Abstract
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease in the Western world, affecting about 1/3 of the US general population and remaining as a significant cause of morbidity and mortality. The hallmark of the disease is the excessive accumulation of fat within the liver cells (hepatocytes), which eventually paves the way to cellular stress, injury and apoptosis. NAFLD is strongly associated with components of the metabolic syndrome and is fast emerging as a leading cause of liver transplant in the USA. Based on clinico-pathologic classification, NAFLD may present as isolated lipid collection (steatosis) within the hepatocytes (referred to as non-alcoholic fatty liver; NAFL); or as the more aggressive phenotype (known as non-alcoholic steatohepatitis; NASH). There are currently no regulatory agency-approved medication for NAFLD, despite the enormous work and resources that have gone into the study of this condition. Therefore, there remains a huge unmet need in developing and utilizing pre-clinical models that will recapitulate the disease condition in humans. In line with progress being made in developing appropriate disease models, this review highlights the cutting-edge preclinical in vitro and animal models that try to recapitulate the human disease pathophysiology and/or clinical manifestations.
Keywords: Non-alcoholic fatty liver disease (NAFLD), Non-alcoholic steatohepatitis (NASH), Organotypic liver system, Lipitoxic system, Diet induced animal model of non-alcoholic fatty liver disease (DIAMOND), Farnesoid X receptor (FXR)
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a leading cause of liver-related morbidity and mortality worldwide and affects almost a third of all American adults [1]. It is strongly related to insulin resistance and its associated clinical conditions such as excess body weight, type 2 diabetes, hypertension, hypertriglyceridemia and low HDL-cholesterol (all of which constitute essential elements in the spectrum of the metabolic syndrome). The NAFLD disease spectrum has two phenotypes, namely, nonalcoholic fatty liver (NAFL) which is characterized by simple steatosis without obvious cellular injury; and nonalcoholic steatohepatitis (NASH), a more aggressive disease marked by cellular injury, inflammatory infiltrates and possible progression to fibrosis or cirrhosis [2]. NASH is increasing as an etiology for end-stage liver disease as well as for hepatocellular cancer-related liver transplantation; and is expected to surpass hepatitis C for this indication in the foreseeable future [3]. The hallmark lesion of NASH is hepatocellular ballooning, which is a marker of hepatocyte injury and constitutes one of the drivers of disease progression into fibrogenic remodeling [4, 5]. Liver biopsy remains the gold standard for the definitive diagnosis of NASH, even though there is significant on-going work at using non-invasive modalities for diagnosis [6].
While data on incident rates are deficient, there are several reports that shed light on the prevalence of NAFLD. A fairly recent meta-analysis estimates the overall global prevalence of NAFLD (as diagnosed via imaging; but not biopsy) at about 25%, with the Middle East and South America (at approximately 32 and 30%, respectively) sharing the highest prevalence rates, while Africa (with about 13%) having the lowest [7]. However, in the specific case of NASH, given that liver biopsies cannot be routinely obtained at the population level, there is an inadequate direct assessment for true incidence or prevalence rates. Therefore, given the limitation in obtaining direct data on NASH, a rough estimate of prevalence in the general population, is thought to be in the range of 1.5–6.5% [7, 8].
The high prevalence of NAFLD in patients with the metabolic syndrome is likewise reciprocated by an increased risk of developing NAFLD in subjects bearing elements of the metabolic syndrome, giving credence to the bidirectional association that bind these two disease entities together [7, 9–11]. Patients with NAFLD have been shown to have higher overall mortality when compared with matched controls from the general population. However, the most common cause of death in NAFLD has been cardiovascular disease, irrespective of any metabolic comorbid conditions. Hence, the joint European Association for the Study of the Liver, European Association for the Study of Diabetes and the European Association for the Study of Obesity (EASL-EASD-EASO) guidelines recommend screening for NAFLD in all patients with persistent abnormal liver enzymes, as well as screening for metabolic syndrome in persons with steatosis, independent of liver enzymes. Likewise, they also recommend routine screening with liver enzymes and/or ultrasound for NAFLD in subjects with obesity or the metabolic syndrome [12]. In contrast, the American Association for the Study of the Liver Practice Guidance in 2017 does not recommend routine screening for NAFLD in high-risk individuals at this time. However, it calls for a high index of suspicion for NAFLD/NASH in subjects with type 2 diabetes and the use of non-invasive means to identify those at risk for advanced fibrosis (these practice guidance statements from the AASLD may be rendered obsolete by the association′s announced 2018 guidelines, which were close to publication at the time of original submission of this manuscript) [6].
Despite the significant toll that NAFLD has on health systems worldwide, the disease still remains without any approved therapies [13]. Therefore, there is an immediate unmet need to better understand the molecular drivers of the disease and its progression, and to develop methods for the best targeting and therapeutic strategies for individual patients. These require developing preclinical models that recapitulate as many aspects of human disease as possible, and allow for translation of findings from simple models to human therapeutics [13, 14]. Here, we will review some recent advances that provide hope for future precision-medicine based approaches for the treatment of NASH.
Human in vitro organotypic models of NAFLD
While numerous animal models have been developed to mimic various aspects of this complex disease spectrum (reviewed below), the use of human primary liver cells to study NAFLD in a meaningful context continues to mature. To address this need, organotypic liver systems aim to incorporate the complexities of the native human liver in vitro, including three-dimensional (3D) architecture, multiple cell types, and the dynamic microenvironment stemming from blood flow through the sinusoids. The development of complex organotypic liver systems has been reviewed elsewhere, and this section of the review will focus on how these organotypic systems have been adapted for studying NAFL and NASH [15–19].
Though immortalized human cell lines, such as HUH7 and HepG2, as well as iPSC-derived hepatocytes, can be induced towards a steatotic phenotype in culture, multiple studies have shown that these cells poorly represent the native liver metabolic function, thus keeping primary human hepatocytes as the gold standard for in vitro studies [20–22]. In addition to hepatocytes, the addition of non-parenchymal cells (NPC), including human hepatic stellate cells (HSCs), resident inflammatory cells (e.g., Kupffer cells, macrophages), and/or sinusoidal endothelial cells (sECs), affect liver biology and are known to contribute to the progression of NAFLD [23–25]. Thus, these additional cell types enable the evaluation of NPC-driven disease mechanisms, such as inflammatory cytokine expression or stellate cell activation, which are hallmark features of NASH and fibrosis.
