Abstract
We examined whether abnormal volumes of several brain regions as well as their mutual associations that have been observed in patients with schizophrenia, are also present in individuals at clinical high-risk (CHR) for developing psychosis.
3T magnetic resonance imaging was acquired in 19 CHR and 20 age- and handedness-matched controls. Volumes were measured for the body and temporal horns of the lateral ventricles, hippocampus and amygdala as well as total brain, cortical gray matter, white matter, and subcortical gray matter volumes. Relationships between volumes as well as correlations between volumes and cognitive and clinical measures were explored. Ratios of lateral ventricular volume to total brain volume and temporal horn volume to total brain volume were calculated. Volumetric abnormalities were lateralized to the left hemisphere. Volumes of the left temporal horn, and marginally, of the body of the left lateral ventricle were larger, while left amygdala but not hippocampal volume was significantly smaller in CHR participants compared to controls. Total brain volume was also significantly smaller and the ratio of the temporal horn/total brain volume was significantly higher in CHR than in controls. White matter volume correlated positively with higher verbal fluency score while temporal horn volume correlated positively with a greater number of perseverative errors.
Together with the finding of larger temporal horns and smaller amygdala volumes in the left hemisphere, these results indicate that the ratio of lateral ventricle the ratio of temporal horns volume to brain volume is abnormal in CHR compared to controls. These abnormalities present in CHR individuals may constitute the biological basis for at least some of the CHR syndrome.
Keywords: Ventricles/Brain volume ratio, temporal horns of lateral ventricles, hippocampus, amygdala, MABS, Verbal fluency, Wisconsin card test, white matter, clinical high risk to develop psychosis
1. Introduction
Morphometric abnormalities in several brain regions are observed in schizophrenia (see review by Shenton et al. 2001). Among these, one of the most replicated findings is larger lateral ventricle’s volume in both chronic and first episode schizophrenia (SZ) compared to healthy controls (Johnstone et al. 1976; DeLisi et al. 1986; Shenton et al. 2001; Nakamura et al. 2007; del Re et al. 2016b). There are a handful of studies that have also investigated lateral ventricles abnormalities in relation to other brain regions. More specifically, larger than normal lateral ventricle volume has been associated with reductions in cortical and subcortical gray matter volume in schizophrenia (Horga et al. 2011) but also with regional volume abnormalities. For example, a higher than normal ventricular/brain ratio has been associated with volume decrease loss in the thalamus, striatum and temporal lobe (Gaser et al. 2004), and with lesser volume of the central corpus callosum (del Re et al. 2016b). In chronic schizophrenia and other major mental illnesses, a higher than normal ratio of lateral ventricle volume to total brain volume (VBR) has been found to be a robust measure that distinguishes healthy controls from patients (van Horn and McManus 1992). VBR has also been shown to separate schizophrenia and bipolar patients from controls better than the individual measures of total brain volume (TBV) or intracranial content (Reite et al. 2010).
In the current study, we investigated individuals at clinical high risk (CHR) for developing psychosis by exploring the relationship between the volumes of the body and temporal horns of the lateral ventricles and selected adjacent structures, i.e. the amygdala and hippocampus (illustrated in Figure 1). These regions of interest (ROI) were also investigated in relation to global brain measures, including total gray and white matter volumes.
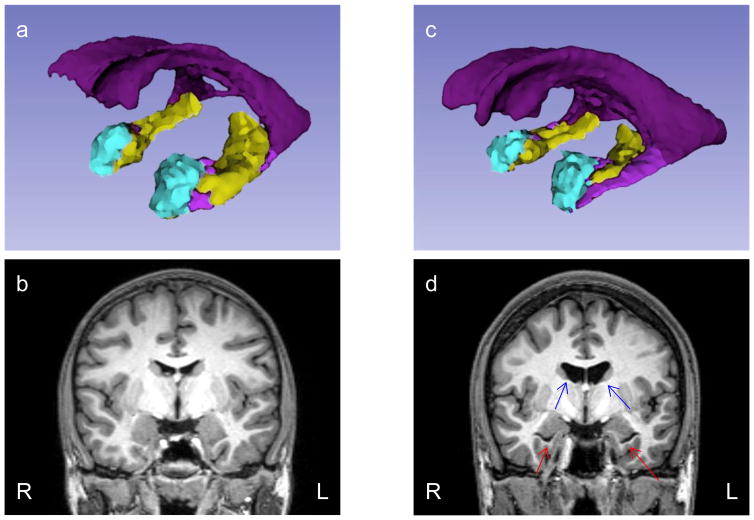
Figure 1. 3D ROIs reconstruction and coronal MRI image of a representative control (a, b) and a representative CHR subject (c, d).
In a and c, the body of lateral ventricles are in dark purple, the temporal horns of the lateral ventricles are in light purple, the amygdala is in cyan blue, and the hippocampus is in light green. 3D reconstruction is based on FreeSurfer segmentation and carried out with 3D Slicer. The red arrows in d indicate enlargement of temporal horns and the blue arrows enlargement of the body of the lateral ventricles.
In the context of a neurodevelopmental view of the CHR syndrome, our hypothesis was that, even in the absence of measurable volume abnormalities in CHR, the relationship between lateral ventricle volume and adjacent subcortical structures, as well as with more global brain measures, will be abnormal in CHR compared to healthy controls. As deficits in neurocognition are an integral part of the CHR syndrome (Seidman et al. 2016; Giuliano et al. 2012) and schizophrenia (Mesholam-Gately et al. 2009; Blokland et al. 2016), we also explored association with two neurocognitive measures, indexing speed of processing, verbal ability and executive function. The hypothesis here was that global brain measures and midline brain structures will index neurocognitive performance, before abnormalities in discreet brain regions are measurable (Johnston et al., 1976; Toulopoulou et al., 2004; Crespo-Facorro et al., 2007; Laywe et al., 2006).
To the best of our knowledge, neither volume of the temporal horn nor the relationship between lateral ventricles and global and local brain measures have previously been investigated in CHR samples.
2. Materials and methods
2.1. Participants and Clinical Procedures
Thirty-nine participants from the Boston Center for Intervention Development and Applied Research (CIDAR) study, entitled “Vulnerability to Progression in Schizophrenia,” provided imaging, clinical and demographic data. The sample comprised 19 individuals at CHR for psychosis and 20 healthy control participants, the latter matched to the CHR group on age, parental socioeconomic status, handedness and gender. Participants were recruited by referrals from clinicians, local hospitals and clinics, advertisements, formal outreach presentations, and word of mouth. The study was approved by the local IRB committees at Harvard Medical School and Beth Israel Deaconess Medical Center, the Cambridge Health Alliance, Massachusetts General Hospital, Brigham and Women’s Hospital and by the Veteran Affairs Boston Healthcare System, Brockton campus. All study participants (or legal guardians for those under 18) gave written informed consent prior to study participation, and subjects received payment for participation.
Clinical diagnoses for all subjects were based on interviews with the Structured Clinical Interview for DSM-IV-TR (SCID), Research Version (First et al. 2002) or the Kid-SCID (Hien et al. 1994) for subjects <18, as well as information from available medical records. In the CHR group, prodromal symptoms were assessed with the Scale of Prodromal Symptoms (SOPS; contained within the Structured Interview of Prodromal Syndromes (SIPS) (Miller et al. 1999). Additionally CHR symptoms were rated on 10 items from the Bonn Scale for the Assessment of Basic Symptoms that were identified as having high predictive validity for the development of psychosis (Klosterkötter et al. 2001) and that are implemented in the Schizophrenia Proneness Instrument, Adult Version (Schultze-Lutter et al. 2007) (See del Re et al. 2015 for details on CHR inclusion criteria used in the CIDAR study and the study by von Hohenberg et al. 2014). Exclusion criteria for the CHR group included the presence of any DSM-IV-TR diagnosis of a psychotic disorder, and substance-induced or other medically induced prodromal-like symptoms.
