Abstract
Introduction
Intravenous preload (delivered before cavernous nerve [CN] injury) of bone marrow–derived mesenchymal stem cells (MSCs) can prevent or decrease postoperative erectile dysfunction (J Sex Med 2015;12:1713–1721). In the present study, the potential therapeutic effects of intravenously administered MSCs on postoperative erectile dysfunction were evaluated in a rat model of CN injury.
Methods
Male Sprague-Dawley rats were randomized into 2 groups after electric CN injury. Intravenous infusion of bone marrow–derived MSCs (1.0 × 106 cells in Dulbecco's modified Eagle's medium 1 mL) or vehicle (Dulbecco's modified Eagle's medium 1 mL) was performed 3 hours after electrocautery-induced CN injury.
Main Outcome Measures
To assess erectile function, we measured intracavernous pressure at 4 weeks after MSC or vehicle infusion. Histologic examinations were performed to investigate neuronal innervation and inhibition of smooth muscle atrophy. Green fluorescent protein–positive bone marrow–derived MSCs were used for cell tracking. To investigate mRNA expression levels of neurotrophins in the major pelvic ganglia (MPGs), quantitative real-time polymerase chain reaction was performed.
Results
The decrease of intracavernous pressure corrected for arterial pressure and area under the curve of intracavernous pressure in the bone marrow–derived MSC group was significantly lower than that in the vehicle group at 4 weeks after infusion (P < .05). Retrograde neuronal tracing indicated that the MSC group had a larger number of FluoroGold-positive neurons in the MPGs compared with the vehicle group. The ratio of smooth muscle to collagen in the MSC group was significantly higher than in the vehicle group. Green fluorescent protein–positive bone marrow–derived MSCs were detected in the MPGs and injured CNs using confocal microscopy, indicating homing of cells to the MPGs and injured CNs. Brain-derived neurotrophic factor and glial cell-derived neurotrophic factor expression levels in the MPGs were significantly higher in the MSC group than in the vehicle group (P < .01).
Conclusion
Intravenous infusion of bone marrow–derived MSCs after CN injury might have therapeutic efficacy in experimental erectile dysfunction.
Matsuda Y, Sasaki M, Kataoka-Sasaki Y, et al. Intravenous Infusion of Bone Marrow–Derived Mesenchymal Stem Cells Reduces Erectile Dysfunction Following Cavernous Nerve Injury in Rats. Sex Med 2018;6:49–57.
Key Words: Mesenchymal Stem Cell, Cavernous Nerve Injury, Erectile Dysfunction
Introduction
Postoperative erectile dysfunction (ED) after radical prostatectomy (RP) can occur due to surgical injury of the cavernous nerves (CNs), which can adversely affect quality of life.1, 2 Although numerous techniques to preserve CNs during RP have been developed using transperineal, retropubic, or laparoscopic approaches and a minimally invasive robotic procedure,3 postoperative ED still occurs in a certain population of patients.4
Takayanagi et al5 reported that intravenous infusion of bone marrow–derived mesenchymal stem cells (MSCs) before crushed CN injury can prevent or decrease postoperative ED in a rat model of CN injury.5 They demonstrated the proof of principle that intravenous infusion of bone marrow–derived MSCs could have therapeutic efficacy for ED from CN injury. The potential mechanisms might include the distribution of preloaded (delivered before CN injury) bone marrow–derived MSCs to the lesion area, where they could provide neuroprotection by secreting neurotrophins and prevent ED after RP.5
In the present study, we tested the hypothesis that intravenous administration of bone marrow–derived MSCs after CN injury would decrease postoperative ED. The MSCs were intravenously infused at 3 hours after CN injury induction. We used an electrocautery-induced CN injury model that might be more clinically relevant compared with mechanical injury models. Evaluation of physiologic changes monitoring intracavernous pressure (ICP) corrected for arterial pressure (AP) was performed to assess the potential inhibition of postoperative ED. Histologic examination including retrograde tracing with FluoroGold (FG) and smooth muscle content also was performed. Quantification of neurotrophic factors using quantitative real-time polymerase chain reaction (RT-PCR) was carried out to assess the potential therapeutic mechanism used by the infused MSCs.
Aim
The aim of this study was to clarify the effect of a cellular therapy to inhibit postoperative ED by systemic administration of MSCs after CN injury in rats.
Methods
All experiments were carried out in accordance with the institutional guidelines of Sapporo Medical University (Sapporo, Japan). The use of animals in this study was approved by the animal care and use committee of Sapporo Medical University.
Preparation of MSCs From Bone Marrow
MSCs were cultured as described in our previous studies.6, 7, 8, 9 Briefly, bone marrow was collected from the femoral bones of adult wild-type and green fluorescence protein (GFP)-expressing rats (W-Tg [CAG-GFP] 184Ys), diluted to 25 mL with Dulbecco's modified Eagle's medium (DMEM; Sigma, St Louis, MO, USA), supplemented with 10% heat-inactivated fetal bovine serum (Thermo Fisher Scientific Inc, Waltham, MA, USA), L-glutamine 2 mmol/L (Sigma), penicillin 100 U/mL, and streptomycin 0.1 mg/mL (Thermo Fisher Scientific), and incubated for 3 days (5% CO2 at 37°C). When the cultures almost reached confluence, the adherent cells were detached using a solution of trypsin and ethylenediaminetetra-acetic acid (Sigma) and sub-cultured on a 150-mm2 tissue culture dish (surface area = 148 cm2; 1030-150; IWAKI, Tokyo, Japan) at 5 × 105 cells/mL in culture medium 14 mL; thus, the plating density was approximately 3.4 × 103/cm2. The surface antigen phenotype of the MSCs was CD45−, CD73+, CD90+, and CD106−.10 The cultured MSCs were used for transplantation after 3 passages. For infusion, the supernatants were washed out and MSCs (1.0 × 106 cells) were suspended in fresh DMEM 1 mL and injected.