Diverse approaches have been utilized to combine these different cell types into 3D tissues in order to mimic the architecture found in the intact liver, including layered co-cultures, spheroids, micro-patterned surfaces, human precision cut liver slices (hPCLS), and bioprinting [15–19, 26]. Further, many of these tissues can be cultured within specialized devices (e.g. microfluidic chambers) to include aspects of flow and transport. Table 1 provides a summary of companies currently developing organotypic liver models who have developed perfused multicellular liver organotypic models.
Table 1.
Overview of liver organotypic systems
| System: | HµRELflow | Regenemed | Emulate | Nortis SQL-SAL | Hepregen |
|
| |||||
| System attributes | |||||
| Base technology | Microfluidic chip | Transwell cultures | Microfluidic chip | Microfluidic chip | Micropatterned cultures |
| Primary application(s) | Clearance, toxicity screen | Toxicity screen | Toxicity screen | Toxicity screen | Clearance, toxicity screen, disease modeling |
| Disease models | No | No | No | No | Hepatitis B/C, malaria |
| Separation of cell signals | No | No | No | No | No |
| Species | Human, rat, dog, monkey | Human, rat | Human | Human | Human, rat, dog, monkey |
| Liver tissue relevance | |||||
| Cell types | Primary: hepatocytes + NPCs | Primary: hepatocytes + NPCs | Primary: hepatocytes + NPCs | Primary hepatocytes + NPC cell lines | Primary hepatocytes + 3T3 mouse fibroblasts |
| Hemodynamic flow and transport | In development | No | Yes | Yes | No |
| Physiological milieu relevance | |||||
| Hormone/growth factors/fatty acids | No public data | No public data | No public data | No public data | Supraphysiological levels of insulin/glucose |
| Validation | |||||
| Morphology/liver function | Yes | Yes | No public data | Yes | Yes |
| CYP & transporter activity | Yes | Yes | No public data | Yes | Yes |
| Drug concentration responsiveness | 10–509× Cmax | 10× Cmax | No public data | 10×–1009× Cmax | 10×–1009× Cmax |
| Disease phenotype benchmark | No public data | No public data | No public data | No public data | No public data |
| NAFLD drug/target evaluation | No public data | No public data | No public data | No public data | No public data |
| References | hurelcorp.com [41] | regenemed.com [42] | emulatebio.com [43] | www.nortisbio.com [44] | hepregen.com [45–47] |
|
| |||||
| System: | Insphero | Organovo ExVive | Human precision-cut liver slices | CN BIO LiverChip | HemoShear |
|
| |||||
| System attributes | |||||
| Base technology | Hanging drop spheroids | 3D bioprinting | Human tissue | Microfluidic chip | Cone-and-plate perfused cocultures |
| Primary application(s) | Toxicity screen, disease modeling | Toxicity screen, disease modeling | Disease, drug, toxicity mechanisms | Clearance, toxicity screen, disease modeling | Disease, drug, toxicity mechanisms |
| Disease models | Drug-induced steatosis, viral hepatitis, cholestasis | Drug-induced steatosis, NAFLD, fibrosis | Based on tissue source (e.g., obese patients) | Hepatitis B/C, malaria, NAFLD | NAFLD, rare diseases |
| Separation of cell signals | No | No | No | No | Yes |
| Species | Human, rat, dog, monkey | Human | Human | Human | Human, rat |
|
| |||||
| System: | Insphero | Organovo ExVive | Human precision-cut liver slices | CN BIO LiverChip | HemoShear |
|
| |||||
| Liver tissue relevance | |||||
| Cell types | Primary: hepatocytes + NPCs | Primary: hepatocytes + NPCs | Primary: hepatocytes + NPCs | Primary: hepatocytes ± NPCs | Primary: hepatocytes + NPCs |
| Hemodynamic flow and transport | No | No | No | Yes | Yes |
| Physiological milieu relevance | |||||
| Hormone/growth factors/fatty acids | No public data | No public data | Supraphysiological levels of glucose | Physiological levels of insulin/glucose; supraphysiological levels of FFAs | Physiological levels of insulin/glucose/FFAs |
| Validation | |||||
| Morphology/liver function | Yes | Yes | No public data | Yes | Yes |
| CYP & transporter activity | Yes | Yes | No public data | Yes | Yes |
| Drug concentration responsiveness | 25×–50× Cmax | 25× Cmax | No public data | 10×–100× Cmax | 1×–10× Cmax |
| Disease phenotype benchmark | No public data | No public data | Obese patient tissue source | No public data | Clinical NASH patient transcriptomics and lipidomics |
| NAFLD drug/target evaluation | No public data | No public data | Obeticholic acid, PPARa agonist | Pioglitazone, metformin | Obeticholic acid, elafibranor, ACC inhibitor, 15+ additional targets |
| References | insphero.com [48, 49] | organovo.com [50] | [26, 51] | cn-bio.com [28, 52, 53] | hemoshear.com [31, 35, 54] |
Attributes that provide a competitive advantage are italicized
Currently, most organotypic models have focused on drug safety and toxicity with few adaptations to mimic other aspects of NAFLD or NASH. In this context, the liver microenvironment includes a number of circulating risk factors that can be incorporated into the media formulation in vitro to promote a NASH-relevant phenotype. These risk factors include endotoxin, inflammatory cytokines, growth factors, glucose, insulin, and free fatty acid (FFA) exposure. In particular, FFAs are known to induce a lipotoxic phenotype and are the most commonly used stimuli in NAFLD in vitro models, as they may also lead to increased levels of additional factors, such as inflammatory cytokines [27].