Controls were drawn from the same geographic region as CHR subjects with comparable age, gender, race and ethnicity, handedness, and parental socioeconomic status (PSES). No controls met criteria for any current major DSM-IV-TR Axis I disorders, or any history of psychosis, Major Depressive Disorder (recurrent), Bipolar Disorder, Obsessive Compulsive Disorder, Post Traumatic Stress Disorder, or developmental disorders. Controls were also excluded if they had any history of psychiatric hospitalizations, prodromal symptoms, schizotypal or other Cluster A personality disorders, first degree relatives with psychosis, or any current or past use of antipsychotics (other past psychotropic medication use was acceptable, but the subjects must have been off medicine for at least 6 months before participating in the study, with the exception of as needed medications including sleeping medications or anxiolytic agents). Exclusion criteria for all participants included: sensory-motor handicaps, neurological disorders, medical illnesses that significantly impair neurocognitive function, diagnosis of mental retardation, education less than 5th grade if <18 or less than 9th grade if ≥18, not fluent in English, DSM-IV-TR substance abuse in the past month, DSM-IV-TR substance dependence (excluding nicotine) in the past 3 months, current suicidality, history of ECT within the past five years for patients and history of ECT ever for controls, or study participation by another family member.
PSES was evaluated using the Hollingshead two-factor index (Hollingshead 1975). Premorbid intellectual abilities were estimated using the Reading subtest from the Wide Range Achievement Test-4 (WRAT-4) (Wilkinson and Robertson 2006) and current intellect was estimated from the Vocabulary and Block Design subtests of the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler 1999). The latter measures were administered by trained research assistants as part of a comprehensive neuropsychological battery. Participants were also evaluated on a number of cognitive domains. Among those, the Category Fluency: Animal Naming test (Spreen and Strauss 1991), that indexes speed of processing, verbal ability and executive function as well as the Wisconsin Card Sorting Test (WCST) that indexes executive functioning/reasoning and problem solving (Heaton 2000; Berg 1948).
All participants were evaluated with the Global Assessment of Functioning scale (GAF) (Jones et al. 1995). All individuals included in the present study were clinically evaluated at baseline and one year following baseline assessment. During 5 years of data acquisition, only one subject converted to psychosis. This subject was included in all analyses (indicated in blue in the Figures).
2.2 MRI Image Acquisition
Images were acquired on a 3 Tesla magnetic resonance imaging (MRI) scanner (Tim Trio, Siemens Medical Solutions, Erlangen, Germany) at Massachusetts General Hospital, Boston, MA, using a standard circular-polarized head coil. T1-weighted structural MRI was acquired with a magnetization prepared rapid gradient echo (MPRAGE) sequence (TR = 2530 ms, TE = 3.39 ms, TI = 1100, 7-degree flip angle, 128 contiguous sagittal slices with 1.33 mm slice thickness, 25.6 cm2 field of view, matrix = 256×256 and 1.33×1×1 mm voxel dimensions).
2.3 MRI Image Processing
Images were visually checked for possible movement artifacts. In order to correct for head tilt, each MRI scan was realigned, horizontally to the anterior commissure - posterior commissure line, and vertically to the sagittal sulcus. Automatic brain masking was conducted using Multi Atlas Brain Segmentation, MABS (del Re et al. 2016a). Segmentation of the scans was executed using FreeSurfer 5.3 (Fischl et al. 2002) and quality of segmentations was determined by visual inspection. Volumes of the body of the lateral ventricles, temporal horns of the lateral ventricles, amygdala, and hippocampi were extracted as well as total brain, and cortical and subcortical gray and white matter. More specifically, total brain volume consisted of all voxels contained in cortical and subcortical regions, but excluded the brain stem, ventricles, CSF, and choroid plexus. Cortical gray matter included the gray matter contained between the pial surface and white matter surface and excluded regions not belonging to the cortex (e.g., hippocampus). White matter included voxels contained inside the white matter surface. Subcortical gray matter included the thalamus, caudate nucleus, hippocampus, amygdala, nucleus accumbens, pallidum, putamen, ventral diencephalon and the substantia nigra. The ratios of lateral ventricles/total brain volume, lateral ventricles/white matter, temporal horns/total brain volume and temporal horns/white matter were multiplied by 100 for all analyses.
2.4 MRI volume analyses
In order to account for differences in head size, intracranial volume was used as a covariate in all analyses of ROI volume effects. FreeSurfer estimates the intracranial volume based on the talairach transform as described in Buckner et al. (2004). As volumes of lateral ventricles, temporal horns, amygdala, and hippocampus substantially differ from one another and hence are not directly comparable, we transformed the data into z-scores using the mean and standard deviation of the control sample. Because the volumes of the ventricles tend to be larger, while volumes of the tissue ROIs, amygdala and hippocampus, tend to be smaller with respect to values of healthy controls, the z-scores of the tissue ROIs were multiplied by minus 1, before entering them into an initial repeated measures Group X Hemisphere X ROI MANCOVA, with Group (diagnosis) as a between-subjects factor and Hemisphere and Brain Regions (n=4), lateral ventricle, temporal horn, amygdala and hippocampus as within-subjects factors. Intracranial volume (ICF) was included as a covariate in the statistical model. Since seven of 19 CHR were medicated with psychotropic medication, the CHR on medication (n=7) were directly compared to the CHR not on medication (n=12) using the same repeated measures MANOVA, in order to determine whether medication status accounts for significant variance in the brain volume measures. For these analyses, medication dosage was estimated using chlorpromazine (CPZ) equivalents, and calculated according to Stoll (2009) and Woods (2003). A significant Hemisphere X Group interaction found in the repeated measures MANCOVA, was followed up by a separate repeated measures MANCOVA for each Hemisphere and where appropriate by univariate between-subjects ANCOVAs for each region. Group differences of global brain measures and of ratios between global measures and lateral ventricles were analyzed with univariate ANOVAs. In all analyses outlier values were defined as >3.0 standard deviations from the mean; no data were outside this range. All statistical analyses were performed with SPSS v.23 (http://www.ibm.com/analytics/us/en/technology/spss) or R v.3.3.0 (www.r-project.org). We note that relative volumes are used in all Figures.
2.5 Correlation analyses
Considering the relatively small sample size, Spearman’s rho was adopted for all correlation analyses, and Bonferroni correction was applied to all correlations to protect against Type 1 error. Clinical and functioning measures included the total SOPS scores for positive, negative, disorganized, and general symptoms, and the GAF score. For the correlations between relative volumes and clinical measures, Bonferroni corrections for multiple correlations were performed with a p-value threshold calculated as follows: 0.05/40 correlations = 0.00093 for eight ROI and five clinical measures. For the correlations between relative volumes and two neurocognitive measures, the WCST perseverative errors and the Category fluency test, the Bonferroni corrected significance threshold for multiple correlations was calculated as follows: 0.05/16=0.003 for eight ROIs and two neurocognitive measures. The Bonferroni corrected significance threshold for correlations among relative volumes was calculated as follows: 0.05/23 correlations = 0.0022. In order to determine whether or not the relationships observed in the CHR and healthy control groups were different, the correlations observed in the two groups were directly compared using the Fisher’s r-to-z test. The results of this test are reported in the Results section.
3. Results
3.1 Participants group characteristics
CHR and control groups did not differ in age, premorbid IQ estimated by WRAT-4 reading score, current IQ, years of education, PSES or number of male and female subjects, as summarized in Table 1. Significant group differences were, however, found for the GAF and the SOPS, as would be expected. There were no Group differences in the Wisconsin perseverative errors score or in the category fluency score. Seven of 19 CHR subjects were medicated with antipsychotic medications. All antipsychotic medications were second-generation: three subjects were on aripiprazole, two were on risperidone, one was on quetiapine, and one was on 3 medications: risperidone, ziprasidone, and aripiprazole. No significant correlations were found between CPZ equivalents and any of the volumes. These findings are summarized in Table 1.
Table 1.
