Abstract
Objective
To assess systemic inflammation in relation to fecundability and anovulation.
Design
Prospective cohort study among participants in the Effects of Aspirin in Gestation and Reproduction (EAGeR) trial who were assigned to the placebo.
Setting
Four US academic medical centers, 2007-2012.
Participants
Healthy, eumenorrheic women (n=572), 18-40 years, with 1-2 prior pregnancy losses, attempting spontaneous pregnancy.
Intervention
Baseline serum high-sensitivity C-reactive protein (hsCRP) values<10 mg/L were categorized into tertiles.
Main Outcome Measures
Discrete Cox proportional hazards models estimated the fecundability odds ratio (FOR) and 95% confidence interval (CI) and adjusted for potential confounders. Log-binomial regression estimated the risk ratio (RR) and 95% CI of anovulation. The algorithm to define anovulation used data on urinary concentrations of human chorionic gonadotropin, pregnanediol-3-glucuronide, and luteinizing hormone; and fertility monitor readings.
Results
Higher hsCRP was associated with reduced fecundability (adjusted FOR=0.74, 95% CI 0.56-1.00; adiposity-adjusted FOR=0.87, 95% CI 0.61-1.24), but not with an increased risk of anovulation (adjusted RR=1.27, 95% CI 0.84-1.93; adiposity-adjusted RR=1.05, 95% CI 0.62-1.76).
Conclusions
Among healthy women attempting pregnancy after 1-2 pregnancy losses, we found preliminary evidence that systemic inflammation is associated with reduced fecundability, but not independently from adiposity. Sporadic anovulation did not appear to drive this association.
Keywords: C-reactive protein, inflammation, anovulation, fertility, prospective studies
Introduction
Appropriate regulation of inflammation is critical for successful ovulation (1, 2) and implantation (3). Peripheral pro-inflammatory cytokines and endometrial inflammation have been shown to be increased among women with recurrent in vitro fertilization implantation failure (4), and aberrant inflammation is involved in the complex pathophysiology and associated infertility of endometriosis (5) and polycystic ovary syndrome (PCOS) (6). Women with PCOS have chronic, increased inflammation independent of obesity (7), and this is thought to contribute to PCOS-associated ovarian dysfunction (2). Recently, we found that, among women with higher systemic inflammation in the Effects of Aspirin in Gestation and Reproduction (EAGeR) Trial, low-dose aspirin (LDA) taken daily while attempting pregnancy restored pregnancy and live birth rates to the levels found among women with lower pre-treatment inflammation (8). Among women with higher pre-treatment hsCRP, the effect of LDA was somewhat stronger among women with body mass index (BMI) <25kg/m2 than BMI≥25 kg/m2, as well as lower versus higher waist-to-hip ratio (8). Given the known positive relationship between adiposity and inflammation (9), these initial findings indicated a potentially complex relationship between adiposity-associated vs. non adiposity-associated inflammation and reproduction.
Further, these prior results suggest that increased inflammation may contribute to subfertility among women without a frank reproductive disorder, since study participants had regular menstrual cycles, no history of infertility, and demonstrated fecundity with a history of only 1-2 pregnancy losses (10). Outside of studies enrolling women treated for endometriosis (5), PCOS (2), or infertility (4, 11), there is scarce evidence from prospective studies on the extent to which sub-acute, systemic inflammation may contribute to subfertility.
In this study, we assessed the association of low-grade inflammation, measured by hsCRP, with fecundability among EAGeR participants. We focused on women assigned to placebo, given the known impact of LDA on pregnancy incidence in women with increased hsCRP, and accounted for adiposity. Because an effect of inflammation on fecundability could be driven by effects on anovulation, we also assessed its association with sporadic anovulation.
Materials and Methods
Study Population
The EAGeR Trial was a multicenter, block-randomized, double-blind, placebo-controlled trial to evaluate the effect of preconception-initiated daily LDA on reproductive outcomes in US women with a history of pregnancy loss, 2007-2012 (10). Participants were attempting pregnancy without the use of fertility treatments, were 18-40 years old, had regular menstrual cycles lasting 21-42 days, and had 1-2 prior pregnancy losses, up to 2 live births, and no known major medical conditions or infertility. Recruitment efforts targeted patients at the four study sites and affiliated medical practices as well as local media outlets. Of 5,485 women assessed for eligibility, 1,228 women were randomized (Supplemental Figure 1). To investigate the relation of systemic inflammation with fecundability and with anovulation, we analyzed data from 572 women assigned to receive placebo after excluding those assigned to receive LDA (n=615), those with hsCRP ≥10 mg/L (n=34), a range which generally indicates acute infection or injury rather than chronic inflammation (12), and those who withdrew before the first follow-up visit (n=7). The institutional review board at each clinical center approved the study protocol, and participants gave informed consent.
Exposure and covariate assessment
Baseline data collection and randomization occurred at a study visit on menstrual cycle day 2-4. Participants provided a blood sample; responded to questionnaires eliciting demographic data, lifestyle habits, reproductive and medical histories; and underwent measurement for height and weight; waist, hip, and upper-arm circumferences; and skinfold measurements (subscapular, suprailiac, and triceps).