Several groups are actively developing these models. For example, Organovo’s bioprinting platform showed evidence of steatosis (drug-induced) and fibrosis, and is being developed as a NASH model. Kostrzewski et al. improved upon a previous microfluidics-based (designed by CellASIC) NAFLD model seeded with HepG2 cells, by incorporating primary human hepatocytes (no NPCs) into CN Bio’s microfluidic liver platform [28, 29]. To generate a NAFLD-relevant phenotype, both studies exposed the hepatocytes to high concentrations of oleic acid (400–660 µM) and palmitic acid (200–330 µM) combinations to create a “fat media”. In keeping with histological observations in NAFLD patients, Kostrzewski et al. reported that the fat media increased lipid accumulation in the cells and altered gene and activity levels of many CYP P450 enzymes. Cytokine levels, such as IL8, were increased in fat media conditions, but other cytokines, such as IL6 were not detected, likely due to the absence of additional cell types, such as Kupffer cells or HSCs [28]. Pioglitazone, which was found in the PIVENS trial to ameliorate fat accumulation and other histological features of NASH in the clinical setting, reduced lipid accumulation in the lipid-loaded hepatocytes, but no additional effects were reported [30].
While the previous model incorporates NASH-associated risk factors in a flow-based system, the lack of additional cell types, functional endpoints and validation confine the model’s potential use for target discovery. Feaver et al. recently developed a lipotoxic organotypic system for studying NAFL and NASH using the HemoShear liver platform [31]. This lipotoxic system incorporates hemodynamic and transport conditions derived from the liver sinusoid in a 3D culture system that contains primary human hepatocytes, macrophages, and HSCs (Fig. 1). The components of the milieu were similar to those of Kostrzewski et al., using physiologically relevant levels of glucose, insulin, oleic acid (65 µM) and palmitic acid (45 µM) derived from circulating levels in NASH patients [32]. Under these lipotoxic conditions, increased intracellular lipid accumulation was observed in agreement with Kostrzewski et al.; and additional functional endpoints were evaluated, including evidence of insulin resistance, increased inflammatory and fibrosis biomarkers, and HSC activation (Fig. 2).
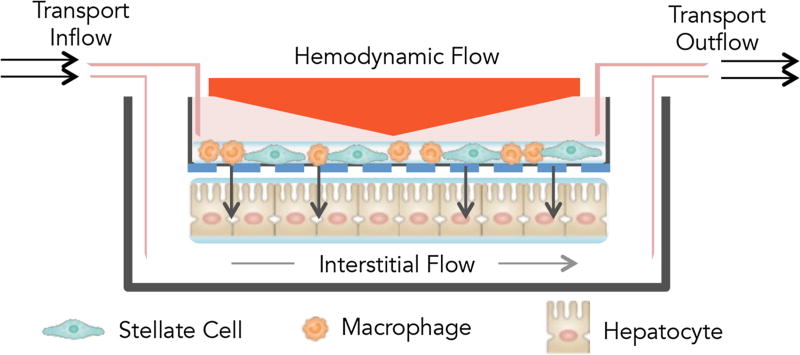
Fig. 1.
Lipotoxic in vitro system utilizing liver sinusoidal hemodynamics in a 3D culture model. Liver sinusoidal hemodynamics are applied to the HemoShear human liver system described by Feaver et al. [23], which uses a cone-and-plate viscometer incorporated into a transwell multi-culture model. Non-parenchymal cells (NPCs, composed of human hepatic stellate cells and macrophages) are plated on the top of transwell while hepatocytes are plated on the bottom of the transwell. Rotation of the cone (orange triangle) imparts shear stress onto the transwell. Media is continually perfused to recapitulate interstitial flow, as indicated by the inflow and outflow ports
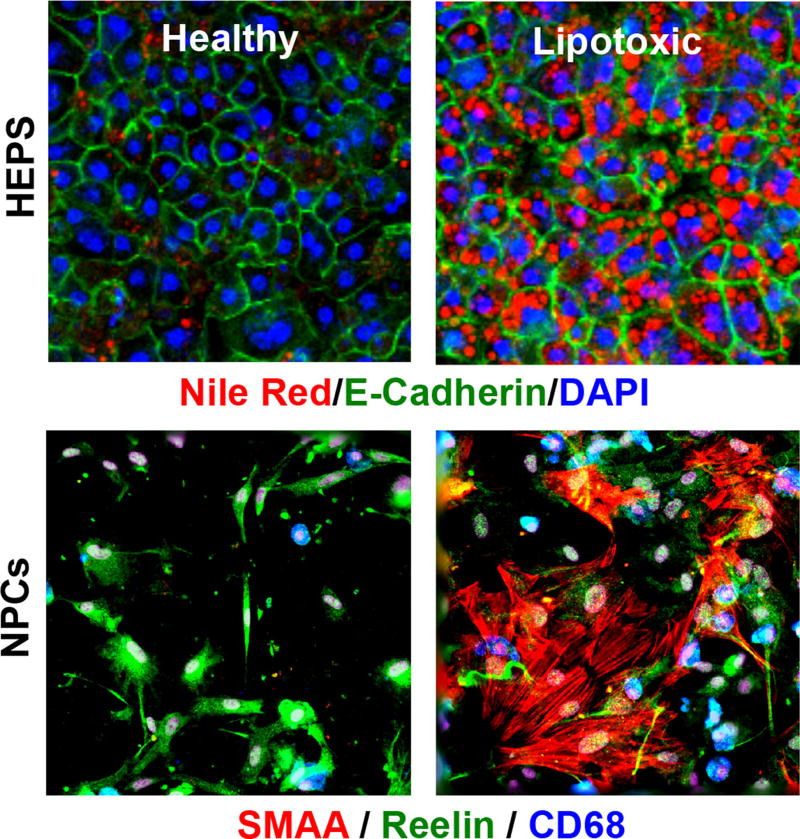
Fig. 2.
Hepatocytes and non-parenchymal cells (NPCs) in the 3D lipotoxic in vitro system demonstrate increased intracellular lipid accumulation as well as inflammatory and fibrotic markers. Representative ×20 photomicrographs of hepatocytes (HEPS) and non-parenchymal cells (NPCs) cultured in the lipotoxic conditions described by Feaver et al. are shown [23]. Hepatocytes were marked by the polarization marker, E-cadherin, and intracellular lipid accumulation was stained by Nile Red. NPCs include hepatic stellate cells (reelin, green) and macrophages (CD68, blue). Activated stellate cells are indicated by smooth muscle actin-α (SMAA, red) staining
A key differentiator was the use of two-omic approaches to more deeply understand the lipotoxic phenotype and directly validate the response to human biopsies [31]. RNA sequencing and lipidomic metabolite signatures from the lipotoxic system and patient biopsies from control and histologically confirmed NASH patients were compared. There was a high degree of concordance between the gene pathways and lipidomic signature induced in the in vitro system and those from patient biopsies [33]. This is the only in vitro model to benchmark -omic responses to those observed in human biopsies at this time.