Electrocautery-Induced CN Injury
Male Sprague-Dawley rats weighing 250 to 350 g were anesthetized by intraperitoneal injection with pentobarbital (45 mg/kg). After the abdomen was shaved, a lower midline incision was made in the supine position. Then, the prostate gland, major pelvic ganglia (MPGs), and CNs were exposed. Bilateral CNs (approximately 5 mm distal to the MPGs) were injured by electrical coagulation for 0.5 second with a bipolar forceps connected to a generator (Kirwan 26-1500 Bipolar Coagulator; Kirwan Surgical Products, Marshfield, MA, USA). The bipolar coagulation was performed carefully at 50 kHz and 16 W to prevent cutting the CNs and complete elimination of erectile function. The abdominal incision was closed with a suture and the rats were allowed to recover from anesthesia.
Experimental Protocol
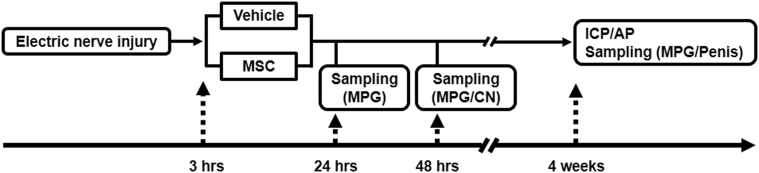
The experimental protocol used is shown in Figure 1. The 1st step was the induction of electrocautery CN injury. 3 hours after electrocautery-induced CN injury, the rats were randomized into MSC and vehicle groups. Rats were intravenously infused with MSCs (1.0 × 106 cells in fresh DMEM 1 mL) or with vehicle (fresh DMEM 1 mL only) through the right external jugular vein. Detection of GFP-expressing bone marrow–derived MSCs (GFP-MSCs) was performed at day 1 and quantitative RT-PCR was performed at day 2. Histologic evaluations were performed after 4 weeks of MSC or vehicle infusion.
Figure 1.
Experimental protocol. Electric CN injury was induced. At 3 hours after CN injury, rats were randomized into 2 groups (vehicle or bone marrow–derived MSC) and intravenously infused with MSCs or vehicle, respectively. Detection of green fluorescent protein–expressing bone marrow–derived MSCs was performed at day 1 and quantitative real-time polymerase chain reaction was performed at day 2. Histologic evaluations were performed after 4 weeks of infusion. AP = arterial pressure; CN = cavernosal nerve; ICP = intracavernous pressure; MPG = major pelvic ganglion; MSC = mesenchymal stem cell.
Evaluation of Erectile Function
Erectile function was evaluated 4 weeks after MSC or vehicle infusion as described previously5 (n = 6 per group). Briefly, under pentobarbital (45 mg/kg) anesthesia, bilateral CNs were re-exposed through a lower abdominal midline incision. A scrotal incision was made and the left penile crus was exposed. To measure ICP, a 23-gauge butterfly needle attached to a PE50 (#427400; Becton Dickinson, Franklin Lakes, NJ, USA) tube with heparinized saline (250 IU/mL) was placed at the left penile crus. AP was monitored through a PE50 tube inserted in the right carotid artery. The exposed CN (approximately 3 mm distal to the MPG) was stimulated with a stainless steel electrode (TF-206-011; Unique Medical Co, Tokyo, Japan) connected to an isolated constant-current electrical stimulation device (20 Hz and 1.5 mA; SEN-3301; Nihon Kohden, Tokyo, Japan) for 60 seconds and the changes in ICP and AP were recorded by a pressure transducer. After 3 minutes, the same procedure was performed for stimulation of the right CN. The averaged values were used for further analysis. In the present study, erectile function was assessed with maximal ICP corrected for AP (ICP/AP) and the area under the curve of ICP plotted for 1 minute of stimulation (ICP-AUC) using LabChart (AD Instruments Inc, Colorado Springs, CO, USA).
Retrograde Tracing Study
FG (H-22845; Thermo Fisher Scientific) injection was performed 1 week before histologic evaluation at 4 weeks after bone marrow–derived MSC or vehicle infusion as previously described.5 Briefly, animals were anesthetized with pentobarbital (45 mg/kg). The skin beside the penis was narrowly opened and FG 4 μL (4.0%) was injected into the penile crus using a 30-gauge Hamilton syringe. At 4 weeks, the MPGs were collected, immediately fixed in 4% paraformaldehyde for approximately 48 hours, and then immersed in phosphate buffer 0.1 mol/L containing 25% sucrose.
Quantification of retrograde-labeled FG-positive cells was performed as previously described.5 Briefly, serial frozen transverse sections (12 μm thick) of the entire MPG were cut with a cryostat. Every 6th section was mounted on 3 aminopropyltriethoxysilane-coated slides (12–20 sections per animal). Images of each MPG were taken under a fluorescent microscope through a wideband ultraviolet filter using a 40× objective (BZ-9000; Keyence, Osaka, Japan). We evaluated all FG-positive neuronal profiles that had nuclei in each MPG section to avoid double-counting of neurons in the sham (n = 5), MSC (n = 6), and vehicle (n = 6) groups. The standardized selection of sections through the entire MPG and counting all FG-positive cells on each section allowed an unbiased assessment of retrograde-labeled cells between the MSC and vehicle groups spanning the entire MPG.
