Abstract
The error-related negativity (ERN) is a negative deflection in the event-related potential waveform that occurs when an individual makes a mistake, and an increased ERN has been proposed as a biomarker for anxiety. However, previous work suggests that fearful children are characterized by a smaller ERN. We have proposed that this may reflect the changing phenomenology of anxiety across development. In the current study, we investigate this possibility using a longitudinal within- subject design. In 271 children, we completed observational measures of fear when the children were 3 years old, and then measured the ERN when the children were 6 and 9 years-old. Fearful children were characterized by a decreased ERN when they were 6-years-old; by age 9, the same children who were fearful at age 3 had increased ERNs—a pattern that closely resembles that of anxious adolescents and adults.
Introduction
An accumulating body of evidence suggests that anxiety begins early in the course of development and often results in chronic impairment. Infants who react negatively to novel stimuli tend to become behaviorally inhibited toddlers (Nathan A. Fox et al., 2005; Nathan A Fox, Snidman, Haas, Degnan, & Kagan, 2015), who, in turn, tend to become anxious children, adolescents, and adults (Chronis-Tuscano et al., 2009; Clauss & Blackford, 2012; McDermott et al., 2009; Rosenbaum et al., 2000).
While the tendency towards anxiety remains relatively stable across development, its expression seems to mirror important developmental transitions. For example, fearfulness in early childhood becomes increasingly associated with inhibition in the presence of others as children age and social interactions become more relevant (Crozier & Burnham, 1990; Jones, Cheek, & Briggs, 2013). As children grow older, anxiety tends to transition from fear of external threat (e.g., the dark, animals, insects, weather) to self-conscious shyness and worry about behavioral competence and social evaluation (i.e., internal threat) (Copeland, Angold, Shanahan, & Costello, 2014; Crozier & Burnham, 1990; Gullone, 2000; Susan H. Spence, Rapee, McDonald, & Ingram, 2001; Vasey, Crnic, & Carter, 1994). This transition between fear of concrete, external stimuli to more abstract, anticipatory, or imaginary stimuli that occurs from infancy to childhood has been proposed to be linked to cognitive development (Gullone, 2000), as well as changing developmental tasks and expectations (e.g., increasing independence from the caregiver and need for self-monitoring). This transition may similarly reflect the growing appreciation for how one’s actions are evaluated by others.
A growing body of research has focused on the error-related negativity (i.e., the ERN) as a biomarker of anxiety. The ERN is a sharp, negative deflection in the event-related potential (ERP) waveform that occurs shortly after individuals make mistakes (Falkenstein, Hohnsbein, Hoormann, & Blanke, 1991; Gehring, Goss, Coles, Meyer, & Donchin, 1993) and is generated in the anterior cingulate cortex (ACC) - a region of the brain that integrates information about pain, threat, and punishment to modify behavioral output (Shackman et al., 2011). An increased ERN has been found in individuals with anxiety-related traits (e.g., worry, punishment sensitivity) and symptoms in over 45 studies to date (see meta-analysis: Jason S Moser, Moran, Schroder, Donnellan, & Yeung, 2013), and we have recently found that an increased ERN predicts the onset of new anxiety disorders in children—even when controlling for baseline anxiety symptoms and other risk factors (Meyer, Hajcak, Torpey-Newman, Kujawa, & Klein, 2015; Meyer, Nelson, Perlman, Klein, & Kotov, Under Review). Considering that the ERN consistently relates to anxiety and similar traits, and has good psychometric properties across development (Meyer, Bress, & Proudfit, 2014), it is an important neural measure for studying the development of anxious processes.
Although the ERN has been linked to “anxiety” in a broad sense, we believe an increased ERN in anxious individuals indexes a particular phenotypic expression of anxiety. Specifically, we proposed that an increased ERN relates to concerns over making mistakes—that is, phenotypic traits that would relate to increased behavioral monitoring. For example, an enhanced ERN is not found in all anxiety and related disorders – adults with phobias and PTSD are not characterized by a potentiated ERN (Jason S. Moser, Hajcak, & Simons, 2005; Rabinak et al., 2013), whereas individuals with GAD and OCD are (Riesel, Endrass, Auerbach, & Kathmann, 2015; Weinberg, Kotov, & Proudfit, 2015). Indeed, some work suggests that the ERN relates to a transdiagnostic phenotype related to anxious apprehension (i.e., cognitive symptoms of anxiety) as opposed to anxious arousal (i.e., acute fear; Jason S. Moser, Moran, & Jendrusina, 2012; Jason S Moser et al., 2013). We recently found that an increased ERN relates to checking behavior when controlling for all other anxiety symptom domains (Weinberg et al., 2016) – a symptom that reflects the tendency to engage in self-monitoring of one’s own behavior (e.g., repeatedly checking to make sure one completed their homework).
