Abstract
Genetically-encoded photosensitizers produce reactive oxygen species (ROS) in response to light. Transgenic expression of fusion proteins can target the photosensitizers to specific cell regions and permit the spatial and temporal control of ROS production. These ROS-generating proteins (RGPs) are widely used for cell ablation, mutagenesis and chromophore-assisted light inactivation of target proteins. However, the species produced by RGPs are unclear due to indirect measures with confounding interpretations. Recently, the RGP mini “Singlet Oxygen Generator” (miniSOG) was engineered from Arabidopsis thaliana phototropin 2. While miniSOG produces singlet oxygen (1O2), the contribution of superoxide (O2•−) to miniSOG-generated ROS remains unclear. We measured the light-dependent O2•− production of purified miniSOG using HPLC separation of dihydroethidium (DHE) oxidation products. We demonstrate that DHE is insensitive to 1O2 and establish that DHE is a suitable indicator to measure O2•− production in a system that produces both 1O2 and O2•−. We report that miniSOG produces both 1O2 and O2•−, as can its free chromophore, flavin mononucleotide. miniSOG produced O2•− at a rate of ~4.0 μmol O2•−/min/μmol photosensitizer for an excitation fluence rate of 5.9 mW/mm2 at 470 ± 20 nm, and the rate remained consistent across fluences (light doses). Overall, the contribution of O2•− to miniSOG phenotypes should be considered.
Keywords: miniSOG, reactive oxygen species, singlet oxygen, superoxide, Flavin mononucleotide
Introduction
Photosensitizers produce reactive oxygen species (ROS) in response to light [1]. Reactive-oxygen-species-generating proteins, or RGPs, are a class of genetically-encoded photosensitizers [1]. These include SuperNova [2], KillerRed [3], KillerOrange [4], and miniSOG [5]. A RGP has the capability to generate different types of ROS including superoxide (O2•−) and singlet oxygen (1O2). mini “Singlet Oxygen Generator” (miniSOG) is unique in that it generates a relatively large quantum yield of 1O2 [5]. This ROS production is attributed to its chromophore flavin mononucleotide (FMN), a well-known 1O2-generating photosensitizer [6, 7]. miniSOG has been used for a variety of applications, such as electron microscopy [5], cell death [8, 9], mutagenesis [10] and target protein inactivation [11, 12]. While miniSOG was successful for these applications, the ROS responsible remained unknown as the 1O2 yield was debated [13, 14].
The disparity between 1O2 yields with different detection methods led to the hypothesis that O2•− may be a species produced by miniSOG [13]. Pimenta et al. measured O2•− production using the fluorescence of dihydroethidium (DHE) oxidation products, a nonspecific measure of O2•−. The fluorescence of DHE oxidation products can be the result of the O2•− specific product, 2-OHE+, and the nonspecific product, E+. These resulting DHE oxidation products are indistinguishable via fluorescence alone, and require HPLC separation to measure O2•− production specifically [15, 16]. Moreover, although based on fluorescence, there are conflicting results and no clear consensus on whether or not 1O2 can react with DHE to form E+ [17–19]. Overall, our goal was to clarify the impact of 1O2 on DHE-oxidation products and confirm if miniSOG generates O2•− by measuring the formation of the O2•− specific DHE oxidation product, 2-OHE+.
Thus, we measured O2•− generated by miniSOG using HPLC separation of the DHE oxidation products to specifically detect O2•−. We characterized the measurement system using Rose Bengal, a chemical photosensitizer that generates both O2•− and 1O2 [20–22]. Detailed DHE oxidation product analysis demonstrates that 1O2 does not react with DHE. Under conditions where miniSOG makes 1O2, it also produces O2•− at a flux that is consistent across fluences (light doses).
Materials and Methods
Singlet oxygen detection using singlet oxygen sensor green
1O2 was measured using singlet oxygen sensor green (SOSG; Molecular Probes). SOSG has a weak blue fluorescence but upon reaction with 1O2 exhibits a strong green fluorescence [23]. SOSG (1μM) baseline fluorescence was measured in a cuvette containing a photosensitizer, Rose Bengal (RB, 2.5 μM; Sigma) or Deuteroporphyrin (DP, 2.5 μM; Frontier Scientific), and SOSG buffer (SB; 120 mM KCl, 25 mM sucrose, 5 mM MgCl2, 5 mM KH2PO4, 1 mM EGTA, 10 mM HEPES, 0.1 mM DPTA, pH 7.3). Where indicated, 20 mM azide or 800 units/mL superoxide dismutase (SOD, Sigma) was present (as illustrated in scheme 1). Temperature was held constant at 25°C. The fluorescence (Ex 488 nm; Em 525 nm; slit width 5 nm) was recorded for 1 minute with constant stirring before and after illumination (560 ± 20 nm, 10.6 mW/mm2) for 0–30 minutes. The change in fluorescence intensity (post minus pre-illumination) was calculated. Experiments using FMN (10 μM) or purified miniSOG (10 μM) were illuminated at 470 ± 20 nm (5.9 mW/mm2). To avoid spectral overlap with FMN, SOSG fluorescence was excited at 504 nm (Em 525 nm; slit width 5 nm). Structures of Rose Bengal and FMN are shown in Supplemental Fig. 1.
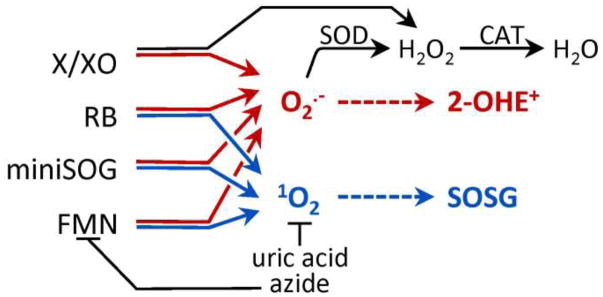
Scheme 1. Overview of ROS detection methods.