Serum concentrations of hsCRP were quantified with an immunoturbidimetric assay using the Roche COBAS 6000 autoanalyzer with a limit of detection (LOD) of 0.15 mg/L (Roche Diagnostics, Indianapolis, IN). hsCRP values below the limit of detection were set equal to LOD/√2 (1.6% of women). Coefficients of variation (CV) were 5.1% at 1.05 mg/L and 6.7% at 3.12 mg/L. Serum leptin concentration was measured with a Quantikine enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN) with a LOD=7.8 pg/mL and CV of 3.3% at 64.5 pg/mL and 3.2% at 621 pg/mL.
Outcome assessment
Follow-up lasted for up to six menstrual cycles while attempting pregnancy. Participants were instructed on the use of the study-issued fertility monitor (Clearblue™, SPD Swiss Precision Diagnostics GMBH, Geneva, Switzerland) and urine pregnancy tests (QuickVue™, Quidel, San Diego, CA). Follow-up clinic visits occurred twice per cycle during the first two cycles of follow-up, and once per cycle thereafter (10).
Anovulation
In cycles 1 and 2, participants collected and froze first-morning urine specimens daily for analysis of hormones. Procedures to select urine specimens timed to the menstrual cycle phase are described in the Supplemental Materials and Methods. Urinary pregnanediol-3-glucuronide (PdG) was measured in urine by competitive chemiluminescence duplex assay that also measured estrone-1-gluconuride and had LOD=45 ng/mL for PdG (Quansys Biosciences, Logan, UT). The interassay laboratory CVs for PdG were 23.2% at 4060 ng/mL and 20.2% at 1604 ng/mL, using an in-house urine control. Urinary luteinizing hormone (LH) was measured via reagent/sandwich immunoassay (Roche Diagnostics, Indianapolis, IN) with an interassay CV of 1.6%. To reduce potential measurement error, hormones were not adjusted for creatinine (13).
After evaluating the phase-specific hormone concentrations, anovulation was determined based on the following step-based algorithm: 1) cycles resulting in pregnancy (n=214 cycles from women eligible for the present analysis) were considered ovulatory; 2) cycles with maximum luteal phase PdG ≥ 5 μg/mL (n=520) were considered ovulatory, while PdG < 5 μg/mL were considered anovulatory (n=125) (14); 3) in the absence of an available luteal-phase specimen, cycles were considered ovulatory if the fertility monitor LH concentration was at least 2.5 times the average of the previous 5 days (n=67) and anovulatory if <2.5 times the average (n=11) (15); 4) if adequate sequential LH data were unavailable from the fertility monitor for step #3, a peak fertility reading on the monitor was considered ovulatory (n=3) (16), and cycles with no peak fertility reading were considered to have missing ovulatory status (n=79; see Missing Data, below).
Pregnancy
Human chorionic gonadotropin (hCG)-positive pregnancies were identified by urine pregnancy tests. Some pregnancies ended very early and were identified using assays for free beta-hCG from urine samples collected at-home and in-clinic (initial test: BioVendor, Asheville, NC, USA; confirmatory test: Diagnostic Automation Inc., Calabasas, CA, USA) (17).
Statistical analysis
For the primary statistical analyses, hsCRP was categorized by tertile, and we estimated the respective associations of the second and third tertiles of hsCRP with the outcome, relative to the first tertile. Categorizing hsCRP into tertiles enhanced precision, whereas the relevance of hsCRP cut-points established for cardiovascular disease risk (18) to reproductive function is uncertain. Additionally, we explored the possibly non-linear relation of hsCRP with fecundability and anovulation non-parametrically by fitting a restricted cubic spline regression (19) with the LGTPHCURV9 macro (20). We conducted additional analyses that stratified the placebo group by BMI<25 vs. ≥25 because of our prior findings that the effect of LDA on pregnancy and live birth rates was stronger among women with BMI<25 and higher pre-treatment inflammation (8). SAS version 9.4 was used for all analyses (SAS Institute, Cary, NC).
Analysis of fecundability
Discrete Cox proportional hazards models estimated the fecundability odds ratio (FOR) and 95% confidence interval (CI) for up to six menstrual cycles while attempting pregnancy, accounting for left truncation (cycles trying before enrollment) and adjusted for potential confounders. The FOR shows the cycle-specific odds of hCG-detected pregnancy in the exposed relative to the unexposed group. A FOR<1.0 indicates that the exposed group has decreased odds of pregnancy.
We employed three adjustment strategies to address the complex relationship of inflammation and adiposity (21). Model 1 accounted for reported cycles attempting pregnancy at baseline; Model 2 additionally adjusted for potential confounders other than adiposity--age at baseline (linear variable), race (white (reference), non-white), marital status (married (reference) vs. living as married/other), and time since last loss (<3 months (reference), ≥3 months); and Model 3 additionally adjusted for body mass index (BMI) (linear variable). As there was no difference in the FOR after further adjustment for number of losses, random glucose, and current regular smoking, these variables were dropped from the final model. Potential confounders were identified through review of published data and causal-graph analysis. To aid interpretation of the observed FORs, we plotted the estimated cumulative percent pregnant by cycles attempting pregnancy in each tertile, adjusted for confounders, by using the BASELINE statement in PROC PHREG and specifying covariate values.
Analysis of anovulation
A potential effect of hsCRP on fecundability may be driven by an increase in sporadic anovulation. Thus, the primary analysis estimated the risk ratio (RR) and 95% confidence interval (CI) of anovulation per cycle. The menstrual cycle was the unit of analysis, with 1-2 menstrual cycles per participant, and multiple cycles addressed by using log-binomial regression models with the generalized estimating equation method.
