Abstract
Introduction
Short-term data on the effect of low-intensity extracorporeal shockwave therapy (Li-ESWT) on erectile dysfunction (ED) have been inconsistent. The suggested mechanisms of action of Li-ESWT on ED include stimulation of cell proliferation, tissue regeneration, and angiogenesis, which can be processes with a long generation time. Therefore, long-term data on the effect of Li-ESWT on ED are strongly warranted.
Aim
To assess the outcome at 6 and 12 months of linear Li-ESWT on ED from a previously published randomized, double-blinded, sham-controlled trial.
Methods
Subjects with ED (N = 126) who scored lower than 25 points in the erectile function domain of the International Index of Erectile Function (IIEF-EF) were eligible for the study. They were allocated to 1 of 2 groups: 5 weekly sessions of sham treatment (group A) or linear Li-ESWT (group B). After a 4-week break, the 2 groups received active treatment once a week for 5 weeks. At baseline and 6 and 12 months, subjects were evaluated by the IIEF-EF, the Erectile Hardness Scale (EHS), and the Sexual Quality of Life in Men.
Main Outcome Measures
The primary outcome measure was an increase of at least 5 points in the IIEF-EF (ΔIIEF-EF score). The secondary outcome measure was an increase in the EHS score to at least 3 in men with a score no higher than 2 at baseline. Data were analyzed by linear and logistic regressions.
Results
Linear regression of the ΔIIEF-EF score from baseline to 12 months included 95 patients (dropout rate = 25%). Adjusted for the IIEF-EF score at baseline, the difference between groups B and A was −1.30 (95% CI = −4.37 to 1.77, P = .4). The success rate based on the main outcome parameter (ΔIIEF-EF score ≥ 5) was 54% in group A vs 47% in group B (odds ratio = 0.67, P = .28). Improvement based on changes in the EHS score in groups A and B was 34% and 24%, respectively (odds ratio = 0.47, P = .82).
Conclusion
Exposure to 2 cycles of linear Li-ESWT for ED is not superior to 1 cycle at 6- and 12-month follow-ups.
Fojecki GL, Tiessen S, Osther PJS. Effect of Linear Low-Intensity Extracorporeal Shockwave Therapy for Erectile Dysfunction—12-Month Follow-Up of a Randomized, Double-Blinded, Sham-Controlled Study. Sex Med 2018;6:1–7.
Key Words: Erectile Dysfunction, Linear Low-Intensity Extracorporeal Shockwave Therapy
Introduction
Restoration of natural erection is the ultimate goal of erectile dysfunction (ED) therapy.1 The introduction of phosphodiesterase type 5 inhibitors (PDE5is) in the late 1990s completely changed the treatment scenario of ED; however, this treatment modality does not represent a cure. Furthermore, most oral medications require planning of sexual intercourse and are associated with, for example, headache, dizziness, or decrease in blood pressure, which can have serious consequences, especially in combination with nitrate preparations.2, 3
Penile low-intensity extracorporeal shockwave therapy (Li-ESWT) was previously reported to be capable of curing ED.4, 5 The underlying mechanisms of action remain elusive. Potential beneficial effects related to ED include stimulation of cell proliferation, tissue regeneration, and angiogenesis.5 In a diabetic rat model, Li-ESWT was shown to promote regeneration of neuronal nitric oxide synthase–positive nerves, endothelium, and smooth muscle cells.6 The effect seemed to be mediated by the recruitment of endogenous mesenchymal stem cells.6 In addition, Li-ESWT showed potential in promoting angiogenesis in a pelvic neurovascular injury rat model.7
Human clinical trials of Li-ESWT have produced inconsistent results.8, 9, 10, 11, 12, 13, 14, 15, 16 A recent systematic review and meta-analysis concluded that Li-ESWT might be especially suitable for men with mild ED5; yet 1 of the included trials only implicated a potential value for severe ED.11 In addition, in several of the trials, an inconsistency was reported of ED outcome measures after Li-ESWT—International Index of Erectile Function (IIEF) vs Erectile Hardness Scale (EHS)—which are difficult to explain.17 One reason for the conflicting results might be that the potential of the Li-ESWT–induced tissue regeneration and angiogenesis, which are inherently slow biological processes, might not have reached its maximum at time of analysis. Thus, the effect of nerve regeneration and angiogenesis on ED might have considerable interindividual variance, and therefore long-term data on the effect of Li-ESWT on ED could better elucidate statistical intervariance.4, 5, 17
In this article, we report on outcomes at 6- and 12-month follow-up from a previously published randomized, sham-controlled clinical trial on linear Li-ESWT (LLi-ESWT) for ED.15
The objective of the study was to evaluate the effects of LLi-ESWT on ED assessed by the IIEF-EF, EHS, and Sexual Quality of Life in Men (SQoL-M) questionnaires.