ROS generators were used to produce superoxide (O2•−) and/or singlet oxygen (1O2). 2-hydroxyethidum (2-OHE+) is a O2•−-selective marker, while 1O2 was detected using Singlet Oxygen Sensor Green (SOSG). Superoxide dismutase (SOD) converts O2•− to hydrogen peroxide (H2O2), which can be removed by catalase (CAT) to water. Both uric acid and azide quench 1O2, while azide additionally quenches the triplet state of FMN. Abbreviations: X (Xanthine), XO (Xanthine oxidase), RB (Rose Bengal), FMN (flavin mononucleotide).
Light intensity was measured using a calibrated thermopile detector (818P-010-12, Newport Corporation, Irvine, CA) connected to an optical power meter (1916-R, Newport Corporation). For all experiments in which the illumination was varied, the intensity of light or fluence rate (watts/area) was held constant. The total fluence (joules/area) was altered by varying the illumination time.
Quantification of xanthine/xanthine oxidase superoxide production using cytochrome c
Xanthine oxidase (XO) catalyzes the oxidation of xanthine (X) to uric acid. During this reaction XO generates O2•− or H2O2 via either a 1 or 2 electron reduction, respectively. The rate of X/XO O2•− formation was measured as the rate of SOD-sensitive cytochrome c reduction. Briefly, at ambient O2, XO (0.1 units/mL) and X (1 mM) were added to a 0.1 cm cuvette containing cytochrome c (800 mM). The rate of cytochrome c reduction was monitored at 550 nm and calculated using an extinction coefficient of 18.7 M−1 cm−1 in the presence or absence of SOD (800 units/mL) [24]. The X/XO reaction predominately generates H2O2 in a pH and oxygen concentration-dependent manner [25]. The percentage of O2•− generated by X/XO was calculated by dividing the O2•− generation by the total electron flux of X to uric acid by XO, as previously described by Kelley et al. [25].
Superoxide detection using HPLC separation of DHE oxidation products
Xanthine/xanthine oxidase (X/XO) system
O2•− was measured using dihydroethidium (DHE; Thermo Fisher Scientific) followed by HPLC separation of the resulting oxidation products [16]. Upon oxidation, DHE forms red fluorescent products, a O2•− specific product, 2-OHE+, and a nonspecific product, E+, which must be separated by HPLC due to overlapping fluorescence spectra [16, 26]. Structures of DHE, 2-OHE+ and E+ are shown in Supplemental Fig. 1. In 1 mL of PBS containing DPTA (DPBS; 7.78 mM Na2HPO4, 2.2 mM KH2PO4, 0.1 mM DPTA, pH 7.4 at 37°C) and DHE (50 μM), 0.1 units/mL XO and 1 mM X were added to generate O2•−. Where indicated, 800 units/mL SOD was present (as illustrated in scheme 1). The solution was incubated at 37°C for 0–20 minutes (as indicated) to generate increasing amounts of O2•−, after which the reaction was stopped with an equal volume of acidified methanol (200 mM HClO4 in methanol). The solution was incubated at −20˚C for 30 minutes and then centrifuged at 17,000 × g, 4°C for 20 minutes. Next, an equal volume of sample was combined with 1 M K+PO4− (pH 2.6). Again, the sample was incubated at −20˚C for 30 minutes and then centrifuged at 17,000 × g for 10 minutes. Samples were separated on a Polar-RP column (Phenomenex, 150 × 2 mm; 4μm) run on Shimadzu HPLC with fluorescence detection (RF-20A). From 0 to 15 minutes the detector was on low sensitivity (channel 1: Ex: 358 nm, Em: 440 nm; channel 2: Ex: 490 nm, Em: 596 nm). After 15 minutes, the sensitivity switched to high (channel 1: Ex: 490 nm, Em: 567 nm; channel 2 remained constant) with a constant flow rate of 0.1 mL/min. Two mobile phases were used (A, 10% ACN with 0.1 %TFA in water; B, 60% ACN with 0.1 %TFA in water) using the following gradient: 0 min, 40% B; 5 min, 40% B; 25 min, 100% B; 30 min, 100% B; 35 min, 40% B; 40min, 40% B. Concentrations of DHE, 2-OHE+ and E+ were measured using standard curves. DHE and E+ standards were commercially available. The 2-OHE+ was made using X/XO generated O2•−. The resulting mixture was separated using HPLC and the fraction containing 2-OHE+ was collected and lyophilized to a dry powder. 2-OHE+ was confirmed using mass spectrometry (URMC Mass Spectrometry Resource Laboratory; m/z = 330.16; the intervals between isotopic peaks = 1.0).
Photosensitizers
Superoxide production from photosensitizers was measured as described above with the following modifications. DHE (50 μM) and RB or DP (2.5 μM) were illuminated (560 ± 20 nm, 17 mW/mm2) in assay buffer (120 mM KCl, 25 mM sucrose, 5 mM MgCl2, 5 mM KH2PO4, 1 mM EGTA, 10 mM HEPES, 0.1 mM DPTA, pH 7.3) for 0–5 minutes. As a control, one cuvette was also incubated in the dark. Where indicated, 20 mM azide or 800 units/mL SOD or 4,200 units/mL of catalase (CAT, 1 mg/mL; from bovine liver or from Corynebacterium glutamicum as indicated, Sigma) or hydrogen peroxide (H2O2, Sigma) was present. The procedure was repeated with 10 μM free FMN (Sigma) or purified miniSOG. Samples were illuminated at 470 ± 20 nm (5.9 mW/mm2).