Model A adjusted for the number of cycles a participant contributed to the analysis to account for the fact that women who became pregnant sooner contributed fewer cycles. Model B additionally adjusted for age (categorized as 18-24 years, 25-34 years (reference), and 35-40 years because anovulation was more common at either end of the age distribution), marital status, and time since last loss, and Model C additionally adjusted for BMI.
Sensitivity analyses
We assessed the robustness of our Model 3 (adiposity-adjusted) results by adjusting for alternative measures of adiposity: predicted percent body fat (Supplemental Materials and Methods), serum leptin concentration, waist circumference, and waist-to-hip ratio. Each adiposity variable was modeled as a linear variable and, if needed, as a restricted cubic spline variable (19) by using the EFFECT statement in PROC PHREG. Similarly, sensitivity analyses for the effect modification of hsCRP by adiposity stratified by waist circumference and waist-to-hip ratio, instead of BMI. Also, we assessed how our results changed when we instead categorized hsCRP by cut-points for cardiovascular disease risk defined by the American Heart Association (AHA) (hsCRP <1 mg/L, 1-2.99 mg/L, 3-9.99 mg/L). A sensitivity analysis of hsCRP and anovulation restricted to cycles with outcome determined by PdG or pregnancy (22). A further sensitivity analysis of hsCRP and anovulation included women assigned to LDA because we previously found that LDA did not affect sporadic anovulation (23) (Supplemental Materials and Methods).
Missing data
Anovulation data were missing for 7.8% of cycles due to mistimed sample collection and missing fertility monitor data. hsCRP values were missing for 2.0% of women due to lack of an analyzable specimen. Height and/or weight were missing for 1.7% of women. A multiple imputation procedure created 20 datasets with imputed missing values (24). The primary analyses of hsCRP tertiles used PROC MIANALYZE to calculate appropriate effect estimates and standard errors. The analyses that included only women with complete data were: the restricted cubic spline regression models, the distribution of baseline characteristics by hsCRP tertile, and the Pearson correlation coefficients with linear hsCRP. Furthermore, we assessed the sensitivity of our main results to imputed data in sub-analyses restricted women with complete data.
Results
At baseline, hsCRP was positively associated with older age, and other variables closely associated with age such as parity and number of previous pregnancy losses, as well as BMI, time since last loss, current smoking, and leptin; it was inversely associated with education (Table 1). We calculated Pearson correlation coefficients between linear hsCRP and: BMI (r=0.56), WHR (0.30), WC (0.54) and predicted percent body fat (r=0.50) (all P-values<0.0001).
Table 1.
Baseline characteristics by hsCRP tertile: 572 women, EAGeR Trial, USA, 2007-2012.a
| Tertiles of hsCRP | ||||
|---|---|---|---|---|
|
| ||||
| Overall Cohort | Tertile 1: <0.70 mg/L | Tertile 2: 0.70-1.94 mg/L | Tertile 3: 1.95-9.9 mg/L | |
| Number of women, n | 572 | 184 | 188 | 188 |
| Category of age, years, % | ||||
| 18-24 | 22 | 26 | 22 | 19 |
| 25-29 | 41 | 47 | 36 | 38 |
| 30-34 | 25 | 20 | 32 | 24 |
| 35-40 | 12 | 7 | 10 | 19 |
| Category of BMI, kg/m2, % | ||||
| Underweight: BMI<18.5 | 5 | 10 | 4 | 1 |
| Normal weight: BMI 18.5-24.9 | 45 | 69 | 54 | 14 |
| Overweight: BMI 25.0-29.9 | 28 | 18 | 28 | 38 |
| Obese: BMI ≥30.0 | 22 | 3 | 14 | 48 |
| Race: white (vs. non-white), % | 96 | 94 | 96 | 97 |
| Married or living with partner, % | 90 | 91 | 92 | 87 |
| Education: > high school, % | 87 | 92 | 87 | 84 |
| Annual household income ≥$75,0002, % | 52 | 51 | 54 | 49 |
| Employed, % | 75 | 74 | 72 | 81 |
| Prior live births, % | ||||
| 0 | 47 | 45 | 49 | 45 |
| 1 | 36 | 37 | 35 | 39 |
| 2 | 17 | 18 | 16 | 16 |
| Previous pregnancy losses, % | ||||
| 1 | 66 | 67 | 68 | 62 |
| 2 | 34 | 33 | 32 | 38 |
| Time from last loss to randomization2, % | ||||
| ≤ 4 months | 53 | 54 | 60 | 47 |
| 5-8 months | 19 | 24 | 18 | 17 |
| 9-12 months | 9 | 5 | 8 | 13 |
| >12 months | 19 | 17 | 15 | 26 |
| hormonal contraceptives use in past 3 months, % | 4 | 6 | 4 | 4 |
| Sexual intercourse frequency per week, mean (SD) | 2.4 (1.8) | 2.6 (1.8) | 2.4 (1.7) | 2.4 (1.8) |
| Current regular smoker, % | 3 | 4 | 4 | 3 |
| Biomarkers, Mean (SD) | ||||
| Waist circumference, cm | 86.9 (14.7) | 78.6 (9.5) | 84.0 (11.4) | 97.3 (15.5) |
| Waist-to-hip ratio | 0.81 (0.070) | 0.79 (0.060) | 0.80 (0.068) | 0.84 (0.075) |
| Leptin, ng/mL | 23.2 (19.6) | 12.1 (10.2) | 20.6 (15.9) | 36.9 (22.1) |
hsCRP, high-sensitivity C-reactive protein; BMI, body mass index, SD, standard deviation
The number of women with missing data by characteristic was: BMI (n=10), employment (n=18), waist circumference (n=4), leptin (n=19).