Demographic and Clinical Information
| HC (n = 20) | CHR (n = 19) | t/χ/F | P | |
|---|---|---|---|---|
| Gender, F/M | 8/12 | 6/13 | 0.30* | 0.584 |
| Age, mean (SD) years | 21.4 (3.7) | 20.9 (4.3) | 0.34 | 0.732 |
| WRAT-4, standardized reading score, mean (SD) | 118.2 (15.8) | 108.8 (16.5) | 1.81 | 0.079 |
| Estimated current IQ, mean (SD) | 121.2 (14.4) | 114.6 (14.2) | 1.43 | 0.160 |
| PSES, mean (SD) | 1.6 (0.8) | 1.9 (0.8) | 1.30 | 0.202 |
| Years of education, mean (SD) | 13.7 (2.7) | 12.6 (2.6) | 1.3 | 0.212 |
| Scale of Prodromal Symptoms, mean (SD) | ||||
| Positive Symptoms | 0.40 (0.82) | 12.79 (4.14) | 12.79 | < 0.001 |
| Negative Symptoms | 0.45 (1.36) | 13.22 (6.52) | 8.15 | < 0.001 |
| Disorganization Symptoms | 0.05 (0.22) | 6.47 (2.86) | 9.78 | < 0.001 |
| General Symptoms | 0.26 (0.93) | 7.33 (3.68) | 7.92 | < 0.001 |
| GAF, mean (SD) | 85.7 (8.2) | 49.1 (10.2) | 12.29 | < 0.001 |
| WCST, perseverative errors | 6.6 (3.5) | 5.7 (1.9) | 1.0+ | 0.32 |
| Category fluency, animals (correct) | 27.1 (5.7) | 24.7 (6.9) | 0.06 | 0.8 |
| Number of Medicated CHR | N.A. | 7/19 | ||
| CPZ equivalents | N.A. | 136.4 (217.2) | ||
Values are mean (SD); HC, Healthy controls; CHR, individuals at clinical high risk to develop psychosis; CPZ Chlorpromazine; All antipsychotics were second generation; CPZ were calculated for CHR on antipsychotic medication; PSES, Parental Socioeconomic Status; N.A., not applicable; WRAT-4, Wide Range Achievement Test-fourth edition; GAF, Global Assessment of Functioning; WCST, Wisconsin Card Sorting Test; CPZ equivalents were calculated according to Stoll (2009) and Woods (2003).
Chi-squared test;
ANOVA test
3.2 Volumetric Analyses
Subcortical measures
A main effect of Group was found in the repeated measures MANCOVA [F(1,36)=8.3; p=0.007], where a higher mean Z-score was present in the CHR group compared to the controls. There was also a significant main effect of Hemisphere [F(1,36)=7.7; p=0.009], where the left hemisphere had higher Z-score than the right hemisphere. A significant interaction of Hemisphere by Group [F(1,360=7.7; p=0.009] was further followed up with a repeated measures Group X ROI MANCOVA model for each Hemisphere, separately.
Left Hemisphere
In the left hemisphere, there was a significant main effect of Group [F(1,36)=9.7; p=0.004] and a significant interaction of ROI by Group [F(3,108)=3.33; p=0.046]. Follow-up univariate ANCOVAs, indicated a significantly larger volume of the left temporal horn in CHR compared to controls [F(1,36)=10.34; p=0.003; Cohen’s d=1.1] as well as a marginally larger volume of the left lateral ventricle in CHR compared to healthy controls [F(1,36)=4.04; p=0.052; Cohen’s d=0.6]. Further, the left amygdala volume was significantly smaller in CHR compared to controls [F(1,36)=6.3; p=0.016; Cohen’s d=0.8] while the volume of the hippocampus did not differ significantly between CHR and controls [F(1,36)=0.38; p=0.54].
Right Hemisphere
In the right hemisphere, there was a main effect of Group [F(1,36)=4.1; p=0.048] but there was no significant interaction of ROIs by Group [F(3,34]=1.3; p=0.28] indicating minor, albeit non-significant, CHR volumetric abnormalities of subcortical measures in the right hemisphere compared to controls.
Global brain measures
Total brain volume, including gray and white matter and excluding ventricles, was significantly smaller in CHR participants compared to controls [F(1,36)=9.6; p=0.004; Cohen’s d=1.0]. The total gray matter volume and the subcortical gray volume did not differ between CHR and control groups [F(1,36)=0.91; p=0.34; and F(1,36)=0.1; p=0.75, respectively], while there was a significant reduction of white matter volume in CHR compared to controls [F(1,36)=6.5; p=0.015] (Figure 3).
Figure 3. Scattergrams of relative volumes of (a) total brain volume (TBV); (b) white matter (WM); (c) gray matter (GM) and (d) subcortical gray matter (SCGM).
The significant TBV decrease in CHR compared to HC (a) was explained by a significant decrease of cortical WM (b). There were no significant differences between groups in the volumes of subcortical GM (SCGM) or cortical GM.
TBV, total brain volume; WM, white matter volume; GM, cortical gray matter; SCGM, subcortical gray matter. HC, healthy control; CHR, individual at clinical high risk of developing psychosis.
Relationship between ventricular volumes and global measures
The ratio of lateral ventricle to total brain volume was significantly higher in CHR than in controls [F(1,37)= 4.3; p=0.046] as was the ratio of temporal horn to total brain volume [F(1,37)=9.96; p=0.003]. There was also a main effect of Group for the lateral ventricle volume/white matter [F(1,37)=4.34; p=0.044] and for temporal horn volume/white matter ratios [F(1,37)=9.83; p=0.003]. These findings are summarized in Table 2 and in Figure 4.
Table 2.
Ratios of Ventricular Volumes to TBV and WM
| HC (n = 20) | CHR (n = 19) | F | P | Effect Size Cohen’s d | |
|---|---|---|---|---|---|
| LV to TBV | 0.93 (0.30) | 1.32 (0.79) | 4.3 | 0.046 | −0.66 |
| LV to WM | 2.31 (0.74) | 3.32 (2.00) | 4.3 | 0.044 | −0.67 |
| TH to TBV | 0.05 (0.02) | 0.07 (0.03) | 10.0 | 0.003 | −1.01 |
| TH to WM | 0.13 (0.05) | 0.19 (0.07) | 9.8 | 0.003 | −1.00 |
HC, healthy controls; CHR, clinical high risk individuals; LV, lateral ventricles; TH, temporal horns; TBV, total brain volume; WM, white matter. All ratios were multiplied by 100.
Figure 4. Ratios of (a) lateral ventricle (LV) to total brain volume (TBV); (b) lateral ventricle (LV) to white matter (WM); (c) temporal horns (TH) to total brain volume (TBV) and temporal horns (TH) to white matter (WM) (d).
In all cases ratios were significantly higher in CHR than in HC. Interestingly, LV/TBV (a) and LV/WM (b) ratios were significantly higher in CHR compared to HC although the relative LV volumes themselves did not significantly differ from those measured in HC as shown in Figure 2a. HC, healthy control; CHR, individual at clinical high risk of developing psychosis.
Impact of medication on volumetric measures
Seven of the 19 CHR were medicated. In order to rule out the possible effect of medication on the volumes of subcortical measures, volumes of medicated and medication-naïve CHR were directly compared using a repeated measures MANCOVA model. No main Group effect or significant interaction of Group X ROI [F(3, 14)=0.83; p=0.49] were present [F(1,16)=0.16; p=0.7].