miniSOG Purification
Recombinant miniSOG with an N-terminal histidine tag was expressed and purified from BL21DE3pLYS cells. Briefly, the coding region of miniSOG (courtesy of Drs. Roger Tsien and Yishi Jin, University of California, San Diego) was inserted in pRSET B using BamHI and EcoRI. The plasmid was electroporated into competent BL21pLyS cells and plated on ampicillin plates (100 μg/mL). A single colony was grown to OD600 of 0.5 and miniSOG expression was induced using IPTG (100 μM). Cells were grown in the dark and lysed after 4 hours of expression with lysis buffer (3% SDS, 50 mM Tris, pH 8.0) and protease inhibitors (Pierce). His-tagged miniSOG was then allowed to bind to Ni-NTA agarose beads (Qiagen) overnight; fluorescence was monitored to ensure protein binding. miniSOG was eluted with 100 μM imidazole and desalted with a PD-10 column (GE Healthcare). The sample was concentrated using a centrifugal filter with a 10 KDa cutoff (Amicon) and stored in 10% glycerol PBS buffer, at 4°C. To ensure that miniSOG ROS generation was only due to miniSOG bound FMN, ROS measurements were taken immediately after concentrating, thus ensuring any free FMN would be removed. The entire purification process was performed in the dark and the protein was protected from light. The resulting protein was characterized by measuring the extinction coefficient, fluorescent quantum yield using fluorescein as standard (ΦF = 0.95) [27], and the absorption and emission spectra. Since the purified miniSOG contained both mature (FMN-containing) and immature (void of FMN) protein, the concentration of mature functional protein was determined using the extinction coefficient of flavin as previously described [13]. Briefly, miniSOG was denatured to release FMN and the concentration of free FMN (ε=12,500 ± 500 M−1 cm−1 at 450 nm) [28, 29] was determined using a standard curve. Since a mature photoactivatable miniSOG contains a single FMN as its chromophore, the concentration of FMN was assumed to equal the concentration of mature miniSOG [5, 13, 14].
Results
Detection of O2•− using HPLC separated DHE-oxidation products
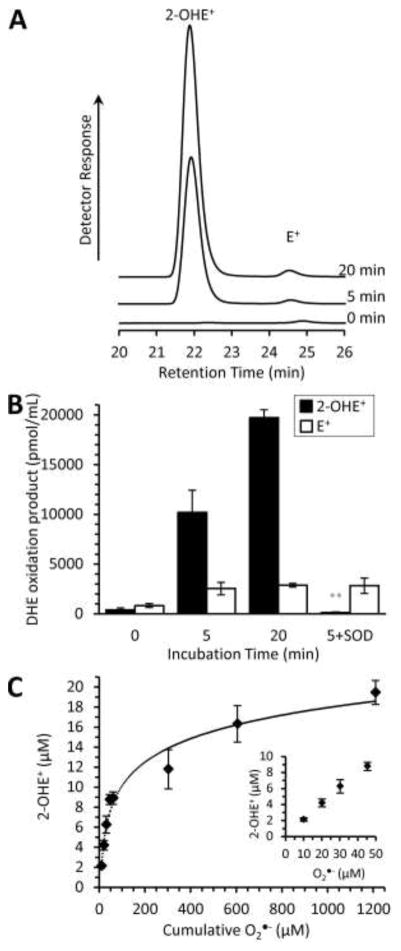
First, we characterized the HPLC separation and fluorescent detection of DHE oxidation products using the X/XO generated O2•− (Fig. 1). Importantly, the X/XO reaction does not generate 1O2 and moreover, a reaction product, uric acid, is an efficient 1O2 scavenger [30]. Thus, we attribute changes in DHE oxidation products to O2•− and resulting dismutation products. We observed an incubation-time-dependent increase in 2-OHE+ (Fig 1A) with a minimal impact on the E+ component. SOD, which scavenges O2•− 1,000 times faster than it can react with the DHE [16], significantly decreased the amount of detectable 2-OHE+, while the E+ component was unaffected (Fig. 1B). We attempted to mimic the inhibitory effect of SOD on 2-OHE+ formation using the chemical O2•− scavenger, TEMPO, but found that TEMPO converted all of the DHE to E+, thereby preventing any possible 2-OHE+ formation (Supplemental Fig. 2). This is consistent with a previous result, which observed an increased fluorescence in DHE products in the presence of TEMPOL [31]. Our findings confirm the E+, not 2-OHE+, contribution to the observed fluorescence signal [19]. We conclude that the HPLC separation of DHE oxidation products can measure O2•− production via 2-OHE+ formation in an SOD-sensitive manner.
Fig. 1.
Characterization of DHE oxidation products derived from xanthine/xanthine oxidase generated ROS. (A) Representative HPLC traces of the fluorescence versus retention time separation of xanthine/xanthine oxidase generated 2-OHE+ and E+. Xanthine (X, 1mM) and xanthine oxidase (XO, 0.1 units/mL) were incubated for increasing amounts of time to generate O2•− in the presence of DHE (50 μM). The resulting DHE oxidation products were separated via HPLC. Using fluorescence detection, peaks at 22.0 and 24.5 minutes correspond to 2-OHE+ and E+, respectively. DHE was not limiting. (B) Peaks were integrated and quantified using a standard curve. The addition of SOD (800 U/mL) to X/XO reduced the 2-OHE+ component while not effecting E+. (C) 2-OHE+ formation is dependent on the concentration of O2•−. Using the cytochrome c reduction assay, X/XO O2•− production was determined to be 60.5 ± 8.7 μM/min. XO and X were incubated for increasing amounts of time in the presence of DHE as in A, B to generate 2-OHE+ as a function of time. The ratio of O2•− to 2-OHE+ was found to be logarithmic (y=3.3838ln(x)–5.4326; R2 = 0.97). Inset is 2-OHE+ formation at the lower O2•− concentrations. All data are means ± SEM, N > 4. ** p < 0.01 versus 5-minute incubation (2-way ANOVA with Tukey correction).
We sought to quantify the superoxide production rate of miniSOG, however, the ratio of 2-OHE+ formation to O2•− is not linear. Given a fourfold difference in X/XO incubation time (5 min vs. 20 min), the 2-OHE+ concentration only doubled (Fig. 1B). A recent report demonstrated that the stoichiometry of O2•− molecules to 2-OHE+ formation varies with the concentration of O2•− [15]. For low concentrations of O2•−, the O2•− to 2-OHE+ ratio is 2:1 and increases nonlinearly with the concentration of O2•− [15]. The increase in the O2•−:2-OHE+ ratio is hypothesized to be the result of increased O2•− dismutation [15]. Therefore, in order to equate 2-OHE+ formation with O2•−, we first determined the O2 •− production capabilities of X/XO using cytochrome c reduction. Secondly, we incubated X/XO with DHE for various incubation times and measured 2-OHE+.