The hypothesis of the study was that LLi-ESWT would improve erectile function at 6 and 12 months, possibly through regenerative processes and angiogenesis.
Methods
Details of the trial (NCT02063061), in which short-term data were reported, were previously published.15 Participants underwent a standard assessment that included medical history, physical examination, and blood testing. Subjects with vasculogenic ED were selected based on inclusion and exclusion criteria (Table 1). Use of any erectogenic therapy was restricted during treatment and short-term follow-up. Furthermore, in subjects previously treated for ED, a 4-week washout period was implemented.
Table 1.
Inclusion and exclusion criteria
| Inclusions | Age > 40 y |
| Complaining of ED > 6 mo | |
| In stable relationship (>3 mo) | |
| Exclusions | Surgery or radiotherapy of pelvic region |
| Treatment with anticoagulants (except acetylsalicylic acid 75 mg) | |
| Treatment with antiandrogens | |
| Anatomic penile deformations or penile prosthesis | |
| Total testosterone level < 8 nmol/dl | |
| Serious heart or lung disease | |
| Psychiatric or neurologic disorder | |
| Pregnant partner | |
| IIEF-EF score ≥ 25 |
ED = erectile dysfunction; IIEF-EF = International Index for Erectile Function erectile function domain.
The study was carried out from February through August 2014 at the Department of Urology at the Hospital of Southern Jutland (Sønderborg, Denmark). This department offers primary urologic care to almost 250,000 inhabitants within a 100-km range.
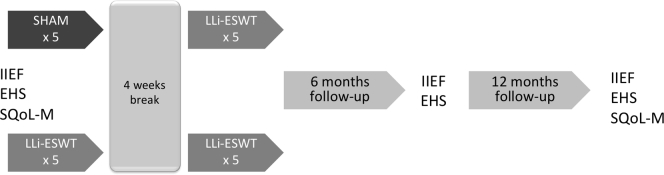
The research secretary generated a random list (www.randomisation.org) with a 1:1 ratio. 126 subjects were allocated to group A or B. The manufacturer of the ESWT device (Richard Wolf GmbH, Knittlingen, Germany) provided 3 identically looking gel pads that were specially designed for this study. In the 1st phase of treatment, a non-penetrable active gel pad was used. Pads were marked A or B, which corresponded to the sham or active group. In the 2nd phase of the trial, all patients received LLi-ESWT using another active gel pad. It had an outer design identical to those used during the 1st phase, allowing for concealed group allocation during the entire study period for investigator and patients. Participants received 5 weekly treatment sessions of LLi-ESWT or sham. After a 4-week break, the 2 groups received LLi-ESWT. We imitated the crossover design from pharmacologic studies. We chose to treat all subjects in the 2nd phase because we expected that treatment would have a prolonged effect. A summary of the study is presented in Figure 1.
Figure 1.
Study design. EHS = Erectile Hardness Scale; IIEF = International Index for Erectile Function; LLi-ESWT = linear low-energy extracorporeal shockwave therapy; SQoL-M = Sexual Quality of Life for Man.
The primary outcome measure was the change in IIEF-EF score from baseline to after 6 or 12 months (ΔIIEF-EF). To enable comparison of our findings with results of other trials,11, 12, 16 changes in IIEF-EF score of at least 5 points were considered clinically relevant. Secondarily, we looked for changes in the EHS score in which an increase to at least 3 indicated improvement. Changes in SQoL-M score from baseline to final follow-up assessment also were recorded. Use of additional pharmacologic treatment for ED was controlled using a national prescription database, which is an online platform that enables physicians to prescribe medicine and monitor whether patients picked up potency enhancers from the pharmacy.