Smooth Muscle-to-Collagen Ratio
The ratio of smooth muscle to collagen in the corpora cavernosa of the penis was evaluated by Masson trichrome staining as described previously with minor modifications11 (n = 6 per group). 4 weeks after MSC or vehicle infusion, rats were perfused transcardially with cold phosphate buffered saline (PBS) followed by 4% paraformaldehyde under deep anesthesia for resection of the penis. The penis was cut transversely from the root with a 3-mm interval, and 4 sections (12 μm thick) per animal were obtained using a cryostat. Color images of sections subjected to Masson trichrome staining were acquired under a microscope (BZ-9000; Keyence). The ratio of smooth muscle to collagen was analyzed for measuring each gross area using ImageJ 1.34j software (National Institutes of Health, Bethesda, MD, USA).
Detection of GFP-Expressing MSCs
48 hours after injection of GFP-MSCs from GFP-expressing rats (n = 4) and non–GFP-MSCs from wild-type Sprague-Dawley rats (n = 4), transcardial perfusion (4% paraformaldehyde) under pentobarbital (45 mg/kg) anesthesia was performed to dissect out the MPGs and CNs. After dissection, MPGs and CNs were fixed in 4% paraformaldehyde for 1 hour at 4°C. Then, frozen embedded MPGs and CNs were cut to 12-μm-thick sections with a cryostat and mounted on glass slides. Slides were washed 3 times in PBS with Tween 20 (0.1%; PBST) and blocked in normal goat serum (10%) and Triton X (0.3%) in PBS at room temperature for 30 minutes. Then, the sections were incubated with the primary antibody (chicken anti-GFP antibody, 1:1,000; ab13970; Abcam, Cambridge, MA, USA) diluted with normal goat serum (5%), Triton X (0.3%), and PBS overnight at 4°C. After 4 washes in PBST, the sections were incubated with the secondary antibody (1:2,000; AF 488-conjugated goat anti-chicken immunoglobulin Y; 150173; Abcam) for 1 hour and counterstained with 4',6-diamidino-2-phenylindole. The sections were examined using a confocal microscope (Ex/Em, 405/488; LSM780 ELYRA System; Carl Zeiss, Oberkochen, Germany).
Real-Time Polymerase Chain Reaction
Quantitative RT-PCR was performed as previously described5 (n = 5 per group). After 24 hours of infusion, animals were anesthetized, and MPGs from each group were collected using a microscope. Total RNA was extracted using an RNeasy Plus Mini kit (#74134; Qiagen, Valencia, CA, USA) according to the manufacturer's instructions.12, 13 The RNA concentration was quantified by determining optical density at 260 nm. RNA (1 μg) was reverse transcribed into cDNA using SuperScript III reverse transcriptase (Qiagen) and oligo-dT. RT-PCR for each sample was performed in triplicate with TaqMan Universal Master Mix II with no UNG (Thermo Fisher Scientific). The following sets of specific primers and TaqMan probes were purchased from Thermo Fisher Scientific: glyceraldehyde-3-phospate dehydrogenase (TaqMan rodent control reagents; Rn01775763-g1) as an endogenous control and brain-derived neurotrophic factor (BDNF; Rn02531967) and glial cell-derived neurotrophic factor (GDNF; Rn00569510) as target genes. The reactions were run on an ABI-StepOne RT-PCR system (Thermo Fisher Scientific) using the 48-well plate format. The cycling conditions included an initial denaturation phase at 95°C for 3 minutes followed by 40 cycles at 95°C for 15 seconds and 55°C for 60 seconds. Relative quantification of target gene expression was performed using the comparative threshold cycle method according to the manufacturer's guidelines.
Statistical Analysis
All statistical analyses were performed using SPSS 18 (SPSS, Inc, Chicago, IL, USA). Comparison was performed using 1-way analysis of variance with the Tukey-Kramer post hoc test or Mann-Whitney U-test.
Results
Erectile Function After Intravenous Infusion of MSCs
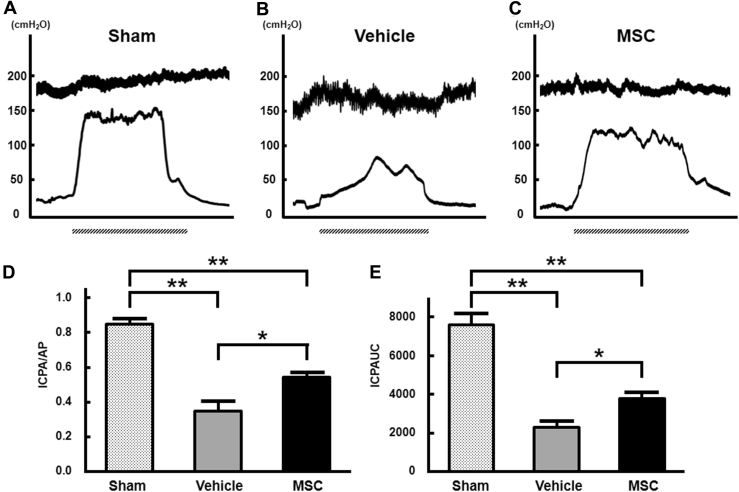
To assess erectile function, a physiologic study was conducted with ICP/AP and ICP-AUC analyses. Representative waveforms of the ICP and AP after 4 weeks of CN injury in the sham group (n = 6; Figure 2A), vehicle group (n = 6; Figure 2B), and bone marrow–derived MSC group (n = 6; Figure 2C) are shown in Figure 2. The ICP/AP in the bone marrow–derived MSC group (0.545 ± 0.021) was higher than that in the vehicle group (0.348 ± 0.057, P < .05; Figure 2D) and the ICP-AUC in the MSC group (3,762 ± 322 cm H2O) was higher than that in the vehicle group (2,294 ± 351 cm H2O) at 4 weeks after transplantation (P < .05; Figure 2E). The ICP/AP was 0.850 ± 0.026 and the ICP-AUC was 7,591 ± 626 cm H2O in the sham group (P < .01).