Consistent with this conceptualization of the ERN as an index of performance monitoring, we view errors as a particular type of threat (Hajcak, 2012). Errors are unpredictable, potentially threatening, internally-generated events that provoke an acute defensive response. For example, after error commission, individuals experience: skin conductance and heart rate deceleration (Hajcak, McDonald, & Simons, 2003), potentiated startle reflex (Hajcak & Foti, 2008; Riesel, Weinberg, Moran, & Hajcak, 2013), amygdala activation (Pourtois et al., 2010), corrugator “frowning” muscle contraction (Lindström, Mattsson-Mårn, Golkar, & Olsson, 2013), pupil dilation (Critchley, Tang, Glaser, Butterworth, & Dolan, 2005), as well as the subjective feeling of distress (Spunt, Lieberman, Cohen, & Eisenberger, 2012). Unlike many other aversive stimuli, errors are self-generated. Therefore, we view variability in the ERN as reflecting the degree to which internally generated threats (i.e., errors) are monitored due to their salience.
Indeed, within-subject experiments that manipulate the salience of errors find significant modulation of the ERN. For example, the ERN is increased when errors are punished, more valuable, or evaluated by other people (Hajcak, Moser, Yeung, & Simons, 2005; Riesel, Weinberg, Endrass, Kathmann, & Hajcak, 2012). Additionally, critical parenting, which may increase the aversiveness of errors for children, is related to an increased ERN in offspring (Brooker & Buss, 2014; Meyer, Proudfit, et al., 2014). Based on this evidence, we have posited that an increased ERN in anxious individuals may reflect differences in reactivity to internally generated potential threat.
In line with this conceptualization, the magnitude of the ERN has also been related to other developmental risk factors for anxiety disorders. For example, the ERN is increased in healthy first degree relatives of individuals with OCD (Carrasco et al., 2013; Riesel et al., 2015), and can predict the onset of new anxiety disorders (Meyer et al., 2015). Moreover, a previous study found that children high in behavioral inhibition (BI) assessed early in childhood were characterized by an increased ERN magnitude in adolescence (McDermott et al., 2009). Another study similarly found that early childhood BI (assessed at 24 and 36 months of age) relates to an enhanced ERN later in childhood (Lahat et al., 2014).
While the majority of studies have focused on the relationship between the ERN and anxiety and risk for anxiety in adults and adolescents, few studies have examined this relationship in young children. However, among 6-year-old children, Torpey et al. (2013) found that temperamental fear related to a smaller ERN. Along similar lines, when we examined the impact of age on the relationship between the ERN and anxiety symptoms in a sample of children and adolescents, a larger ERN was related to increased anxiety among older children; however, among younger children, the relationship was in the opposite direction - a smaller ERN related to increased anxiety symptoms (Meyer, Weinberg, Klein, & Hajcak, 2012). Results suggested that the relationship between anxiety symptoms and the ERN varied as a function of age, and were consistent with the data from Torpey et al. (2013) who found temperamental fearfulness related to a smaller ERN in 6-year-olds.
These results were interpreted as tracking the changing phenomenology of anxiety across development. It is possible that anxious younger children are more focused on external threat during the ERN assessment (e.g., the darkness of the room, the experimenter, being separated from their parent, etc.), whereas anxious older children may begin to monitor more for internal signals of threat (e.g., performing well on the task, evaluation of performance by the experimenter, etc.). Our previous study examined the between-subject relationship between age and the anxiety/ERN relationship. A prediction that follows from this study is: anxious children characterized by a decreased ERN in early childhood should undergo developmental changes so that by later childhood, those same children should be characterized by an increased ERN. In other words, we should observe this same trajectory within-subjects. To test this hypothesis in the current study, we examined the ERN at age 9 among 271 children previously reported in Torpey et al. (2013). We hypothesized that the same temperamentally fearful children who were characterized by a decreased ERN at Age 6 would now be characterized by an increased ERN at the Age 9 assessment.
Method
Participants
The current study included a subset of participants (N = 271) from a larger longitudinal study, who had observational measures of temperamental fear from the Age 3 laboratory assessment, as well as ERP data from the Age 6 and Age 9 assessments. Participants were originally recruited through a commercial mailing list. Eligible families had a child without a significant medical condition or developmental disability, and at least one English-speaking biological parent. Of families who were eligible, 66.4% entered the study. Families who agreed and declined participation did not differ significantly on child sex, race, ethnicity, parental marital status, education, or employment status. Census data suggest the sample is reasonably representative of the surrounding county (Bufferd, Dougherty, Carlson, & Klein, 2011; Olino, Klein, Dyson, Rose, & Durbin, 2010). Overall, 87 children were excluded based on EEG data from the age 6 assessment (69 due to committing 5 or fewer errors, 16 due to having 5 or fewer artifact-free error trials, 1 due to technical error, and 1 due to having an ERP value more than 3 standard deviations from the overall mean. In addition, 5 children were excluded from the current analysis due to poor quality EEG recordings at age 9, and 10 were excluded for poor performance (i.e., accuracy less than 55%) during the age 9 flanker task. Children who were excluded from the study did not differ from the rest of the sample on demographic variables or any of the main study variables, all ps > .10. Of the 271 children included in the current study, 124 were female. Average child age at the initial assessment was 3.56, SD = 0.26, at the second assessment was 6.11, SD = 0.44, and at the third assessment was 9.21, SD = .43. Most children were Caucasian (94.5%). The study was approved by the Stony Brook Institutional Review Board and completed with consent of parents and assent of participants.