Treatment sessions consisted of 600 shockwave (SW) pulses with an energy flux density (EFD) of 0.09 mJ/mm2 and a frequency of 5 Hz delivered within 15 minutes. SWs were given in 3 areas: 300 impulses were administered to the corpora cavernosa in the upright position and 150 impulses were administered to each penile crus in the lithotomy position. The ESWT device was equipped with a piezoelectric linear therapy source (FBL10; Richard Wolf GmbH). Penetration depth in this device is adjusted by applying different gel pads. In our study we used a 0-mm gel pad that allowed treatment of an organ area 1 cm deep and 5 cm wide. The number of impulses used was chosen based on a previous trial reporting positive outcomes after applying focused Li-ESWT, taking into consideration that a linear probe delivers SWs to a wider area of the penis.9
Outcomes were assessed using questionnaires (IIEF-EF, EHS, and SQoL-M) at baseline and at 6 and 12 months. Before the 1st treatment session, subjects completed questionnaires on a tablet in a separate room and the research nurse assisted on request. During follow-up, patients received an e-mail with a link that allowed them to submit questionnaires from their own electronic devices at home. All questionnaires had an identical form and layout. Answers were collected on a server (www.surveyexact.dk). Subjects who did not have access to a computer (n = 21) received questionnaires sent by mail with a return envelope. The investigator with the assistance of a secretary transferred those results to the server.
The project was registered at www.clinicaltrials.gov (NCT02063061). The study was approved by the regional ethics committee (ID-20120028), the Danish Ministry of Health (2013073909; CIV-13-07-011546), and the Regional Data Protection Agency. The Good Clinical Practice unit at the University of Southern Denmark monitored the complete research process. The investigator acquired written informed consent before the study.
Statistics Including Power of Study
We included 63 patients in each group, which was required to detect a minimum 5-point change in IIEF-EF score as the primary end point. We assumed a type 1 error of 5%, power of 80%, and common SD of 9.3. We expected 10% dropouts. Outcome measurements were summarized at baseline and 6 months and 12 months after completing the treatment protocol. We present our results as the number of subjects, means, SDs, and 95% CIs. The change from baseline to 12 months was compared between groups using linear regression adjusting for baseline measurements. Adherence to the normality assumption was checked by visual inspection of QQ plots. The change from baseline over time was analyzed by a mixed-effects linear regression with random effects given by the patients and interaction with time. A P value less than 0.05 was considered significant. Statistical analysis was performed with STATA 14 (StataCorp, College Station, TX, USA).
Results
From February through May 2014, we screened 184 patients. 126 participants were found eligible for the study and randomized in 2 groups at the ratio of 1:1. Baseline characteristics are presented in Table 2.
Table 2.
Patients' basic characteristics
| Population (N = 126) | Group A (n = 43) | Group B (n = 52) | |
|---|---|---|---|
| Age (y) | 64.9 (10.5) | 64.4 (8.3) | 66.8 (8.2) |
| BMI (kgm2) | 27.4 (3.6) | 27.6 (3.1) | 27.3 (3.8) |
| Total testosterone (nmol/dL) | 14.0 (4.4) | 13.1 (4.1) | 14.4 (4.9) |
| Smoking status | 22 (17.5%) | 12 (27.9%) | 6 (10.9%) |
| Myocardial infarction | 15 (11.9%) | 6 (13.9%) | 5 (11.7%) |
| Hypercholesterolemia | 93/54∗ (73.8%) | 33/20∗ (76.7%) | 35/22∗ (67.3%) |
| Peripheral artery disease | 11 (8.7%) | 2 (4.6%) | 8 (15%) |
| Hypertension | 54 (42.8%) | 15 (34.9%) | 22 (42.3%) |
| Diabetes | 15 (11.9%) | 7 (16.3%) | 3 (5.8%) |
| Treatment with PDE5i | — | 24 (56%) | 30 (58.0%) |
BMI = body mass index; PDE5i = phosphodiesterase type 5 inhibitor.
Diagnosed during screening.