Fecundability
In total, 2,022 cycles and 348 pregnancies were observed among 572 women assigned to placebo (median=3 cycles). Higher hsCRP (i.e., third tertile) was associated with lower fecundability after adjusting for demographic and reproductive variables (Model 2 FOR= 0.74, 95% CI 0.56-1.00), while further adjustment for BMI weakened the FOR and the 95% CI crossed the null (Model 3 FOR=0.87, 95% CI 0.61-1.24) (Figure 2). Similarly, the median number of cycles to pregnancy among the third tertile of hsCRP was greater than among the first tertile when adjusted for Model 2 (4 cycles vs. 3 cycles), but not Model 3 (3 cycles versus 3 cycles). The spline regression plot displayed a linear decrease in the FOR with increasing hsCRP before adjustment for BMI (not shown), and a flat, imprecise relation after adjustment (Supplemental Figure 2). Noting the linear relation before adjusting for BMI, we calculated the FOR associated with a 1 mg/L increase in hsCRP: Model 2 FOR=0.94 (95% CI 0.89, 1.00); Model 3 FOR=0.98 (95% CI 0.91, 1.05).
Figure 2.
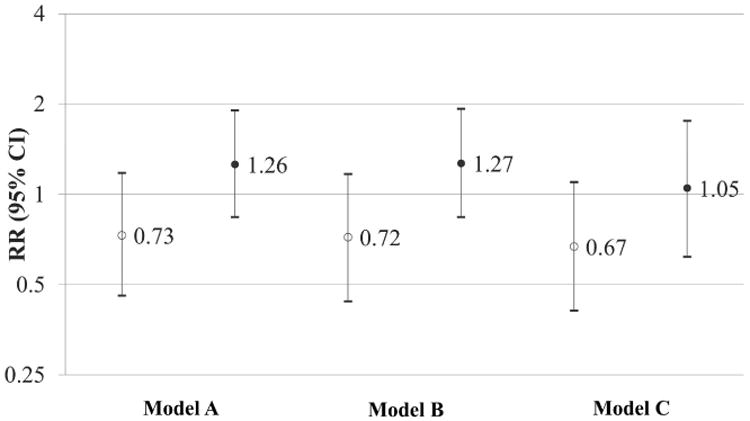
The Risk Ratio (RR) and 95% CI for anovulation for the second tertile (open circle) and third tertile (closed circle) of hsCRP relative to the first tertile. Results are restricted to 572 women assigned to placebo. Model A is adjusted for cycles of follow-up. Model B is adjusted for cycles of follow-up, age (18-24 years, 25-34, 35-40), marital status (married, living as married or other) and the time from last pregnancy loss to study enrollment (≤4 months, 5-8 months, 9-12 months, >12 months). Model C is adjusted for cycles of follow-up, age (18-24 years, 25-34, 35-40), marital status (married, living as married or other) the time from last pregnancy loss to study enrollment (≤4 months, 5-8 months, 9-12 months, >12 months), and BMI (linear variable).
Stratified analyses
In a stratified analysis by BMI category, higher hsCRP was non-significantly associated with fecundability after adjusting for demographic and reproductive history variables (Model 2: BMI<25 kg/m2, FOR=0.85, 95% CI 0.47, 1.54; BMI≥25 kg/m2, FOR=0.85, 95% CI 0.51, 1.42; Supplemental Table 1). Further adjustment for linear BMI made a somewhat greater change in the FOR among women with BMI≥25 kg/m2 than among women with BMI<25 kg/m2 (Model 3: BMI<25 kg/m2, FOR=0.86, 95% CI 0.47, 1.58; BMI≥25 kg/m2, FOR=0.96, 95% CI 0.55, 1.67; Supplemental Table 1).
In exploratory analyses stratified by waist and by WHR, there were sporadic associations observed between higher hsCRP and fecundability (Supplemental Table 1). Sensitivity analyses applying AHA categories of hsCRP produced essentially the same results as the analysis of hsCRP tertiles (data not shown). Adjusting for BMI may not completely control confounding by adiposity, but sensitivity analyses that adjusted Model 3 for various alternative adiposity measures produced results consistent with those from the primary analysis. Of these sensitivity analyses, adjusting for the restricted-cubic spline variable for WHR produced the strongest association (FOR=0.77, 95% CI 0.57, 1.04), and adjusting for the restricted cubic spline variable for serum leptin produced the weakest association (FOR=0.94, 95% CI 0.65, 1.35).
Sensitivity analyses
Women had been attempting pregnancy for 0 to 55 cycles at baseline, with 98% of women attempting for ≤12 cycles. Results from the main analysis were essentially unchanged after excluding 65 women who had been trying for >6 cycles, and who thus had a greater chance of having an undiagnosed problem with subfertility that was unrelated to hsCRP (3rd tertile vs. 1st tertile, Model 2 FOR=0.72, 95% CI: 0.53, 0.97; Model 3 FOR=0.89, 95% CI: 0.61, 1.30). Also, during follow-up, 21 women in our analysis reported at an end-of-cycle study that they had abstained from sexual intercourse in that cycle, and these women were disproportionately in tertile 3 (6.9% vs. 2.2% in tertile 1, P=0.02). However, a sensitivity analysis that excluded menstrual cycles with report of no sexual intercourse found almost no difference in the results (hsCRP tertile 3, Model 2: FOR=0.75, 95% CI=0.76-1.01; Model 3 FOR=0.88, 95% CI=0.61-1.26).