XO produces both H2O2 and O2•− in a pH and oxygen dependent manner [25]. The rate of O2•− production was measured via cytochrome c reduction. Under conditions that mimic DHE experiments, the O2•− flux of X/XO was 60.5 ± 8.7 μM/min. This rate demonstrates that 30.3% of total XO electron flux results in O2•− and is consistent with a previous report [25]. Next, we measured 2-OHE+ formation as a function of time to mimic photosensitizer O2•− formation (Fig. 1C). The cumulative amount of 2-OHE+ generated during a period was plotted as a function of cumulative O2•− generated. The stoichiometry of O2•− to 2-OHE+ is in agreement with previous reports [15] and follows a logarithmic relationship. Using X/XO O2•− generation as a standard, we can quantify O2•− production using 2-OHE+ formation.
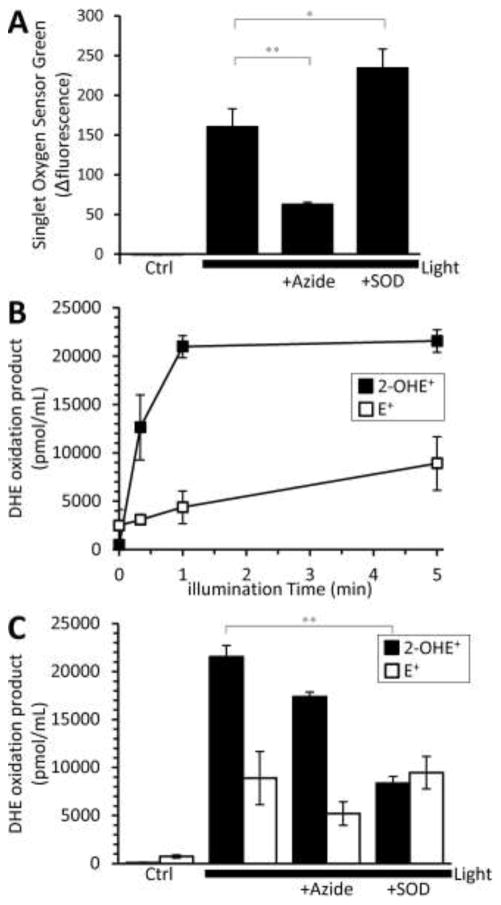
Next, to determine whether DHE is suitable for measuring photosensitizer-generated ROS, we measured DHE oxidation products resulting from the illumination of Rose Bengal (RB). Since RB is known to generate large quantities of 1O2 and to a lesser amount O2•− [6, 7, 22], it served as a control to determine if 1O2 has confounding effects on the DHE oxidation products. Additionally, DHE is light-sensitive and can undergo photo-oxidation [32]. The illumination of Singlet Oxygen Sensor Green (SOSG) or DHE alone did not prevent the detection of photosensitizer-generated 1O2 or O2•− (see figure legends for each wavelength). When RB was illuminated, 1O2 was detected (Fig. 2 A) and we found the rapid formation of DHE-oxidation products (Fig. 2B). Notably, both the 2-OHE+ and E+ components were insensitive to the 1O2 scavenger azide (Fig. 2C, p = 0.16; p = 0.13; 5 min light vs 5 min light + azide for 2-OHE+ and E+, respectively), which suggests that DHE is insensitive to 1O2. Consistent with the X/XO data, SOD specifically reduced RB generated 2-OHE+, suggesting we can detect O2•− despite the presence of 1O2. Similar results were found using another chemical photosensitizer, deuteroporphyrin (DP, Supplemental Fig. 3). Collectively, these results demonstrate that the formation of the O2•−-selective DHE oxidation product 2-OHE+ is SOD-sensitive and that azidesensitive 1O2 does not impact its formation, as seen in scheme 1.
Fig. 2.
Detection of Rose Bengal (RB) O2•− production by DHE. (A) RB was illuminated (560 ± 20 nm, 10.6 mW/mm2) for 5 minutes in the presence of Singlet Oxygen Sensor Green (SOSG). 1O2 production was determined by the change in SOSG fluorescence intensity. The 1O2 scavenger, azide (20 mM) or O2•− scavenger superoxide dismutase (SOD, 800 U/mL) was present where indicated. Azide or SOD in the presence of SOSG in the dark alone had no effect. SOSG illuminated for 5 minutes in the absence of photosensitizer yielded a change in fluorescence of −6.28 ± 1.4 a.u. Since RB has a large 1O2 quantum yield, we wanted to determine if we were still able to detect O2•− with dihydroethidium (DHE). (B, C) RB (2.5 μM) was illuminated under the same conditions for increasing amounts of time in the presence of DHE (50 μM). Peaks, 2-OHE+ and E+, were integrated and quantified using a standard curve. The formation of the O2•− specific product, 2-OHE+, is sensitive to SOD, as the addition of SOD (800 U/mL) reduced the 2-OHE+ component while not effecting the E+ component. Azide (20 mM) had no effect on either component, 2-OHE+ or E+. DHE illuminated for 5 minutes resulted in 258 ± 186 pmol/mL 2-OHE+ and 607 ± 423 pmol/mL E+. All data are means ± SEM, N > 4. ** p < 0.01 versus 5-minute light (2-way ANOVA with Tukey correction).
miniSOG 1O2 generation
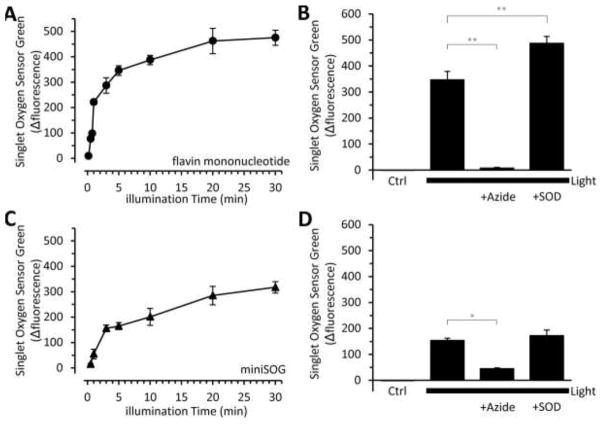
Given that the HPLC separation of DHE oxidation products can measure O2•− in the presence of 1O2, we next sought to determine if miniSOG produces O2•−. We purified miniSOG and the resulting protein had an extinction coefficient of 15.15 ± 0.9 × 103 M−1 cm−1, fluorescence quantum yield of 0.35 ± 0.017, and an expected molecular weight (Supplemental Fig. 4) as previously reported [5, 13]. miniSOG is a small protein that encapsulates a flavin mononucleotide (FMN), which acts as the photosensitizer. In order to understand its ROS production, we therefore compared miniSOG to its free chromophore, FMN. Both FMN and miniSOG generate 1O2 in response to light [5]. We first determined if miniSOG was functional by detecting 1O2 via Singlet Oxygen Sensor Green (SOSG).