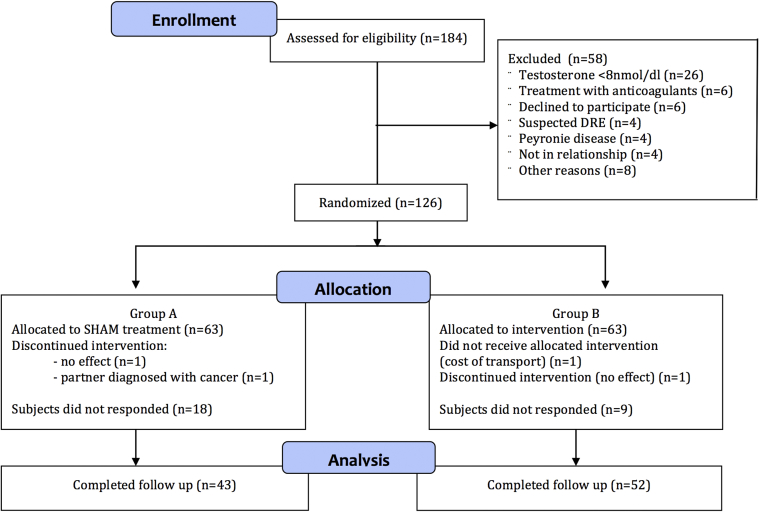
43 subjects (68%) from group A (sham; LLI-ESWT 5 times) and 52 (82%) from group B (LLI-ESWT 10 times) completed the questionnaires 6 and 12 months after treatment. Patients who were found ineligible after randomization (IIEF-EF score > 25, n = 4), those who dropped out during treatment phase (n = 4), and those who did not return questionnaires (n = 23) were excluded from final analysis. A flowchart presenting the inclusion process, according to the Consolidated Standards of Reporting Trials (CONSORT) statement,18 is shown in Figure 2.
Figure 2.
Patient flow diagram. DRE = digital rectal examination; IIEF-EF = International Index for Erectile Function erectile function domain.
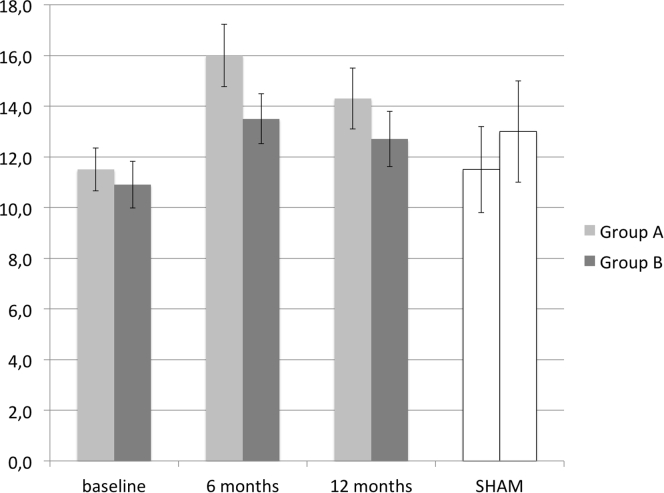
Mean IIEF-EF scores in group B were 10.9 (SD = 7.1) at baseline 13.5 (SD = 9.2) at 6 months, and 12.8 (SD = 9.4) at 12 months. Mean scores in group A were 11.2 (SD = 6.6), 16 (SD = 9.8), and 14.3 (SD = 9.9), respectively. Results at 4-week follow-up after 5 sham treatment sessions (group A) from a previously published article15 are presented in Figure 3. Linear regression of ΔIIEF-EF score from baseline to 12 months included 95 patients. Adjusting for IIEF-EF score at baseline, the difference between groups B and A was −1.30 (CI = −4.37 to 1.77, P = .4). The success rate based on the main outcome parameter (ΔIIEF-EF ≥ 5) was 54% in group A vs 47% in group B (odds ratio = 0.67, P = .28). The improvement based on changes in EHS score in groups A and B was 34% and 24%, respectively (odds ratio = 0.47, P = .82).
Figure 3.
Predicted change of International Index for Erectile Function erectile function domain score (mean and 95% CI) over time. SHAM = effect assessed 4 weeks after 5 simulated treatment sessions.
Analysis of IIEF-EF changes over time showed a significant increase from baseline only in group A (P = .001); however, it should be noted that it did not reach the predefined minimal clinically important difference. The difference between groups was not significant (P = .18).
The values of SQoL-M were 37% in group A and 35% in group B at 1-year follow-up. The change from baseline was −6.1% in group A and −6.6% in group B (P = .82).