Anovulation
In the first two cycles of follow-up, the 572 women contributed 1,019 cycles during the first two cycles of follow-up; 14.5% of cycles were anovulatory. Although higher hsCRP was associated with lower fecundability in observations described above, higher hsCRP was not appreciably associated with increased risk of anovulation (Model 2 RR=1.27, 95% CI: 0.84, 1.93; Model 3 RR=1.05, 95% CI: 0.62-1.76). The RR (95% CI) for each mg/L increase in hsCRP was 1.09 (1.01-1.18) in Model 2 and 1.06 (0.96-1.17) in Model 3. The analysis of hsCRP in AHA categories produced similar results (data not shown). After restricting the analyses to 940 cycles with complete data, results remained similar to the analyses with imputed data (data not shown). Furthermore, results were essentially unchanged when we restricted the analysis to 859 cycles with observed outcome determined by PdG or pregnancy (data not shown).
Discussion
In a prospective cohort study of women attempting pregnancy after a history of 1-2 pregnancy losses, we observed reduced fecundability among women with higher pre-treatment hsCRP after adjusting for potential demographic and reproductive history confounders. Further adjustment for BMI attenuated the association of inflammation and fecundability and widened the confidence interval, indicating that BMI is either a confounder or potentially a mediator of the relationship between low-grade inflammation and fecundability. Analysis of hsCRP and risk of sporadic anovulation suggested that the observed association between hsCRP and fecundability was not attributable to an effect on anovulation.
The causal relationships linking inflammation, adiposity, and fecundability are complex. Given that our found associations were attenuated by adjusting for adiposity, our results are consistent with two potential causal mechanisms: the effect of inflammation on fecundability may be confounded by adiposity, or it may be mediated by adiposity. First, it is well established that obesity promotes hepatic CRP production through adipocyte production of adipokines (25). Also, the adiposity milieu that contributes to elevated hsCRP may impact fecundability through other pathways besides inflammation. While prior research implicates female obesity in anovulatory infertility (26), adiposity may additionally decrease fecundability through altered oocyte morphology, reduced fertilization, impaired embryo quality, and altered endometrial receptivity (27), all factors that could reduce fecundability irrespective of an inflammatory milieu. This biological rationale for adiposity acting as a confounder is illustrated in Figure 3A. The presence of substantial confounding coupled with the small number of events resulted in imprecision of the adjusted effect estimate, indicating there were insufficient numbers to detect a potential, small effect of higher systemic inflammation independent of adiposity. Never the less, a small inverse association remained after adjustment for various measures of adiposity—such as BMI, leptin, and waist-to-hip ratio—although the loss of precision with this adjustment varied with the measure used.
Figure 3.
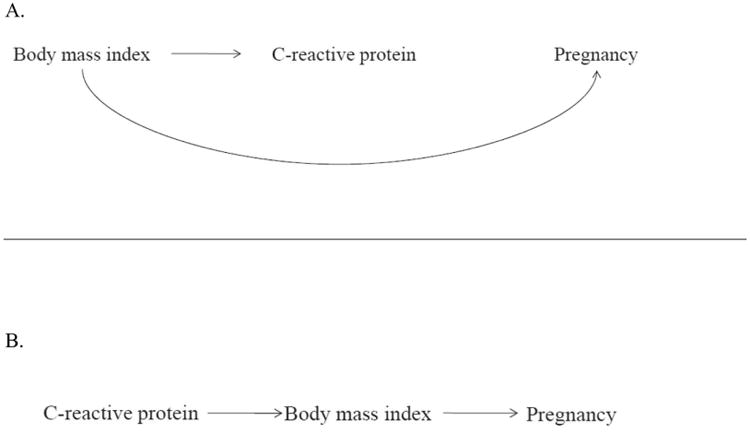
Simplified directed acyclic graphs to illustrate the potential causal structure of C-reactive protein, body mass index, and pregnancy. Figure 3A: Body mass index is a common cause of C-reactive protein and pregnancy, and therefore can be considered a confounder of the relationship between inflammation and fecundability. Figure 3B: Body mass index is an intermediate on the causal path from C-reactive protein to pregnancy, and therefore adjustment for adiposity would partially obscure the total effect of inflammation on fecundability.
Regarding the second potential mechanism that is consistent with our findings, if BMI partially mediates the relationship between inflammation and fecundability, as illustrated in Figure 3B, adjusting for adiposity would also be expected to attenuate the effect. Studies in experimental models indicate that hsCRP directly impairs leptin signaling, contributing to leptin resistance and dysregulation of energy balance (28), mechanisms known to underlie weight gain. Indeed, in mice, adipose tissue gain was induced by experimentally increased systemic (29) or hypothalamic (30) inflammation and oxidative stress. While prospective epidemiologic studies of inflammation leading to weight gain are scarce, higher hsCRP at mid-pregnancy has been associated with greater subsequent gestational weight gain (31), suggesting that inflammation could be a precursor to excess weight gain. Considering both of these causal scenarios is paramount for interpreting the complex relations between inflammation, adiposity, and fecundability. Irrespective of the causal structure, hsCRP modifies the effect of LDA on live birth, as previously reported (8), and this inflammatory biomarker is an indicator of women at risk of poorer fecundability.