Lastly, an additional and vital validation step required for in vitro systems to be utilized in drug discovery is to provide evidence of clinical translation. As previously mentioned, Kostrzewski et al. evaluated the impact of pioglitazone and showed anti-steatotic effects. While hPCLS fail to incorporate flow aspects, disease-associated risk factors, and have short time windows before the tissue begins to decline in function, they excel at incorporating the full complement of liver cell types and are freshly isolated from the human liver. Ljssennagger et al. used hPCLS to evaluate the farnesoid X receptor (FXR)-agonist, obeticholic acid (OCA, 1 µM for 24 h), which is currently in Phase 3 clinical trials for the treatment of NASH, and reported that gene expression profiling revealed on-target engagement of FXR [26]. Similarly, the lipotoxic system developed by Feaver et al. observed several clinically-relevant effects with 0.5-µM OCA treatment, including on-target effects and reductions in steatosis, inflammatory cytokines, fibrotic biomarkers, and triglycerides—all of which were observed in the Phase 2b clinical trial [31, 34, 35]. OCA was found in the clinic to raise LDL, a risk not observed in pre-clinical animal studies. Both hPCLS and the lipotoxic system reported transcriptional signatures that may explain the cholesterol endpoints observed in the clinical trials for OCA [26, 35]. Additionally, in the lipotoxic system, OCA treatment induced high secretion of ApoB (the primary apoprotein associated with LDL) and increased intracellular levels of cholesterol, suggesting dysregulation of this pathway, in agreement with what was observed in the clinic [31, 34]. Thus, while not perfect, substantial progress has been made in development of in vitro models that capture many aspects of NASH at a functional, molecular, transcriptomic and metabolomic levels that provide opportunities to test specific compounds, or combinations of compounds, for their anti-NASH efficacy.
Animal models of NAFLD/NASH
The liver has a substantial capacity to synthesize as well as store fat. It is therefore not surprising that a variety of manipulations targeting the metabolic machinery of the liver alters lipid flux and leads to accumulation of fat in the liver. This induces cell stress and may also lead to modest inflammation [36, 37]. These findings have fueled intense interest in studying pre-clinical models of human NAFLD. However, the great majority of animal models, especially those with specific gene knockouts or unusual diets do not come close to recapitulating human disease [38].
An ideal animal model must meet the following criteria in order to be considered representative of human disease. It must recapitulate human NAFLD by: (1) not requiring a specific gene knockout since human NAFLD is not marked by these gene-specific deficiencies, (2) induction by diet-induced obesity, (3) use of diets with at least a macronutrient composition similar to those consumed by most humans, (4) development of adiposity, insulin resistance and dyslipidemia similar to that in humans, (5) demonstrating a systemic inflammatory state with visceral adipose tissue inflammation and increased systemic inflammatory cytokines, (6) activation of cell signaling pathways that are relevant to human disease and disease progression, (7) transcriptomic concordance with human disease, (8) lipidomic concordance with human disease, and (9) histological concordance with human disease and progression to advanced fibrosis and hepatocellular cancer.
A variety of animal models have been described using many species from zebrafish and sandflies to rodents and even large mammals. They generally involve either dietary manipulation or genetic manipulation. Diets have, however, been largely inconsistent with what is known in humans. For example, the majority of human diets are not choline-deficient [38], and while choline deficient diets can induce steatohepatitis, its translatability to the human state is low, especially since the animals do not develop insulin resistance and can actually lose weight. Other diets that include 2% cholesterol are simply unphysiologic. The ALIOS model and the high-fat diet with ad lib use of glucose/fructose described by Tetri et al. and others come closest to mimicking the average macronutrient composition of humans with NAFLD in the West [39, 40].
These limitations of existing models led us to work on a mouse model of NAFLD that, to a reasonable extent, answers to important elements of the criteria outlined above [14]. The discovery of this model was made by serendipity, but over the course of 10 years and extensive validation, we believe this model is perhaps the most rigorously evaluated and meets many of the quality metrics for an optimal representation of NAFLD. This Diet-Induced Animal Model Of NAFLD (DIAMOND) is an isogenic strain of a C57Bl/J and a S129/V mouse with 60% of genetic materials from the C57Bl/J. A key feature is that, while neither parent strain develops a phenotype, this mouse develops a consistent phenotype even after rederivation in three different environments. Upon initiation of a high fat (40% calories from fat and 0.1% cholesterol) diet with ad lib glucose–fructose in drinking water, the mice sequentially develop fatty liver and then steatohepatitis with classical hepatocellular ballooning (Mallory–Denk bodies); and then progressive fibrosis resulting in bridging fibrosis and early nodule formation (Fig. 3). In some mice, this disease will progress to hepatocellular cancer, as in humans. Disease progression is generally accompanied by obesity, adipose tissue inflammation, elevated liver enzymes and activation of lipogenic, oxidative stress, unfolded protein response, inflammatory and apoptotic pathways. At a transcriptomic and lipidomic level, it has a strong concordance with human NASH [14].