Age 3 child temperamental fear
At age 3, children participated in 12 age-appropriate episodes from the Laboratory Temperament Assessment Battery (Lab-TAB; Goldsmith, Reilly, Lemery, Longley, & Prescott, 1995) that were designed to elicit a range of temperament-relevant behaviors and emotions (described in detail in Olino et al., 2010). During each video-taped episode, instances of facial, vocal, and bodily fear were rated on a four-point intensity scale and then summed across each episode. These ratings were then averaged across the 12 episodes to yield a composite score for fear (α = .63). Interrater reliability of the fear composite, assessed using the intraclass correlation coefficient (ICC), was .64 (N = 35).
Error-related brain activity
At age 6, children were administered a developmentally appropriate Go/No-Go task with Presentation software (Neurobehavioral Systems, Inc.) to measure the ERN (previously described: Meyer et al., 2013; Torpey, Hajcak, Kim, Kujawa, & Klein, 2011). The stimuli were green equilateral triangles presented in one of four different orientations for 1,200 ms in the middle of the monitor. On 60% of the trials, triangles were vertically aligned and pointed up, 20% were vertically aligned and pointed down, 10% were tilted slightly to the left, and 10% were tilted slightly to the right. Children were told to respond to upward-pointing triangles by pressing a button, and to withhold a response to all other triangles. Following the presentation of the triangle and prior to the start of the next trial, a small gray fixation cross was displayed in the middle of the monitor for a random interval that ranged from 300 to 800 ms. Children completed four blocks of 60 trials each. Participants were told that they earned points which could be exchanged for money for every correct button press and for every correct withholding of a button press. Children were instructed that they could win up to $5.00 and speed of the response was also emphasized. At the end of each block, the number of points won by the participant was displayed in white numbers. Following completion of the task, all children were told they won $5.00.
At age 9, children completed an arrow version of the flanker task (Eriksen & Eriksen, 1974) that was administered using Presentation software (Neurobehavioral Systems, Inc., Albancy, CA, USA) to control the presentation and timing of all stimuli. Each stimulus was displayed on a 19 in (48.3 cm) monitor. On each trial, five horizontally aligned arrowheads were presented for 200 ms, followed by an ITI that varied randomly between 2300 to 2800 ms. Half of the trials were compatible (“<<<<<” or “>>>>>”) and half were incompatible (“<<><<” or “>><>>”); the order of trials was randomly determined. Participants were told to press the right mouse button if the center arrow was facing to the right and to press the left mouse button if the center arrow was facing to the left. After a practice block of 30 trials, participants completed 11 blocks of 30 trials (330 trials total) with each block initiated by the participant. Participants received feedback based on their performance at the end of each block. If performance was 75% correct or lower, the message “Please try to be more accurate” was displayed; if performance was above 90% correct, the message “Please try to respond faster” was displayed; otherwise the message “You’re doing a great job” was displayed.
The Active Two system (Biosemi, Amersterdam, Netherlands) was used to acquire EEG data and 32 Ag/AgCI-tipped electrodes (34 at the Age 9 assessment) were used with a small amount of electrolyte gel (Signa Gel; Bio-Medical Instruments Inc., Warren, Michigan) at each electrode position. Electrooculogram (EOG) generated from eye movements and eyeblinks was recorded with four facial electrodes; horizontal eye movements were measured by two electrodes located approximately 1 cm outside the outer edge of the right and left eyes. Vertical eye movements and blinks were measured by two electrodes placed approximately 1 cm above and below the right eye. The EEG signal was pre-amplified at the electrode to improve the signal-to-noise ratio and amplified with a gain of one by a BioSemi Active Two system (BioSemi, Amsterdam). All data were sampled at 512 Hz at the age 6 assessment and 1024 Hz at the age 9 assessment. The ground electrode during acquisition was formed by the common mode sense (CMS) active electrode and the driven right leg passive (DRL) electrode.
Data was processed offline with Brain Vision Analyzer (Brain Products, Gilching, Germany). EEG data was re-referenced to the nose at the age 6 assessment, and to the average of the left and right mastoids at the age 9 assessment, and high and low pass filtered at 0.1 Hz and 30 Hz, respectively. For the age 6 assessment, EEG segments of 1,300 ms were extracted from the continuous EEG, beginning 500 ms prior to responses. For the age 9 assessment, the EEG was segmented for each trial beginning 500 ms prior to the response and continued for 800 ms after the response. Data was then corrected for eye-movements and blinks (Gratton, Coles, & Donchin, 1983) and artifacts were rejected if any of the following criteria were met: a voltage step of more than 50 microvolts between data points, a voltage difference of 300 microvolts within a single trial, or a voltage difference of less than 0.5 microvolts within 100 ms intervals. After this, data were visually inspected for remaining artifacts. ERP averages were created for error and correct trials and a baseline of the average activity from −500 to −300 ms prior to the response was subtracted from each data point.
Age 6 ERP and behavioral results in the full sample have been previously reported (Torpey et al., 2011). For both the age 6 and age 9 assessments, the error-related negativity (ERN) and correct-related negativity (CRN) were scored as the average voltage in the window between 0 ms and 100 ms after response commission on error and correct trials, respectively; the CRN and ERN were quantified at midline electrodes, Fz and Cz at the age 6 assessment1, and Fz, Cz, and FCz at the age 9 assessment. The delta ERN (ΔERN), thought to reflect error-specific activity, was calculated by subtracting the CRN from the ERN.