We found that both FMN and miniSOG behaved similarly, with SOSG fluorescence steadily increasing with illumination time until a peak value was reached (Fig. 3A, C). Notably, FMN produced a larger amount of detectable 1O2 at a faster rate than miniSOG (99.5 a.u./min versus 24.6 a.u./min, FMN vs. miniSOG, respectively). This supports the lower published 1O2 quantum yield of miniSOG [13, 14]. However, both reached asymptotic levels following prolonged irradiation (Fig. 3A, C). Azide prevented the FMN- and miniSOG-mediated SOSG fluorescence increase, suggesting a 1O2-dependent mechanism (Fig. 3B, D). As expected, fluorescence was not lost with SOD (Fig. 3B, D). These results demonstrate that the purified miniSOG is functional and, like FMN, can generate 1O2.
Fig. 3.
Singlet oxygen sensor green (SOSG) detection of flavin mononucleotide (FMN) and miniSOG generated 1O2. (A) Free flavin mononucleotide (FMN) and (C) purified miniSOG were illuminated (470 ± 20 nm; 5.9 mW/mm2) for the indicated time in the presence of SOSG. 1O2 production was determined by the change in SOSG fluorescence intensity. Note: SOSG was excited at 504 nm. Where indicated, the 1O2 scavenger, azide (20 mM) or O2•− scavenger, SOD (800 U/mL) were present when (B) free FMN or (D) miniSOG were illuminated for 5 minutes. SOSG illuminated for 5 minutes yielded a change in fluorescence of −1.53 ± 0.3 a.u. All data are means ± SEM, N > 4. *p<0.05, **p<0.01 versus 5-minute light (2-way ANOVA with Tukey correction).
miniSOG O2•− generation
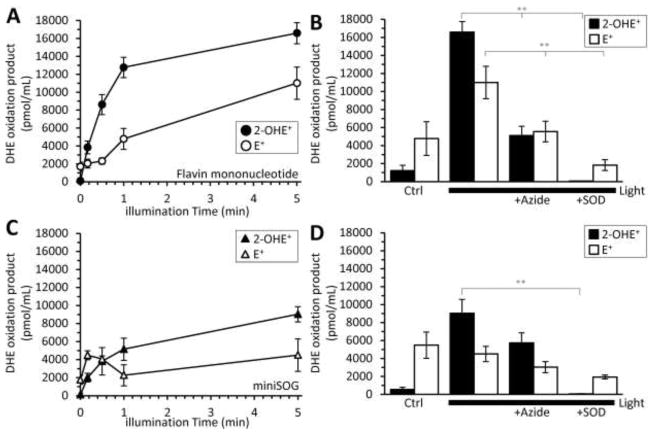
Similar to RB, FMN generates both 1O2 (Fig. 3) and O2•− in response to light [6, 7]. However, the precise mechanism of light-induced miniSOG ROS production was unclear. We hypothesized that miniSOG could produce both 1O2 and O2•− in a manner similar to its free photosensitizer, FMN. Alternatively, miniSOG’s protein encapsulation of the FMN could alter its ROS production properties to favor a particular photosensitization mechanism. Thus, we next measured the O2•− production of FMN and miniSOG using HPLC separation of DHE oxidation products, as seen in scheme 1. FMN and miniSOG were incubated with DHE and illuminated for various amounts of time (Fig. 4A, C). Both FMN and miniSOG produced 2-OHE+, but miniSOG did so to a lesser extent. SOD significantly reduced the 2-OHE+ components in both systems (p < 0.001, Fig. 4B, D). While azide had no effect on miniSOG-mediated 2-OHE+ formation (p=0.22, 5 min light vs 5 min light + azide, Fig. 4D), azide significantly reduced the 2-OHE+ component in the FMN system (Fig. 4B). We hypothesize that the reduction is due to azide directly interacting with the excited state of FMN [33] rather than scavenging ROS. Light excites FMN to its triplet state, which can then interact with O2 to form either O2•− or 1O2. Azide can prevent the generation of ROS by interacting with FMN’s excited state, thus preventing FMN’s ability to react with O2 [33]. Azide quenches 1O2 with KQ ~ 2 to 4 × 108 M−1s−1; however, azide additionally quenches the triplet state of FMN at a rate of ~ 2 to 5 ×109 M−1s−1 [7]. Thus, the decrease in FMN light-dependent 2-OHE+ formation in the presence of azide may be the result of azide preventing ROS formation rather than scavenging it (Fig. 4B). In support of this hypothesis, uric acid, a different 1O2 scavenger significantly decreased FMN 1O2 production with no effect on O2•− generation (Supplemental Fig. 5). Azide did not have the same impact on 2-OHE+ generation for miniSOG as it did with free FMN (Fig. 4D).
Fig. 4.
Detection of FMN and miniSOG light-dependent O2•− generation using HPLC separation of DHE oxidation products. (A) free FMN (10 μM) or (C) purified miniSOG (10 μM) were illuminated (470 ± 20 nm; 5.9 mW/mm2) for increasing amounts of time in the presence of DHE (50 μM). The resulting 2-OHE+ or E+ peaks were integrated and quantified using a standard curve. Where indicated, the 1O2 scavenger, azide (20 mM), O2•− scavenger, SOD (800 U/mL) were present when (B) free FMN or (D) miniSOG were illuminated for 5 minutes. DHE illuminated for 5 minutes resulted in 50 ± 17 pmol/mL 2-OHE+ and 673 ± 142 pmol/mL E+. All data are means ± SEM, N > 4. *p<0.05, ** p <0.01 versus 5-minute light (2-way ANOVA with Tukey correction.