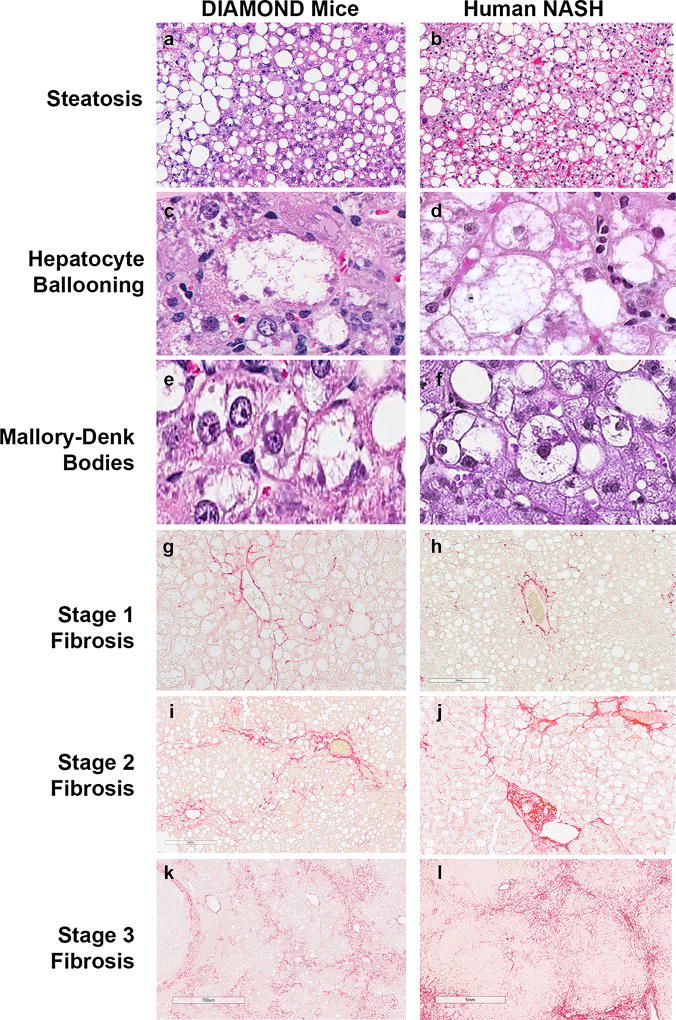
Fig. 3.
Main histological features of DIAMOND mice are comparablec ► to human NASH. Representative images of liver histology from DIAMOND mice (a, c, e, g, i, k) or human NASH (b, d, f, h, j, l) depicting steatosis (a, b; H&E; original magnification, ×5), Hepatocyte ballooning (c, d; H&E; original magnification, ×40), Mallory-Denk bodies (as indicated by dark arrows) (e, f; H&E; original magnification, ×40), Stage 1 fibrosis (g, h; Sirius Red; original magnification, ×20), Stage 2 fibrosis (i, j; Sirius Red; original magnification, ×20), Stage 3 fibrosis (k, l; Sirius Red; original magnification, ×10). CV central vein; PT portal tract. (Adapted from [14] with permission)
This model, which has close resemblance to human NAFLD with respect to the features noted above, provides an opportunity to evaluate the evolution of the disease and its progression to cirrhosis at a molecular level while longitudinal human transcriptomic data are awaited. Interrogation of the transcriptome identified early activation of metabolic pathways including PPAR-α and FXR, both known to be relevant in human disease. Interestingly, despite continuing on the same diet, with disease progression, there was a decrease in many metabolic pathways with the exception of FXR [14]. There was, however, progressive activation of inflammatory pathways. Furthermore, at a transcriptomic level, fibrogenic signaling peaked when the disease activity and inflammatory activation was the greatest. Together, these provide a molecular signature of disease evolution. Also, it demonstrated that, even though the histology showed steatohepatitis with some fibrosis, at different points in time, there was differential activation levels of metabolic, cell stress, apoptosis, inflammatory and fibrosis related pathways. If also true for the human state, this may provide insights into why only some patients respond to a given therapy.
As may be expected from any animal model, the DIAMOND also has its limitations, such as the prolonged period of time to develop steatosis (16 weeks) and bridging fibrosis (36 weeks) and the relatively high HCC frequency. Furthermore, and as a departure from the human disease state, there appears to be suppression of cholesterol synthesis in the DIAMOND [14]. The true implication of this difference is yet unclear.
Translating from preclinical models to human therapeutics
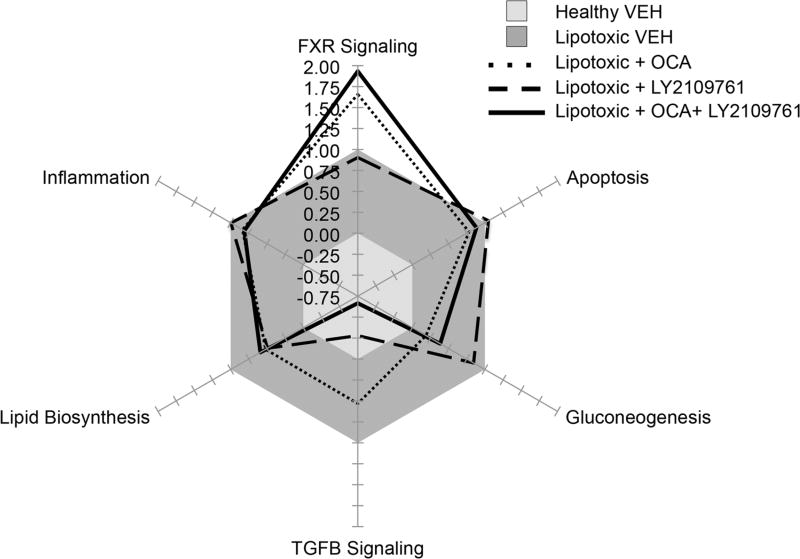
Despite the high interest in NASH, there continues to be a lack of common therapeutic strategy, as evidenced by the over 30 different targets currently in development. This result likely stems from a lack of disease understanding and experimental models for identifying and validating targets. Preclinical models that show human translation can provide powerful platforms for gaining deeper mechanistic understanding of not only disease progression, but also therapeutic response mechanisms. The ability to develop in vitro and mouse models of NAFLD that recapitulate many features of human disease allow conceptualization of seamless drug development where the effects of one or more drugs on the key pathways relevant for disease progression in humans can be assessed. For example, recently a detailed description of FXR pharmacology has been described using such an approach in vitro [23, 27]. As a proof-of-concept, Fig. 4 illustrates the utility of in vitro systems for evaluating the impact of individual drugs or drug combinations (Feaver et al., unpublished data). The radar plot provides a visualization of how OCA, a TGFβR inhibitor (LY2109761), or the combination of both impact gene expression of specific pathways relative to vehicle controls in the lipotoxic system described by Feaver et al. above [31]. The impact of on-target signaling for each compound can be observed where OCA potently activated FXR signaling (Fig. 4, dotted line), while the TGFβR strongly inhibited TGFβ signaling (Fig. 4, dashed line). Used in combination (Fig. 4, solid line), an additive effect was observed yielding a therapeutic treatment that could simultaneously activate FXR and inhibit TGFβ. Thus, in vitro systems could aid in the discovery of drug/target combinations that may synergistically ameliorate key aspects of the NASH disease phenotype for future development.