Statistical Analyses
All statistical analyses were conducted using SPSS (Version 17.0) General Linear Model Software, with Greenhouse-Geisser correction applied to p values with multiple-df, repeated-measures comparisons when necessitated by violation of the assumption of sphericity. Repeated-measures ANOVAs were conducted to examine error-related brain activity at both the age 6 and age 9 assessments. Multiple regressions were conducted to examine the relationship between child temperamental fear at age 3, and error-related brain activity at age 6 and 9. Furthermore, to examine the relationship between child temperamental fear and the change in error-related brain activity from age 6 to age 9, we conducted a repeated-measures ANCOVA wherein age 6 ΔERN and age 9 ΔERN were entered as within-subject variables, and temperamental fear was entered as a between-subject variable. Follow-up analyses were completed, utilizing a median-split on child temperamental fear.
Results
Behavioral data
During the Go/NoGo age 6 assessment, reaction time varied as a function of trial type, F(1, 268) = 940.21, p < .001, ηp2 = .78, such that children were faster on error trials, M = 509.21, SD = 87.67, compared to correct trials, M = 627.98, SD = 73.37. Children were slower on Go trials that followed error trials, M = 655.31, SD = 119.34, compared to all Go trials, M = 627.98, SD = 73.37, F(1, 268) = 30.18, p < .001, ηp2 = .10. During the Go/NoGo task, children made an average of 25.65, SD = 13.41, errors of commission (going on a NoGo trial) and had an overall accuracy rate of 87.41%, SD = 8.06. Neither reaction time on error or correct trials, post-error slowing, nor accuracy rates related to temperamental fear, all ps > .80.
During the flanker age 9 assessment, reaction time varied as a function of trial type, F(1, 268) = 944.58, p < .001, ηp2 = .78, such that children were faster on error trials, M = 429.74, SD = 72.11, compared to correct trials, M = 586.37, SD = 106.70. Children were slower on trials that followed error trials, M = 565.34, SD = 111.14, compared to trials that followed correct trials, M = 555.57, SD = 99.72, F(1, 268) = 11.49, p < .001, ηp2 = .04. During the flankers task, children made an average of 60.31 errors and had an overall accuracy rate of 81.15%, SD = 7.22. Neither reaction time on error or correct trials, post-error slowing, nor accuracy rates related to temperamental fear, all ps > .10.
Error-related brain activity
As previously reported (Torpey et al., 2013), at age 6, errors were associated with an increased negativity relative to correct trials at midline electrodes, F(1, 270) = 298.22, p < .001, ηp2 = .53. The ΔERN was maximal at Cz, M = −9.29, SD = 8.86, compared to Fz, M = −5.19, SD = 8.21, therefore all subsequent analyses regarding the age 6 ΔERN focus on Cz2.
At age 9, error trials were also associated with increased negativity at midline electrodes, F(1, 270) = 193.45, p < .001, ηp2 = .42. The interaction between electrode and response was significant, F(2, 540) = 7.48, p < .001, ηp2 = .03, suggesting that the ΔERN was maximal at FCz, M = −5.04, SD = 5.83, compared to Cz, M = −4.47, SD = 5.86, and Fz, M = −4.17, SD = 5.77, therefore all subsequent analyses regarding the age 9 ΔERN focus on FCz.
To examine the relationship between temperamental fear and age 6 ΔERN magnitude, we regressed age 6 ΔERN on temperamental fear. Consistent with our previous report (Torpey et al., 2013), children high in temperamental fear were characterized by a smaller (i.e., less negative) ΔERN, F(1, 270) = 5.13, p < .05, R2 = .02, β = .14. Furthermore, the relationship between age 6 ΔERN and temperamental fear remained significant after controlling for age 6 accuracy, age 6 average reaction time on correct go trials, age 6 child age, and child sex, β = .16, p < .01.
To examine the relationship between temperamental fear and age 9 ΔERN magnitude, we regressed age 9 ΔERN on temperamental fear. Results suggested that children high in temperamental fear were characterized by a larger (i.e., more negative) ΔERN, F(1, 270) = 6.72, p < .01, R2 = .02, β = -.16. Furthermore, the relationship between age 9 ΔERN and temperamental fear remained significant after controlling for age 9 accuracy, average reaction time on correct trials during the flankers task, age 9 child age, and child sex, β = -.15, p < .01.
Next, we completed a repeated-measures ANOVA wherein age 6 ΔERN and age 9 ΔERN were entered as within-subject variables, and temperamental fear was entered as a between-subject variable. The ΔERN differed between the age 6 and age 9 assessments, F(1, 269) = 9.73, p < .01, ηp2 = .04. Moreover, there was a significant interaction between temperamental fear and difference between age 6 and age 9 ΔERN, F(1, 269) = 12.65, p < .001, ηp2 = .05. To conduct follow-up analyses, a median-split was performed on temperamental fear. Paired-sample t-tests suggested that amongst children low in temperamental fear, the magnitude of the ΔERN decreased from age 6 to age 9, t(129) = -.232, p < .05. Furthermore, amongst children high in temperamental fear, the magnitude of the ΔERN increased from age 6 to age 9, t(134) = 2.36, p < .05. This pattern of results is depicted in Figure 1. For the purposes of presentation, the ΔERN values were z-scored. Figure 2 depicts waveforms for error and correct trials, as well as the difference (error minus correct) for high and low fear groups (based on a median-split) for the age 6 and age 9 assessments. Topographical headmaps are also presented for these groups wherein activity during correct trials was subtracted from error trials, 0 to 100 ms after response commission.
