Abstract
Purpose
Patients presenting with nodal Merkel cell carcinoma without an identifiable (unknown) primary lesion (MCC-UP) are nearly twice as likely to survive compared to similarly staged patients with known primary lesions (MCC-KP). The basis of this previously reported finding is unclear.
Experimental Design
Survival analyses and markers of immunity were evaluated in 123 patients with advanced MCC. Whole exome sequence data was analyzed from 16 tumors.
Results
As in prior studies, patients with nodal MCC-UP had strikingly improved MCC-specific survival as compared to MCC-KP patients (HR 0.297, p < 0.001). Surprisingly, patients presenting with distant metastatic MCC-UP also had significantly improved survival (HR 0.296, p = 0.038). None of the 72 patients with MCC-UP were immunosuppressed as compared to 12 of the 51 (24%) patients with MCC-KP (p < 0.001). Merkel polyomavirus oncoprotein antibody median titer was higher in MCC-UP patients (26,229) than MCC-KP patients (3,492; p < 0.001). Additionally, the median number of nonsynonymous exome mutations in MCC-UP tumors (688 mutations) was markedly higher than MCC-KP tumors (10 mutations, p = 0.016).
Conclusions
This is the first study to our knowledge to explore potential underlying immune-mediated mechanisms of MCC-UP presentation. In this cohort, MCC-UP patients were never immune suppressed, had higher oncoprotein antibody titers, and higher tumor mutational burdens. Additionally, we show that nodal tumors identified in MCC-UP patients did indeed arise from primary skin lesions as they contained abundant UV-signature mutations. These findings suggest that stronger underlying immunity against MCC contributes to primary lesion elimination and improved survival.
INTRODUCTION
Merkel cell carcinoma (MCC) is a highly aggressive skin cancer with a relative mortality of 46% (1), making this disease ~3 times as deadly as malignant melanoma on a per case basis. While rare (~2,000 new cases per year in the US), the incidence has dramatically risen over the past 25 years due to improved detection methods and increased prevalence of risk-factors for MCC (2-4). Among patients presenting with palpable or scan-detectable regional lymph nodes at the time of MCC diagnosis (macroscopic nodal disease; stage IIIB), one-third to half of patients do not have a detectable skin primary. Several studies have documented that among stage IIIB patients with MCC, those presenting with an unknown primary tumor (MCC-UP) have significantly improved survival as compared to stage IIIB patients with known primary tumors (MCC-KP) (5-9). The magnitude of this survival benefit ranges from 60%-70% decreased chance of death if no primary lesion is present (5,6,8).
Several reports postulate that regression of the primary lesion may be attributable to immune-mediated mechanisms (5,8,10), however, limited evidence has been published to support this notion. Importantly, despite two etiologically distinct mechanisms (11) to MCC development (viral versus ultraviolet carcinogenesis), nearly all MCCs are highly immunogenic. In the majority of cases (80%), the Merkel cell polyomavirus (MCPyV) is clonally integrated in MCC tumors and persistent expression of the immunogenic MCPyV large and small T-antigens drive oncogenesis in these virus-positive tumors (12). The 20% of MCCs that are MCPyV-negative are induced via UV-mediated mutagenesis and harbor very high mutational burdens with UV-signatures (10,11,13). In multiple malignancies, high mutational burdens have been associated with immunogenicity and response to immunotherapy, likely through generation of neoepitopes (14). Importantly, both virus-positive and -negative MCCs have shown remarkable response rates to immune checkpoint inhibitor therapy, providing the strongest evidence that both virus-positive and -negative MCCs are immunogenic and responsive to immune mediated regression (15).
In this study, we report significantly improved survival of patients presenting with both virus-positive and –negative MCC-UP and we probe the relationship between immunity and MCC-UP presentation. We demonstrate that MCC-UP patients have enhanced immune function and significantly higher tumor mutation burdens than MCC-KP patients.
METHODS
Patient selection criteria
All studies were performed in accordance with Helsinki principles and approved by the Institutional Review Board at the Fred Hutchinson Cancer Research Center (IRB # 6585). All patients included in this study provided informed consent for enrollment in this IRB-approved database.
In our repository of 1,099 MCC patients, 407 were enrolled within 180 days of diagnosis of histologically confirmed MCC between June 1st, 2006 and December 9th, 2015 (Fig. 1). The median overall survival was significantly reduced and disease-specific death was increased in patients referred to UW more than 180 days after initial diagnosis, therefore to prevent selection bias, patients enrolled > 180 days after diagnosis were excluded from analysis. Additionally, we have previously reported improved outcomes among MCC-UP patients from a separate de-identified Kaiser Permanente Northern California cohort of patients. There is 1 patient (<1%) that we are aware of that was included in both cohorts, and while additional overlap is possible as patients were de-identified from the Kaiser Permanente group, we estimate that this number does not exceed 5 (~4%). Staging was performed as per AJCC 7th edition guidelines (1). The analysis was then restricted to 123 patients diagnosed with regional nodal (stage IIIB) and distant metastatic (stage IV) MCC and who had a primary status, diagnosis date, and date of last follow-up. As per guidelines, patients were classified as stage IIIB if they presented with clinically evident (via scan or physical exam) nodal involvement from skin-draining nodal basins without evidence of distant disease. Patients were classified as stage IV if they presented with clinically evident nodal disease in non-skin draining lymph nodes or with visceral metastatic disease. All patients received at least two comprehensive skin exams, including one at the Seattle Cancer Care Alliance and at least one or more at outside facilities in order to determine primary status presentation.
Figure 1. Enrollment criteria for patients with stage IIIB or IV MCC.