Fig. 4.
Lipotoxic in vitro model comparing obeticholic acid and a TGFβR inhibitor (LY2109761). The effect of obeticholic acid (OCA, dotted line) alone, a TGFβR inhibitor (LY2109761, dashed line) alone, or a combination of both treatments (solid line) were evaluated on key signaling pathways associated with NASH in the Feaver et al. lipotoxic in vitro model [23]. The axes represent the average gene pathway fold change response relative to the lipotoxic vehicle (VEH) response (gray, set to equal 1) and a healthy control response (light gray, set to equal 0)
There is currently an unmet need to assess the status of the hepatic transcriptome in ways that are easy to implement in clinical practice. There are ongoing efforts to reverse-engineer the transcriptomic profile to predict its impact on the metabolome and capture that signature in circulation. However, much more work needs to be done before these approaches become reality. It is hoped that, with continuing innovation and translation of scientific discovery in preclinical stages, as well as with the growing number of relevant animal models, the specific drivers of disease in a given patient will be readily identifiable and specific therapies can be brought to bear on these targets.
Acknowledgments
Support: RO1 DK 105961 (National Institute of Diabetes and Digestive and Kidney Diseases). T32 DK007150/DK/NIDDK NIH HHS/United States [National Institute of Diabetes and Digestive and Kidney Diseases (US)]. R44 DK115301 (SBIR, HemoShear Therapeutics).
Dr. Sanyal is President of Sanyal Biotechnology and has stock options in Genfit, Akarna, Tiziana, Indalo, Durect, Exhalenz and Hemoshear. He has served as a consultant to AbbVie, Astra Zeneca, Nitto Denko, Ardelyx, Conatus, Nimbus, Amarin, Salix, Tobira, Takeda, Fibrogen, Jannsen, Gilead, Boehringer, Lilly, Zafgen, Novartis, Pfizer, Jannsen and Genfit. He has been an unpaid consultant to Intercept, Echosens, Immuron, Galectin, Fractyl, Syntlogic, Novo Nordisk, Affimune, Chemomab, Nordic Bioscience and Bristol Myers Squibb. His institution has received grant support from Gilead, Salix, Tobira, Bristol Myers, Shire, Intercept, Merck, Astra Zeneca, Malinckrodt, Cumberland and Novartis. He receives royalties from Elsevier and UptoDate.
Footnotes
Compliance with ethical standards
Conflict of interest Abdul M. Oseini, MD No conflicts to disclose, Danny Issa, MD No conflicts to disclose, Ryan E. Feaver, PhD Employee of HemoShear, Banumathi K. Cole, PhD Employee of HemoShear.
Contributor Information
Abdul M. Oseini, Email: abdul.oseini@vcuhealth.org.
Arun J. Sanyal, Email: arun.sanyal@vcuhealth.org.
References
- 1.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10(11):686–90. doi: 10.1038/nrgastro.2013.171. https://doi.org/10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 2.Oseini AM, Sanyal AJ. Therapies in non-alcoholic steatohepatitis (NASH) Liver Int. 2017;37(Suppl 1):97–103. doi: 10.1111/liv.13302. https://doi.org/10.1111/liv.13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141(4):1249–53. doi: 10.1053/j.gastro.2011.06.061. https://doi.org/10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 4.Hirsova P, Gores GJ. Ballooned hepatocytes, undead cells, sonic hedgehog, and vitamin E: therapeutic implications for nonalcoholic steatohepatitis. Hepatology. 2015;61(1):15–7. doi: 10.1002/hep.27279. https://doi.org/10.1002/hep.27279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedossa P. Histological assessment of NAFLD. Dig Dis Sci. 2016;61(5):1348–55. doi: 10.1007/s10620-016-4062-0. https://doi.org/10.1007/s10620-016-4062-0. [DOI] [PubMed] [Google Scholar]
- 6.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2017 doi: 10.1002/hep.29367. https://doi.org/10.1002/hep.29367. [DOI] [PubMed]
- 7.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. https://doi.org/10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 8.Golabi P, Sayiner M, Fazel Y, Koenig A, Henry L, Younossi ZM. Current complications and challenges in nonalcoholic steatohepatitis screening and diagnosis. Expert Rev Gastroenterol Hepatol. 2016;10(1):63–71. doi: 10.1586/17474124.2016.1099433. https://doi.org/10.1586/17474124.2016.1099433. [DOI] [PubMed] [Google Scholar]
- 9.Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62(1 Suppl):S47–64. doi: 10.1016/j.jhep.2014.12.012. https://doi.org/10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Argo CK, Caldwell SH. Epidemiology and natural history of non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13(4):511–31. doi: 10.1016/j.cld.2009.07.005. https://doi.org/10.1016/j.cld.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9(6):524. doi: 10.1016/j.cgh.2011.03.020. https://doi.org/10.1016/j.cgh.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 12.European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD): European Association for the Study of Obesity (EASO) EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. Diabetologia. 2016;59(6):1121–40. doi: 10.1007/s00125-016-3902-y. https://doi.org/10.1007/s00125-016-3902-y. [DOI] [PubMed] [Google Scholar]