Figure 1.
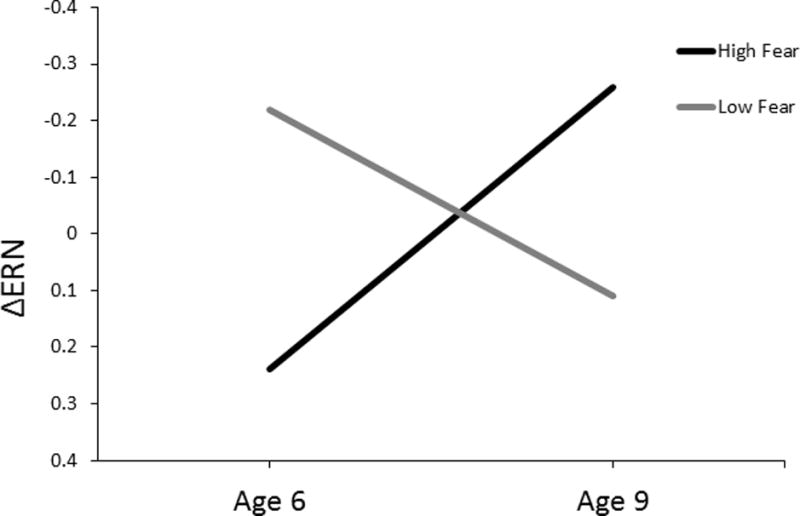
A depiction of the developmental trajectory of the ERN in high and low fear children (based on a median-split). Children high in fear were characterized by a decreased ERN at age 6 and an increased ERN at age 9. The opposite pattern is found in children low in fear – they are characterized by a relatively increased ERN at age 6 and a decreased ERN at age 9. For the purposes of presentation, the ΔERN values were z-scored.
Figure 2.
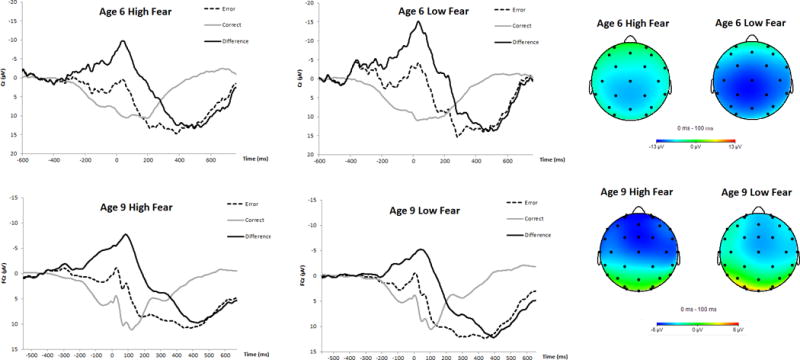
Depicts waveforms for error and correct trials, as well as the difference (error minus correct) for high and low fear groups (based on a median-split) for the age 6 and age 9 assessments. Topographical headmaps are also depicted for these groups wherein activity during correct trials was subtracted from error trials, 0 to 100 ms after response commission.
Discussion
Results from the current longitudinal study support our hypothesis that the relationship between temperamental fear and error-related neural activity changes across development from early to middle childhood within the same individuals. Children high in temperamental fear were characterized by decreased error-related brain activity when they were approximately 6 years old (Torpey et al., 2013). However, at the age 9 assessment, these same temperamentally fearful children were characterized by an increased ERN. That is, by age 9, the relationship between error-related brain activity and temperamental fearfulness more closely resembled that typically found for anxious adolescents and adults. These findings are consistent with a previous cross-sectional study indicating that age moderated the relationship between anxiety and the ERN (Meyer et al., 2012). The current study extended previous work by investigating this developmental trajectory within-subjects. Indeed, among fearful children, the ERN increased from age 6 to 9, whereas the ERN decreased across this same period among children who were low in temperamental fear.
The current findings add nuance to a growing literature on the development of error processing in children and adolescents. Although previous work has noted a developmental increase in both the ERN magnitude and error-related ACC activity in individuals between the ages of 7 and 27 years old (Tamnes, Walhovd, Torstveit, Sells, & Fjell, 2013), these studies have investigated this question using a between-subjects design and none have examined developmental changes in young children. The current study further suggests the early-emerging individual differences in temperamental fear alter the developmental trajectory of error-related brain activity.