3.3 Correlations between volumes
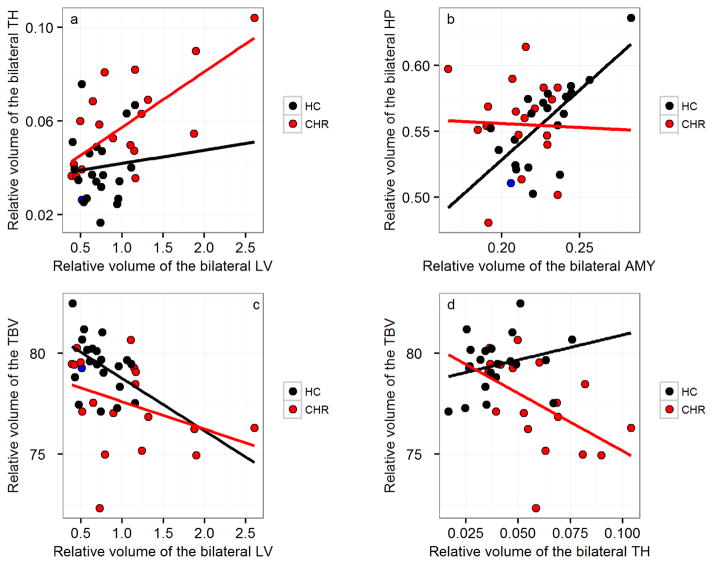
Investigation of the relationship between the following bilateral structures: lateral ventricle, temporal horn, amygdala, and hippocampus volumes with global measures in CHR and controls, indicated a strong inverse correlation in CHR subjects between lateral ventricle and total brain volume (rho=−0.60, p=0.007) such that the larger the ventricles, the smaller the total brain volume. Such an inverse correlation was also observed in CHR subjects between temporal horn and total brain volume (rho=−0.61, p=0.006), such that the larger the temporal horns, the smaller the total brain volume. These correlations were not significant in controls (rho=−0.39, p=0.09; and rho=0.29, p=0.21; respectively). The Fisher’s r-to-z test was carried out in order to determine whether or not the volumetric relationships observed in the CHR group differed from those observed in the healthy control group. Findings showed that the relationship between temporal horns and total brain volume differed significantly between the CHR and healthy control groups (z=2.9; p=0.002). In contrast, albeit the inverse correlation between lateral ventricle and total brain volume was significant in CHR and not significant in healthy controls, the Fisher r-to-z test results did not confirm that the relationship between the volumes was significantly different between the two groups (z=0.853; p=0.197). A strong positive correlation was, however, observed between increased lateral ventricle and increased temporal horn in CHR subjects (rho=0.60, p=0.007). This latter correlation was also not present in controls (rho=−0.006, p=0.98). The Fisher r-to-z test confirmed the temporal horn-lateral ventricle volumetric relationship observed in CHR to be significantly different from the one found in controls (z=−2.18; p=0.03). Interestingly, in controls, amygdala and hippocampus were significantly and positively correlated (rho=0.66, p=0.0014), but the same relationship was not found in CHR (rho=−0.03, p=0.90). The Fisher r-to-z test confirmed that the relationship between amygdala and hippocampus observed in CHR and healthy controls, differed (z=2.41; p=0.008) (Figure 5).
Figure 5. Correlations between volumes.
(a) A strong positive correlation between bilateral lateral ventricle (LV) and bilateral temporal horns (TH) is found in CHR (rho=0.60, p=0.007), but not in controls (rho=−0.006, p=0.98). (b) A significant correlation present between bilateral amygdala (AMY) and hippocampus (HP) is found in HC (rho=0.66, p=0.0014), but not in CHR (rho=−0.03, p=0.91). (c) Strong inverse correlation trends between bilateral lateral ventricle (LV) and total brain volume (TBV) (rho=−0.60, p=0.007) and (d) between temporal horns (TH) and total brain volume (TBV) (rho=−0.61, p=0.006) in CHR, but not in HC (rho=−0.39, p=0.09; and rho=0.29, p=0.21; respectively). HC, healthy control; CHR, individual at clinical high risk of developing psychosis.
3.4 Correlation between volumes and clinical scores in CHR
There was a trend level positive correlation between bilateral lateral ventricle volume and negative symptoms score (rho=0.54, p=0.02) (Figure 6a), i.e., the larger the bilateral ventricle volume, the higher the negative symptoms; while the volume of the bilateral hippocampus was inversely correlated with disorganization symptoms (rho=−0.49, p=0.032), i.e., the smaller the hippocampus, the more the disorganized symptoms (Figure 6b). None of the other associations were significant.
Figure 6. Correlations between volumes and clinical and neurocognitive measures in CHR.
Correlations at baseline did not survive Bonferroni correction for multiple comparisons, nonetheless at trend level there was (a) a positive correlation (rho=−0.49) between bilateral lateral ventricle (LV) volume and the total SOPS score for negative symptoms; and (b), an inverse correlation (rho=0.54) between disorganization symptoms and relative volumes of bilateral hippocampus (HP). White matter volume was positively correlated (rho=0.62) with the Category fluency test, animal naming score (c). The one CHR that converted at one-year clinical follow-up is marked in blue. CHR, individual at clinical high risk of psychosis; LV, lateral ventricle; HIP, hippocampus; ANC, Category fluency test, animal naming.
3.5. Correlation between volumes and WCST perseverative errors and Category fluency in CHR
The white matter volume was positively correlated with the Category fluency test, animal naming score where larger white matter volume corresponded to a higher test score (rho=0.62, p=0.004; r=0.61) (Figure 6c). The volume of the temporal horns was positively correlated to the score of perseverative errors on the WCST test, where the larger the volume of the temporal horns, the higher the number of perseverative errors (rho=0.6, p=0.008; r=0.55). None of the associations remained significant after strict Bonferroni correction (cut-off value of p=0.003). Nonetheless, effect sizes, reported as r (see above) were very large. In the healthy control group, there were no statistically significant correlations between white matter volume and Category fluency test (rho=−0.32, p=0.17; r=−0.29), nor between perseverative errors of the WCST or volume of temporal horns (rho=0.12, p=0.62; r=−0.14). The Fisher r-to-z test confirmed that the correlations between white matter volume and the Category fluency test (z=2.89; p=0.002) and between temporal horn volumes and WCST (z=−1.54; p=0.01) found in within each of the two groups were significantly different.
4. Discussion
In the present investigation of CHR individuals, we examined the volumes of subcortical structures that are anatomically adjacent to the body and the temporal horns of the lateral ventricles, i.e., the amygdala and hippocampus, and their interrelationships and association with global brain measures, including total brain volume and volumes of the cortical gray matter, subcortical gray matter and white matter. In the context of a neurodevelopmental view of the CHR syndrome we hypothesized that, even in the absence of measurable volume differences in CHR compared to controls, the relationships between lateral ventricle volume and adjacent subcortical structures, as well as global brain measures, would be abnormal in CHR compared to controls.
4.1 Lateral Ventricle and Temporal Horn
The results indicated lateralized, left hemisphere abnormalities in CHR, with larger volumes of the left temporal horn and marginally of the left lateral ventricle in the presence of smaller volume of the left amygdala in CHR subjects compared with controls. The hippocampal volume was not found to be significantly different in CHR compared to controls It appeared that the significantly smaller total brain volume in CHR compared to controls was associated with less white matter volume and not cortical or subcortical gray matter. The ratio between lateral ventricular volume to total brain volume or VBR as well as the ratio between lateral ventricular volume to white matter volume were significantly higher in CHR subjects than in controls. The ratios between the temporal horns volume to total brain volume as well as the temporal horns to white matter volume were also significantly higher in CHR compared to controls.
VBR as well as the ratio between temporal horn volume and total brain volume are measured within each individual. These ratios thus represent measures that adjusts and correct for brain and head size variability among individuals. VBR increase in chronic schizophrenia is a consistent finding (see for example Reite et al. 2010; and reviews by McCarley et al. 1999; Shenton et al. 2001; and the meta-analysis by van Horn and McManus 1992).
This novel finding of increased VBR in CHR is particularly important because research focused on CHR is largely unconfounded by factors such as medication and chronicity (e.g. Addington et al. 2015; Fusar-Poli et al. 2013; Goncalves et al. 2016; von Hohenberg et al. 2014), that may be present in studies on schizophrenia per se. Moreover, considering that the CHR population might include young, often still developing individuals, VBR might be especially important in capturing dynamic structural brain changes and changing interactions between brain regions, before volumetric abnormalities become measurable during the course of disease.
Typically, temporal horn and lateral ventricle volumes are significantly larger in chronic schizophrenia compared to healthy control subjects (see for example comprehensive review by Shenton et al. 2001; and also Kempton et al. 2010; Nakamura et al. 2007; van Erp et al. 2015; Chance et al. 2003; Shenton et al. 1992; Yotsutsuji et al. 2003), and in the very early stages of the disease (Rosa et al. 2010; del Re et al., 2016b). Additionally, larger left than right ventricle volume abnormalities have often been reported in schizophrenia (e.g. Shenton et al., 2001; Buchsbaum et al., 1997; Mathalon et al., 2001).