Adjusting for use of erectogenic drugs did not result in any statistically significant differences between groups. Mean IIEF-EF scores in group A were 11.4 (95% CI = 7.8–15) at baseline, 10.9 (95% CI = 7–14.8) at 6 months, and 9.5 (95% CI = 6–13) at 12 months. Corresponding results in group B were 12.9 (95% CI = 9.5–16.3), 13.1 (95% CI = 8.4–17.8), and 12.6 (95% CI = 8.4–16.8).
Safety
We did not see any serious adverse events of LLi-ESWT during treatment or follow-up. One patient from group A was diagnosed with Peyronie disease (PD) 6 months after the treatment.
Using a national prescription database to assess the number of patients who were taking medications for ED during follow-up, we found that 24 men (56%) in group A and 30 men (58%) in group B were using a PDE5i and 1 man (2%) in group B was injecting alprostadil.
Discussion
There is an imperative requirement for more long-term data on the effect of Li-ESWT on ED.4, 5, 17 In this report we present 6- and 12-month data from a randomized, sham-controlled trial on LLi-ESWT for ED. In line with our previously published short-term results,15 we did not find a clinically significant effect of LLI-ESWT on ED at our time points. 2 controlled trials on Li-ESWT reporting long-term data on ED have been published.12, 16 Srini et al16 reported on 12-month follow-up after focused Li-ESWT and found significant increases in the IIEF-EF and EHS domains. However, these results are seriously flawed by a very high dropout rate (58% and 42% in sham and active treated groups, respectively) and therefore should be interpreted with caution. Olsen et al,12 who initially reported positive short-term results of focused LI-ESWT in the EHS but not in the IIEF-EF domain, found no significant effects at 6 months for either outcome measure. Thus, the overall long-term clinical effects of Li-ESWT, whether focused or linear SW delivery, seem to be doubtful. Indeed, there is reason to doubt the short-term clinical effects of Li-ESWT, because results from randomized trials have been inconsistent, and only 1 of 3 systematic reviews and meta-analyses on Li-ESWT for ED has documented increases in IIEF-EF4, 5, 19 to what is considered the minimal clinically important difference (ΔIIEF-EF score > 4).20 Whether repeated treatment sessions over a longer period might stimulate cellular and molecular mechanisms, which are believed to be involved in angiogenesis and nerve regeneration translating into significant clinical effects, still needs to be evaluated in controlled trials. Treatment with LLi-ESWT in 2 5-week sessions with a 4-week break (group B) did not result in any positive improvements in IIEF-EF, EHS, or SQoL-M scores. In fact, group A, which received only 5-week treatment, had a better outcome during follow-up, although not reaching the predefined clinically relevant end point (ΔIIEF-EF ≥ 5), suggesting that extending treatment sessions beyond 5 weeks might not achieve superior results.
There could be several reasons for the conflicting results on the effect of Li-ESWT in the literature. Among these are differences in SW technology (piezoelectric, electromagnetic, electrohydraulic), differences in SW delivery (focused, linear), and differences in number of SWs used, which potentially confuse comparison of studies and thus lower the validity of meta-analyses. In our study the number of SWs delivered with the linear probe was calculated based on data from a previous trial using focused ESWT9 with an identical EFD (0.09 mJ/mm2).