Figure 2.
ICP response to electrostimulation of cavernous nerves. Panels A to C show representative waveforms of ICP and AP in the sham, vehicle, and MSC groups, respectively. Panels D and E show quantitative analyses of erectile function in the sham (n = 6), vehicle (n = 6), and bone marrow–derived MSC (n = 6) groups as evidenced by ICP/AP and ICPAUC, respectively. ICP/AP and ICPAUC at 4 weeks after cavernosal nerve injury in the bone marrow–derived MSC group were significantly increased compared with ICP/AP after cavernosal nerve injury in the vehicle group (*P < .05). Dashed bars under the x-axis indicate 1 minute of stimulation. *P < .05; **P < .01. AP = arterial pressure; ICP/AP = intracavernous pressure corrected for arterial pressure; ICPAUC = area under the curve for intracavernous pressure; MSC = mesenchymal stem cell.
FG-Positive Cells in MPGs
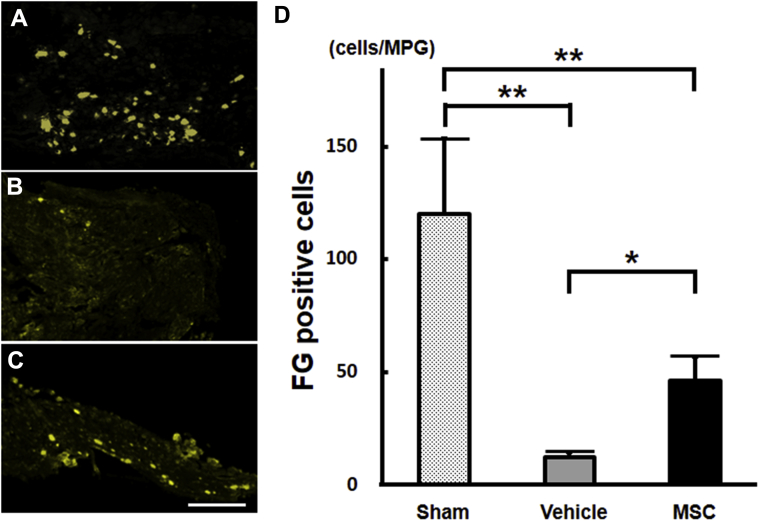
To assess the degree of neural connectivity, a retrograde tracing study was performed using FG. Representative MPG sections, as described in the Methods section, from the sham (Figure 3A), vehicle (Figure 3B), and MSC (Figure 3C) animals are shown. The mean number of FG-positive cells (46.2 ± 10.8 cells/MPG) in the MSC group (n = 6) was compared with that in the vehicle group (12.2 ± 2.5 cells/animal, n = 6; P < .01) at 4 weeks after CN injury (Figure 3D). This comparison indicated that there were 278.7% more FG-positive neurons in bone marrow–derived MSC-treated rats. The mean number of FG-positive cells in the sham group was 119.8 ± 33.2 cells/MPG (n = 5).
Figure 3.
Panels A to C show FG+ neurons in MPGs after infusion in the sham, vehicle, and bone marrow–derived MSC groups, respectively. Panel D shows quantification of FG+ neurons in the sham (n = 5), vehicle (n = 6), and MSC (n = 6) groups. *P < .05; **P < .01. Scale bar = 300 μm. FG = FluoroGold; MPG = major pelvic ganglion; MSC = mesenchymal stem cell.
Smooth Muscle-to-Collagen Ratio
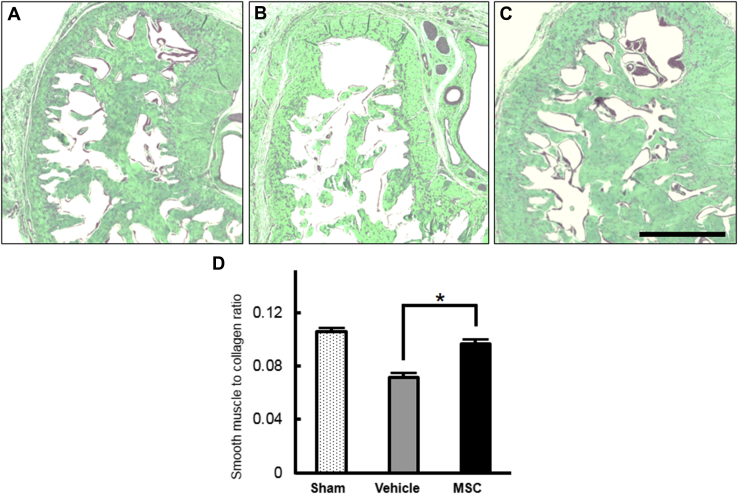
The content of smooth muscle and collagen in the corpus cavernosum was determined by Masson trichrome staining. The average smooth muscle-to-collagen ratios in the sham (n = 6; Figure 4A), vehicle (n = 6; Figure 4B), and bone marrow–derived MSC (n = 6; Figure 4C) groups were 10.6%, 7.2%, and 9.6%, respectively. The smooth muscle-to-collagen ratio in the bone marrow–derived MSC group was significantly higher than that in the vehicle group (Figure 4D).