Buchsbaum and colleagues (1997) previously described a larger volume of the left temporal horn in schizotypal personality disorder (SPD, reviewed by Dickey et al. 2002), in the absence of lateral ventricle volume enlargement. Schizotypal personality disorder is a disorder that shares genetic factors with schizophrenia but without the occurrence of overt psychosis (Dickey et al. 2002). It is also an independent risk syndrome for psychosis (Woods et al. 2009). This finding is similar to what we found in CHR. That is, while the larger volume of the left lateral ventricle in CHR was marginally significant, the volume of the left temporal horn was larger in CHR compared to controls with very large effect size. This suggests that large volume of the left temporal horn may be the first abnormality observed prior to abnormalities in volume of the lateral ventricles, indicating a progression that might well predict conversion in those CHR who convert to schizophrenia. Indeed, whereas in CHR, volume of the lateral ventricle is typically not enlarged (Brent et al. 2013; Thermenos et al. 2013), and temporal horn volume has rarely been assessed, progressive increase of lateral ventricular volume is present across post onset schizophrenia (see comprehensive study by Kempton et al. 2010 and Nakamura et al. 2007). This will need to be followed up in future studies (see also below).
There is currently no consensus on the root cause of lateral ventricle enlargement in schizophrenia since its first description on computed tomography (CT) scans in 1976 (Johnstone et al. 1976). While both neurodevelopmental and/or neurodegenerative aspects might contribute to abnormal ventricle volume, the finding of significantly larger VBR and temporal horn volume to brain volume ratio in CHR, coexisting with more limited subcortical regional aberrations, suggests strong neurodevelopmental aspects. These findings further indicate that in addition to global factors, the local environment might have an effect on volumes that share the same anatomical space. Our findings of an almost perfect positive correlation between hippocampus and amygdala volumes in controls that is completely absent in CHR, as well as a very significant inverse correlation between TH and total brain volume in CHR that is not present in controls, as verified by the Fisher r-to-z test, are in accord with our suggestion that abnormal neurodevelopmental factors will affect relationships between adjacent subcortical structures.
4.2 Global Brain Measures
In terms of global measures, the majority of neuroimaging studies in CHR samples have focused on regional gray matter volume loss and cortical thickness (see meta-analyses by Fusar-Poli et al. 2011; and also Bartholomeusz et al. 2016; Cannon et al. 2015; Chung et al. 2015; Mechelli et al. 2011) while a few studies have reported decreased volume of white matter in regional areas (frontal, temporal, limbic, see extensive review by Kubicki et al. 2007). Velakoulis and colleagues (2006) reported a significant decrease of total brain volume in a study of a large number of CHR individuals. Decreased total white matter in CHR compared to controls has also been reported (Ziermans et al. 2012) although Smieskowa and colleagues (2010) reported increased volume of white matter in CHR individuals transitioning to psychosis compared to both healthy controls and non-transitioning CHR. Typically in schizophrenia, gray matter volume deficits are prominent compared to deficits of white matter (Zipursky et al., 1992; Cannon et al., 2015). Most probably the different pattern of deficits found in CHR versus established schizophrenia is reflective of the clinical diversity of the CHR population, with only 20–35% of CHR transitioning to psychosis within two years time (Cannon et al., 2016).
The results of the present investigation further advance previous investigations in that we show for the first time that abnormalities of global brain measures are related to abnormalities of the ventricular system in CHR.
Our finding of higher ventricular volume to white matter volume in CHR is of interest in terms of possible white matter associations with clinical findings. Our results showed CHR had lower scores in verbal fluency associated with decreased white matter volume. Integrity of white matter is essential to language functions (see for example review by Friederici 2009; and Seitz et al., 2016); these functions are significantly affected in CHR (Giuliano et al. 2012; Seidman et al. 2016) and have been related to diffusion tensor abnormalities in CHR (Kubicki et al. 2013).
4.3 Amygdala and Hippocampus
Amygdala and hippocampus are in close anatomical proximity to each other and are also adjacent to the ventricles where the hippocampal gyrus forms the walls of the temporal horn of the lateral ventricles. The amygdala comprises several nuclei in the medial temporal lobe where it shapes the end of the temporal horn (Kiernan et al. 2012) (Figure 1). Lesser volumes of the amygdala and the hippocampus compared to controls have been reported in chronic schizophrenia (e.g. Bogerts et al. 1990; Rossi et al. 1994; Tamminga et al. 2010), in early onset schizophrenia (Frazier et al.2008) and in familial high risk individuals including those < 30 years of age, largely the same age range as CHR samples (Thermenos et al. 2013). Significantly lesser bilateral volume of the amygdala and hippocampus (Seidman et al. 1999) or of the left amygdala (Keshavan et al., 2002) in familial CHR compared to healthy controls has been reported. Nonetheless, in line with the findings of the present study, many studies in CHR have reported hippocampus volumes comparable to those of controls (e.g. McDonald et al. 2006; Velakoulis et al. 2006; Wood et al. 2008). Inconsistent results strongly indicate substantial variability in the composition of the CHR population. Results also suggest that abnormalities in amygdala volume in CHR might preceed abnormalities of hippocampal volume, again indicating a progression that might well predict conversion to schizophrenia.
4.4 Clinical Findings
At trend level, our results indicate that larger lateral ventricle volume corresponds to a higher score on measures of negative symptoms, and that smaller volumes of the hippocampus correspond to higher disorganization symptoms. The correlation between lateral ventricle volume and negative symptoms has long been described in patients affected by chronic schizophrenia (e.g. Kemali et al. 1987; Klausner et al. 1992; Nakamura et al. 2007; Nesvåg et al. 2012; Pearlson et al. 1989; Williams et al. 1985), while in schizophrenia, improvement of disorganization symptoms has been shown to be inversely related to hippocampal volume (Molina et al. 2003). These trend level correlations will need to be replicated in larger CHR populations as they recapitulate findings in schizophrenia.
Volume of the temporal horns in CHR correlated positively with increased perseverative errors measured during the Wisconsin Card Sorting Test. In schizophrenia patients, performance on the test, and especially on the set shifting portion of it, is significantly affected and related to frontal dysfunction (Everett et al. 2001). Giuliano and colleagues (2012) have reported small-moderate effect size for executive function measured on the Wisconsin Card Sorting Test between CHR and controls. Here, number of perseverative errors was not significantly different between CHR and controls, but the correlation between perseveration and temporal horn volume, was large. In line with our findings, several studies have shown a significant correlation between volume of the ventricles and executive function (Johnston et al., 1976; Toulopoulou et al., 2004; Crespo-Facorro et al., 2007). Laywer and colleagues (2006) using a Bayesian regression have also shown that ventricular volume provides more power than diagnosis for visuo-motor speed, vocabulary, and executive function in schizophrenia.
It is thus possible that the temporal horn enlargement observed in this study is an indicator of cognitive impairment (Laywer et al., 2006). In light of neurodevelopmental mechanisms of schizophrenia and considerable previous research (Gilmore et al., 2008), there might be predictive diagnostic value for this finding. This novel result concerning the temporal horn in CHR in relation to executive function will need to be replicated in a larger CHR population in order to determine its significance and predictive ability.
4.5 Summary and Implications for CHR Syndrome
Some limitations to this study are the following. First, although most of the large number of comparisons were controlled by strict Bonferroni corrections, results will need to be confirmed in larger populations. Second, even though we group-matched on sex, there was an insufficient number of female subjects to conduct meaningful comparisons, comparisons that are likely important (see comprehensive review by Goldstein et al. 2013). Although most CHR were medication naïve, seven were receiving anti-psychotic medications at baseline. However, direct comparison of medication-naïve CHR and CHR on psychotropic medication in a MANCOVA analysis indicated a lack of effect of medication on volumes and their interrelationships. However, it is possible that medication effects would be present in a larger population of CHR receiving medication.
In summary, in this study we have shown that local and global volumes are affected in CHR compared with controls, with abnormalities of subcortical brain regions limited to the left hemisphere. Further, we have shown a significantly larger VBR and temporal horn to total brain volume ratio distinguishing CHR from HC. Additionally, we have shown that relationships between brain regions differ significantly between the CHR and healthy control groups. An important tentative conclusion is that the findings reported here may form the biological basis for at least some of the CHR syndrome and its deficits. CHR individuals, irrespective of conversion, are characterized by neurocognitive deficits and lower functioning and quality of life (Cannon et al. 2016; Fusar-Poli et al. 2012; First et al. 2002; Seidman et al. 2016). These deficits might be indexed by abnormalities of the ventriclular system before development of overt psychosis (this study and Johnston et al., 1976; Toulopoulou et al., 2004; Crespo-Facorro et al., 2007).