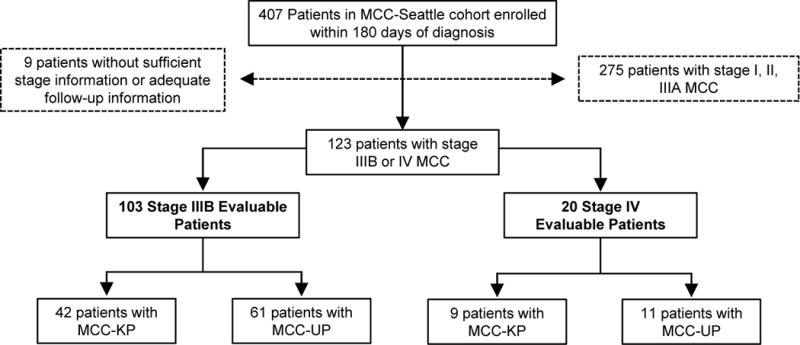
Patients included in the analysis were enrolled within 180 days of their diagnosis of stage IIIB or IV MCC as defined by AJCC 7th ed. criteria. All patients had clinical information on the presence or absence of a primary lesion and the time points necessary to calculate survival. 123 patients met all selection criteria with breakdowns as shown in terms of stage and primary lesion status.
Serological evaluation, viral status, sample preparation and tumor whole exome sequencing
Serological testing for antibodies against the MCPyV T-antigen oncoproteins was performed on 103 patients as previously described (16) at the University of Washington Clinical Immunology Laboratory and these results are shown in Table 1. Only patients with virus-positive tumors produce these antibodies (16-18), therefore all tumors from patients who tested serologically positive (n = 57) were considered virus-positive. The remaining 46 patients tested were serologically negative, however, because roughly half of seronegative MCC patients do in fact have virus-positive tumors (16,17), additional testing was done on patients with available tumor samples (n = 21). Viral status was evaluated in these patients using qPCR detection of viral DNA and immunohistochemical staining (IHC) using the CM2B4 (SC136172; Santa Cruz Biotechnology (19) and Ab3 antibodies (a generous gift from James DeCaprio, Dana-Farber Cancer Institute (20) targeting the MCPyV large T-antigen as previously described (18) (Table 1).
Table 1.
Patient demographic details by stage and primary status
| Characteristic | Stage IIIB | Stage IV | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| MCC-KP (n = 42) | MCC-UP (n = 61) | MCC-KP (n = 9) | MCC-UP (n = 11) | |||
|
| ||||||
| No. (%) | No. (%) | P | No. (%) | No. (%) | P | |
| Sex | ||||||
| Male (n=85) | 30 (71.4) | 40 (65.6) | 0.668 | 8 (88.9) | 7 (63.6) | 0.319 |
| Female (n=38) | 12 (28.6) | 21 (34.4) | 1 (11.1) | 4 (36.4) | ||
|
| ||||||
| Age at Dx | ||||||
| ≥65 (n=67) | 23 (54.8) | 32 (52.5) | 0.843 | 4 (44.4) | 8 (72.7) | 0.362 |
| <65 (n=56) | 19 (45.2) | 29 (47.5) | 5 (55.6) | 3 (27.3) | ||
|
| ||||||
| Immune Suppressed** | ||||||
| Yes (n=12) | 11 (26.2) | 0 (0.0) | <0.001 | 1 (11.1) | 0 (0.0) | 0.450 |
| No (n=111) | 31 (73.8) | 61 (100.0) | 8 (88.9) | 11 (100.0) | ||
|
| ||||||
| MCPyV Viral Status | ||||||
| Positive (n=68) | 22 (95.7) | 36 (85.7) | 0.406 | 4 (80.0) | 6 (75.0) | 1.000 |
| Negative (n=10) | 1 (4.3) | 6 (14.3) | 1 (20.0) | 2 (25.0) | ||
| * Unknown (n=45) | 19 | 19 | 4 | 3 | ||
|
| ||||||
| MCPyV Oncoprotein Serostatus | ||||||
| Positive (n=57) | 17 (48.5) | 32 (59.3) | 0.385 | 3 (60.0) | 5 (55.6) | 1.000 |
| Negative (n=46) | 18 (51.5) | 22 (40.1) | 2 (40.0) | 4 (44.4) | ||
| * Unknown (n=18) | 7 | 7 | 4 | 2 | ||
|
| ||||||
| Received Radiation Therapy? | ||||||
| Yes (n=106) | 37 (90.2) | 58 (98.3) | 0.156 | 4 (44.4) | 7 (70.0) | 0.370 |
| No (n=13) | 4 (9.8) | 1 (1.7) | 5 (55.6) | 3 (30.0) | ||
| * Unknown (n=4) | 1 | 2 | 0 | 1 | ||
|
| ||||||
| Received Chemotherapy? | ||||||
| Yes (n=31) | 6 (14.3) | 13 (22.4) | 0.439 | 5 (55.6) | 7 (63.6) | 1.000 |
| No (n=89) | 36 (85.7) | 45 (77.6) | 4 (44.4) | 4 (36.4) | ||
| * Unknown (n=3) | 0 | 3 | ||||
Unknown values were excluded from percentage calculations
Causes of immunosuppression were HIV (n=4), Chronic lymphocytic leukemia (n=3), solid organ transplant (n=2), mycosis fungoides (n=1), methotrexate treatment (n=1)
A previous study by Goh et al. (11) performed whole exome sequencing on 16 tumors (10 from MCC-UP and 6 from MCC-KP) enrolled in our cohort and determination of the number of somatic nonsynonymous mutations was performed as previously described. UV and age-related mutational signatures were defined according to Alexandrov et al. (21). C to T transitions that are characteristic of UV-induced mutational signatures were counted as follows. The fastq files were aligned with ELAND, and somatic mutations were called using previously published algorithms (11). Each mutation, such as a C>T, was called accordingly. C>T’s that occur on neighboring nucleotides were noted as CC>TT transitions. Aside from UV- and age-associated mutational signatures, several other signatures were identified, however, none were consistently represented across samples and therefore these were condensed into ‘other’ as described previously (11).