Figure 4.
Histologic changes in smooth muscle-to-collagen ratios in the corpus cavernosum. Panels A to C show representative light micrographs of Masson trichrome-stained frozen sections of penile mid-shaft specimens in the sham, vehicle, and bone marrow–derived MSC groups, respectively, to assess the composition of smooth muscle (red) and collagen (green). Panel D shows quantification of smooth muscle-to-collagen ratios in penile tissue in the sham (n = 6), vehicle (n = 6), and bone marrow–derived MSC (n = 6) groups. Note that the ratio of smooth muscle to collagen in the MSC group is significantly higher than that in the vehicle group. *P < .05. Scale bar = 500 μm. MSC = mesenchymal stem cell.
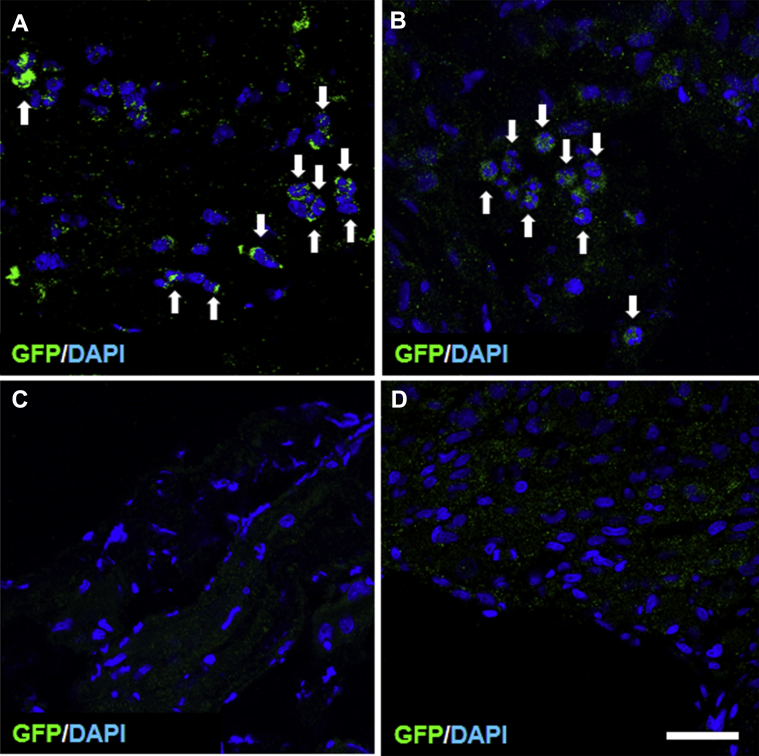
Distribution of Transplanted GFP-Labeled MSCs in MPGs and Injured Lesion Site of CN
GFP-MSCs were easily identified in the MPGs and injured CNs by their green fluorescence (n = 4). Many infused GFP-MSCs were widely distributed. The distribution of the transplanted GFP-MSCs showed a homing effect into the MPGs (Figure 5A) and CN lesions (Figure 5B) after systemic delivery. To confirm whether the bone marrow–derived MSCs showed autofluorescence at wavelengths used to study GFP fluorescence, we examined these sections in animals infused with non–GFP-MSCs derived from wild-type Sprague-Dawley rats (n = 4).9 No GFP-positive cells were observed in these lesions (Figure 5C, D).
Figure 5.
Panels A and B show transverse sections of injured cavernosal nerves and injured major pelvic ganglia, respectively, after intravenous infusion of GFP-MSCs (green; white arrows). Endogenous cells were stained with DAPI (blue). Panels C and D show that after infusion of non–GFP-MSCs from wild-type Sprague-Dawley rats, no GFP+ cells were observed in the cavernosal nerves and major pelvic ganglia, respectively. Scale bar = 150 μm. DAPI = 4',6-diamidino-2-phenylindole; GFP = green fluorescent protein.
Expression of BDNF and GDNF mRNA After MSC Infusion
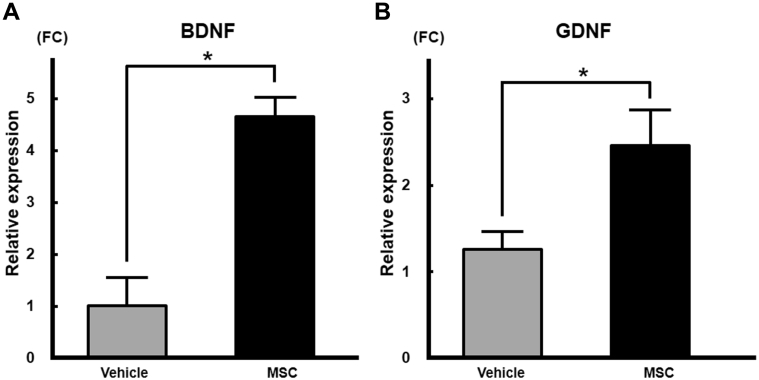
To investigate expression levels of BDNF and GDNF mRNA in MPGs, we performed quantitative RT-PCR. The relative expression levels of BDNF mRNA (Figure 6A) in MPGs were significantly higher (4.6-fold) in the bone marrow–derived MSC group (4.65 ± 0.21, n = 5) compared with the vehicle group (1.02 ± 0.26, n = 5; P < .01). The relative expression level of GDNF mRNA (Figure 6B) in MPGs also was significantly higher (2.0-fold) in the bone marrow–derived MSC group (2.46 ± 0.20) compared with the vehicle group (1.26 ± 0.10; P < .05).