In the future we plan to expand this research to a larger CHR population to investigate further our findings of the association between the ventricular system and global measures, lateralization of morphometric abnormalities in CHR, as well as other biological measures, including their relationship to clinical findings.
Figure 2. Scattergrams of relative volumes of (a) left and right lateral ventricles (LV); (b) left and right temporal horns (TH); (c) left and right amygdala (AMY); (d) left and right hippocampus (HP). All significant Group differences were found in the left hemisphere such as that (a) the left LV volume of CHR was marginally larger than the left LV of HC; (b) the left TH volume of CHR was significantly bigger than the left TH of HC; (c) the left amygdala volume of CHR was significantly smaller than the left amygdala volume of HC.
No significant CHR-HC differences were found for the for the HP volumes. HC, healthy control; CHR, individual at clinical high risk of developing psychosis; LV, lateral ventricles; TH, temporal horns; AMY, amygdala; HP, hippocampus..
Acknowledgments
We are grateful to the study participants, the investigators, the research assistants and the PIs of the CIDAR study for making this project possible. We also thank the clinical and data management staff from the Boston CIDAR study, including Caitlin Bryant, BS, Ann Cousins, PhD, APRN, Grace Francis, PhD, Molly Franz, BA, Michelle Friedman-Yakoobian, PhD, Lauren Gibson, EdM, Anthony Giuliano, PhD, Andréa Gnong-Granato, MSW, Maria Hiraldo, PhD, Sarah Hornbach, BA, Kristy Klein, PhD, Grace Min, EdM, Corin Pilo-Comtois, LMHC, Janine Rodenhiser-Hill, PhD, Julia Schutt, BA, Shannon Sorenson, BA, Reka Szent-Imry, BA, Alison Thomas, BA, Lynda Tucker, Chelsea Wakeham, BA, and Joanne D. Wojcik, PhD, APRN. Finally, we are grateful for the hard work of many research volunteers, including Zach Feder, Elizabeth Piazza, Julia Reading, Devin Donohoe, Sylvia Khromina, Alexandra Oldershaw, Elena Molokotos, and Olivia Schanz.
Funding
This study was conducted in part by the P50 MH080272 Boston Center for Intervention Development and Applied Research (Boston CIDAR; RWM PI, MES, LJS, RI-M-G, JG, TLP), entitled: “Longitudinal Assessment and Monitoring of Clinical Status and Brain Function in Adolescents and Adults.” This work was also supported in part by R01MH40799 (RWM) and R01MH092380 (TLP) from National Institutes of Health, by the Commonwealth Research Center (SCDMH82101008006, LJS), by a VA Merit Award (MES), and by a Clinical Translational Science Award UL1RR025758 to Harvard University and Beth Israel Deaconess Medical Center from the National Center for Research Resources (LJS). This work was also supported in part by R21 MH109819 (ECdR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Compliance with ethical standards
Conflict of interests
No conflict of interest has been reported by any of the authors of the study.
Informed consent and ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments (1975), and the applicable revisions at the time of the investigation. Informed consent was obtained from all patients for being included in the study.
References
- Addington J, Liu L, Buchy L, Cadenhead KS, Cannon TD, Cornblatt BA, et al. North American Prodrome Longitudinal Study (NAPLS 2) The Journal of Nervous and Mental Disease. 2015;203:328–335. doi: 10.1097/NMD.0000000000000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomeusz CF, Cropley VL, Wannan C, Di Biase M, McGorry PD, Pantelis C. Structural neuroimaging across early-stage psychosis: Aberrations in neurobiological trajectories and implications for the staging model. Aust N Z J Psychiatry. 2016 doi: 10.1177/0004867416670522. [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro Benedicto, Barbadillo Laura, Pelayotera José Maria, Rodriguez-Sanchez José Manuel. Neuropsychological functioning and brain structure in schizophrenia. International Review of Psychiatry. 2007;19(4):325–336. doi: 10.1080/09540260701486647. [DOI] [PubMed] [Google Scholar]
- Berg EA. A simple objective technique for measuring flexibility in thinking. J Gen Psychol. 1948;39:15–22. doi: 10.1080/00221309.1948.9918159. [DOI] [PubMed] [Google Scholar]
- Blokland GA, Mesholam-Gately RI, Toulopoulou T, Del Re EC, Lam M, DeLisi LE, et al. Heritability of neuropsychological measures in schizophrenia and nonpsychiatric populations: A systematic review and meta-analysis. Schizophr Bull. 2016 doi: 10.1093/schbul/sbw146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokland GA, de Zubicaray GI, McMahon KL, Wright MJ. Genetic and environmental influences on neuroimaging phenotypes: a meta-analytical perspective on twin imaging studies. Twin Res Hum Genet. 2012;15(3):351–371. doi: 10.1017/thg.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogerts B, Ashtari M, Degreef G, Alvir JM, Bilder RM, Lieberman JA. Reduced temporal limbic structure volumes on magnetic resonance images in first episode schizophrenia. Psychiatry Res. 1990;35(1):1–13. doi: 10.1016/0925-4927(90)90004-p. [DOI] [PubMed] [Google Scholar]
- Brent BK, Thermenos HW, Keshavan MS, Seidman LJ. Gray matter alterations in schizophrenia high-risk youth and early-onset schizophrenia: a review of structural MRI findings. Child and adolescent psychiatric clinics of North America. 2013;22:689–714. doi: 10.1016/j.chc.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum MS, Yang S, Hazlett E, Siegel BV, Jr, Germans M, Haznedar M, et al. Ventricular volume and asymmetry in schizotypal personality disorder and schizophrenia assessed with magnetic resonance imaging. Schizophr Res. 1997;27(1):45–53. doi: 10.1016/S0920-9964(97)00087-X. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23(2):724–38. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Cannon TD. Brain biomarkers of vulnerability and progression to psychosis. Schizophr Bull. 2016;42(Suppl 1):S127–132. doi: 10.1093/schbul/sbv173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Chung Y, He G, Sun D, Jacobson A, van Erp TG, et al. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry. 2015;77(2):147–157. doi: 10.1016/j.biopsych.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, van Erp TG, Bearden CE, Loewy R, Thompson P, Toga AW, et al. Early and late neurodevelopmental influences in the prodrome to schizophrenia: contributions of genes, environment, and their interactions. Schizophr Bull. 2003;29(4):653–669. doi: 10.1093/oxfordjournals.schbul.a007037. [DOI] [PubMed] [Google Scholar]
- Chance SA, Esiri MM, Crow TJ. Ventricular enlargement in schizophrenia: a primary change in the temporal lobe? Schizophr Res. 2003;62(1–2):123–131. doi: 10.1016/s0920-9964(02)00344-4. [DOI] [PubMed] [Google Scholar]
- Chung Y, Jacobson A, He G, van Erp TGM, McEwen S, Addington J, et al. Prodromal Symptom Severity Predicts Accelerated Gray Matter Reduction and Third Ventricle Expansion Among Clinically High Risk Youth Developing Psychotic Disorders. Molecular neuropsychiatry. 2015;1:13–22. doi: 10.1159/000371887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo-Facorro Benedicto, Barbadillo Laura, Maria Pelayotera José, Rodriguez-Sanchez José Manuel. Neuropsychological functioning and brain structure in schizophrenia. International Review of Psychiatry. 2007;19(4):325–336. doi: 10.1080/09540260701486647. [DOI] [PubMed] [Google Scholar]