- 13.Sanyal AJ, Friedman SL, McCullough AJ, Dimick-Santos L. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: findings and recommendations from an American Association for the Study of Liver Diseases-US Food and Drug Administration Joint Workshop. Hepatology. 2015;61(4):1392–405. doi: 10.1002/hep.27678. https://doi.org/10.1002/hep.27678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asgharpour A, Cazanave SC, Pacana T, Seneshaw M, Vincent R, Banini BA, et al. A diet-induced animal model of non-alcoholic fatty liver disease and hepatocellular cancer. J Hepatol. 2016;65(3):579–88. doi: 10.1016/j.jhep.2016.05.005. https://doi.org/10.1016/j.jhep.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bale SS, Vernetti L, Senutovitch N, Jindal R, Hegde M, Gough A, et al. In vitro platforms for evaluating liver toxicity. Exp Biol Med (Maywood) 2014;239(9):1180–91. doi: 10.1177/1535370214531872. https://doi.org/10.1177/1535370214531872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol. 2014;32(8):760–72. doi: 10.1038/nbt.2989. https://doi.org/10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 17.Chang SY, Weber EJ, Ness KV, Eaton DL, Kelly EJ. Liver and kidney on chips: microphysiological models to understand transporter function. Clin Pharmacol Ther. 2016;100(5):464–78. doi: 10.1002/cpt.436. https://doi.org/10.1002/cpt.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dash A, Blackman BR, Wamhoff BR. Organotypic systems in drug metabolism and toxicity: challenges and opportunities. Expert Opin Drug Metab Toxicol. 2012;8(8):999–1014. doi: 10.1517/17425255.2012.693161. https://doi.org/10.1517/17425255.2012.693161. [DOI] [PubMed] [Google Scholar]
- 19.LeCluyse EL, Witek RP, Andersen ME, Powers MJ. Organotypic liver culture models: meeting current challenges in toxicity testing. Crit Rev Toxicol. 2012;42(6):501–48. doi: 10.3109/10408444.2012.682115. https://doi.org/10.3109/10408444.2012.682115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao X, Liu Y. A transcriptomic study suggesting human iPSC-derived hepatocytes potentially offer a better in vitro model of hepatotoxicity than most hepatoma cell lines. Cell Biol Toxicol. 2017;33(4):407–21. doi: 10.1007/s10565-017-9383-z. https://doi.org/10.1007/s10565-017-9383-z. [DOI] [PubMed] [Google Scholar]
- 21.Hart SN, Li Y, Nakamoto K, Subileau EA, Steen D, Zhong XB. A comparison of whole genome gene expression profiles of HepaRG cells and HepG2 cells to primary human hepatocytes and human liver tissues. Drug Metab Dispos. 2010;38(6):988–94. doi: 10.1124/dmd.109.031831. https://doi.org/10.1124/dmd.109.031831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez-Antona C, Donato MT, Boobis A, Edwards RJ, Watts PS, Castell JV, et al. Cytochrome P450 expression in human hepatocytes and hepatoma cell lines: molecular mechanisms that determine lower expression in cultured cells. Xenobiotica. 2002;32(6):505–20. doi: 10.1080/00498250210128675. https://doi.org/10.1080/00498250210128675. [DOI] [PubMed] [Google Scholar]
- 23.Guguen-Guillouzo C, Clement B, Campion JP, Brechot C, Fauchet R, Brissot P, et al. Excretion of HBs and HBe antigens by normal human adult hepatocytes infected in vitro by hepatitis B virus. Presse Med. 1983;12(19):1232–3. [PubMed] [Google Scholar]
- 24.Krause P, Saghatolislam F, Koenig S, Unthan-Fechner K, Probst I. Maintaining hepatocyte differentiation in vitro through co-culture with hepatic stellate cells. Vitro Cell Dev Biol Anim. 2009;45(5–6):205–12. doi: 10.1007/s11626-008-9166-1. https://doi.org/10.1007/s11626-008-9166-1. [DOI] [PubMed] [Google Scholar]
- 25.Takayama G, Taniguchi A, Okano T. Identification of differentially expressed genes in hepatocyte/endothelial cell co-culture system. Tissue Eng. 2007;13(1):159–66. doi: 10.1089/ten.2006.0143. https://doi.org/10.1089/ten.2006.0143. [DOI] [PubMed] [Google Scholar]
- 26.Ljssennagger N, Janssen AW, Milona A, Ramos Pittol JM, Hollman DA, Mokry M, et al. Gene expression profiling in human precision cut liver slices in response to the FXR agonist obeticholic acid. J Hepatol. 2016;64(5):1158–66. doi: 10.1016/j.jhep.2016.01.016. https://doi.org/10.1016/j.jhep.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 27.Alkhouri N, Dixon LJ, Feldstein AE. Lipotoxicity in nonalcoholic fatty liver disease: not all lipids are created equal. Expert Rev Gastroenterol Hepatol. 2009;3(4):445–51. doi: 10.1586/egh.09.32. https://doi.org/10.1586/egh.09.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kostrzewski T, Cornforth T, Snow SA, Ouro-Gnao L, Rowe C, Large EM, et al. Three-dimensional perfused human in vitro model of non-alcoholic fatty liver disease. World J Gastroenterol. 2017;23(2):204–15. doi: 10.3748/wjg.v23.i2.204. https://doi.org/10.3748/wjg.v23.i2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gori M, Simonelli MC, Giannitelli SM, Businaro L, Trombetta M, Rainer A. Investigating nonalcoholic fatty liver disease in a liver-on-a-chip microfluidic device. PLoS ONE. 2016;11(7):e0159729. doi: 10.1371/journal.pone.0159729. https://doi.org/10.1371/journal.pone.0159729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, et al. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-alpha and -delta, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology. 2016;150(5):1147–1159. doi: 10.1053/j.gastro.2016.01.038. https://doi.org/10.1053/j.gastro.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 31.Feaver RE, Cole BK, Lawson MJ, Hoang SA, Marukian S, Blackman BR, et al. Development of an in vitro human liver system for interrogating nonalcoholic steatohepatitis. JCI Insight. 2016;1(20):e90954. doi: 10.1172/jci.insight.90954. https://doi.org/10.1172/jci.insight.90954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Almeida IT, Cortez-Pinto H, Fidalgo G, Rodrigues D, Camilo ME. Plasma total and free fatty acids composition in human non-alcoholic steatohepatitis. Clin Nutr. 2002;21(3):219–23. doi: 10.1054/clnu.2001.0529. [DOI] [PubMed] [Google Scholar]