The adjusted associations between higher hsCRP and anovulation were weak and highly imprecise. Even so, we were unable to conclude that hsCRP had no effect on anovulation because there may have been too few anovulatory events to detect a potential small effect on anovulation. Urine specimens were collected for hormonal assays during the first two cycles, out of a possible six cycles of attempting pregnancy. Although an inflammation-anovulation link has been reported in the pathophysiology of PCOS (2, 6), this may not extend to healthy, eumenorrheic women. The few studies that have investigated the relation of hsCRP and ovarian function among eumenorrheic women have shown that peri-ovulatory hsCRP was positively associated with ovulation (32, 33), and progesterone was positively associated with luteal hsCRP after adjusting for BMI (34). These reports may be more indicative of the transient tissue remodeling and inflammation that accompany ovulation, as opposed to an effect of systemic inflammation on ovulation. If higher inflammation does not affect ovulatory function, other potential mechanisms that explain our findings of lower fecundability include inflammation's impact on embryonic survival or implantation (4).
Our study has numerous strengths for studying the association of hsCRP and fecundability. One is the standardized timing of hsCRP concentration in the follicular phase and restriction to hsCRP<10 mg/L, a range that is used clinically to represent chronic inflammation (18). Available data on covariates included measured waist and hip circumferences; height; weight; leptin; and predicted percent body fat, enhancing our ability to more completely examine the role of adiposity in the relationship between inflammation and fecundability. We used an algorithm for classifying urinary PdG to define anovulation, and urinary PdG has been shown to have high sensitivity and specificity for defining anovulation (14) relative to the gold standard, ultrasound detection. Furthermore, we found our results were not sensitive to the inclusion of cycles in which anovulation was determined by the fertility-monitor reading (7.9% of cycles) or multiple imputation (6.4%). Time-to-pregnancy was also measured accurately due to systematic use of clinical pregnancy tests and additional urinary hCG assay. However, our study was subject to some limitations. Notably, higher chronic inflammation may have been misclassified due to our use of a single measure of hsCRP obtained during menses. CRP varies across the menstrual cycle; the mean and variance are highest during menses (34). Thus, we expect that misclassification of chronic inflammation would likely take the form of misclassifying women in higher categories when they have truly low inflammation. Such misclassification would tend to attenuate a true, harmful effect of higher inflammation on fecundability and anovulation, and would not be expected to explain our findings. All participants took folic acid supplements, which may have influenced their inflammatory response (35), and possibly limited the generalizability of our findings to only women who are taking a prenatal vitamin. Also, participants predominantly self-identified as white, and this also may limit the generalizability of these findings. Additionally, while we used a multi-step approach for evaluating anovulation that took into account pregnancy status via hCG assays, phase-specific urinary PdG and LH concentrations, and fertility monitor readings, we may have potentially misclassified cycles. This could be due to urinary PdG and LH cut-offs that may miss a true ovulation.
Conclusions
In summary, among healthy women attempting pregnancy after 1-2 pregnancy losses, we found preliminary evidence that sub-acute inflammation is associated with reduced fecundability, but not after adjusting for adiposity. Increased risk of anovulation did not appear to drive this association. In combination with our prior findings of LDA restoring pregnancy rates among women with higher inflammation, these collective results indicate hsCRP is a marker of who would benefit from LDA's effect on pregnancy, even though hsCRP's causal role is uncertain. The role of adiposity in a potentially causal relationship between inflammation and fecundability is complex, and further investigation is needed to disentangle the role of obesity-associated inflammation from inflammation triggered by other causes and their respective impacts on reproduction.
Supplementary Material
Supplemental Table 1. Baseline high-sensitivity C-reactive protein and fecundability among 572 women assigned to the placebo: EAGeR Trial, USA, 2007-2012.
Supplemental Table 2. High-sensitivity C-reactive protein and risk of anovulation: 1,150 women assigned to low-dose aspirin and placebo (2,019 cycles)- EAGeR Trial- USA- 2007-2012.
Supplemental Figure 1. Flow diagram showing numbers of women at each stage of the study.
Figure 1.
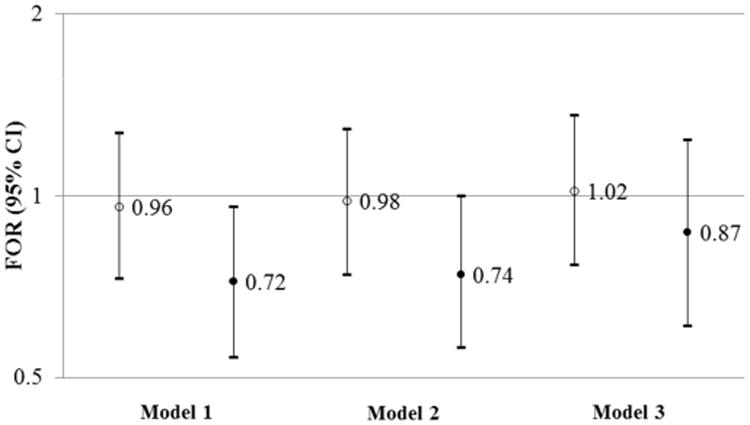
The Fecundability Odds Ratio (FOR) and 95% CI for the second tertile (open circle) and third tertile (closed circle) of hsCRP relative to the first tertile. Results are restricted to 572 women assigned to placebo. Model 1 is adjusted for cycles trying at enrollment. Model 2 is adjusted for cycles trying at enrollment, age (linear variable), race (white, non-white), marital status (married, living as married or other) and the time from last pregnancy loss to study enrollment (≤4 months, 5-8 months, 9-12 months, >12 months). Model 3 is adjusted for cycles trying at enrollment, age (linear variable), race (white, non-white), marital status (married, living as married or other) the time from last pregnancy loss to study enrollment (≤4 months, 5-8 months, 9-12 months, >12 months), and BMI (linear variable).