Statistical analysis
Analyses were completed using STATA software, version 11.0 and Prism software, version 6 with a statistical significance threshold of 5%. Comparisons of ordinal variables between MCC-KP and MCC-UP groups were performed using the Mann-Whitney test. Comparison of categorical variables in Table 1 were performed using the Fisher’s exact test. MCC-specific survival was defined as the length of time between the date of diagnosis (defined as date of first biopsy confirming MCC) and the date of death caused by MCC. Fine and Gray’s proportional sub-hazards model was used to evaluate competing-risks and calculate MCC-specific survival significance and hazard ratios in both the univariate and multivariate setting. The competing-risk was death by all causes except MCC. Overall and recurrence-free survival were defined as the length of time between the date of diagnosis (defined as date of first biopsy confirming MCC) and the date of death by any cause or the development of recurrent disease. Overall and recurrence-free survival was analyzed using a Cox-proportional hazards model. Patients for all survival analyses were censored by date of last contact. Multivariate analyses for stage IIIB patients controlled for age at diagnosis, sex, MCPyV oncoprotein antibody serological status and having received radiation therapy or chemotherapy. For stage IV patients (n = 20), multivariate analysis was limited to age at diagnosis and sex because of the small samples size and the fact that not all characteristics could be assessed on all 20 patients.
RESULTS
Characteristics of MCC-UP and MCC-KP patients
Among the 123 evaluable patients who were diagnosed with stage IIIB and stage IV MCC, 51 (41%) presented with MCC-KP and 72 (59%) presented with MCC-UP (Table 1). These 123 patients were followed for a collective 471.5 person-years and a median of 1.5 years per patient following diagnosis. When evaluating potential demographic characteristics associated with MCC-UP and MCC-KP presentation, we found no statistically significant difference in sex, age at diagnosis, MCPyV oncoprotein serological status, MCPyV viral status, treatment with radiation therapy, or treatment with chemotherapy between MCC-UP and MCC-KP patients (Table 1).
Differentiation of regional versus distant metastatic MCC without a primary
The definition of regional (stage III) versus distant (stage IV) disease in MCC-UP patients who present with only nodal involvement (i.e. no visceral metastasis) has not been clearly established to the best of our knowledge. In this study, we defined MCC-UP patients presenting with nodal disease within skin-draining lymph node basins as stage IIIB (regional), while patients presenting with deeper, non-skin-draining nodal disease were classified as stage IV (distant; Fig. 2A). Notably, skin-draining lymph nodes could potentially be sites of distant metastases, however, in the absence of a detectable primary tumor it is impossible to determine whether these lesions represent regional or distant disease. Using this classification, among MCC-UP patients presenting with only nodal disease, stage IIIB patients had significantly improved MCC-specific survival (HR=3.98; p=0.003) relative to stage IV MCC-UP patients (Fig. 2B), suggesting this dichotomy identified a meaningful difference in risk.
Figure 2. Patients with skin-draining lymph nodes have improved survival compared with patients with nodal disease in non-skin-draining nodes.
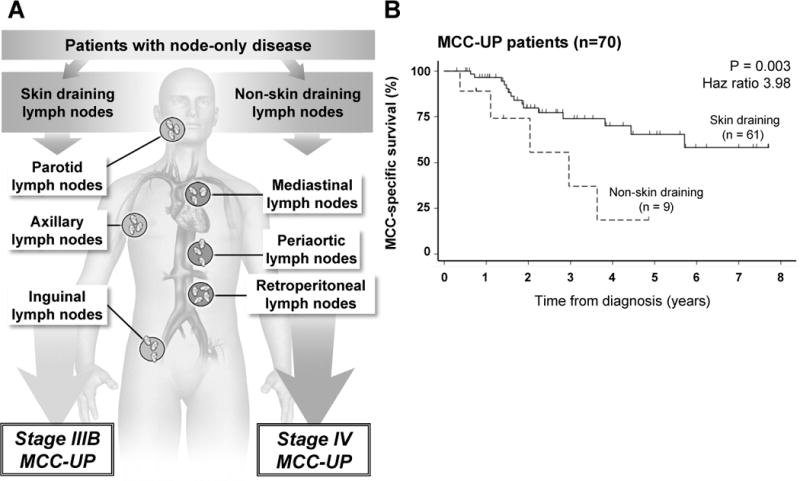
Panel A depicts representative skin-draining lymph nodes that were classified as stage IIIB and non-skin draining lymph nodes that were classified as stage IV. Panel B depicts MCC-specific survival for patients presenting with node-only disease. Sixty one patients were classified as stage IIIB with skin-draining lymph nodes and unknown primary lesions while 9 patients were classified as stage IV with non-skin draining lymph nodes and no primary lesions (2 stage IV MCC-UP patients were excluded due to presentation with visceral metastasis).