Figure 6.
Panels A and B shows mRNA expression of BDNF and GDNF, respectively, in major pelvic ganglia after intravenous infusion of bone marrow–derived MSCs. The relative expression levels of BDNF and GDNF mRNA in major pelvic ganglia were significantly higher in the bone marrow–derived MSC group (P < .05). BDNF = brain-derived neurotrophic factor; FC = fold change; GDNF = glial cell-derived neurotrophic factor; MSC = mesenchymal stem cell.
Discussion
The primary objective of the present study was to establish a new approach to decrease postoperative ED by intravenous infusion of MSCs after CN injury in rats. Erectile function in MSC-infused animals as assessed using ICP/AP and ICP-AUC analysis was significantly preserved compared with that in the vehicle group. A retrograde tracing study using FG also demonstrated that the number of FG-positive cells in the MPG was larger in the bone marrow–derived MSC group compared with the vehicle group. The ratio of smooth muscle to collagen was significantly higher in the bone marrow–derived MSC infused group. Accumulated GFP-MSCs were found in the MPGs and injured CNs after intravenous infusion of GFP-MSCs. We observed significantly higher expression of BDNF and GDNF mRNA in the MPGs from the bone marrow–derived MSC group by quantitative RT-PCR. These results suggest that intravenous infusion of bone marrow–derived MSCs preserves erectile function through neuroprotection in a rat model of CN injury.
Accumulation of GFP-MSCs at 48 hours after intravenous infusion as observed in the MPG neurons and injured CNs might provide neuroprotection.5 In this study, we observed in increase in BDNF and GDNF expression in the MPGs from the bone marrow–derived MSC group. BDNF enhances recovery of erectile function after CN injury.11 GDNF also restores erectile function after CN injury.14 Recent studies have suggested paracrine mechanisms of injected bone marrow–derived MSCs. Paracrine effects might play an important role in erectile function by which infused MSCs improve ED after CN injury.5, 15, 16 Because we focused on the acute neurotrophic effects by the infused MSCs, we measured GDNF and BDNF mRNA at 48 hours after MSC infusion in this study. If protein levels of these neurotrophins are increased after infused bone marrow–derived MSCs, this could contribute to the inhibition of ED after CN injury.
Primary nerve damage by CN injury was evaluated to count FG-positive neurons in the MPG. We found that the number of FG-positive neurons in the MPG was larger in the bone marrow–derived MSC group compared with the vehicle group, indicating greater neural survival. CN injury also leads to damage of subtunical smooth muscle cells.11, 17 The ratio of smooth muscle content to collagen was maintained in the MSC group, suggesting that preservation of neural survival protected the corpus cavernosum structure by inhibiting smooth muscle from atrophy and collagen from fiber deposition. Collectively, these observations could contribute to the therapeutic efficacy of infused bone marrow–derived MSCs after CN injury.
In this study, we infused bone marrow–derived MSCs at 3 hours after CN injury and found that the bone marrow–derived MSC-infused group displayed less ED after 4 weeks. Erectile function in the bone marrow–derived MSC group was significantly greater than that in the vehicle group as evaluated by physiologic analyses, suggesting that postoperative ED could be inhibited by infusion of bone marrow–derived MSCs after CN injury. Because RP surgery, unlike trauma, can be scheduled, preparation of MSCs before surgery is possible. It might be feasible to perform systemic administration of bone marrow–derived MSCs after RP with a careful pathologic examination in a clinical setting.
In the present study, we developed a new method of electrocautery-induced CN injury using a bipolar forceps. Electrocautery devices are widely used during RP and can damage the CNs at the time of hemostasis and dissection during surgery. Although most experimental animal models of CN injury are mechanically created trauma models such as “crush,” “freezing,” and ligation,18 thermal damage also could be a potential cause of ED.19 We applied electrical coagulation to the bilateral CNs for 0.5 second at 50 kHz and 16 W using a bipolar forceps. This condition provided a stable platform to study the therapeutic effects of infused bone marrow–derived MSCs after CN injury.
Whether intravenous administration of MSCs promotes tumorigenesis or progression of residual prostate cancer remains controversial. Although there are several reports evaluating progression of prostate cancer, most have concluded that infused MSCs have no adverse effects on progression of experimental prostate cancer.20, 21 In addition, it is possible to select patients for infusion of MSCs by combined information from magnetic resonance imaging, biopsy Gleason score, and prostate-specific antigen level to diagnose as low risk. In patients with localized prostate cancer detected during the early era of prostate-specific antigen testing, RP did not significantly increase mortality through at least 12 years of follow-up.22 Although we agree that intravenous infusion of MSCs would be contraindicated for aggressive cancers,23 MSC infusion after RP for low-risk patients would not have negative effects on the prognosis. We also examined tumorigenesis under Good Laboratory Practices standards before our clinical trial using MSCs for stroke (phase III, JMACCT ID JMA-IIA00117 and JMA-IIA00118) and spinal cord injury (phase II, JMACCT ID JMA-IIA00154) and found that our MSCs are safe in patients.
For the detection of expressed factors from the MPG, we observed significantly higher expression of BDNF and GDNF mRNA in the MPG from the MSC group by quantitative RT-PCR (P < 0.01) based on previous studies from our group5, 13, 24, 25, 26 and others.27, 28 However, the mRNA levels are not necessarily equal to the protein levels. It is not known how these molecules are upregulated in protein levels by MSCs (directly or indirectly) and whether GDNF and BDNF are actually involved in the neuroprotection in these rats treated by MSCs. We did not measure protein levels in this study, and future studies will be performed to examine protein levels in the MPGs after intravenous infusion of MSCs in our rat model of CN injury.