Acknowledgments
The authors thank Dr. Aijun Ye for his assistance with data management and Ms. Laurie Lesher, Dr. Anne Lynch, Dr. Jean Wactawski-Wende, Dr. Janet Townsend, Dr. Noya Galai, and Dr. David Faraggi, for their assistance with study management and operations. The authors appreciate the outstanding commitment of the EAGeR participants and study staff.
Funding Support: Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland (Contract Nos. HHSN267200603423, HHSN267200603424, HHSN267200603426).
Footnotes
Conflicts of interest: Dr. Pollack reports payment from ICF Global Consulting for work performed while consulting on a project about preterm birth and spontaneous abortion for the US Environmental Health and Protection Agency and payment from Triway Chinese Delegation for development of an educational presentation on environmental factors affecting female reproductive health. All other authors report no potential conflicts of interest.
Clinical Trial Registration Number: ClinicalTrials.gov, NCT00467363
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sirois J, Sayasith K, Brown KA, Stock AE, Bouchard N, Dore M. Cyclooxygenase-2 and its role in ovulation: a 2004 account. Hum Reprod Update. 2004;10:373–85. doi: 10.1093/humupd/dmh032. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez F, Mather K, Considine R, Pardue S, Acton A. Suppression of nutrient-induced inflammation with a nonsteroidal anti-inflammatory agent ameliorates ovarian dysfunction in lean insulin-sensitive women with polycystic ovary syndrome (PCOS) Fertil Steril. 2015;104:e21. [Google Scholar]
- 3.Macklon NS, Brosens JJ. The human endometrium as a sensor of embryo quality. Biol Reprod. 2014;91:98. doi: 10.1095/biolreprod.114.122846. [DOI] [PubMed] [Google Scholar]
- 4.Galgani M, Insabato L, Cali G, Della Gatta AN, Mirra P, Papaccio F, et al. Regulatory T cells, inflammation, and endoplasmic reticulum stress in women with defective endometrial receptivity. Fertil Steril. 2015;103:1579–86.e1. doi: 10.1016/j.fertnstert.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 5.Halme J, Becker S, Haskill S. Altered maturation and function of peritoneal macrophages: Possible role in pathogenesis of endometriosis. Am J Obstet Gynecol. 1987;156:783–9. doi: 10.1016/0002-9378(87)90333-4. [DOI] [PubMed] [Google Scholar]
- 6.Kelly CCJ, Lyall H, Petrie JR, Gould GW, Connell JMC, Sattar N. Low Grade Chronic Inflammation in Women with Polycystic Ovarian Syndrome. J Clin Endocrinol Metab. 2001;86:2453–5. doi: 10.1210/jcem.86.6.7580. [DOI] [PubMed] [Google Scholar]
- 7.Vgontzas AN, Trakada G, Bixler EO, Lin HM, Pejovic S, Zoumakis E, et al. Plasma interleukin 6 levels are elevated in polycystic ovary syndrome independently of obesity or sleep apnea. Metabolism. 2006;55:1076–82. doi: 10.1016/j.metabol.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Sjaarda LA, Radin RG, Silver RM, Mitchell EM, Mumford SL, Wilcox B, et al. Preconception low-dose aspirin restores diminished pregnancy and live birth rates in women with low grade inflammation: a secondary analysis of a randomized trial. J Clin Endocrinol Metab. 2017;102:1495–1504. doi: 10.1210/jc.2016-2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wisse BE, Kim F, Schwartz MW. An Integrative View of Obesity. Science. 2007;318:928–9. doi: 10.1126/science.1148032. [DOI] [PubMed] [Google Scholar]
- 10.Schisterman EF, Silver RM, Perkins NJ, Mumford SL, Whitcomb BW, Stanford JB, et al. A randomised trial to evaluate the effects of low-dose aspirin in gestation and reproduction: design and baseline characteristics. Paediatr Perinat Epidemiol. 2013;27:598–609. doi: 10.1111/ppe.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson S, Pemberton P, Laing I, Nardo LG. Low grade inflammation, as evidenced by basal high sensitivity CRP, is not correlated to outcome measures in IVF. J Assist Reprod Genet. 2008;25:383–8. doi: 10.1007/s10815-008-9253-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ridker PM. C-Reactive Protein: A Simple Test to Help Predict Risk of Heart Attack and Stroke. Circulation. 2003;108:e81–5. doi: 10.1161/01.CIR.0000093381.57779.67. [DOI] [PubMed] [Google Scholar]
- 13.Zacur H, Kaufman SC, Smith B, Westhoff C, Helbig D, Lee YJ, et al. Does creatinine adjustment of urinary pregnanediol glucuronide reduce or introduce measurement error? Gynecol Endocrinol. 1997;11:29–33. doi: 10.3109/09513599709152314. [DOI] [PubMed] [Google Scholar]
- 14.Johnson S, Weddell S, Godbert S, Freundl G, Roos J, Gnoth C. Development of the first urinary reproductive hormone ranges referenced to independently determined ovulation day. Clin Chem Lab Med. 2015;53:1099–108. doi: 10.1515/cclm-2014-1087. [DOI] [PubMed] [Google Scholar]