Although the expression of anxiety changes across development, making a broad shift from the fear of external stimuli to more self-conscious fears, few studies have investigated underlying neural correlates of this transition. Work by Crozier suggests that fearfulness in 5 year old children expands to include self-conscious shyness by age 10 (Crozier & Burnham, 1990). Worry about behavioral competence and social evaluation increases from early to late childhood (Gullone, 2000; Susan H Spence & McCathie, 1993; Vasey et al., 1994) and these changes may reflect cognitive development, as well as changing demands in an individual’s environment (Gullone, 2000). The ERN may be a particularly useful neural marker for studying this transition because it appears to index individual differences in sensitivity related to monitoring one’s own behavior (Weinberg et al., 2016). Future work should explore to what extent developmental changes in the ERN relate to changes in specific facets of fear. For example, using a fear inventory, (e.g., the Fear Survey Schedule for Children (Ollendick, 1983), future work could examine whether the ERN relates to developmental changes in factors that assess fear of failure and criticism versus fear of animals.
The current investigation had several limitations. One limitation is that a fear inventory was not included in the current study, so we were unable to link the development of the ERN to specific types of fear. Another limitation is the fact that different tasks were used to elicit the ERN at the two assessments. Additionally, while both tasks provided block by block feedback, the nature of the feedback differed (points displayed versus text regarding performance) While previous work suggests the these versions of the Go/No-Go and flankers ERN share a substantial amount of variance in children (Meyer, Bress, et al., 2014), and the relationship between the ERN and anxiety does not seem to vary as a function of task (Jason S Moser et al., 2013), we cannot be certain that the changing relationship between the ERN and fear across development was not related to task or feedback differences. Although it is unclear why temperamental fear would differentially predict changes in task-related neural differences (i.e., positive versus negative differences as a function of task), future work should investigate this possibility.
The current study extended previous work by examining the developmental trajectory of the ERN from early to middle childhood in the same individuals as function of early temperamental fear. These findings highlight significant fear-related differences in the developmental course of error-related brain activity—and such developmental changes may be important for understanding the transition from normative to pathological anxiety. Insofar as early intervention is important, future work might extend this work to even younger populations, and examine clinical outcomes as a function of developmental changes in the ERN. Additionally, future studies should further characterize the specific phenotypic expression of anxiety across development, in relation to the ERN—and the extent to which the ERN can be modulated by environmental alterations or targeted interventions. For example, previous work suggests the ERN in children may be impacted by parenting styles (Brooker & Buss, 2014; Meyer, Proudfit, et al., 2014), and that this relates to clinical outcomes. Future work might explore to what extent modifying the ERN early in development may alter trajectories of ERN and resulting anxiety disorders.
Research Highlights.
In 271 children, we completed observational measures of fear when the children were 3 years -old
We measured the ERN when these children were 6 years-old and then again when they were 9 years-old
Fearful children were characterized by a decreased ERN when they were 6 years-old
These same fearful children were characterized by an increased ERN when they were 9 years-old
Footnotes
It should be noted that electrode FCz was not measured for the age 6 assessment.
The ERN has been shown to be more parietally distributed in young children. For more information regarding this, please see: (Torpey et al., 2011; Torpey et al., 2013).
References
- Brooker RJ, Buss KA. Harsh parenting and fearfulness in toddlerhood interact to predict amplitudes of preschool error-related negativity. Developmental Cognitive Neuroscience. 2014;9:148–159. doi: 10.1016/j.dcn.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufferd SJ, Dougherty LR, Carlson GA, Klein DN. Parent-reported mental health in preschoolers: findings using a diagnostic interview. Comprehensive Psychiatry. 2011;52(4):359–369. doi: 10.1016/j.comppsych.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Harbin SM, Nienhuis JK, Fitzgerald KD, Gehring WJ, Hanna GL. Increased Error-Related Brain Activity In Youth With Obsessive-Compulsive Disorder And Unaffected Siblingseased Error-Related Brain Activity In Youth With Obsessive-Compulsive Disorder And Unaffected Siblings. Depression and anxiety. 2013;30(1):39–46. doi: 10.1002/da.22035. [DOI] [PubMed] [Google Scholar]
- Chronis-Tuscano A, Degnan KA, Pine DS, Perez-Edgar K, Henderson HA, Diaz Y, Fox NA. Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48(9):928–935. doi: 10.1097/CHI.0b013e3181ae09df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss JA, Blackford JU. Behavioral inhibition and risk for developing social anxiety disorder: a meta-analytic study. Journal of the American Academy of Child & Adolescent Psychiatry. 2012;51(10):1066–1075.e1061. doi: 10.1016/j.jaac.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland WE, Angold A, Shanahan L, Costello EJ. Longitudinal patterns of anxiety from childhood to adulthood: The great smoky mountains study. Journal of the American Academy of Child & Adolescent Psychiatry. 2014;53(1):21–33. doi: 10.1016/j.jaac.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Tang J, Glaser D, Butterworth B, Dolan RJ. Anterior cingulate activity during error and autonomic response. NeuroImage. 2005;27(4):885–895. doi: 10.1016/j.neuroimage.2005.05.047. [DOI] [PubMed] [Google Scholar]