In summary, our results indicate that intravenous infusion of bone marrow–derived MSCs after CN injury decreases postoperative ED in a rat model of CN injury. The potential mechanisms involved include distribution of infused bone marrow–derived MSCs to the affected region, where they could release neurotrophins that might provide neuroprotection and prevent neurapraxia.
Statement of authorship
Category 1
-
(a)Conception and Design
- Yohei Matsuda; Masanori Sasaki; Yuko Kataoka-Sasaki; Akio Takayanagi; Ko Kobayashi; Shinichi Oka; Masahito Nakazaki; Naoya Masumori; Jeffery D. Kocsis; Osamu Honmou
-
(b)Acquisition of Data
- Yohei Matsuda; Masanori Sasaki; Yuko Kataoka-Sasaki; Akio Takayanagi; Ko Kobayashi; Shinichi Oka; Masahito Nakazaki; Naoya Masumori; Jeffery D. Kocsis; Osamu Honmou
-
(c)Analysis and Interpretation of Data
- Yohei Matsuda; Masanori Sasaki; Yuko Kataoka-Sasaki; Akio Takayanagi; Ko Kobayashi; Shinichi Oka; Masahito Nakazaki; Naoya Masumori; Jeffery D. Kocsis; Osamu Honmou
Category 2
-
(a)Drafting the Article
- Yohei Matsuda; Masanori Sasaki; Yuko Kataoka-Sasaki; Akio Takayanagi; Ko Kobayashi; Shinichi Oka; Masahito Nakazaki; Naoya Masumori; Jeffery D. Kocsis; Osamu Honmou
-
(b)Revising It for Intellectual Content
- Yohei Matsuda; Masanori Sasaki; Yuko Kataoka-Sasaki; Akio Takayanagi; Ko Kobayashi; Shinichi Oka; Masahito Nakazaki; Naoya Masumori; Jeffery D. Kocsis; Osamu Honmou
Category 3
-
(a)Final Approval of the Completed Article
- Yohei Matsuda; Masanori Sasaki; Yuko Kataoka-Sasaki; Akio Takayanagi; Ko Kobayashi; Shinichi Oka; Masahito Nakazaki; Naoya Masumori; Jeffery D. Kocsis; Osamu Honmou
Acknowledgment
We are grateful to Mr Arai (Fuji Electric Co, Tokyo, Japan). We also thank the National BioResource Project—Rat (http://www.anim.med.kyoto-u.ac.jp/NBR/) for providing this strain of rat (W-Tg [CAG-GFP] 184Ys).
Footnotes
Conflicts of Interest: The authors report no conflicts of interest.
Funding: The Japanese Ministry of Education, Culture, Sports, Science and Technology, the Grant-in-Aid for Scientific Research (grant 25462516 to A.T. and grant 16K11052 to Y.M.), the Rehabilitation Research and Development Service of the Department of Veterans Affairs (grants B7335R and B9260L), the National Multiple Sclerosis Society, and the CT Stem Cell Research Program (12-SCB-Yale-05).
References
- 1.Burnett A.L., Aus G., Canby-Hagino E.D. Erectile function outcome reporting after clinically localized prostate cancer treatment. J Urol. 2007;178:597–601. doi: 10.1016/j.juro.2007.03.140. [DOI] [PubMed] [Google Scholar]
- 2.Namiki S., Ishidoya S., Nakagawa H. The relationships between preoperative sexual desire and quality of life following radical prostatectomy: a 5-year follow-up study. J Sex Med. 2012;9:2448–2456. doi: 10.1111/j.1743-6109.2012.02788.x. [DOI] [PubMed] [Google Scholar]
- 3.Yiou R., De Laet K., Hisano M. Neurophysiological testing to assess penile sensory nerve damage after radical prostatectomy. J Sex Med. 2012;9:2457–2466. doi: 10.1111/j.1743-6109.2012.02793.x. [DOI] [PubMed] [Google Scholar]
- 4.Mulhall J.P., Bivalacqua T.J., Becher E.F. Standard operating procedure for the preservation of erectile function outcomes after radical prostatectomy. J Sex Med. 2013;10:195–203. doi: 10.1111/j.1743-6109.2012.02885.x. [DOI] [PubMed] [Google Scholar]
- 5.Takayanagi A., Sasaki M., Kataoka-Sasaki Y. Intravenous preload of mesenchymal stem cells rescues erectile function in a rat model of cavernous nerve injury. J Sex Med. 2015;12:1713–1721. doi: 10.1111/jsm.12957. [DOI] [PubMed] [Google Scholar]
- 6.Morita T., Sasaki M., Kataoka-Sasaki Y. Intravenous infusion of mesenchymal stem cells promotes functional recovery in a model of chronic spinal cord injury. Neuroscience. 2016;335:221–231. doi: 10.1016/j.neuroscience.2016.08.037. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura H, Sasaki Y, Sasaki M, et al. Elevated brain derived neurotrophic factor (BDNF) levels in plasma but not serum reflect in vivo functional viability of infused mesenchymal stem cells after middle cerebral artery occlusion in rat. J Neurosurg Scihttps://doi.org/10.23736/S0390-5616.17.03989-3. E-pub ahead of print. [DOI] [PubMed]
- 8.Nakazaki M., Sasaki M., Kataoka-Sasaki Y. Intravenous infusion of mesenchymal stem cells inhibits intracranial hemorrhage after recombinant tissue plasminogen activator therapy for transient middle cerebral artery occlusion in rats. J Neurosurg. 2017;127:917–926. doi: 10.3171/2016.8.JNS16240. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki Y., Sasaki M., Kataoka-Sasaki Y. Synergic effects of rehabilitation and intravenous infusion of mesenchymal stem cells after stroke in rats. Phys Ther. 2016;96:1791–1798. doi: 10.2522/ptj.20150504. [DOI] [PubMed] [Google Scholar]