- 15.Park SJ, Goldsmith LT, Skurnick JH, Wojtczuk A, Weiss G. Characteristics of the urinary luteinizing hormone surge in young ovulatory women. Fertil Steril. 2007;88:684–90. doi: 10.1016/j.fertnstert.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 16.Behre HM, Kuhlage J, Gaβner C, Sonntag B, Schem C, Schneider HPG, et al. Prediction of ovulation by urinary hormone measurements with the home use ClearPlan® Fertility Monitor: comparison with transvaginal ultrasound scans and serum hormone measurements. Hum Reprod. 2000;15:2478–82. doi: 10.1093/humrep/15.12.2478. [DOI] [PubMed] [Google Scholar]
- 17.Schisterman EF, Mumford SL, Schliep KC, Sjaarda LA, Stanford JB, Lesher LL, et al. Preconception low dose aspirin and time to pregnancy: findings from the effects of aspirin in gestation and reproduction randomized trial. J Clin Endocrinol Metab. 2015;100:1785–91. doi: 10.1210/jc.2014-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Iii, Criqui M, et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the centers for disease control and prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 19.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–61. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 20.Li R, Hertzmark E, Louie M, Chen L, Spiegelman D. The SAS LGTPHCURV9 Macro. Boston, MA: Channing Division of Network Medicine; 2011. [Google Scholar]
- 21.Thaler JP, Schwartz MW. Minireview: Inflammation and obesity pathogenesis: the hypothalamus heats up. Endocrinology. 2010;151:4109–15. doi: 10.1210/en.2010-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynch KE, Mumford SL, Schliep KC, Whitcomb BW, Zarek SM, Pollack AZ, et al. Assessment of anovulation in eumenorrheic women: comparison of ovulation detection algorithms. Fertil Steril. 2014;102:511–8.e2. doi: 10.1016/j.fertnstert.2014.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radin RG, Sjaarda LA, Perkins NJ, Silver RM, Chen Z, Lesher LL, et al. Low-dose aspirin and sporadic anovulation in the EAGeR randomized trial. J Clin Endocrinol Metab. 2017;82:86–92. doi: 10.1210/jc.2016-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–42. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 25.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 26.Clark AM, Thornley B, Tomlinson L, Galletley C, Norman RJ. Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Hum Reprod. 1998;13:1502–5. doi: 10.1093/humrep/13.6.1502. [DOI] [PubMed] [Google Scholar]
- 27.Practice Committee of the American Society for Reproductive Medicine. Obesity and reproduction: a committee opinion. Fertil Steril. 2015;104:1116–26. doi: 10.1016/j.fertnstert.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 28.Chen K, Li F, Li J, Cai H, Strom S, Bisello A, et al. Induction of leptin resistance through direct interaction of C-reactive protein with leptin. Nature Med. 2006;12:425–32. doi: 10.1038/nm1372. [DOI] [PubMed] [Google Scholar]
- 29.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–72. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perng W, Rifas-Shiman SL, Rich-Edwards JW, Stuebe AM, Oken E. Inflammation and weight gain in reproductive-aged women. Ann Hum Biol. 2016;43:91–5. doi: 10.3109/03014460.2014.968619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Capobianco G, de Muro P, Cherchi GM, Formato M, Lepedda AJ, Cigliano A, et al. Plasma levels of C-reactive protein, leptin and glycosaminoglycans during spontaneous menstrual cycle: differences between ovulatory and anovulatory cycles. Arch Gynecol Obstet. 2010;282:207–13. doi: 10.1007/s00404-010-1432-2. [DOI] [PubMed] [Google Scholar]
- 33.Clancy KB, Baerwald AR, Pierson RA. Systemic inflammation is associated with ovarian follicular dynamics during the human menstrual cycle. PloS one. 2013;8:e64807. doi: 10.1371/journal.pone.0064807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaskins AJ, Wilchesky M, Mumford SL, Whitcomb BW, Browne RW, Wactawski-Wende J, et al. Endogenous reproductive hormones and C-reactive protein across the menstrual cycle: the BioCycle Study. Am J Epidemiol. 2012;175:423–31. doi: 10.1093/aje/kwr343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chambers JC, Ueland PM, Obeid OA, Wrigley J, Refsum H, Kooner JS. Improved vascular endothelial function after oral B vitamins: An effect mediated through reduced concentrations of free plasma homocysteine. Circulation. 2000;102:2479–83. doi: 10.1161/01.cir.102.20.2479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Baseline high-sensitivity C-reactive protein and fecundability among 572 women assigned to the placebo: EAGeR Trial, USA, 2007-2012.
Supplemental Table 2. High-sensitivity C-reactive protein and risk of anovulation: 1,150 women assigned to low-dose aspirin and placebo (2,019 cycles)- EAGeR Trial- USA- 2007-2012.
Supplemental Figure 1. Flow diagram showing numbers of women at each stage of the study.