Patients with regional nodal (stage IIIB) MCC-UP have improved survival
To determine survival differences between MCC-UP and MCC-KP patients, Kaplan-Meier curves were used to evaluate MCC-specific, overall and recurrence-free survival for stage IIIB (Fig. 3A–C). Among living stage IIIB patients, the median follow-up time was 2.2 years for MCC-UP and 1.4 years for MCC-KP patients. This difference in follow-up time is largely due to a significant difference in survival between these two groups. Indeed, the MCC-specific survival among stage IIIB patients was dramatically improved for MCC-UP patients as compared to MCC-KP patients at 2 years (80% vs 45%) and 5-years (66% vs 30%; p <0.001; Fig. 3A) with an overall reduced risk of death by MCC of 70% (HR = 0.297, P < 0.001; Table 2). Similarly, overall (Fig. 3B) and recurrence-free survival (Fig. 3C) were also significantly improved for MCC-UP patients. Specifically, stage IIIB MCC-UP patients had a 70% reduction in the risk of death from any cause or MCC (HR = 0.300; p < 0.001) and a 64% reduced risk of recurrence when compared to MCC-KP (HR = 0.358; p = 0.001; Table 2). This clinically and statistically significant improvement in survival among MCC-UP patients persisted on multivariate analyses controlling for age at diagnosis, sex, MCPyV-oncoprotein serological status, treatment with radiation therapy, and treatment with chemotherapy (Table 2).
Figure 3. MCC-UP status predicts better survival among patients with either stage IIIB or IV disease.
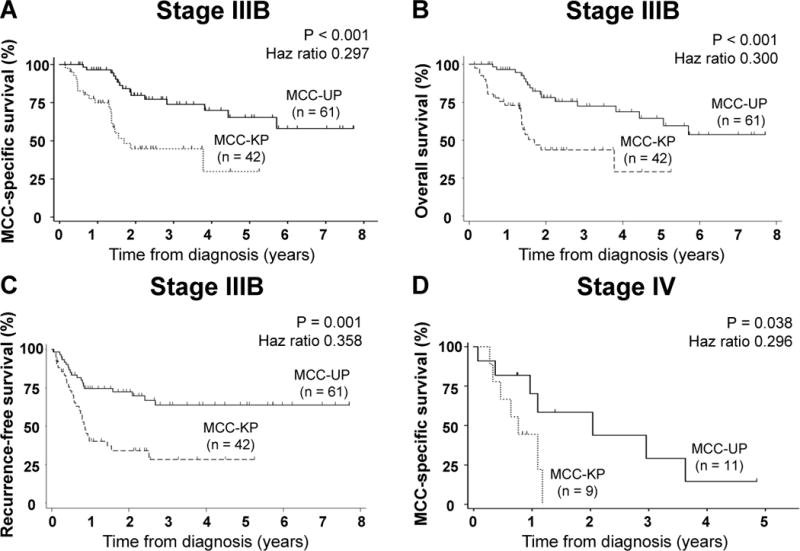
Panel A depicts MCC-specific survival, B illustrates overall survival and C indicates recurrence-free survival for 103 patients with stage IIIB MCC by unknown primary status. Panel D describes MCC-specific survival for 20 patients with stage IV MCC. MCC-specific survival analyses was completed using Fine and Gray’s proportional sub-hazards model to evaluate competing risks for MCC-specific survival analyses. For overall and recurrence-free survival analyses, we used a cox proportional hazard model.
Table 2.
Merkel cell carcinoma survival and unknown primary status
| Stage IIIB (n = 103) | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| MCC-specific survival | 0.297 | 0.152 to 0.582 | <0.001 | 0.257 | 0.117 to 0.542 | < 0.001 |
| Overall survival | 0.300 | 0.156 to 0.578 | <0.001 | 0.287 | 0.137 to 0.592 | 0.001 |
| Recurrence-free survival | 0.358 | 0.199 to 0.646 | 0.001 | 0.363 | 0.188 to 0.700 | 0.002 |
|
| ||||||
| Stage IV (n = 20) | ||||||
|
| ||||||
| MCC-specific survival | 0.296 | 0.093 to 0.935 | 0.038 | 0.219 | 0.049 to 0.968 | 0.045 |
| Overall survival | 0.296 | 0.093 to 0.935 | 0.038 | 0.190 | 0.038 to 0.945 | 0.042 |
| Recurrence-free survival | 0.618 | 0.243 to 1.570 | 0.312 | 0.667 | 0.200 to 3.383 | 0.512 |
Patients with distant metastatic MCC-UP also have improved MCC-specific survival
A dramatic survival difference was also observed in patients with distant metastatic MCC without a primary lesion, with MCC-UP having improved MCC-specific survival as compared to MCC-KP patients at 2 years (59% vs 0%; p = 0.038; Fig. 3D). A 5-year follow-up time point was not reached. The median follow-up time for stage IV MCC-UP was 1.5 years as compared to 0.8 years for MCC-KP. On multivariate competitive-risks regression also accounting for age at diagnosis and sex, presenting with stage IV MCC-UP was associated with a remarkable 79% decreased risk of MCC-specific death when compared with presenting with stage IV MCC-KP (HR = 0.219; p = 0.045; Table 2). MCC-UP patients also had significantly improved overall survival despite a similar rate of recurrence (Supplemental Fig. 1).
Patients with MCC-UP have intact immune function and higher oncoprotein antibody titers
Within our cohort, 12 patients presented with profound immune suppression (i.e. HIV, CLL, organ transplant). Among those without immune suppression, 72 of the 111 patients (65%) presented with MCC-UP at diagnosis whereas among those with immune suppression, 0 of 12 (0%; p < 0.001) presented without a primary lesion (Fig. 4A). Given the variable nature of human disease, we were unable to control for the relative degree of immune suppression between various immune-suppressed patients and could not determine the relative impact of various forms of immune suppression on survival. However, in order to verify that the disproportionately higher number of MCC-KP patients presenting with immune suppression was not the underlying cause of the reduced survival we observed, survival analyses were also performed excluding all cases of immune suppression (n= 92; Supplemental Fig. 2). Survival analyses for stage IIIB patients excluding those with immunosuppression retained statistical significance on univariate and multivariate analysis (Supplementary Fig. 2; Supplementary Table 1). For stage IV patients, overall and MCC-specific survival retained statistical significance on univariate analysis but became only marginally non-significant on multivariate analysis (n = 19; MCC-specific survival: p = 0.071; overall survival: p = 0.069; Supplementary Table 1). Overall, these data strongly suggest that immune competence correlates with MCC-UP presentation and immunosuppression does not appear to explain the difference in prognosis between MCC-UP and MCC-KP patients.