There are several reports of positive outcomes of LLi-ESWT for ED.14, 21, 22, 23 3 trials applied an electromagnetic device (Renova, Direx Systems GmbH, Wiesbaden, Germany).21, 22, 23 Bechara et al21 and Reisman et al22 treated their patients with 3,600 SWs and an EFD of 0.09 mJ/mm2 in 4 weeks, and Pelayo-Nieto et al23 applied the same energy level but their treatment protocol consisted of 4 treatments of 5,000 SWs. Motil and Sramkova14 tested the same piezoelectric therapy source used in the present study. Their treatment protocol involved 4 weekly sessions of 4,000 SWs, an EFD of 0.16 mJ/mm2, and a penetration depth of 10 to 15 mm. Positive clinical effects were reported in all cited trials; however, only the study of Bechara et al21 was designed as a randomized controlled trial and the others were open-label trials.14, 22, 23 Considering those encouraging outcomes and absence of serious adverse effects, it seems safe to proceed with planning LLi-ESWT trials using a larger number of SWs. The advantage of linear ESWT is that usage of the linear probe delivers SWs to a wider area of the corpora cavernosa, thus limiting movements of the probe during treatment compared with focused ESWT, which could decrease user dependency.14 However, before clinical trials on ESWT in ED are performed, dose-finding studies defining the right protocol settings for the specific device tested should be mandatory, which to a large extent has been neglected in previous trials including ours. Recommending Li-ESWT as a treatment option for ED needs to be properly scientifically evaluated, because offering the treatment without evidence carries serious ethical issues.24
In general, health care professionals should be aware that ED might be an indicator of endothelial dysfunction that precedes vascular events.25 Therefore, when applicable, lifestyle changes should always be recommended to decrease vascular risk factors, which have been shown to improve IIEF-EF score.26, 27
Limitations
We estimated treatment dose based on the trial of Vardi et al,9 in which a focused transducer was used. In our trial we used a linear probe, and the lack of a dose-finding study can be considered a limitation of our study. Furthermore, owing to our study design, we could assess only short-term effects of sham treatment, because all participants received active treatment in the 2nd phase. Results obtained 4 weeks after 5 simulated treatment sessions (sham) served as a control.
In our short-term data we had a very low dropout rate (3%).15 In the present report, 25% of patients were lost to follow-up, which might have introduced selection bias.28 The difference in dropout rate between groups A (32%) and B (17%) might explain the better outcome in patients who received only 5 treatment sessions (group A), because an uneven dropout can introduce potential bias favoring positive outcomes.29 Furthermore, a larger proportion of men in group B complained of peripheral artery disease, which could be indicative of more severe endothelial dysfunction, and thus they would be expected to have a poorer response. Differences in tobacco usage and prevalence of diabetes between the 2 groups also could have affected the outcome.
We did not apply objective measures for diagnosis of ED etiology. Usage of duplex ultrasonography to confirm vascular insufficiency for patient selection might have resulted in a different outcome.
The gel pad applied in our study was originally developed for treatment of skin wounds. In this configuration, some of the acoustic energy is restricted to the transducer and thus might not be effectively transmitted to the site of need. Different gel pad designs might be more effective, and in future studies a greater penetration depth of SWs could be a target of interest.
Results of previous trials9, 12, 14 suggested that PDE5i responders showed significantly improved erectile function. Our trial was not specifically powered to make distinctions between PDE5i responders and non-responders.
Safety
There has been concern that repeated Li-ESWT treatments can result in fibrosis of the corpora cavernosa and eventually the development of PD.30 In our series 1 patient developed PD with classic plaques and 30° angulation 6 months after treatment. The patient was in group A, meaning that he received ESWT only during the 2nd round of treatment. Men in group B, who received 2 rounds of ESWT, had no long-term complaints, suggesting that the case of PD was coincidental and not related to the SW effects.
Strengths
Using a national prescription database, we could reliably identify patients who were using pharmacologic treatment for ED during the follow-up period. The proportion of men using medication for ED during follow-up was comparable between groups. During the complete trial, data collection and management were monitored by an independent Good Clinical Practice unit, which also could be considered a strength of the study.
Conclusion
This study showed that 2 cycles of LLi-ESWT for ED (10 treatment sessions) were not superior to 1 cycle (5 sessions) at 6- and 12-month follow-up. Targets for future Li-ESWT research could be increasing the penetration depth and the number of SWs. Because the effect of Li-ESWT on ED is questionable, treatment of patients with ED with this therapeutic modality preferably should be confined to controlled clinical trials.