- Crozier WR, Burnham M. Age-related differences in children’s understanding of shyness. British Journal of Developmental Psychology. 1990;8(2):179–185. doi: 10.1111/j.2044-835X.1990.tb00832.x. [DOI] [Google Scholar]
- Eriksen B, Eriksen C. Effects of noise letters upon the identification of a target letter in a nonsearch task. Attention, Perception, & Psychophysics. 1974;16(1):143–149. doi: 10.3758/bf03203267. [DOI] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalography and clinical neurophysiology. 1991;78(6):447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Fox NA, Nichols KE, Henderson HA, Rubin K, Schmidt L, Hamer D, Pine DS. Evidence for a Gene-Environment Interaction in Predicting Behavioral Inhibition in Middle Childhood. Psychological Science. 2005;16(12):921–926. doi: 10.1111/j.1467-9280.2005.01637.x. [DOI] [PubMed] [Google Scholar]
- Fox NA, Snidman N, Haas SA, Degnan KA, Kagan J. The relations between reactivity at 4 months and behavioral inhibition in the second year: Replication across three independent samples. Infancy. 2015;20(1):98–114. doi: 10.1111/infa.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A Neural system for error detection and compensation. Psychological Science. 1993;4(6):385–390. [Google Scholar]
- Goldsmith HH, Reilly J, Lemery KS, Longley s, Prescott A. Preschool Laboratory Temperament Assessment Battery. University of Wisconsin; 1995. Unpublished instrument. [Google Scholar]
- Gratton G, Coles MGH, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and clinical neurophysiology. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Gullone E. The development of normal fear: A century of research. Clinical Psychology Review. 2000;20(4):429–451. doi: 10.1016/s0272-7358(99)00034-3. [DOI] [PubMed] [Google Scholar]
- Hajcak G. What we’ve learned from mistakes. Current Directions in Psychological Science. 2012;21(2):101–106. doi: 10.1177/0963721412436809. [DOI] [Google Scholar]
- Hajcak G, Foti D. Errors Are Aversive: Defensive Motivation and the Error-Related Negativity. Psychological Science (Wiley-Blackwell) 2008;19(2):103–108. doi: 10.1111/j.1467-9280.2008.02053.x. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. To err is autonomic: Error-related brain potentials, ANS activity, and post-error compensatory behavior. Psychophysiology. 2003;40(6):895–903. doi: 10.1111/1469-8986.00107. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Yeung N, Simons RF. On the ERN and the significance of errors. Psychophysiology. 2005;42(2):151–160. doi: 10.1111/j.1469-8986.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- Jones WH, Cheek JM, Briggs SR. Shyness: Perspectives on research and treatment. Springer Science & Business Media; 2013. [Google Scholar]
- Lahat A, Lamm C, Chronis-Tuscano A, Pine DS, Henderson HA, Fox NA. Early behavioral inhibition and increased error monitoring predict later social phobia symptoms in childhood. Journal of the American Academy of Child & Adolescent Psychiatry. 2014 doi: 10.1016/j.jaac.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström BR, Mattsson-Mårn IB, Golkar A, Olsson A. In your face: risk of punishment enhances cognitive control and error-related activity in the corrugator supercilii muscle. PloS one. 2013;8(6):e65692. doi: 10.1371/journal.pone.0065692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott JM, Perez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, Fox NA. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biological Psychiatry. 2009;65(5):445–448. doi: 10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Bress J, Proudfit GH. Psychometric Properties of the Error-Related Negativity in Children and Adolescents. Psychophysiology. 2014 doi: 10.1111/psyp.12208. [DOI] [PubMed] [Google Scholar]
- Meyer A, Hajcak G, Torpey-Newman DC, Kujawa A, Klein DN. Enhanced error-related brain activity in children predicts the onset of anxiety disorders between the ages of 6 and 9. Journal of Abnormal Psychology. 2015;124(2):266. doi: 10.1037/abn0000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Hajcak G, Torpey DC, Kujawa A, Kim J, Bufferd S, Klein DN. Increased error-related brain activity in six-year-old children with clinical anxiety. Journal of Abnormal Child Psychology. 2013;41(8):1257–1266. doi: 10.1007/s10802-013-9762-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Nelson BD, Perlman G, Klein D, Kotov R. Enhanced error-related brain activity (ERN) predicts the onset of generalized anxiety disorder in a large sample of adolescent females. Biological Psychiatry. doi: 10.1111/jcpp.12922. Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Proudfit GH, Bufferd SJ, Kujawa AJ, Laptook RS, Torpey DC, Klein DN. Self-reported and observed punitive parenting prospectively predicts increased error-related negativity in six-year-old children. Journal of Abnormal Child Psychology. 2014 doi: 10.1007/s10802-014-9918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Weinberg A, Klein DN, Hajcak G. The development of the error-related negativity (ERN) and its relationship with anxiety: Evidence from 8 to 13 year-olds. Developmental Cognitive Neuroscience. 2012;2(1):152–161. doi: 10.1016/j.dcn.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser JS, Hajcak G, Simons RF. The effects of fear on performance monitoring and attentional allocation. Psychophysiology. 2005;42(3):261–268. doi: 10.1111/j.1469-8986.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- Moser JS, Moran TP, Jendrusina AA. Parsing relationships between dimensions of anxiety and action monitoring brain potentials in female undergraduates. Psychophysiology. 2012;49(1):3–10. doi: 10.1111/j.1469-8986.2011.01279.x. [DOI] [PubMed] [Google Scholar]