- 10.Kim S., Honmou O., Kato K. Neural differentiation potential of peripheral blood- and bone-marrow-derived precursor cells. Brain Res. 2006;1123:27–33. doi: 10.1016/j.brainres.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piao S., Kim I.G., Lee J.Y. Therapeutic effect of adipose-derived stem cells and BDNF-immobilized PLGA membrane in a rat model of cavernous nerve injury. J Sex Med. 2012;9:1968–1979. doi: 10.1111/j.1743-6109.2012.02760.x. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi K., Kato R., Hisasue S. Animal model for the study of the relationship between lower urinary tract symptoms/bladder outlet obstruction and erectile dysfunction. Int J Urol. 2011;18:710–715. doi: 10.1111/j.1442-2042.2011.02823.x. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita S., Kato R., Kobayashi K. Inhibition of interleukin-6 attenuates erectile dysfunction in a rat model of nerve-sparing radical prostatectomy. J Sex Med. 2011;8:1957–1964. doi: 10.1111/j.1743-6109.2011.02283.x. [DOI] [PubMed] [Google Scholar]
- 14.May F., Buchner A., Schlenker B. Schwann cell-mediated delivery of glial cell line-derived neurotrophic factor restores erectile function after cavernous nerve injury. Int J Urol. 2013;20:344–348. doi: 10.1111/iju.12078. [DOI] [PubMed] [Google Scholar]
- 15.Albersen M., Fandel T.M., Lin G. Injections of adipose tissue-derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med. 2010;7:3331–3340. doi: 10.1111/j.1743-6109.2010.01875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiu X., Lin H., Wang Y. Intracavernous transplantation of bone marrow-derived mesenchymal stem cells restores erectile function of streptozocin-induced diabetic rats. J Sex Med. 2011;8:427–436. doi: 10.1111/j.1743-6109.2010.02118.x. [DOI] [PubMed] [Google Scholar]
- 17.Lue T.F. Erectile dysfunction. N Engl J Med. 2000;342:1802–1813. doi: 10.1056/NEJM200006153422407. [DOI] [PubMed] [Google Scholar]
- 18.Chung E., De Young L., Brock G.B. Investigative models in erectile dysfunction: a state-of-the-art review of current animal models. J Sex Med. 2011;8:3291–3305. doi: 10.1111/j.1743-6109.2011.02505.x. [DOI] [PubMed] [Google Scholar]
- 19.Song L.-J., Zhu J.-Q., Xie M.-K. Electrocautery-induced cavernous nerve injury in rats that mimics radical prostatectomy in humans. BJU Int. 2014;114:133–139. doi: 10.1111/bju.12348. [DOI] [PubMed] [Google Scholar]
- 20.Brennen W.N., Denmeade S.R., Isaacs J.T. Mesenchymal stem cells as a vector for the inflammatory prostate microenvironment. Endocr Relat Cancer. 2013;20:R269–R290. doi: 10.1530/ERC-13-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J.H., Lee H.J., Song Y.S. Stem cell based gene therapy in prostate cancer. Biomed Res Int. 2014;2014:549136. doi: 10.1155/2014/549136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilt T.J., Brawer M.K., Jones K.M. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203–213. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zimmerlin L., Park T.S., Zambidis E.T. Mesenchymal stem cell secretome and regenerative therapy after cancer. Biochimie. 2013;95:2235–2245. doi: 10.1016/j.biochi.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hisasue S., Kato R., Kobayashi K. Alteration of glial cell line-derived neurotrophic factor family receptor alpha-2 mRNA expression and its co-expression with neuronal nitric oxide synthase in pelvic ganglia following unilateral cavernous nerve injury. Int J Urol. 2008;15:82–86. doi: 10.1111/j.1442-2042.2007.01917.x. [DOI] [PubMed] [Google Scholar]
- 25.Hisasue S., Kato R., Suetomi T. Age-related alteration of neurturin receptor GFRa2 and nNOS in pelvic ganglia. Neurobiol Aging. 2006;27:1524–1530. doi: 10.1016/j.neurobiolaging.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 26.Kato R., Kiryu-Seo S., Sato Y. Cavernous nerve injury elicits GAP-43 mRNA expression but not regeneration of injured pelvic ganglion neurons. Brain Res. 2003;986:166–173. doi: 10.1016/s0006-8993(03)03249-9. [DOI] [PubMed] [Google Scholar]
- 27.Albersen M., Berkers J., Dekoninck P. Expression of a distinct set of chemokine receptors in adipose tissue-derived stem cells is responsible for in vitro migration toward chemokines appearing in the major pelvic ganglion following cavernous nerve injury. Sex Med. 2013;1:3–15. doi: 10.1002/sm2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park K.S., Cha S.K., Kim M.J. An alpha3beta4 subunit combination acts as a major functional nicotinic acetylcholine receptor in male rat pelvic ganglion neurons. Pflugers Arch. 2006;452:775–783. doi: 10.1007/s00424-006-0086-1. [DOI] [PubMed] [Google Scholar]