In order to quantify miniSOG O2•− rates, we used the ratio of O2•−:2-OHE+ calculated from the X/XO system (Fig. 1C). A previous report had demonstrated that miniSOG’s 1O2 quantum yield is light dose-dependent [14]. Thus, we determined the O2•− flux-dependence on light dose (Supplemental Fig. 6). miniSOG’s O2•− remained constant at ~4.0 μmol O2•−/min/μmol photosensitizer for an excitation fluence rate of 5.9 mW/mm2 at 470 ± 20 nm and was independent of light dose (Supplemental Fig. 6).
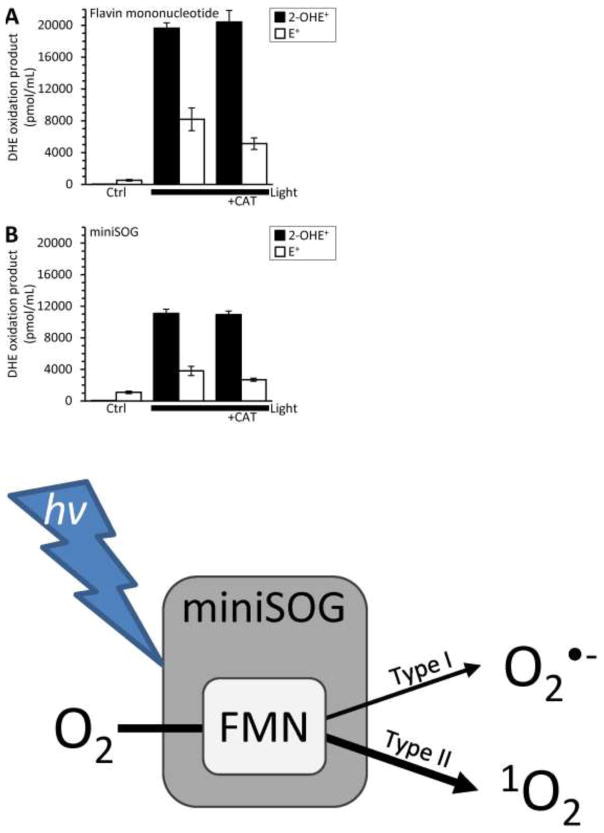
A previous study utilized catalase-sensitive DHE fluorescence to elude to miniSOG-mediated O2•− formation [13]. Catalase decomposes hydrogen peroxide (H2O2) to water and oxygen and has no direct effect on O2•−. Moreover, H2O2 does not directly react with DHE [19]. Thus, we examined the impact of H2O2 and catalase on DHE oxidation products. We found that H2O2 increased E+ formation in a catalase-dependent manner and the addition of catalase alone increased E+ formation through an unknown mechanism (Supplemental Fig. 7). Impurities in the H2O2 solution or the catalase preparation may contribute the increased E+ formation. However, catalase from Corynebacterium glutamicum yielded a finding similar to catalase from bovine liver (DHE alone, 112 ± 30 pmol/mL 2-OHE+ and 1583 ± 423 pmol/mL E+; DHE plus C. glutamicum catalase, 317 ± 31 pmol/mL 2-OHE+ and 7405 ± 1397 pmol/mL E+). Despite the impact of catalase on E+ generation (Supplemental Fig. 7), we sought to confirm if catalase could decrease miniSOG generated 2-OHE+ formation [13]. The addition of catalase to the FMN or miniSOG photosensitization reaction had no effect on the 2-OHE+ component (Fig. 5B, C) and is contrary to a previous report [13]. We suggest that the difference can be attributed to analyzing fluorescence alone as opposed to in combination with HPLC separation.
Fig. 5.
H2O2 scavenger catalase (CAT) does not impact FMN or miniSOG generated O2·−. (A) Free FMN (10 μM) or (B) purified miniSOG (10 μM) were illuminated (470 ± 20 nm; 5.9 mW/mm2) for 5 minutes in the presence of DHE (50 μM) and CAT (1 mg/mL). DHE illuminated for 5 minutes (no photosensitizer) resulted in 73 ± 37 pmol/mL 2-OHE+ and 642 ± 37 pmol/mL E+. CAT had no significant effect on either the 2-OHE+ or E+ component (2-way ANOVA with Tukey correction). All data are means ± SEM, N > 4.
Discussion
Genetically-encoded photosensitizers contain a chromophore, which when illuminated can generate ROS. The miniSOG chromophore, FMN, is relatively well-studied, has a 1O2 quantum yield of 0.51 and generates O2•− [5, 34]. However, by encapsulating this chromophore, miniSOG’s ROS-producing capabilities may be altered. miniSOG’s 1O2-generating capabilities are reported elsewhere [5, 13, 14]; however, it was unclear if it could produce O2•−, since previous studies relied on indirect measures of O2 •−and used catalase as a scavenger [13].
While DHE is a commonly used O2•− detector, fluorescence alone does not indicate the presence of O2•− due to the formation of a nonspecific oxidation product [15, 16]. Additionally, catalase, an enzyme that removes H2O2 from the system, should not impact O2•− (scheme 1). Lastly, it was unknown whether or not 1O2 could impact the oxidation of DHE [17–19]. Consequently, a report that miniSOG generates O2•− based on catalase-sensitive fluorescence spectra was inconclusive [13]. We improve upon these measurements by isolating the O2•−-specific product by HPLC separation. The data presented herein demonstrate that despite the presence of both 1O2 and O2•−, we found no evidence that 1O2 impacted the O2•−-selective DHE oxidation product (Fig. 2C). We demonstrate that miniSOG generates O2 •− in an SOD-dependent and azide/catalase-independent manner. miniSOG’s O2•− production rate was lower than its free chromophore and insensitive to light dose. Interestingly, while azide directly interacted with the excited state of FMN to prevent ROS formation, azide did not have the same effect on miniSOG. This is consistent with a lack of access to FMN in the protein or a shift in the triplet state energy of bound FMN, rendering the quenching by azide less efficient. Together, these data demonstrate that the surrounding protein can influence ROS flux from miniSOG.