Figure 4. Patients with MCC-UP have intact immune function including robust oncoprotein antibody titers.
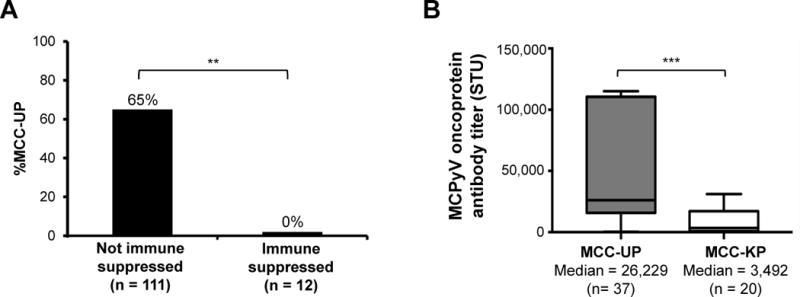
Panel A: Among the 123 patients with stage IIIB and IV MCC, no MCC patients with MCC-UP presented with immune suppression (**p < 0.001) whereas 65% of non-immune suppressed patients presented with MCC-UP. Panel B: MCC-UP patients presented with a significantly higher oncoprotein antibody titer (median 26,229 STU) as compared to MCC-KP patients (median 3,492 STU; ***p < 0.001). The median oncoprotein titers are indicated by the horizontal black lines and the P value was calculated using the Mann-Whitney test.
An additional marker of an MCPyV-specific immune response is the presence of antibodies specific to the MCPyV oncoproteins which can be detected in most virus-positive MCC patients (but are almost never present in healthy controls) (17). Among MCC patients who produce MCPyV oncoprotein antibodies (n = 57), MCC-UP patients had significantly higher median antibody titers (26,229) compared to seropositive MCC-KP patients (3,492, p < 0.001; Fig. 4B), suggesting that MCC-UP patients experienced more robust humoral immune responses than MCC-KP patients.
MCC-UP patients have a higher tumor mutational burden than patients with MCC-KP
It has been documented that higher mutational loads within tumors (including melanoma, colorectal, and several types of lung cancer) are associated with an increased prevalence of tumor-associated neoantigens, enhanced immunogenicity and ultimately improved response to immune-based therapies (14). We hypothesized that the improved survival advantage observed among MCC-UP patients may be correlated with higher tumor mutation burdens resulting in increased neoantigen presentation and immunogenicity as compared to tumors from MCC-KP patients. Previously, whole exome sequencing (WES) was performed on 16 tumors, which included 10 MCC-UP and 6 MCC-KP patients enrolled in our cohort (11). Analysis of these cases revealed that MCC-UP tumors harbor a significantly higher median number of nonsynonymous mutations (688/tumor) than MCC-KP tumors (10/tumor, p =0.016; Fig. 5A). As anticipated, virus-negative tumors (filled in symbols) overall harbor significantly higher mutation burdens than virus-positive tumors (open symbols). When evaluating mutation burden among virus-positive cases independently, patients presenting with MCC-UP have higher mutational loads than MCC-KP tumors (25 vs 7 nSSNV’s per tumor respectively; p = 0.029). This trend was also observed among virus-negative tumors with MCC-UP tumors having a median of 1,041 nSSNV’s per tumor as compared to MCC-KP tumors with a median of 310 nSSNV’s per tumor. While this comparison in virus-negative tumors did not achieve statistical significance, potentially due to low sample numbers, the 3-fold difference observed between these two subgroups strongly suggests that this is a meaningful distinction.
Figure 5. Relationships of mutational burden, MCPyV status and unknown primary status in MCC.
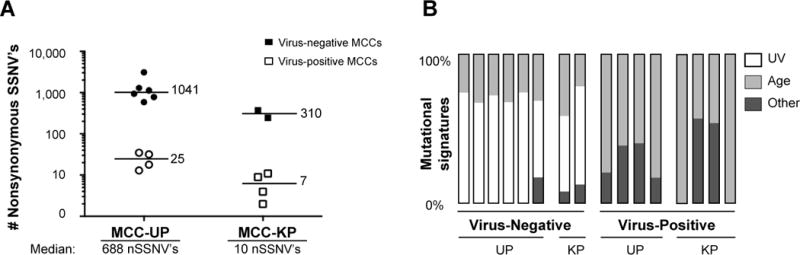
Panel A: Number of nonsynonymous single somatic nucleotide variations (nSSNV’s) among virus-negative cases MCC-UP (n=10) and MCC-KP (n=6). Median values for virus-positive and -negative subgroups are denoted adjacent to horizontal black lines. The median number of nSSNV’s for MCC-UP and MCC-KP patients are denoted below the X-axis. The Mann-Whitney test was performed to characterize the difference between the median values (p =0.016). Panel B: Relative frequency of an ultraviolet light or age-induced mutational signature grouped by viral and primary status. This panel is modified from data presented in Goh et al.