Statement of authorship
Category 1
-
(a)Conception and Design
- Grzegorz Lukasz Fojecki; Stefan Tiessen; Palle Jørn Sloth Osther
-
(b)Acquisition of Data
- Grzegorz Lukasz Fojecki
-
(c)Analysis and Interpretation of Data
- Grzegorz Lukasz Fojecki; Stefan Tiessen; Palle Jørn Sloth Osther
Category 2
-
(a)Drafting the Article
- Grzegorz Lukasz Fojecki
-
(b)Revising It for Intellectual Content
- Grzegorz Lukasz Fojecki; Palle Jørn Sloth Osther
Category 3
-
(a)Final Approval of the Completed Article
- Grzegorz Lukasz Fojecki; Stefan Tiessen; Palle Jørn Sloth Osther
Footnotes
Conflicts of Interest: The authors report no conflicts of interest.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Hatzichristou D. Low-intensity extracorporeal shock waves therapy (LI-ESWT) for the treatment of erectile dysfunction: where do we stand? Eur Urol. 2017;71:234–236. doi: 10.1016/j.eururo.2016.06.037. [DOI] [PubMed] [Google Scholar]
- 2.Lim P.H., Moorthy P., Benton K. The clinical safety of Viagra. Ann N Y Acad Sci. 2002;962:378–388. doi: 10.1111/j.1749-6632.2002.tb04082.x. [DOI] [PubMed] [Google Scholar]
- 3.Giuliano F., Jackson G., Montorsi F. Safety of sildenafil citrate: review of 67 double-blind placebo-controlled trials and the postmarketing safety database. Int J Clin Pract. 2010;64:240–255. doi: 10.1111/j.1742-1241.2009.02254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clavijo R.I., Kohn T.P., Kohn J.R. Effects of low-intensity extracorporeal shockwave therapy on erectile dysfunction: a systematic review and meta-analysis. J Sex Med. 2017;14:27–35. doi: 10.1016/j.jsxm.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Lu Z., Lin G., Reed-Maldonado A. Low-intensity extracorporeal shock wave treatment improves erectile function: a systematic review and meta-analysis. Eur Urol. 2016;71:223–233. doi: 10.1016/j.eururo.2016.05.050. [DOI] [PubMed] [Google Scholar]
- 6.Qiu X., Lin G., Fau-Xin Z. Effects of low-energy shockwave therapy on the erectile function and tissue of a diabetic rat model. J Sex Med. 2013;10:738–746. doi: 10.1111/jsm.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li H., Matheu M.P., Sun F. Low-energy shock wave therapy ameliorates erectile dysfunction in a pelvic neurovascular injuries rat model. Transl Androl Urol. 2016;5:977–979. doi: 10.21037/tau.2016.11.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vardi Y., Appel B., Jacob G. Can low-intensity extracorporeal shockwave therapy improve erectile function? A 6-month follow-up pilot study in patients with organic erectile dysfunction. Eur Urol. 2010;58:243–248. doi: 10.1016/j.eururo.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Vardi Y., Appel B., Kilchevsky A. Does low intensity extracorporeal shock wave therapy have a physiological effect on erectile function? Short-term results of a randomized, double-blind, sham controlled study. J Urol. 2012;187:1769–1775. doi: 10.1016/j.juro.2011.12.117. [DOI] [PubMed] [Google Scholar]
- 10.Gruenwald I., Appel B., Vardi Y. Low-intensity extracorporeal shock wave therapy—a novel effective treatment for erectile dysfunction in severe ED patients who respond poorly to PDE5 inhibitor therapy. J Sex Med. 2012;9:259–264. doi: 10.1111/j.1743-6109.2011.02498.x. [DOI] [PubMed] [Google Scholar]
- 11.Yee C.H., Chan E.S., Hou S.S. Extracorporeal shockwave therapy in the treatment of erectile dysfunction: a prospective, randomized, double-blinded, placebo controlled study. Int J Urol. 2014;21:1041–1045. doi: 10.1111/iju.12506. [DOI] [PubMed] [Google Scholar]
- 12.Olsen A.B., Persiani M., Boie S. Can low-intensity extracorporeal shockwave therapy improve erectile dysfunction? A prospective, randomized, double-blind, placebo-controlled study. Scand J Urol. 2015;49:329–333. doi: 10.3109/21681805.2014.984326. [DOI] [PubMed] [Google Scholar]
- 13.Kitrey N.D., Gruenwald I., Appel B. Penile low intensity shock wave treatment is able to shift pde5i nonresponders to responders: a double-blind, sham controlled study. J Urol. 2016;195:1550–1555. doi: 10.1016/j.juro.2015.12.049. [DOI] [PubMed] [Google Scholar]
