for cancer therapy. Biochem Pharmacol. 2014;92:90–101. doi: 10.1016/j.bcp.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Wondrak GT. Redox-directed cancer therapeutics: molecular mechanisms and opportunities. Antioxid Redox Signal. 2009;11:3013–3069. doi: 10.1089/ars.2009.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pei S, Minhajuddin M, Callahan KP, et al. Targeting aberrant glutathione metabolism to eradicate human acute myelogenous leukemia cells. J Biol Chem. 2013;288:33542–33558. doi: 10.1074/jbc.M113.511170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gregory MA, D’Alessandro A, Alvarez-Calderon F, et al. ATM/G6PDdriven redox metabolism promotes FLT3 inhibitor resistance in acute myeloid leukemia. Proc Natl Acad Sci USA. 2016;113:E6669–E6678. doi: 10.1073/pnas.1603876113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Auclair D, Miller D, Yatsula V, et al. Antitumor activity of sorafenib in FLT3-driven leukemic cells. Leukemia. 2007;21:439–445. doi: 10.1038/sj.leu.2404508. [DOI] [PubMed] [Google Scholar]
- 11.Quentmeier H, Martelli MP, Dirks WG, et al. Cell line OCI/AML3 bears exon-12 NPM gene mutation-A and cytoplasmic expression of nucleophosmin. Leukemia. 2005;19:1760–1767. doi: 10.1038/sj.leu.2403899. [DOI] [PubMed] [Google Scholar]
- 12.Alvarez-Calderon F, Gregory MA, Pham-Danis C, et al. Tyrosine kinase inhibition in leukemia induces an altered metabolic state sensitive to mitochondrial perturbations. Clin Cancer Res. 2015;21:1360–1372. doi: 10.1158/1078-0432.CCR-14-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nemkov T, Hansen KC, D’Alessandro A. A three-minute method for high-throughput quantitative metabolomics and quantitative tracing experiments of central carbon and nitrogen pathways. Rapid Commun Mass Spectrom. 2017;31:663–673. doi: 10.1002/rcm.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Alessandro A, Amelio I, Berkers CR, et al. Metabolic effect of TAp63alpha: enhanced glycolysis and pentose phosphate pathway, resulting in increased antioxidant defense. Oncotarget. 2014;5:7722–7733. doi: 10.18632/oncotarget.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willems L, Jacque N, Jacquel A, et al. Inhibiting glutamine uptake represents an attractive new strategy for treating acute myeloid leukemia. Blood. 2013;122:3521–3532. doi: 10.1182/blood-2013-03-493163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacque N, Ronchetti AM, Larrue C, et al. Targeting glutaminolysis has antileukemic activity in acute myeloid leukemia and synergizes with BCL-2 inhibition. Blood. 2015;126:1346–1356. doi: 10.1182/blood-2015-01-621870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matre P, Velez J, Jacamo R, et al. Inhibiting glutaminase in acute myeloid leukemia: metabolic dependency of selected AML subtypes. Oncotarget. 2016;7:79722–79735. doi: 10.18632/oncotarget.12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goto M, Miwa H, Shikami M, et al. Importance of glutamine metabolism in leukemia cells by energy production through TCA cycle and by redox homeostasis. Cancer Invest. 2014;32:241–247. doi: 10.3109/07357907.2014.907419. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.