The 1O2 quantum yield of miniSOG is lower than that of FMN [13, 14]. One of the contributing factors to the difference in yield is miniSOG’s protein encapsulation of FMN. Ruiz-Gonzalez et al. monitored the time-resolved phosphorescence of miniSOG-generated 1O2 with increasing fluence (0–400 J/cm2) and found a ~10-fold increase in 1O2 quantum yield [14]. The authors hypothesize that this increase in yield is due to inactivation of 1O2 quenching amino acid residues in miniSOG [14]. Thus, the 1O2 quantum yield of miniSOG ranges from 0.03 to ~0.30 and is directly related to fluence [13, 14]. In contrast to 1O2, we found no change in the O2•− production rate with increasing light dose (0–177 J/cm2, Supplemental Fig. 6), suggesting that O2•− is able to freely diffuse out of the protein.
Each ROS has unique chemical properties, which may permit preference towards a biological target [35, 36]. For example, O2•− has a high reaction rate with iron-sulfur clusters, while 1O2 by comparison has a more indiscriminate reactivity [35, 36]. miniSOG is used in various applications ranging from electron microscopy to photoablation [5, 8–12]. Understanding the type and amount of biologically available ROS produced by miniSOG can improve the experimental design and interpretation of the results.
Conclusion
Genetically-encoded photosensitizers that produce ROS are important tools for redox biology. Proteins such as miniSOG, offer spatial and temporal control over ROS production [1]. However, a complete study of their ROS-generating capability is limited and hampers further development of the optogenetic toolbox. Our results demonstrate that, in addition to 1O2, miniSOG generates O2•− in response to light, much like its FMN chromophore. The O2•− production rate was determined using HPLC separation and fluorescence detection of the O2•−-selective product, 2-OHE+. These results addressed the confounding interpretations using nonspecific readouts of fluorescence alone. The contribution of each ROS to the overall output of miniSOG is light dose-dependent and should be considered in future studies.
Supplementary Material
Highlights.
Oxidation of dihydroethidium establishes miniSOG-mediated O2•− generation
HPLC separation of O2•−-selective marker, 2-hydroethidium quantified miniSOG O2•−
miniSOG O2•− production rate was consistent over a range of fluences
Acknowledgments
We thank Paul Brookes and members of the University of Rochester Medical Center’s Mitochondrial Research Group for helpful discussions. This work was supported by the National Institutes of Health to APW (R01 NS092558) and an Institutional Ruth L. Kirschstein National Research Service Award (GM068411) to MB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wojtovich AP, Foster TH. Optogenetic control of ROS production. Redox Biol. 2014;2:368–76. doi: 10.1016/j.redox.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takemoto K, Matsuda T, Sakai N, Fu D, Noda M, Uchiyama S, Kotera I, Arai Y, Horiuchi M, Fukui K, Ayabe T, Inagaki F, Suzuki H, Nagai T. SuperNova, a monomeric photosensitizing fluorescent protein for chromophore-assisted light inactivation. Sci Rep. 2013;3:2629. doi: 10.1038/srep02629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bulina ME, Chudakov DM, Britanova OV, Yanushevich YG, Staroverov DB, Chepurnykh TV, Merzlyak EM, Shkrob MA, Lukyanov S, Lukyanov KA. A genetically encoded photosensitizer. Nat Biotechnol. 2006;24(1):95–9. doi: 10.1038/nbt1175. [DOI] [PubMed] [Google Scholar]
- 4.Sarkisyan KS, Zlobovskaya OA, Gorbachev DA, Bozhanova NG, Sharonov GV, Staroverov DB, Egorov ES, Ryabova AV, Solntsev KM, Mishin AS, Lukyanov KA. KillerOrange, a Genetically Encoded Photosensitizer Activated by Blue and Green Light. Plos One. 2015;10(12) doi: 10.1371/journal.pone.0145287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shu X, Lev-Ram V, Deerinck TJ, Qi Y, Ramko EB, Davidson MW, Jin Y, Ellisman MH, Tsien RY. A genetically encoded tag for correlated light and electron microscopy of intact cells, tissues, and organisms. PLoS Biol. 2011;9(4):e1001041. doi: 10.1371/journal.pbio.1001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silva E, Edwards AM, Pacheco D. Visible light-induced photooxidation of glucose sensitized by riboflavin. J Nutr Biochem. 1999;10(3):181–5. doi: 10.1016/s0955-2863(98)00093-x. [DOI] [PubMed] [Google Scholar]
- 7.Spikes JD, Shen HR, Kopeckova P, Kopecek J. Photodynamic crosslinking of proteins. III. Kinetics of the FMN- and rose bengal-sensitized photooxidation and intermolecular crosslinking of model tyrosine-containing N-(2-hydroxypropyl)methacrylamide copolymers. Photochem Photobiol. 1999;70(2):130–7. [PubMed] [Google Scholar]
- 8.Qi YB, Garren EJ, Shu X, Tsien RY, Jin Y. Photo-inducible cell ablation in Caenorhabditis elegans using the genetically encoded singlet oxygen generating protein miniSOG. Proc Natl Acad Sci U S A. 2012;109(19):7499–504. doi: 10.1073/pnas.1204096109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu S, Chisholm AD. Highly efficient optogenetic cell ablation in C. elegans using membrane-targeted miniSOG. Sci Rep. 2016;6:21271. doi: 10.1038/srep21271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noma K, Jin Y. Optogenetic mutagenesis in Caenorhabditis elegans. Nat Commun. 2015;6:8868. doi: 10.1038/ncomms9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wojtovich AP, Wei AY, Sherman TA, Foster TH, Nehrke K. Chromophore-Assisted Light Inactivation of Mitochondrial Electron Transport Chain Complex II in Caenorhabditis elegans. Sci Rep. 2016;6:29695. doi: 10.1038/srep29695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makhijani K, To TL, Ruiz-Gonzalez R, Lafaye C, Royant A, Shu X. Precision Optogenetic Tool for Selective Single- and Multiple-Cell Ablation in a Live Animal Model System. Cell Chem Biol. 2017;24(1):110–119. doi: 10.1016/j.chembiol.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pimenta FM, Jensen RL, Breitenbach T, Etzerodt M, Ogilby PR. Oxygen-dependent photochemistry and photophysics of “miniSOG,” a protein-encased flavin. Photochem Photobiol. 2013;89(5):1116–26. doi: 10.1111/php.12111. [DOI] [PubMed] [Google Scholar]