- 14.Motil I.K., Sramkova T. Treatment of vasculogenic erectile dysfunction with Piezowave2 device. Application of low intensity shockwaves using novel linear shockwave tissue coverage (LSTC-ED®) technique. A prospective, multicentric, placebo-controlled study. Adv Sex Med. 2016;6:15–18. [Google Scholar]
- 15.Fojecki G.L., Tiessen S., Osther P.J. Effect of low-energy linear shockwave therapy on erectile dysfunction—a double-blinded, sham-controlled, randomized clinical trial. J Sex Med. 2017;14:106–112. doi: 10.1016/j.jsxm.2016.11.307. [DOI] [PubMed] [Google Scholar]
- 16.Srini V.S., Reddy R.K., Shultz T. Low intensity extracorporeal shockwave therapy for erectile dysfunction: a study in an Indian population. Can J Urol. 2015;22:7614–7622. [PubMed] [Google Scholar]
- 17.Fojecki G.L., Tiessen S., Osther P.J. Extracorporeal shock wave therapy (ESWT) in urology: a systematic review of outcome in Peyronie's disease, erectile dysfunction and chronic pelvic pain. World J Urol. 2017;35:1–9. doi: 10.1007/s00345-016-1834-2. [DOI] [PubMed] [Google Scholar]
- 18.Schulz K.F., Altman D.G., Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Med. 2010;7:e1000251. doi: 10.1371/journal.pmed.1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angulo J.C., Arance I., de Las Heras M.M. Efficacy of low-intensity shock wave therapy for erectile dysfunction: a systematic review and meta-analysis. Actas Urol Esp. 2017;41:479–490. doi: 10.1016/j.acuro.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Rosen R.C., Allen K.R., Ni X. Minimal clinically important differences in the erectile function domain of the International Index of Erectile Function scale. Eur Urol. 2011;60:1010–1016. doi: 10.1016/j.eururo.2011.07.053. [DOI] [PubMed] [Google Scholar]
- 21.Bechara A., Casabé A., De Bonis W. Twelve-month efficacy and safety of low-intensity shockwave therapy for erectile dysfunction in patients who do not respond to phosphodiesterase type 5 inhibitors. Sex Med. 2016;4:e225–e232. doi: 10.1016/j.esxm.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reisman Y., Hind A., Varaneckas A. Initial experience with linear focused shockwave treatment for erectile dysfunction: a 6-month follow-up pilot study. Int J Impot Res. 2015;27:108–112. doi: 10.1038/ijir.2014.41. [DOI] [PubMed] [Google Scholar]
- 23.Pelayo-Nieto M., Linden-Castro E., Alias-Melgar A. Linear shock wave therapy in the treatment of erectile dysfunction. Actas Urol Esp. 2015;39:456–459. doi: 10.1016/j.acuro.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Fode M., Lowenstein L., Reisman Y. Low-intensity extracorporeal shockwave therapy in sexual medicine: a questionnaire-based assessment of knowledge, clinical practice patterns, and attitudes in sexual medicine practitioners. Sex Med. 2017;5:e94–e98. doi: 10.1016/j.esxm.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong J.Y., Zhang Y.H., Qin L.Q. Erectile dysfunction and risk of cardiovascular disease: meta-analysis of prospective cohort studies. J Am Coll Cardiol. 2011;58:1378–1385. doi: 10.1016/j.jacc.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 26.Giugliano F., Esposito K., Di Palo C. Erectile dysfunction associates with endothelial dysfunction and raised proinflammatory cytokine levels in obese men. J Endocrinol Invest. 2004;27:665–669. doi: 10.1007/BF03347500. [DOI] [PubMed] [Google Scholar]
- 27.Esposito K., Giugliano F., Maiorino M.I. Dietary factors, Mediterranean diet and erectile dysfunction. J Sex Med. 2010;7:2338–2345. doi: 10.1111/j.1743-6109.2010.01842.x. [DOI] [PubMed] [Google Scholar]
- 28.Dettori J.R. Loss to follow-up. Evid Based Spine Care J. 2011;2(1):7–10. doi: 10.1055/s-0030-1267080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lane P. Handling drop-out in longitudinal clinical trials: a comparison of the LOCF and MMRM approaches. Pharm Stat. 2008;7:93–106. doi: 10.1002/pst.267. [DOI] [PubMed] [Google Scholar]
- 30.Hatzichristodoulou G., Meisner C., Gschwend J.E. Extracorporeal shock wave therapy in Peyronie's disease: results of a placebo-controlled, prospective, randomized, single-blind study. J Sex Med. 2013;10:2815–2821. doi: 10.1111/jsm.12275. [DOI] [PubMed] [Google Scholar]