DISCUSSION
Here we report that among patients presenting with nodal disease, those with MCC with an unknown primary (MCC-UP) had a striking 70% reduced risk of death from MCC as compared to MCC-KP patients. We show that unknown primary status is also relevant for outcomes among patients presenting with distant metastatic (stage IV) disease. Additionally, we examined the relationship between MCC-UP presentation and immune function. MCC-UP patients never presented with immune suppression, had elevated MCPyV oncoprotein antibody titers and presented with a strikingly higher median number of tumor-associated nonsynonymous exome mutations as compared to patients presenting with MCC-KP. Mutational analyses further revealed UV-signature mutations in virus-negative tumors even among patients presenting with MCC-UP, indicating that these nodal lesions did arise from primary skin disease. These findings collectively suggest that enhanced immune function may underlie the development of MCC-UP through elimination of the primary skin lesion.
Our findings indicating improved survival among nodal MCC-UP patients are highly consistent with several previous reports which also indicate a 60-70% reduced risk of death from MCC (5,6,8). Other reports have speculated that regression of the primary lesion may be immune-mediated (5,8,10), however, there has been little evidence to support this theory. Therefore, we investigated differences in immune function and tumor immunogenicity between MCC-UP and MCC-KP patients. We found a statistically significant difference in the incidence of immunosuppression among MCC-UP and MCC-KP patients. This suggests that immune function is protective in MCC and may be contributing to regression of the primary lesion. While we saw no examples of MCC-UP arising in immunosuppressed patients (among 72 patients), there are in fact isolated cases in the literature. These include 2 reported cases of MCC-UP occurring in patients who received organ transplantation, and 3 with HIV (7,9). These five cases were reported among a total of 90 that were drawn from largely independent case reports and therefore likely reflect a publication bias that might tend to over emphasize this less common scenario in which MCC-UP can develop in patients with suppressed immune function (7,9).
Additionally, our finding that MCC-UP patients have higher oncoprotein antibody titers at the time of diagnosis may reflect a more robust immune response against MCC (16). Notably, serological status was included as a parameter in our multivariate survival analyses and overall oncoprotein seropositivity was not found to be statistically different between MCC-UP and MCC-KP patients, indicating that simply the presence of an antibody response is not associated with MCC-UP presentation. Rather, the magnitude of the response as reflected by the antibody titer is associated with MCC-UP presentation, suggesting that these antibody titers reflect augmented immunity.
Indicating that the tumors themselves may be more immunogenic in MCC-UP patients, we found that these tumors harbor significantly higher mutational burdens than MCC-KP tumors. High mutational burdens have been shown to elicit robust immune responses against neoantigens in several tumor types (14). Therefore, higher mutational loads among MCC-UP tumors may reflect enhanced neoantigen presentation, thereby enabling immune-mediated clearance of the primary lesions and improving survival. Notably, higher mutational loads among MCC-UP tumors relative to MCC-KP tumors were observed among both virus-negative and virus-positive subsets of MCC, though statistical significance was only achieved within the virus-positive group. This was surprising in the setting of virus-positive tumors because they have a much lower mutational burden (median 11 per tumor) than virus-negative tumors (864.5 per tumor). This finding suggests that the presence of even these small numbers of neoantigens within the virus-positive MCCs (median 25 for MCC-UP and 7 for MCC-KP) may significantly enhance immune activity even for these MCC tumors known to express highly antigenic viral oncoproteins. Future investigation into differences in T cell infiltration and function between tumors from MCC-UP and MCC-KP patients could provide additional insight into the immunological underpinnings of unknown primary presentation.
Our study also has important implications relating to the origin of MCC-UP tumors. It has been proposed that nodal disease observed in MCC-UP patients originated within the nodal basin instead of on the skin (22). Here we provide strong evidence that virus-negative MCC-UP tumors are skin derived based on the finding that when these tumors present in a lymph node they have high-levels of UV-signature mutations (21) (namely C to T transitions: Fig. 5B).
Notably, MCC is not the only cancer in which unknown primary presentation is associated with improved survival. A recent systematic review of melanoma presenting with an unknown primary (MUP) reported a reduced risk of disease-specific death among stage III and stage IV disease (17% and 15% reduction respectively) (23). Like MCC, it is postulated that MUP presentation is immune mediated. While there is currently limited evidence to link immune function and MUP presentation, one study indicated that MUP patients were 1.9-fold more likely to either present or develop vitiligo during follow-up than patients with a known primary site (24). This suggests that a specific anti-melanocytic immune response is correlated with clearance of the primary tumor (24). Importantly, the markedly reduced relative risk of dying from MCC observed among MCC-UP (70%) as compared to MUP (17%) suggests that MCC may be a more immune-responsive disease. This notion is supported by the higher response rates to checkpoint inhibition observed in MCC (15,25).
Importantly, we do not believe that the survival advantage observed among MCC-UP patients is attributable to differences in initial treatment including immune-based therapies. In all but one case, initial treatment was via standard therapies (surgery, radiation and/or chemotherapy) and these parameters are included within our multivariate analyses that indicated no significant difference in initial treatment between MCC-UP and MCC-KP patients. Notably, we did not include recurrent disease treatment modalities within our multivariate analysis because the probability of developing a recurrence is significantly affected by the initial presentation of a primary lesion (i.e. MCC-KP patients were significantly more likely to recur). Of note, 17 patients within our cohort who developed recurrent disease received various immune-based therapies (Supplementary Table 2). However, there was no association between receiving immunotherapy and presentation with a primary lesion (24.2% of MCC-UP and 23.7% of MCC-KP patients received immunotherapy for their subsequent recurrence). To date, the most effective immunotherapies for treating MCC are PD-1 checkpoint inhibitors (15,25) and of the 6 patients treated with these agents, all 6 presented with a known primary lesion. Therefore, any benefit that immunotherapy had on improving survival in this cohort would potentially reduce the survival advantage associated with MCC-UP presentation.