- del Re EC, Gao Y, Eckbo R, Petryshen TL, Blokland GA, Seidman LJ, et al. A New MRI Masking Technique Based on Multi-Atlas Brain Segmentation in Controls and Schizophrenia: A Rapid and Viable Alternative to Manual Masking. J Neuroimaging. 2016a;26(1):28–36. doi: 10.1111/jon.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Re EC, Konishi J, Bouix S, Blokland GA, Mesholam-Gately RI, Goldstein J, et al. Enlarged lateral ventricles inversely correlate with reduced corpus callosum central volume in first episode schizophrenia: association with functional measures. Brain Imaging Behav. 2016b;10(4):1264–1273. doi: 10.1007/s11682-015-9493-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Re EC, Spencer KM, Oribe N, Mesholam-Gately RI, Goldstein J, Shenton ME, et al. Clinical high risk and first episode schizophrenia: auditory event-related potentials. Psychiatry Res. 2015;231(2):126–133. doi: 10.1016/j.pscychresns.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisi LE, Goldin LR, Hamovit JR, Maxwell ME, Kurtz D, Gershon ES. A family study of the association of increased ventricular size with schizophrenia. Arch Gen Psychiatry. 1986;43(2):148–153. doi: 10.1001/archpsyc.1986.01800020058007. [DOI] [PubMed] [Google Scholar]
- Dickey CC, McCarley RW, Shenton ME. The brain in schizotypal personality disorder: a review of structural MRI and CT findings. Harv Rev Psychiatry. 2002;10(1):1–15. doi: 10.1080/10673220216201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett J, Lavoie K, Gagnon JF, Gosselin N. Performance of patients with schizophrenia on the Wisconsin Card Sorting Test (WCST) J Psychiatry Neurosci. 2001;26(2):123–130. [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version. New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Frazier JA, Hodge SM, Breeze JL, Giuliano AJ, Terry JE, Moore CM, et al. Diagnostic and sex effects on limbic volumes in early-onset bipolar disorder and schizophrenia. Schizophr Bull. 2008;34:37–46. doi: 10.1093/schbul/sbm120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD. Pathways to language: fiber tracts in the human brain. Trends Cogn Sci. 2009;13(4):175–181. doi: 10.1016/j.tics.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69(3):220–229. doi: 10.1001/archgenpsychiatry.2011.1472. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Borgwardt S, Bechdolf A, Addington J, Riecher-Rossler A, Schultze-Lutter F, et al. The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry. 2013;70(1):107–120. doi: 10.1001/jamapsychiatry.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Borgwardt S, Crescini A, Deste G, Kempton MJ, Lawrie S, et al. Neuroanatomy of vulnerability to psychosis: A voxel-based meta-analysis. Neuroscience & Biobehavioral Reviews. 2011;35:1175–1185. doi: 10.1016/j.neubiorev.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Gaser C, Nenadic I, Buchsbaum BR, Hazlett EA, Buchsbaum MS. Ventricular enlargement in schizophrenia related to volume reduction of the thalamus, striatum, and superior temporal cortex. Am J Psychiatry. 2004;161(1):154–156. doi: 10.1176/appi.ajp.161.1.154. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Smith LC, Wolfe HM, Hertzberg BS, Smith JK, Chescheir NC, Evans DD, Kang C, Hamer RM, Lin W, Gerig G. Prenatal mild ventriculomegaly predicts abnormal development of the neonatal brain. Biol Psychiatry. 2008;64:1069–76. doi: 10.1016/j.biopsych.2008.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano AJ, Li H, Mesholam-Gately RI, Sorenson SM, Woodberry KA, Seidman LJ. Neurocognition in the psychosis risk syndrome: a quantitative and qualitative review. Curr Pharm Des. 2012;18(4):399–415. doi: 10.2174/138161212799316019. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Cherkerzian S, Petryshen TL. Sex differences in the genetic risk for schizophrenia: History of the evidence for sex-specific and sex-dependent effects. Amer J of Med Genetic Part B: Neuropsychiatric Genetics. 2013;162B(7):698–710. doi: 10.1002/ajmg.b.32159. [DOI] [PubMed] [Google Scholar]
- Goncalves AM, de Dantas CR, Banzato CE. Values and DSM-5: looking at the debate on attenuated psychosis syndrome. BMC Med Ethics. 2016;17(1):7. doi: 10.1186/s12910-016-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK. WCST-64: Computer Version3 for Windows-Research Edition. Odessa, Fl: Psychological Assessment Resources; 2000. [Google Scholar]
- Hien D, Matzner FJ, First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-child edition (Version 1.0) New York: Columbia University; 1994. [Google Scholar]
- Hollingshead AB. Two-Factor Index of Social Position. New Haven, CT: Yale University Press; 1975. [Google Scholar]
- Horga G, Bernacer J, Dusi N, Entis J, Chu K, Hazlett EA, et al. Correlations between ventricular enlargement and gray and white matter volumes of cortex, thalamus, striatum, and internal capsule in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2011;261(7):467–476. doi: 10.1007/s00406-011-0202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone EC, Crow TJ, Frith CD, Husband J, Kreel L. Cerebral ventricular size and cognitive impairment in chronic schizophrenia. Lancet (London, England) 1976;2:924–926. doi: 10.1016/s0140-6736(76)90890-4. [DOI] [PubMed] [Google Scholar]
- Jones SH, Thornicroft G, Coffey M, Dunn G. A brief mental health outcome scale-reliability and validity of the Global Assessment of Functioning (GAF) Br J Psychiatry. 1995;166:654–659. doi: 10.1192/bjp.166.5.654. [DOI] [PubMed] [Google Scholar]
- Kemali D, Maj M, Galderisi S, Salvati A, Starace F, Valente A, et al. Clinical, biological, and neuropsychological features associated with lateral ventricular enlargement in DSM-III schizophrenic disorder. Psychiatry Res. 1987;21:137–149. doi: 10.1016/0165-1781(87)90071-0. [DOI] [PubMed] [Google Scholar]
- Kempton MJ, Stahl D, Williams SCR, DeLisi LE. Progressive lateral ventricular enlargement in schizophrenia: A meta-analysis of longitudinal MRI studies. Schizophr Res. 2010;120:54–62. doi: 10.1016/j.schres.2010.03.036. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Dick E, Mankowski I, Harenski K, Montrose DM, Diwadkar V, DeBellis M. Decreased left amygdala and hippocampal volumes in young offspring at risk for schizophrenia. Schizophrenia research. 2002;58(2):173–183. doi: 10.1016/s0920-9964(01)00404-2. [DOI] [PubMed] [Google Scholar]
- Kiernan JA. Anatomy of the temporal lobe. Epilepsy research and treatment. 2012;2012:176157. doi: 10.1155/2012/176157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner JD, Sweeney JA, Deck MD, Haas GL, Kelly AB. Clinical correlates of cerebral ventricular enlargement in schizophrenia. Further evidence for frontal lobe disease. The Journal of nervous and mental disease. 1992;180:407–412. doi: 10.1097/00005053-199207000-00001. [DOI] [PubMed] [Google Scholar]
- Klosterkötter J, Hellmich M, Steinmeyer EM, Schultze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry. 2001;58:158–164. doi: 10.1001/archpsyc.58.2.158. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Fennema-Notestine C, Eyler LT, Panizzon MS, Chen CH, Franz CE, et al. Genetics of brain structure: contributions from the Vietnam Era Twin Study of Aging. Am J Med Genet B Neuropsychiatr Genet. 2013;162B(7):751–761. doi: 10.1002/ajmg.b.32162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Shenton ME, Maciejewski PK, Pelavin PE, Hawley KJ, Ballinger T, et al. Decreased axial diffusivity within language connections: a possible biomarker of schizophrenia risk. Schizophr Res. 2013;148(1–3):67–73. doi: 10.1016/j.schres.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, McCarley R, Westin CF, Park HJ, Maier S, Kikinis R, et al. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007;41(1–2):15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laywer G, Nyman H, Agartz I, Arnborg S, Jönsson EG, Sedvall GC, Hall H. Morphological correlates to cognitive dysfunction in schizophrenia as studied with Bayesian regression. BMC Psychiatry. 2006;10(6):31. doi: 10.1186/1471-244X-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalon DH, Sullivan EV, Lim KO, Pfefferbaum A. Progressive brain volume changes and the clinical course of schizophrenia in men: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:148–57. doi: 10.1001/archpsyc.58.2.148. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JJ, Fischer IA, et al. MRI anatomy of schizophrenia. Biol Psychiatry. 1999;45(9):1099–1119. doi: 10.1016/s0006-3223(99)00018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald C, Marshall N, Sham PC, Bullmore ET, Schulze K, Chapple B, et al. Regional brain morphometry in patients with schizophrenia or bipolar disorder and their unaffected relatives. Am J Psychiatry. 2006;163(3):478–487. doi: 10.1176/appi.ajp.163.3.478. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Riecher-Rössler A, Meisenzahl EM, Tognin S, Wood SJ, Borgwardt SJ, et al. Neuroanatomical abnormalities that predate the onset of psychosis: a multicenter study. Arch Gen Psychiatry. 2011;68:489–495. doi: 10.1001/archgenpsychiatry.2011.42. [DOI] [PubMed] [Google Scholar]
- Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23(3):315–336. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Woods SW, Stein K, Driesen N, Corcoran CM, et al. Symptom assessment in schizophrenic prodromal states. The Psychiatric quarterly. 1999;70:273–287. doi: 10.1023/a:1022034115078. [DOI] [PubMed] [Google Scholar]
- Molina V, Reig S, Sarramea F, Sanz J, Francisco Artaloytia J, Luque R, et al. Anatomical and functional brain variables associated with clozapine response in treatment-resistant schizophrenia. Psychiatry Res. 2003;124:153–161. doi: 10.1016/s0925-4927(03)00108-2. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Salisbury DF, Hirayasu Y, Bouix S, Pohl KM, Yoshida T, et al. Neocortical gray matter volume in first-episode schizophrenia and first-episode affective psychosis: a cross-sectional and longitudinal MRI study. Biological Psychiatry. 2007;62:773–783. doi: 10.1016/j.biopsych.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesvåg R, Bergmann Ø, Rimol LM, Lange EH, Haukvik UK, Hartberg CB, et al. A 5-year follow-up study of brain cortical and subcortical abnormalities in a schizophrenia cohort. Schizophr Res. 2012;142:209–216. doi: 10.1016/j.schres.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Pearlson GD, Kim WS, Kubos KL, Moberg PJ, Jayaram G, Bascom MJ, et al. Ventricle-brain ratio, computed tomographic density, and brain area in 50 schizophrenics. Arch Gen Psychiatry. 1989;46:690–697. doi: 10.1001/archpsyc.1989.01810080020003. [DOI] [PubMed] [Google Scholar]
- Reite M, Reite E, Collins D, Teale P, Rojas DC, Sandberg E. Brain size and brain/intracranial volume ratio in major mental illness. BMC Psychiatry. 2010;10:79. doi: 10.1186/1471-244X-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A, Stratta P, Mancini F, Gallucci M, Mattei P, Core L, et al. Magnetic resonance imaging findings of amygdala-anterior hippocampus shrinkage in male patients with schizophrenia. Psychiatry Res. 1994;52:43–53. doi: 10.1016/0165-1781(94)90119-8. [DOI] [PubMed] [Google Scholar]
- Schultze-Lutter F, Addington J, Ruhrmann S, Klosterkötter J. The Schizophrenia Proneness Instrument, Adult Version (SPI-A) Rome, Italy: Giovanni Fioriti Editore; 2007. [Google Scholar]
- Seidman LJ, Shapiro DI, Stone WS, Woodberry KA, Ronzio A, Cornblatt BA, et al. Association of Neurocognition With Transition to Psychosis: Baseline Functioning in the Second Phase of the North American Prodrome Longitudinal Study. JAMA Psychiatry. 2016 doi: 10.1001/jamapsychiatry.2016.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Faraone SV, Goldstein JM, Goodman JM, Kremen WS, Toomey R, … Tsuang MT. Thalamic and amygdala–hippocampal volume reductions in first-degree relatives of patients with schizophrenia: an MRI-based morphometric analysis. Biological psychiatry. 1999;46(7):941–954. doi: 10.1016/s0006-3223(99)00075-x. [DOI] [PubMed] [Google Scholar]
- Seitz J, Zuo JX, Lyall AE, Makris N, Kikinis Z, Bouix S, … Kubicki M. Tractography Analysis of 5 White Matter Bundles and Their Clinical and Cognitive Correlates in Early-Course Schizophrenia. Schizophr Bull. 2016;42(3):762–71. doi: 10.1093/schbul/sbv171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, et al. Abnormalities of the left temporal lobe and thought disorder in schizophrenia. A quantitative magnetic resonance imaging study. N Engl J Med. 1992;327(9):604–612. doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]
- Smieskova R, Fusar-Poli P, Allen P, Bendfeldt K, Stieglitz RD, Drewe J, et al. Neuroimaging predictors of transition to psychosis--a systematic review and meta-analysis. Neuroscience and Biobehavioral Reviews. 2010;34:1207–1222. doi: 10.1016/j.neubiorev.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A Compendium of Neuropsychological Tests. New York: Oxford University Press; 1991. [Google Scholar]
- Stoll AL. The Psychopharmacology Reference Card, 1989–2009. Belmont, MA: McLean Hospital; 2009. [Google Scholar]
- Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. Am J Psychiatry. 2010;167:1178–1193. doi: 10.1176/appi.ajp.2010.09081187. [DOI] [PubMed] [Google Scholar]
- Thermenos HW, Keshavan MS, Juelich RJ, Molokotos E, Whitfield-Gabrieli S, Brent BK, Seidman LJ. A review of neuroimaging studies of young relatives of persons with schizophrenia: a developmental perspective from schizotaxia to schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2013;162:604–635. doi: 10.1002/ajmg.b.32170. [DOI] [PubMed] [Google Scholar]
- Toulopoulou T, Grech A, Morris RG, Schulze K, McDonald C, Chapple B, Rabe-Hesketh S, Murray RM. The relationship between volumetric brain changes and cognitive function: a family study on schizophrenia. Biol Psychiatry. 2010;56(6):447–53. doi: 10.1016/j.biopsych.2004.06.026. [DOI] [PubMed] [Google Scholar]
- van Erp TGM, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Horn JD, McManus IC. Ventricular enlargement in schizophrenia. A meta-analysis of studies of the ventricle:brain ratio (VBR) Br J Psychiatry. 1992;160:687–697. doi: 10.1192/bjp.160.5.687. [DOI] [PubMed] [Google Scholar]
- Velakoulis D, Wood SJ, Wong MTH, McGorry PD, Yung A, Phillips L, et al. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry. 2006;63:139–149. doi: 10.1001/archpsyc.63.2.139. [DOI] [PubMed] [Google Scholar]
- von Hohenberg CC, Pasternak O, Kubicki M, Ballinger T, Vu MA, Swisher T, et al. White Matter Microstructure in Individuals at Clinical High Risk of Psychosis: A Whole-Brain Diffusion Tensor Imaging Study. Schizophr Bull. 2014;40:895–903. doi: 10.1093/schbul/sbt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. 1999. [Google Scholar]
- Wilkinson GS, Robertson GJ. Wide range achievement test. 4 2006. [Google Scholar]
- Williams AO, Reveley MA, Kolakowska T, Ardern M, Mandelbrote BM. Schizophrenia with good and poor outcome. II: Cerebral ventricular size and its clinical significance. Br J Psychiatry. 1985;146:239–246. doi: 10.1192/bjp.146.3.239. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Pantelis C, Velakoulis D, Yücel M, Fornito A, McGorry PD. Progressive changes in the development toward schizophrenia: studies in subjects at increased symptomatic risk. Schizophr Bull. 2008;34:322–329. doi: 10.1093/schbul/sbm149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Woods SW, Addington J, Cadenhead KS, Cannon TD, Cornblatt BA, Heinssen R, … McGlashan TH. Validity of the Prodromal Risk Syndrome for First Psychosis: Findings From the North American Prodrome Longitudinal Study. Schizophrenia Bulletin. 2009;35(5):894–908. doi: 10.1093/schbul/sbp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsutsuji T, Saitoh O, Suzuki M, Hagino H, Mori K, Takahashi T, et al. Quantification of lateral ventricular subdivisions in schizophrenia by high-resolution three-dimensional magnetic resonance imaging. Psychiatry Res. 2003;122(1):1–12. doi: 10.1016/s0925-4927(02)00105-1. [DOI] [PubMed] [Google Scholar]
- Ziermans TB, Schothorst PF, Schnack HG, Koolschijn PC, Kahn RS, van Engeland H, et al. Progressive structural brain changes during development of psychosis. Schizophr Bull. 2012;38(3):519–530. doi: 10.1093/schbul/sbq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipursky, et al. 1992 [Google Scholar]