- Moser JS, Moran TP, Schroder HS, Donnellan MB, Yeung N. On the relationship between anxiety and error monitoring: a meta-analysis and conceptual framework. Frontiers in human neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olino TM, Klein DN, Dyson MW, Rose SA, Durbin CE. Temperamental emotionality in preschool-aged children and depressive disorders in parents: associations in a large community sample. Journal of Abnormal Psychology. 2010;119(3):468. doi: 10.1037/a0020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollendick TH. Reliability and validity of the revised fear survey schedule for children (FSSC-R) Behaviour Research and Therapy. 1983;21(6):685–692. doi: 10.1016/0005-7967(83)90087-6. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Vocat R, N’diaye K, Spinelli L, Seeck M, Vuilleumier P. Errors recruit both cognitive and emotional monitoring systems: simultaneous intracranial recordings in the dorsal anterior cingulate gyrus and amygdala combined with fMRI. Neuropsychologia. 2010;48(4):1144–1159. doi: 10.1016/j.neuropsychologia.2009.12.020. [DOI] [PubMed] [Google Scholar]
- Rabinak CA, Holman A, Angstadt M, Kennedy AE, Hajcak G, Phan KL. Neural response to errors in combat-exposed returning veterans with and without post-traumatic stress disorder: A preliminary event-related potential study. Psychiatry Research: Neuroimaging. 2013;213(1):71–78. doi: 10.1016/j.pscychresns.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Riesel A, Endrass T, Auerbach LA, Kathmann N. Overactive Performance Monitoring as an Endophenotype for Obsessive-Compulsive Disorder: Evidence From a Treatment Study. American Journal of Psychiatry. 2015;0(0) doi: 10.1176/appi.ajp.2014.14070886. appi.ajp.2014.14070886. [DOI] [PubMed] [Google Scholar]
- Riesel A, Weinberg A, Endrass T, Kathmann N, Hajcak G. Punishment has a lasting impact on error-related brain activity. Psychophysiology. 2012;49(2):239–247. doi: 10.1111/j.1469-8986.2011.01298.x. [DOI] [PubMed] [Google Scholar]
- Riesel A, Weinberg A, Moran T, Hajcak G. Time course of error-potentiated startle and its relationship to error-related brain activity. Journal of Psychophysiology. 2013;27(2):51. [Google Scholar]
- Rosenbaum JF, Biederman J, Hirshfeld-Becker DR, Kagan J, Snidman N, Friedman D, Faraone SV. A Controlled Study of Behavioral Inhibition in Children of Parents With Panic Disorder and Depression. Am J Psychiatry. 2000;157(12):2002–2010. doi: 10.1176/appi.ajp.157.12.2002. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience. 2011;12(3):154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence SH, McCathie H. The Stability of Fears in Children: a Two-Year Prospective Study: a Research Note. Journal of Child Psychology and Psychiatry. 1993;34(4):579–585. doi: 10.1111/j.1469-7610.1993.tb01037.x. [DOI] [PubMed] [Google Scholar]
- Spence SH, Rapee R, McDonald C, Ingram M. The structure of anxiety symptoms among preschoolers. Behaviour Research and Therapy. 2001;39(11):1293–1316. doi: 10.1016/s0005-7967(00)00098-x. [DOI] [PubMed] [Google Scholar]
- Spunt RP, Lieberman MD, Cohen JR, Eisenberger NI. The phenomenology of error processing: the dorsal ACC response to stop-signal errors tracks reports of negative affect. Journal of Cognitive Neuroscience. 2012;24(8):1753–1765. doi: 10.1162/jocn_a_00242. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Walhovd KB, Torstveit M, Sells VT, Fjell AM. Performance monitoring in children and adolescents: A review of developmental changes in the error-related negativity and brain maturation. Developmental Cognitive Neuroscience. 2013;6(0):1–13. doi: 10.1016/j.dcn.2013.05.001. http://dx.doi.org/10.1016/j.dcn.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torpey DC, Hajcak G, Kim J, Kujawa A, Klein DN. Electrocortical and behavioral measures of response monitoring in young children during a Go/No-Go task. Developmental Psychobiology. 2011 doi: 10.1002/dev.20590. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torpey DC, Hajcak G, Kim J, Kujawa AJ, Dyson MW, Olino TM, Klein DN. Error-related brain activity in young children: associations with parental anxiety and child temperamental negative emotionality. Journal of Child Psychology and Psychiatry. 2013 doi: 10.1111/jcpp.12041. no-no. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasey M, Crnic K, Carter W. Worry in childhood: A developmental perspective. Cognitive Therapy and Research. 1994;18(6):529–549. doi: 10.1007/bf02355667. [DOI] [Google Scholar]
- Weinberg A, Kotov R, Proudfit GH. Neural indicators of error processing in generalized anxiety disorder, obsessive-compulsive disorder, and major depressive disorder. Journal of Abnormal Psychology. 2015;124(1):172. doi: 10.1037/abn0000019. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Meyer A, Hale-Rude E, Perlman G, Kotov R, Klein DN, Hajcak G. Error-related negativity (ERN) and sustained threat: Conceptual framework and empirical evaluation in an adolescent sample. Psychophysiology. 2016;53(3):372–385. doi: 10.1111/psyp.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]