Our study had several limitations. Because of the retrospective nature of this study, some patients’ records were not complete or could not be obtained. Notably, there was likely referral and self-selection bias due to the tertiary, highly specialized nature of our multidisciplinary program. As a result, our cohort has a slightly higher proportion of MCC-UP (59% of stage IIIB) as compared to other cohorts (32%-55%) (5,6,26). The classification of MCC-UP status was based upon at least two comprehensive skin exams, including one by the initially diagnosing physician and one at the referral or tertiary site. It is possible, however, that diagnoses of other skin cancers were in fact missed cases of MCC. Based upon our prior experience with reviewing pathology records and pathological evaluation of other tumor biopsies at the time of MCC diagnosis, we estimate that misdiagnosis of other skins cancers as MCC occurs in fewer than 5% of cases. Importantly, the survival data for stage IIIB patients in our cohort closely resembles previously published reports (5-7,26,27), indicating that the survival difference observed between MCC-UP and MCC-KP patients is likely not attributable to recruitment bias or consistent misdiagnoses of other skin lesions within this cohort.
Additionally, although MCC is increasing in incidence it remains an uncommon disease. Therefore, while our study size of 123 is large for advanced MCC, only 20 patients presented with stage IV disease, limiting conclusions that can be drawn from this small subgroup. Most notably, when evaluating the presence of visceral disease among stage IV patients, 3 of 11 (27%) MCC-UP patients presented with visceral involvement, while 7 of 9 (78%) MCC-KP presented with visceral disease. Therefore, we cannot conclude whether presentation with an UP versus a KP affects survival when accounting for the presence of visceral disease because of the small sample size. Ideally, unknown primary status would be evaluated among patients with stage IV node-only disease separately from stage IV patients with visceral disease, however, the size of our study prevents this distinction. A reasonable interpretation is that KP disease is more likely to spread and persist successfully in key organs, however, further evaluation of these findings in a larger cohort is necessary.
Importantly, there are several clinically relevant implications of these findings. Multiple independent groups have corroborated that patients presenting with nodal MCC-UP have significantly improved survival. Therefore, unknown primary status is now being used to prognostically stratify patients in the recently released AJCC 8th edition staging system to more accurately reflect their improved outcomes(10).
Our results also support additional changes for future staging revisions. Firstly, we show that there is a statistically significant survival difference between patients presenting with nodal involvement of skin-draining basins only as compared to those presenting with non-skin draining nodes. We therefore propose that MCC-UP patients presenting with only skin-draining nodal involvement should be classified as regional (stage III) while those with involvement of non-skin draining nodes should be classified as distant metastatic (stage IV) disease. Secondly, further investigation into the survival advantage observed among stage IV MCC-UP patients may improve prognostic accuracy for patients with distant metastatic disease.
Lastly, it is possible these findings may have implications for the appropriate management of patients presenting with MCC-UP. While there are limited therapeutic options for late stage MCC patients, the use and availability of immune-based therapies is rapidly increasing. Checkpoint inhibitors, including anti-PD-1, have remarkable efficacy in treating both virus-positive and -negative MCC (15). The likely link between immune function and unknown primary status suggests that unknown primary status and response to immune therapies should be examined in future studies.
Supplementary Material
TRANSLATIONAL RELEVANCE.
Numerous reports show that Merkel cell carcinoma (MCC) patients presenting with nodal disease without detectable (unknown) primary skin lesions have ~50% improved survival as compared to similarly staged patients with skin lesions. This finding will be incorporated into the new staging system for MCC (active as of January 1st, 2018). Here we also show a significant survival difference among MCC-unknown primary (MCC-UP) patients presenting with distant metastatic disease. Additionally, this is the first report to our knowledge to explore potential mechanisms underlying MCC-UP presentation. Here we found that MCC-UP patients have higher levels of tumor-specific antibodies and higher tumor mutational burdens suggesting enhanced tumor immunogenicity and immune-mediated clearance of primary skin lesions. In the era of immune checkpoint blockade therapy, it may be that MCC-UP patients respond differently to these immune-based agents and therefore should be examined in future studies.
Acknowledgments
J.C. Is the Ruth K. Freinkel Research Professor and is supported by a Foglia family grant.