- 33.Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46(4):1081–90. doi: 10.1002/hep.21763. https://doi.org/10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 34.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385(9972):956–65. doi: 10.1016/S0140-6736(14)61933-4. https://doi.org/10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dash A, Figler RA, Blackman BR, Marukian S, Collado MS, Lawson MJ, et al. Pharmacotoxicology of clinically-relevant concentrations of obeticholic acid in an organotypic human hepatocyte system. Toxicol In Vitro. 2017;39:93–103. doi: 10.1016/j.tiv.2016.11.014. https://doi.org/10.1016/j.tiv.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perla FM, Prelati M, Lavorato M, Visicchio D, Anania C. The role of lipid and lipoprotein metabolism in non-alcoholic fatty liver disease. Children (Basel) 2017;4(6):46. doi: 10.3390/children4060046. https://doi.org/10.3390/children4060046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mota M, Banini BA, Cazanave SC, Sanyal AJ. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism. 2016;65(8):1049–61. doi: 10.1016/j.metabol.2016.02.014. https://doi.org/10.1016/j.metabol.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hardy T, Oakley F, Anstee QM, Day CP. Nonalcoholic fatty liver disease: pathogenesis and disease spectrum. Annu Rev Pathol. 2016;11:451–96. doi: 10.1146/annurev-pathol-012615-044224. https://doi.org/10.1146/annurev-pathol-012615-044224. [DOI] [PubMed] [Google Scholar]
- 39.Tetri LH, Basaranoglu M, Brunt EM, Yerian LM, Neuschwander-Tetri BA. Severe NAFLD with hepatic necroinflammatory changes in mice fed trans fats and a high-fructose corn syrup equivalent. Am J Physiol Gastrointest Liver Physiol. 2008;295(5):G987–95. doi: 10.1152/ajpgi.90272.2008. https://doi.org/10.1152/ajpgi.90272.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Charlton M, Krishnan A, Viker K, Sanderson S, Cazanave S, McConico A, et al. Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. Am J Physiol Gastrointest Liver Physiol. 2011;301(5):G825–34. doi: 10.1152/ajpgi.00145.2011. https://doi.org/10.1152/ajpgi.00145.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Novik E, Maguire TJ, Chao P, Cheng KC, Yarmush ML. A microfluidic hepatic coculture platform for cell-based drug metabolism studies. Biochem Pharmacol. 2010;79(7):1036–44. doi: 10.1016/j.bcp.2009.11.010. https://doi.org/10.1016/j.bcp.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kostadinova R, Boess F, Applegate D, Suter L, Weiser T, Singer T, et al. A long-term three dimensional liver co-culture system for improved prediction of clinically relevant drug-induced hepatotoxicity. Toxicol Appl Pharmacol. 2013;268(1):1–16. doi: 10.1016/j.taap.2013.01.012. https://doi.org/10.1016/j.taap.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 43.Huh D, Kim HJ, Fraser JP, Shea DE, Khan M, Bahinski A, et al. Microfabrication of human organs-on-chips. Nat Protoc. 2013;8(11):2135–57. doi: 10.1038/nprot.2013.137. https://doi.org/10.1038/nprot.2013.137. [DOI] [PubMed] [Google Scholar]
- 44.Vernetti LA, Senutovitch N, Boltz R, DeBiasio R, Shun TY, Gough A, et al. A human liver microphysiology platform for investigating physiology, drug safety, and disease models. Exp Biol Med (Maywood) 2016;241(1):101–14. doi: 10.1177/1535370215592121. https://doi.org/10.1177/1535370215592121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khetani SR, Bhatia SN. Microscale culture of human liver cells for drug development. Nat biotechnol. 2008;26(1):120–6. doi: 10.1038/nbt1361. https://doi.org/10.1038/nbt1361. [DOI] [PubMed] [Google Scholar]
- 46.Khetani SR, Kanchagar C, Ukairo O, Krzyzewski S, Moore A, Shi J, et al. Use of micropatterned cocultures to detect compounds that cause drug-induced liver injury in humans. Toxicol sci. 2013;132(1):107–17. doi: 10.1093/toxsci/kfs326. https://doi.org/10.1093/toxsci/kfs326. [DOI] [PubMed] [Google Scholar]
- 47.March S, Ramanan V, Trehan K, Ng S, Galstian A, Gural N, et al. Micropatterned coculture of primary human hepatocytes and supportive cells for the study of hepatotropic pathogens. Nat protoc. 2015;10(12):2027–53. doi: 10.1038/nprot.2015.128. https://doi.org/10.1038/nprot.2015.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bell CC, Hendriks DF, Moro SM, Ellis E, Walsh J, Renblom A, et al. Characterization of primary human hepatocyte spheroids as a model system for drug-induced liver injury, liver function and disease. Sci Rep. 2016;6:25187. doi: 10.1038/srep25187. https://doi.org/10.1038/srep25187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Messner S, Agarkova I, Moritz W, Kelm JM. Multi-cell type human liver microtissues for hepatotoxicity testing. Arch Toxicol. 2013;87(1):209–13. doi: 10.1007/s00204-012-0968-2. https://doi.org/10.1007/s00204-012-0968-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nguyen DG, Funk J, Robbins JB, Crogan-Grundy C, Presnell SC, Singer T, et al. Bioprinted 3D primary liver tissues allow assessment of organ-level response to clinical drug induced toxicity in vitro. PLoS ONE. 2016;11(7):e0158674. doi: 10.1371/journal.pone.0158674. https://doi.org/10.1371/journal.pone.0158674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Janssen AW, Betzel B, Stoopen G, Berends FJ, Janssen IM, Peijnenburg AA, et al. The impact of PPARalpha activation on whole genome gene expression in human precision cut liver slices. BMC genomics. 2015;16:760. doi: 10.1186/s12864-015-1969-3. https://doi.org/10.1186/s12864-015-1969-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Domansky K, Inman W, Serdy J, Dash A, Lim MH, Griffith LG. Perfused multiwell plate for 3D liver tissue engineering. Lab Chip. 2010;10(1):51–8. doi: 10.1039/b913221j. https://doi.org/10.1039/b913221j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sarkar U, Rivera-Burgos D, Large EM, Hughes DJ, Ravindra KC, Dyer RL, et al. Metabolite profiling and pharmacokinetic evaluation of hydrocortisone in a perfused three-dimensional human liver bioreactor. Drug Metab Dispos. 2015;43(7):1091–9. doi: 10.1124/dmd.115.063495. https://doi.org/10.1124/dmd.115.063495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chapman KA, Collado MS, Figler RA, Hoang SA, Armstrong AJ, Cui W, et al. Recapitulation of metabolic defects in a model of propionic acidemia using patient-derived primary hepatocytes. Mol Genet Metab. 2016;117(3):355–62. doi: 10.1016/j.ymgme.2015.12.008. https://doi.org/10.1016/j.ymgme.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]