- 14.Ruiz-Gonzalez R, Cortajarena AL, Mejias SH, Agut M, Nonell S, Flors C. Singlet oxygen generation by the genetically encoded tag miniSOG. J Am Chem Soc. 2013;135(26):9564–7. doi: 10.1021/ja4020524. [DOI] [PubMed] [Google Scholar]
- 15.Michalski R, Michalowski B, Sikora A, Zielonka J, Kalyanaraman B. On the use of fluorescence lifetime imaging and dihydroethidium to detect superoxide in intact animals and ex vivo tissues: a reassessment. Free Radic Biol Med. 2014;67:278–84. doi: 10.1016/j.freeradbiomed.2013.10.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zielonka J, Vasquez-Vivar J, Kalyanaraman B. Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat Protoc. 2008;3(1):8–21. doi: 10.1038/nprot.2007.473. [DOI] [PubMed] [Google Scholar]
- 17.Bilski PJ, Karriker B, Chignell CF. Quenching and generation of singlet oxygen by hydroethidine and related chromophores. Chemical Physics Letters. 2009;475(1–3):116–119. [Google Scholar]
- 18.Bindokas VP, Jordan J, Lee CC, Miller RJ. Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J Neurosci. 1996;16(4):1324–36. doi: 10.1523/JNEUROSCI.16-04-01324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zielonka J, Kalyanaraman B. Hydroethidine- and MitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: another inconvenient truth. Free Radic Biol Med. 2010;48(8):983–1001. doi: 10.1016/j.freeradbiomed.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gottschalk P, Paczkowski J, Neckers DC. Factors Influencing the Quantum Yields for Rose-Bengal Formation of Singlet Oxygen. Journal of Photochemistry. 1986;35(3):277–281. [Google Scholar]
- 21.Lamberts JJM, Neckers DC. Rose-Bengal Derivatives as Singlet Oxygen Sensitizers. Tetrahedron. 1985;41(11):2183–2190. [Google Scholar]
- 22.Lee PC, Rodgers MA. Laser flash photokinetic studies of rose bengal sensitized photodynamic interactions of nucleotides and DNA. Photochem Photobiol. 1987;45(1):79–86. doi: 10.1111/j.1751-1097.1987.tb08407.x. [DOI] [PubMed] [Google Scholar]
- 23.Gollmer A, Arnbjerg J, Blaikie FH, Pedersen BW, Breitenbach T, Daasbjerg K, Glasius M, Ogilby PR. Singlet Oxygen Sensor Green(R): photochemical behavior in solution and in a mammalian cell. Photochem Photobiol. 2011;87(3):671–9. doi: 10.1111/j.1751-1097.2011.00900.x. [DOI] [PubMed] [Google Scholar]
- 24.Margoliash E, Frohwirt N. Spectrum of horse-heart cytochrome c. Biochem J. 1959;71(3):570–2. doi: 10.1042/bj0710570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelley EE, Khoo NK, Hundley NJ, Malik UZ, Freeman BA, Tarpey MM. Hydrogen peroxide is the major oxidant product of xanthine oxidase. Free Radic Biol Med. 2010;48(4):493–8. doi: 10.1016/j.freeradbiomed.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao H, Joseph J, Fales HM, Sokoloski EA, Levine RL, Vasquez-Vivar J, Kalyanaraman B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc Natl Acad Sci U S A. 2005;102(16):5727–32. doi: 10.1073/pnas.0501719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magde D, Wong R, Seybold PG. Fluorescence quantum yields and their relation to lifetimes of rhodamine 6G and fluorescein in nine solvents: improved absolute standards for quantum yields. Photochem Photobiol. 2002;75(4):327–34. doi: 10.1562/0031-8655(2002)075<0327:fqyatr>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 28.Drepper T, Eggert T, Circolone F, Heck A, Krauss U, Guterl JK, Wendorff M, Losi A, Gartner W, Jaeger KE. Reporter proteins for in vivo fluorescence without oxygen. Nat Biotechnol. 2007;25(4):443–5. doi: 10.1038/nbt1293. [DOI] [PubMed] [Google Scholar]
- 29.Mukherjee A, Walker J, Weyant KB, Schroeder CM. Characterization of flavin-based fluorescent proteins: an emerging class of fluorescent reporters. PLoS One. 2013;8(5):e64753. doi: 10.1371/journal.pone.0064753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sueishi Y, Hori M, Ishikawa M, Matsu-Ura K, Kamogawa E, Honda Y, Kita M, Ohara K. Scavenging rate constants of hydrophilic antioxidants against multiple reactive oxygen species. J Clin Biochem Nutr. 2014;54(2):67–74. doi: 10.3164/jcbn.13-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burnaugh L, Sabeur K, Ball BA. Generation of superoxide anion by equine spermatozoa as detected by dihydroethidium. Theriogenology. 2007;67(3):580–9. doi: 10.1016/j.theriogenology.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 32.Zielonka J, Vasquez-Vivar J, Kalyanaraman B. The confounding effects of light, sonication, and Mn(III)TBAP on quantitation of superoxide using hydroethidine. Free Radic Biol Med. 2006;41(7):1050–7. doi: 10.1016/j.freeradbiomed.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 33.Heelis PF. The Photophysical and Photochemical Properties of Flavins (Isoalloxazines) Chem Soc Rev. 1982;11(1):15–39. [Google Scholar]
- 34.Redmond RW, Gamlin JN. A compilation of singlet oxygen yields from biologically relevant molecules. Photochem Photobiol. 1999;70(4):391–475. [PubMed] [Google Scholar]
- 35.Bachi A, Dalle-Donne I, Scaloni A. Redox proteomics: chemical principles, methodological approaches and biological/biomedical promises. Chem Rev. 2013;113(1):596–698. doi: 10.1021/cr300073p. [DOI] [PubMed] [Google Scholar]
- 36.D’Autreaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8(10):813–24. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.