Funding Support:
NIH K24CA139052, NIH R01CA162522, NIH T32ES007032
Footnotes
Conflict of Interest:
The authors declare no potential conflicts of interest
References
- 1.Lemos BD, Storer BE, Iyer JG, Phillips JL, Bichakjian CK, Fang LC, et al. Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: analysis of 5823 cases as the basis of the first consensus staging system. J Am Acad Dermatol. 2010;63(5):751–61. doi: 10.1016/j.jaad.2010.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albores-Saavedra J, Batich K, Chable-Montero F, Sagy N, Schwartz AM, Henson DE. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol. 2010;37(1):20–7. doi: 10.1111/j.1600-0560.2009.01370.x. [DOI] [PubMed] [Google Scholar]
- 3.Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49(5):832–41. doi: 10.1067/S0190. [DOI] [PubMed] [Google Scholar]
- 4.Hodgson NC. Merkel cell carcinoma: changing incidence trends. J Surg Oncol. 2005;89(1):1–4. doi: 10.1002/jso.20167. [DOI] [PubMed] [Google Scholar]
- 5.Foote M, Veness M, Zarate D, Poulsen M. Merkel cell carcinoma: the prognostic implications of an occult primary in stage IIIB (nodal) disease. J Am Acad Dermatol. 2012;67(3):395–9. doi: 10.1016/j.jaad.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Chen KT, Papavasiliou P, Edwards K, Zhu F, Perlis C, Wu H, et al. A better prognosis for Merkel cell carcinoma of unknown primary origin. Am J Surg. 2013;206(5):752–7. doi: 10.1016/j.amjsurg.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Tarantola TI, Vallow LA, Halyard MY, Weenig RH, Warschaw KE, Weaver AL, et al. Unknown primary Merkel cell carcinoma: 23 new cases and a review. J Am Acad Dermatol. 2013;68(3):433–40. doi: 10.1016/j.jaad.2012.07.035. [DOI] [PubMed] [Google Scholar]
- 8.Asgari MM, Sokil MM, Warton EM, Iyer J, Paulson KG, Nghiem P. Effect of host, tumor, diagnostic, and treatment variables on outcomes in a large cohort with Merkel cell carcinoma. JAMA Dermatol. 2014;150(7):716–23. doi: 10.1001/jamadermatol.2013.8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotteas EA, Pavlidis N. Neuroendocrine Merkel cell nodal carcinoma of unknown primary site: management and outcomes of a rare entity. Crit Rev Oncol Hematol. 2015;94(1):116–21. doi: 10.1016/j.critrevonc.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Harms KL, Healy MA, Nghiem P, Sober AJ, Johnson TM, Bichakjian CK, et al. Analysis of Prognostic Factors from 9387 Merkel Cell Carcinoma Cases Forms the Basis for the New 8th Edition AJCC Staging System. Ann Surg Oncol. 2016;23(11):3564–71. doi: 10.1245/s10434-016-5266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goh G, Walradt T, Markarov V, Blom A, Riaz N, Doumani R, et al. Mutational landscape of MCPyV-positive and MCPyV-negative Merkel cell carcinomas with implications for immunotherapy. Oncotarget. 2016;7(3):3403–15. doi: 10.18632/oncotarget.6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319(5866):1096–100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong SQ, Waldeck K, Vergara IA, Schroder J, Madore J, Wilmott JS, et al. UV-associated mutations underlie the etiology of MCV-negative Merkel cell carcinomas. Cancer Res. 2015;75(24):5228–34. doi: 10.1158/0008-5472.CAN-15-1877. [DOI] [PubMed] [Google Scholar]
- 14.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 15.Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N Engl J Med. 2016;374(26):2542–52. doi: 10.1056/NEJMoa1603702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paulson KG, Lewis CW, Redman MW, Simonson WT, Lisberg A, Ritter D, et al. Viral oncoprotein antibodies as a marker for recurrence of Merkel cell carcinoma: A prospective validation study. Cancer. 2017;123(8):1464–74. doi: 10.1002/cncr.30475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paulson KG, Carter JJ, Johnson LG, Cahill KW, Iyer JG, Schrama D, et al. Antibodies to merkel cell polyomavirus T antigen oncoproteins reflect tumor burden in merkel cell carcinoma patients. Cancer Res. 2010;70(21):8388–97. doi: 10.1158/0008-5472.CAN-10-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moshiri AS, Doumani R, Yelistratova L, Blom A, Lachance K, Shinohara MM, et al. Polyomavirus-Negative Merkel Cell Carcinoma: A More Aggressive Subtype Based on Analysis of 282 Cases Using Multimodal Tumor Virus Detection. J Invest Dermatol. 2017;137(4):819–27. doi: 10.1016/j.jid.2016.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shuda M, Feng H, Kwun HJ, Rosen ST, Gjoerup O, Moore PS, et al. T antigen mutations are a human tumor-specific signature for Merkel cell polyomavirus. Proc Natl Acad Sci U S A. 2008;105(42):16272–7. doi: 10.1073/pnas.0806526105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodig SJ, Cheng J, Wardzala J, DoRosario A, Scanlon JJ, Laga AC, et al. Improved detection suggests all Merkel cell carcinomas harbor Merkel polyomavirus. J Clin Invest. 2012;122(12):4645–53. doi: 10.1172/JCI64116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–21. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan Z, Chen YY, Wu X, Trisal V, Wilczynski SP, Weiss LM, et al. Merkel cell carcinoma of lymph node with unknown primary has a significantly lower association with Merkel cell polyomavirus than its cutaneous counterpart. Mod Pathol. 2014;27(9):1182–92. doi: 10.1038/modpathol.2013.250. [DOI] [PubMed] [Google Scholar]
- 23.Bae JM, Choi YY, Kim DS, Lee JH, Jang HS, Lee JH, et al. Metastatic melanomas of unknown primary show better prognosis than those of known primary: a systematic review and meta-analysis of observational studies. J Am Acad Dermatol. 2015;72(1):59–70. doi: 10.1016/j.jaad.2014.09.029. [DOI] [PubMed] [Google Scholar]
- 24.Savoia P, Fava P, Osella-Abate S, Nardo T, Comessatti A, Quaglino P, et al. Melanoma of unknown primary site: a 33-year experience at the Turin Melanoma Centre. Melanoma Res. 2010;20(3):227–32. [PubMed] [Google Scholar]
- 25.Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D’Angelo SP, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17(10):1374–85. doi: 10.1016/S1470-2045(16)30364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fields RC, Busam KJ, Chou JF, Panageas KS, Pulitzer MP, Allen PJ, et al. Five hundred patients with Merkel cell carcinoma evaluated at a single institution. Ann Surg. 2011;254(3):465–73. doi: 10.1097/SLA.0b013e31822c5fc1. discussion 73–5. [DOI] [PubMed] [Google Scholar]
- 27.Deneve JL, Messina JL, Marzban SS, Gonzalez RJ, Walls BM, Fisher KJ, et al. Merkel cell carcinoma of unknown primary origin. Ann Surg Oncol. 2012;19(7):2360–6. doi: 10.1245/s10434-011-2213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


